Abstract
High-density electrocorticography (ECoG) arrays are promising interfaces for high-resolution neural recording from the cortical surface. Commercial options for high-density arrays are limited, and historically tradeoffs must be made between spatial coverage and electrode density. However, thin-film technology is a promising alternative for generating electrode arrays capable of large area coverage and high channel count, with resolution on the order of cortical columns in the functional surface unit of a human gyrus. Here, we evaluate the sensing performance of a high-density thin-film 128-electrode array designed specifically for recording the distributed neural activity of a single human cortical gyrus. We found robust field potential responses throughout the superior temporal gyrus evoked by speech sounds, and clear phonetic feature selectivity at the resolution of 2 mm inter-electrode distance. Decoding accuracy improved with increasing density of electrodes over all three patients tested. Thin-film ECoG has significant potential for high-density neural interface applications at the scale of a human gyrus.
Index terms: electrocorticography, micro-ECoG, brain machine interface, neural device design, spatial resolution, neural decoding, thin film, high density
I. Introduction
Electrocorticography (ECoG) is a method of recording electrical neural signals from the cortical surface. Traditionally, ECoG has been used to localize the seizure focus in patients with refractory epilepsy for surgical planning. Most currently available clinical electrode arrays have one centimeter center-to-center electrode spacing. There are many emerging applications, such as brain-computer interfaces (BCIs), in which sensing resolution on a much finer scale is required. In particular, there is a need for new neural interface devices that are capable of recording neural activity on the order of cortical columns, an important spatial unit of cortical organization.
Micro-electrocorticography (μECoG) refers to ultra high-density recordings with spacing on the order of microns to millimeters. An important feature of μECoG is the potential to record high-density neural activity safely, while also conforming to a specific anatomic region-of-interest. The technology used to make these multi-electrode arrays must necessarily be different than that for hand-made, commercially available electrode arrays.
We have utilized thin-film MEMS technology to fabricate a new μECoG multi-electrode array for human application. The array was designed to leverage the safety and low impedance properties of disk-type electrodes for the cortical surface, while also increasing density. The technological platform is a flexible one, so the same manufacturing technique can be applied to a variety of array configurations of surface area, electrode size, electrode shape, array pattern, electrode number, and density. Here we show high-resolution recording and discrimination of speech sounds using the thin-film array in human subjects as a demonstration of their potential for neural interface applications.
II. Experimental Methods
A. μECoG design and fabrication
The μECoG array consists of 128 electrodes (8×16), 1.2 mm in diameter with a center-to-center spacing of 2mm, designed specifically to span a human cortical gyrus. The thin film arrays were designed and fabricated in a similar manner as detailed in [1]. Polyimide layers provided insulation and structure between each metal layer, making the array a total thickness of approximately 20μm. Thin-film Ti/Au/Ti was sputter-deposited and patterned using contact lithography and wet etching to form conductive traces. Vias were etched in the polyimide using oxygen plasma to provide electrical contact between the electrode metal layer and the trace metal. The electrode metal layer was added by sputter depositing 250nm of Pt over a Ti adhesion layer and patterning the metal using contact lithography and reactive-ion etching. Finally, oxygen plasma was used to etch openings on top of electrodes and connector pads. Each μECoG array was wire-bonded to a silicon interconnect board that was subsequently potted in medical grade epoxy. Electrode impedances at 1kHz were in the range of 800–900 kΩ.
B. Experimental setup
Experimental procedures were approved by the Committee for Human Research at the University of California, San Francisco and the Lawrence Livermore National Laboratory Institutional Review Board. All participants provided written informed consent. Three patients undergoing an awake craniotomy with language mapping prior to resection of epileptic foci volunteered to participate in this research study. In all three patients, the human auditory speech cortex, superior temporal gyrus (STG), was exposed as part of the clinical procedure. The sterilized thin-film μECoG device was placed on the STG, where cortical responses can be evoked in response to speech sounds[2], [3]. A needle electrode in the scalp served as the reference electrode and ground. The subjects listened to 51 pseudo-randomized consonant-vowel (CV) syllables played through free-field speakers. The inter-stimulus interval was 2.25 seconds with a jitter of up to 250ms. Presentation of experimental stimuli was synchronized to neural recordings.
C. Recording and data processing
Signals were acquired at a sampling rate of 3051.8 Hz and were amplified and digitized using a Tucker-Davis Technologies neural recording system. Manual artifact and channel rejection was performed, followed by common average referencing (CAR) [4]. The CAR consists of the mean across 64-channel banks of non-rejected electrodes that are spatially arranged in four rows that span 3.5cm × 1.2cm. The CAR approximates the activity contributed by the original reference, so subtracting the CAR from each channel largely removes contributions of the original reference electrode and electrical artifacts that are often present in groups of 64 channels due to shared connectors and cabling (see Crone et al. 2001 for a discussion of CAR in ECoG recordings).
III. Analytical methods
A. Signal processing
The signal from each electrode channel was filtered into 56 frequency bands using an FFT followed by a Hilbert transform with logarithmically increasing center frequencies (range of CF = 4.07 Hz – 944.83 Hz). The analytic amplitude, also known as the envelope, was calculated for each [5]. The filtered frequencies were then averaged over high gamma (70 – 150 Hz) bands. The natural log of this signal was then z-scored with respect to rest (no-task) epochs within the same recording.
Electrodes were chosen for analysis using a bootstrap t-test to find electrodes that responded significantly to speech over silence. The threshold p-value for inclusion was 0.001 over 10,000 bootstrap iterations.
B. Decoding analysis
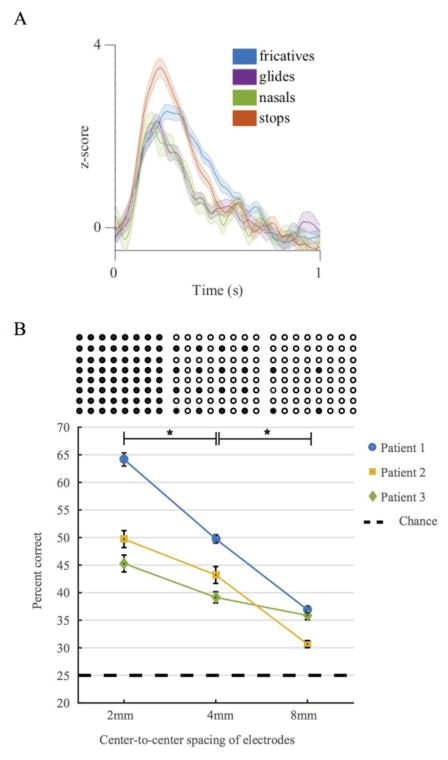
To test decoding performance, the z-scored log of the high gamma analytic amplitude was used as the signal for significantly modulated electrodes (defined above). Classification was done using Linear Discriminant Analysis (LDA) and trained on four phonetic feature categories of sounds for which the STG neurons are known to express selectivity [2]: ‘fricatives’, ’nasals’, ’glides’, and ‘stops.’ A leave-one-out cross validation was employed to generate performance metrics. High gamma responses were taken for ten evenly spaced time points 200ms to 300ms after the onset of the syllable and associated with a phonetic category. The average of the correct predicted categories for each ten-time-point representation of the CV was used as the percent correct for each CV syllable prediction. These were averaged together to get a final metric of ‘percent correct.’
The electrode array was spatially subsampled at 4mm and 8mm inter-electrode spacing for all possible subsample variations. The variation in performance between spatial shifts of the same decimation factor was small, and results are reported as an average over all possible spatial subsamples for 4 and 8mm.
IV. Results
The goal of our study was to functionally validate the sensing performance of a novel thin-film μECoG device for human recordings. We verified that evoked responses can be successfully recorded in the frequency range of interest for neural recordings. We then compared decoding accuracy using all responsive electrodes and decimated subsets of those electrodes.
In contrast to commercially available high-density grids for surface recordings, the thin-film μECoG has not only large surface area but also high density. The surface area and density are highlighted in Fig. 1. The thin-film array covers approximately the width of a human gyrus. It is low profile and extremely flexible to be conformal to the brain’s surface.
Figure 1.
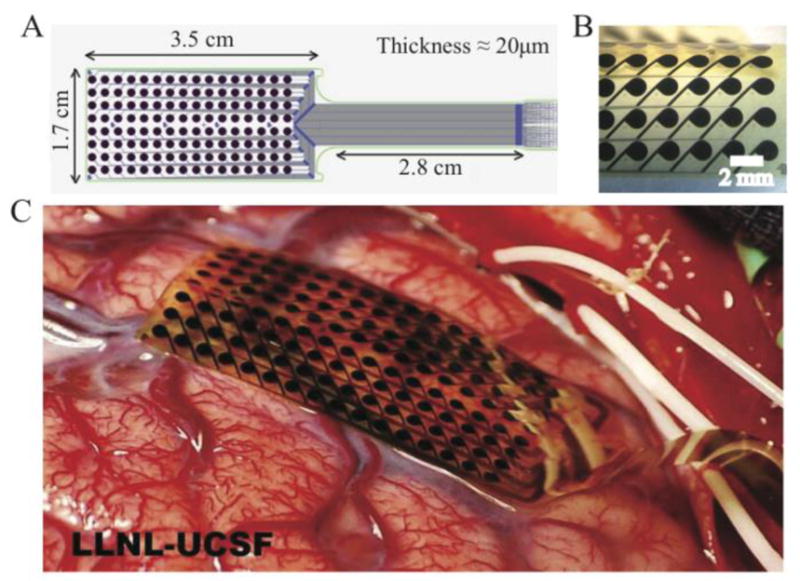
LLNL-UCSF thin-film array for human cortical recordings. (A) Schematic showing array layout. (B) Magnified view of thin-film μECoG array. Center-to-center spacing of electrodes is 2mm. Exposed electrode diameter is 1.2 mm. The array is thin and flexible, and the surface texture is smooth. Electrode positioning is precise to the order of microns. (C) Thin-film μECoG array on the surface of the human brain.
A schematic of the array on the surface of the STG is shown in Fig. 2. Fig. 2B shows a typical single-electrode response to an acoustic stimulus. Robust evoked neural responses are recorded across the array, as shown in Fig. 2C. Several electrodes did not record good responses, possibly due to placement over small cortical blood vessels or faulty electrodes.
Figure 2.
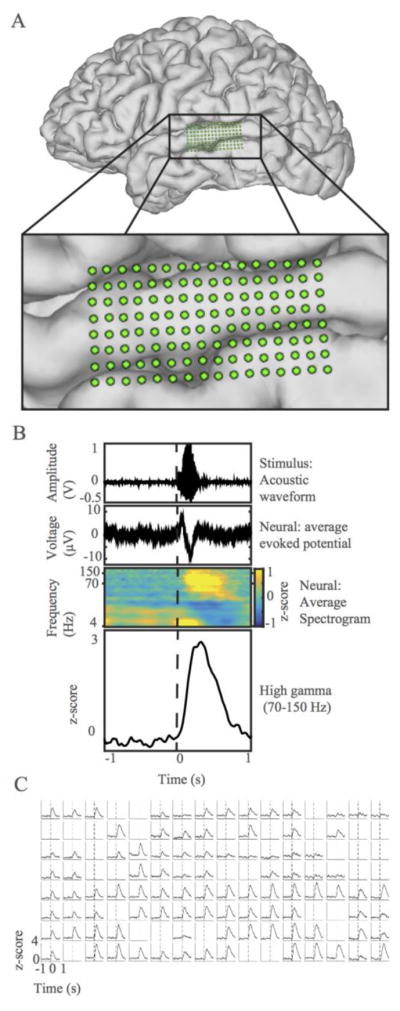
Thin-film array records robust evoked potentials. (A) MRI reconstruction of one subject’s brain with μECoG array overlaid. Inset zooms in on μECoG on STG (B) Single electrode response to acoustic stimulus, time-locked to acoustic stimulus presentation. Dotted line marks stimulus onset. Top: Representative acoustic waveform of consonant-vowel stimulus. Second: Average raw evoked potential of neural signal. Third: Event-related cortical spectrogram. Each frequency band was individually z-scored on silence. Bright yellow areas show time points with relatively high activity in comparison to silence. Fourth: Evoked potential traces in time for z-scored high gamma band. (C) Robust high gamma responses are shown for each electrode site on the array. Each subplot represents the average high gamma response of the electrode at that location to the stimulus. Standard error is represented as a shaded area around each line and is very low. Dotted lines mark stimulus onset. Rejected channels are left blank.
Single electrodes show selectivity for phonetic features. An example is shown in Fig. 3A, where one electrode’s mean responses to the four phonetic categories tested is plotted. This electrode is selective for stops. We wanted to determine if, for the same available surface area of the human STG, a higher density of electrodes increases decoding accuracy. Results of this analysis are shown in Fig. 4. With chance at 25%, we see that neural activity for all center-to-center spacings boosts predictive performs over chance. Beyond this observation, decoding performance increases as spatial density increases.
Figure 3.
Single electrode selectivity and population decoding. A. High gamma phonetic feature selectivity for a representative electrode. (Average evoked potential - solid line; standard error of the mean - shaded). ERPs are color coded by phonetic category. B. Population decoding accuracy increases with higher density electrode sampling within the same surface area. Example electrodes included in each variation of the spacing designated on the x-axis are shown above the graph in agreement with the x-axis labels. Percent of correctly classified phonetic categories is presented as a function of electrode spacing. Chance performance is 25% and is plotted as a dotted line. Mean is plotted with error bars for percent correct at each density, subsampled from the same array. There is a significant increase in decoding accuracy as center-to-center spacing decreases. This is true for all three patients and is designated with a star at the top of the graph between the relevant center-to-center spacings.
V. Discussion
Our goal was to evaluate the human-based physiological recording properties of a high-density ECoG device fabricated using MEMS-based thin-film technology. Here we show that thin-film μECoG can record robust human cortical signals. We showed that there are differences in phoneme feature selectivity at single electrodes. Across all three patients, we see an improvement in decoding accuracy with increased spatial resolution in the same available area.
In the last decade, thin-film MEMS-based electrode arrays have proven their utility in animal ECoG studies [6]–[12]. The technology is appealing because the arrays are highly conformal to the brain’s surface, and the fabrication platform allows for scalable, customizable designs. The micron-level precision enables the fabrication of extremely small electrodes and very dense center-to-center spacing. Some groups have capitalized on the flexibility of the thin-films, making arrays that can be bent to record within a sulcus [7], [12]. A recent study used thin-film devices in humans intra-operatively to record spontaneous surface potentials [13]. However, to our knowledge, this represents the first validation of a thin-film array that demonstrates recording of functional evoked activity from human cortex. Additionally, our array highlights the advantages of maintaining broad spatial coverage while increasing number of electrodes within an anatomical area.
Our combination of large surface area and dense spacing of electrodes allows us to interrogate a functionally related area on a human gyrus at high resolution. Spatial resolution improvements are obviously meaningful, as increased density improves decoding performance. This could be due to boosting of the signal-to-noise ratio from pooled estimates of a signal over electrodes. Alternatively, it could mean that the brain actually represents information at the scale of 2mm or less, information that is not captured with low-density sampling. We have evidence that higher density improves decoding, and with even higher density we may see further decoding benefits.
VI. Conclusions
We have concluded that the thin-film μECoG array is a viable technology for recording neural signals from the human cortical surface. This technology is a good alternative to commercially available high-density options, as the arrays are safe, conformal, and relatively low impedance. The thin-film arrays are capable of recording robust and discrete high gamma signals even at a spacing of 2mm center-to-center. Higher density recordings enhance neural decoding capability within single patients, and the limit to this improvement has not yet been reached.
Acknowledgments
The authors would like to thank M. Leonard, L. Hamilton, E. Edwards, D. Moses, C. Tang, M. Sjerps, B. Dichter, D. Conant, L. Frank, C. Schreiner, D. Lowenstein, and M. Maharbiz for helpful discussion on this work.
This work was supported by grants from the NIH and the National Institute on Deafness and Other Communication Disorders (R01 DC01237904, to EFC, DARPA SUBNETS W911NF-14-2-0043, and a University of California Office of the President Research Grant. Work was performed under the auspices of the U.S. Department of Energy by the Lawrence Livermore National Laboratory under contract number DE-AC52-07NA27344.
References
- 1.Tooker A, Tolosa V, Shah GK, Sheth H, Felix S, Delima T, Pannu S. Optimization of multi-layer metal neural probe design. Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS; 2012. pp. 5995–5998. [DOI] [PubMed] [Google Scholar]
- 2.Mesgarani N, Cheung C, Johnson K, Chang EF. Phonetic feature encoding in human superior temporal gyrus. Science. 2014;343(6174):1006–10. doi: 10.1126/science.1245994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang EF, Rieger JW, Johnson K, Berger MS, Barbaro NM, Knight RT. Emergence of Categorical Speech Representation in the Human Superior Temporal Gyrus. Nat Neurosci. 2010;13(11):1428–1432. doi: 10.1038/nn.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crone N, Boatman D, Gordon B, Hao L. Induced electrocorticographic gamma activity during auditory perception. Clinical Neurophysiology. 2001 doi: 10.1016/s1388-2457(00)00545-9. [DOI] [PubMed] [Google Scholar]
- 5.Edwards E, Soltani M, Kim W, Dalal SS, Nagarajan SS, Berger MS, Knight RT. Comparison of time-frequency responses and the event-related potential to auditory speech stimuli in human cortex. J Neurophysiol. 2009;102:377–386. doi: 10.1152/jn.90954.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubehn B, Bosman C, Oostenveld R, Fries P, Stieglitz T. A MEMS-based flexible multichannel ECoG-electrode array. J Neural Eng. 2009;6:036003. doi: 10.1088/1741-2560/6/3/036003. [DOI] [PubMed] [Google Scholar]
- 7.Matsuo T, Kawasaki K, Osada T, Sawahata H, Suzuki T, Shibata M, Miyakawa N, Nakahara K, Iijima A, Sato N, Kawai K, Saito N, Hasegawa I. Intrasulcal electrocorticography in macaque monkeys with minimally invasive neurosurgical protocols. Front Syst Neurosci. 2011;5:34. doi: 10.3389/fnsys.2011.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukushima M, Saunders RC, Mullarkey M, Doyle AM, Mishkin M, Fujii N. An electrocorticographic electrode array for simultaneous recording from medial, lateral, and intrasulcal surface of the cortex in macaque monkeys. J Neurosci Methods. 2014;233:155–165. doi: 10.1016/j.jneumeth.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ledochowitsch P, Felus RJ, Gibboni RR, Miyakawa A, Bao S, Maharbiz MM. Fabrication and testing of a large area, high density, parylene MEMS microECoG array. 2011 IEEE 24th Int. Conf. Micro Electro Mech. Syst; 2011. pp. 1031–1034. [Google Scholar]
- 10.Ledochowitsch P, Koralek aC, Moses D, Carmena JM, Maharbiz MM. Sub-mm functional decoupling of electrocortical signals through closed-loop BMI learning. Proc Annu Int Conf IEEE Eng Med Biol Soc EMBS. 2013:5622–5625. doi: 10.1109/EMBC.2013.6610825. [DOI] [PubMed] [Google Scholar]
- 11.Thongpang S, Richner TJ, Brodnick SK, Schendel a, Kim J, Wilson Ja, Hippensteel J, Krugner-Higby L, Moran D, Ahmed aS, Neimann D, Sillay K, Williams JC. A Micro-Electrocorticography Platform and Deployment Strategies for Chronic BCI Applications. Clin EEG Neurosci. 2011;42(4):259–265. doi: 10.1177/155005941104200412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viventi J, Kim D-H, Vigeland L, Frechette ES, Blanco JA, Kim Y-S, Avrin AE, Tiruvadi VR, Hwang S-W, Vanleer AC, Wulsin DF, Davis K, Gelber CE, Palmer L, Van der Spiegel J, Wu J, Xiao J, Huang Y, Contreras D, Rogers JA, Litt B. Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo. Nature Neuroscience. 2011;14:1599–1605. doi: 10.1038/nn.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khodagholy D, Gelinas JN, Thesen T, Doyle W, Devinsky O, Malliaras GG, Buzsaki G. NeuroGrid: recording action potentials from the surface of the brain. Nat Neurosci. 2015 Feb;18(2):310–315. doi: 10.1038/nn.3905. [DOI] [PMC free article] [PubMed] [Google Scholar]