Abstract
Background
An increasing body of literature is supporting the safety of minimally invasive pancreaticoduodenectomy compared to open pancreaticoduodenectomy, but there are limited comparative studies between laparoscopic and robotic pancreaticoduodenectomy. The aim of this study was to compare the rate of postoperative 30-day overall complications between laparoscopic and robotic pancreaticoduodenectomy.
Methods
Patients who underwent laparoscopic and robotic pancreaticoduodenectomy were abstracted from the 2014–2015 pancreas-targeted American College of Surgeons National Surgical Quality Improvement Program. A multivariable logistic regression model was developed to determine if the type of minimally invasive approach was associated with 30-day overall complications.
Results
We identified 428 minimally invasive pancreaticoduodenectomy cases, of which 235 (55%) were performed laparoscopically and 193 (45%) robotically. Patients who underwent the robotic approach were more likely to be white compared to those who underwent laparoscopic, and less likely to have pulmonary disease, undergo preoperative radiotherapy, and have vascular and multivisceral resection. On multivariable analysis, we found that the type of minimally invasive approach, whether laparoscopic or robotic, was not associated with overall complications. The predictors of 30-day overall complications were higher body mass index (odds ratio [OR], 1.05; 95% confidence interval [CI], 1.02–1.09), vascular resection (OR, 2.10; 95% CI, 1.23–3.58), and longer operative time (OR, 1.002; 95% CI, 1.001–1.004).
Conclusions
Robotic pancreaticoduodenectomy was associated with a similar 30-day overall complication rate to laparoscopic pancreaticoduodenectomy. Further studies are needed to corroborate these findings and to establish the best approach to perform this complex operation.
Keywords: Minimally invasive, laparoscopic, robotic, pancreaticoduodenectomy, NSQIP
Introduction
The adoption of minimally invasive pancreaticoduodenectomy (MIPD) has been cautious due to the complexity of the operation, the need to perform multiple delicate anastomoses, the concern for suboptimal oncological outcomes, and the high morbidity of the operation, even when performed in an open approach.[1] Recently, multiple studies have suggested that MIPD is safe and feasible, especially in high-volume centers, with inferior outcomes in low-volume hospitals. [2–14] Most of these studies were either a case series of laparoscopic or robotic operations or observational comparative studies between MIPD and open pancreaticoduodenectomy (OPD). [2–14] The majority of these reports showed that MIPD has at least equivalent postoperative and oncological outcomes compared to OPD but few studies have compared laparoscopic (LPD) versus robotic pancreaticoduodenectomy (RPD) to determine which minimally invasive platform may be best to adopt more broadly. [14–19]
Currently the surgery field is divided between centers supporting LPD or RPD without objective evidence of the superiority of one approach over the other. The advocates of LPD propose that laparoscopy is already engrained in surgical training and thus its adoption to another operation such as pancreaticoduoedenectomy (PD) should be easier than adopting the robotic platform, which is not usually used by residents or even fellows.[6] The proponents of RPD suggest that the improved visual perception and ergonomics of the robotic platform allow for easier dissemination of this platform and possibly better outcomes, yet there are no large comparative studies evaluating the impact of the minimally invasive platform utilized on postoperative outcomes.[20]
The primary aim of this study was to compare RPD and LPD with respect to 30-day overall complication rates using the pancreas-targeted American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database.
Materials and Methods
Study Design
We used the 2014–2015 pancreas-targeted ACS-NSQIP database to perform a retrospective study comparing robotic and laparoscopic cases. The NSQIP program collects more than 150 variables from 500 participating hospitals, including preoperative, intraoperative, and 30-day postoperative mortality and morbidity outcomes.[21] The pancreas-targeted component has an additional 26 variables specific to pancreatectomy in comparison to the general NSQIP database and is only available at 120 hospitals. The ACS-NSQIP database is maintained by trained and certified surgical clinical reviewers who collect and enter the data, and the web-based database is audited periodically to ensure the highest quality.[21]
Patient Selection
After merging the pancreas-targeted NSQIP participant user data files with the general database, we selected the following current procedural terminology (CPT) codes: 48150, 48152 (classic Whipple-type procedure with and without pancreatojejunostomy), 48153, and 48154 (pylorus-preserving PD [PPPD] with and without pancreatojejunostomy). The following patients were excluded (Figure 1):
Figure 1.
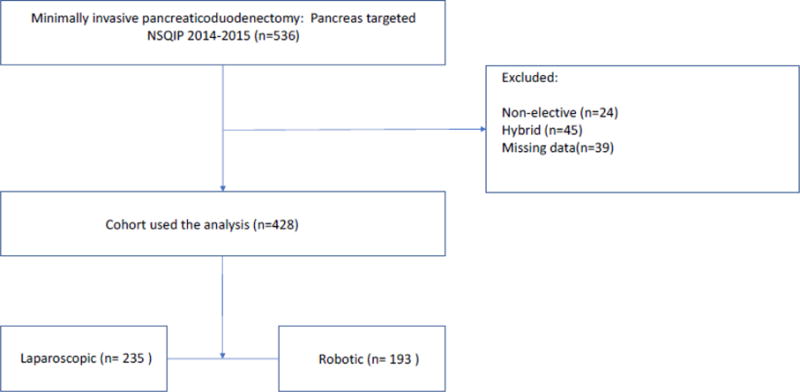
CONSORT diagram
Patients who
Underwent a nonelective procedure.
Underwent a hybrid procedure. The NSQIP defines a hybrid procedure as “a combination of approaches not otherwise specified” making it unclear how these operations were performed.
Had missing data.
The diagnosis group was divided into pancreatitis, T0-T2 malignant, T3-T4 malignant, ≤5 cm benign, and >5 cm benign lesions. We defined multivisceral resection as a colonic, hepatic, and/or intestinal resection performed with MIPD. MIPD was defined as LPD or RPD.
Outcomes
The primary outcome of this study was the 30-day overall complication rate, and the secondary outcome was the conversion rate. A patient who had any of the following was considered to have a major complication: pneumonia, unplanned intubation, pulmonary embolism, on ventilator for >48 hours, deep surgical site infection, organ space surgical site infection, dehiscence, bleeding requiring transfusion within the first 72 hours of surgery start time, deep vein thrombosis/thrombophlebitis, cerebrovascular accident, cardiac arrest, myocardial infarction, sepsis/septic shock, renal failure, or postoperative pancreatic fistula.
Statistical Analysis
We used SPSS version 24 to perform all statistical analysis. Categorical variables were presented as counts and proportions and continuous variables were presented as means with standard deviations or medians. We performed a t test or univariate logistic regression for continuous variables and a chi-squared or Fischer’s exact or univariate logistic regression test, when appropriate, for categorical variables. To determine the association of the type of minimally invasive technique with overall complications, we adjusted for pre- and intraoperative factors using forward multivariable logistic regression. As a secondary analysis, we determined the predictors of conversion by using a forward multivariable logistic regression. Variables with p < 0.25 on univariate analysis were explored in both models and only statistically significant variables were kept in the final models. Two-tailed tests were used with the significance level set at <0.05.
Results
Baseline Characteristics of RPD and LPD
We identified 428 MIPD cases of which 235 (55%) were performed laparoscopically and 193 (45%) robotically (Figure 1). Patients who underwent RPD were more likely to be white compared to those who underwent LPD (88.6% vs 78.7%; p = 0.024), less likely to have dyspnea (2.1% vs 6.0%; p = 0.046), and less likely to undergo preoperative radiotherapy (2.6% vs 9.4%; p = 0.004). In addition, RPD patients were less likely to undergo vascular resection (12.4% vs 23.4%; p = 0.004) and multivisceral resection (4.7% vs 12.3%; p = 0.005), but were more likely to have drains placed (99.0% vs 92.8%; p = 0.002). All other baseline characteristics were similar (Table 1).
Table 1.
Patient, tumor, and operative characteristics
| Laparoscopic PD n (%) | Robotic PD n (%) | p value | |
|---|---|---|---|
| Total patients | 235 (54.9) | 193 (45.1) | |
| Gender | |||
| Female | 106 (45.1) | 92 (47.7) | 0.597 |
| Male | 129 (54.9) | 101 (52.3) | |
| Age (mean, SD years) | 63.4 (11.6) | 63.5 (11.9) | 0.962 |
| Race | 0.024 | ||
| White | 185 (78.7) | 171 (88.6) | |
| African American | 25 (10.6) | 12 (6.2) | |
| Others/unknown | 25 (10.6) | 10 (5.2) | |
| Body mass index (mean, SD kg/m2) | 27.6 (6.6) | 27.8 (5.3) | 0.682 |
| Obstructive jaundice | 0.306 | ||
| No | 143 (60.9) | 108 (56.0) | |
| Yes | 92 (39.1) | 85 (44.0) | |
| Weight loss | 0.376 | ||
| ≤10% loss | 211 (89.8) | 168 (87.0) | |
| >10% loss | 24 (10.2) | 25 (13.0) | |
| ASA class | 0.162 | ||
| Class I | 1 (0.4) | 3 (1.6) | |
| Class II | 56 (23.8) | 42 (21.8) | |
| Class III | 172 (73.2) | 136 (70.5) | |
| Class IV | 6 (2.6) | 12 (6.2) | |
| Diabetes mellitus | 54 (32.0) | 47 (24.4) | 0.739 |
| Hypertension | 113 (48.1) | 103 (53.4) | 0.277 |
| Dyspnea | 14 (6.0) | 4 (2.1) | 0.046 |
| Diagnosis group | 0.912 | ||
| ≤5 cm, benign | 29 (12.3) | 28 (14.5) | |
| >5 cm, benign | 7 (3.0) | 4 (2.1) | |
| T0–T2, malignant | 50 (21.3) | 44 (22.8) | |
| T3–T4, malignant | 138 (58.7) | 109 (56.5) | |
| Pancreatitis | 11 (4.7) | 8 (4.1) | |
| Neoadjuvant chemotherapy | 38 (16.2) | 42 (21.8) | 0.140 |
| Neoadjuvant radiotherapy | 22 (9.4) | 5 (2.6) | 0.004 |
| Surgery type | |||
| Whipple | 181 (77.0) | 144 (74.6) | 0.562 |
| PPPD | 54 (23.0) | 49 (25.4) | |
| Vascular resection | 55 (23.4) | 24 (12.4) | 0.004 |
| Multivisceral resection | 29 (12.3) | 9 (4.7) | 0.005 |
| Intraoperative drains | 218 (92.8) | 191 (99.0) | 0.002 |
Abbreviatio ns: ASA, American Society of Anesthesiologists; PD, pancreaticoduoedenectomy; PPPD, pylorus-preserving pancreaticoduoedenectomy; SD, standard deviation
Perioperative Outcomes of RPD and LPD
An unadjusted comparison showed that RPD was associated with a lower conversion rate compared to LPD (11.4% vs 26.0%; p = 0.004; Table 2). There was no difference in operative time, reoperation rate, length of stay, 30-day mortality, and overall and major complication rates. RPD was associated with increased superficial surgical site infections (9.3% vs 3.8%; p = 0.020) but there was no difference in all other complications, including postoperative pancreatic fistula and delayed gastric emptying.
Table 2.
Unadjusted perioperative outcomes of robotic and laparoscopic cases
| Laparoscopic PD | Robotic PD | p value | |
|---|---|---|---|
| Total patients (%) | 235 (54.9) | 193 (45.1) | |
| Mean (median) | |||
| Operative time (minutes) | 429 (424) | 422 (399) | 0.588 |
| Length of stay (days) | 10.6 (7.0) | 10.7 (8.0) | 0.904 |
| Frequency (%) | |||
| Return to operating room | 18 (7.7) | 13 (6.7) | 0.714 |
| 30-day mortality | 6 (2.6) | 2(1.0) | 0.303a |
| Readmission | 38 (16.2) | 43(22.3) | 0.108 |
| Discharge to nonhome | 20 (8.7) | 20 (10.5) | 0.544 |
| Conversion | 61 (26.0) | 22 (11.4) | <0.001 |
| Overall complication | 115 (48.9) | 106 (54.9) | 0.218 |
| Major complication | 96 (40.9) | 81 (42.0) | 0.815 |
| Superficial SSI | 9 (3.8) | 18 (9.3) | 0.020 |
| Deep SSI | 2 (0.9) | 4 (2.1) | 0.416a |
| Organ space SSI | 30 (12.8) | 28 (14.5) | 0.600 |
| Dehiscence | 5 (2.1) | 1 (0.5) | 0.229a |
| Pneumonia | 8 (3.4) | 2 (1.0) | 0.196a |
| Unplanned intubation | 12 (5.1) | 8 (4.1) | 0.639 |
| Pulmonary embolism | 4 (1.7) | 4 (2.1) | >0.999a |
| Ventilator for >48 hours | 11 (4.7) | 5 (2.6) | 0.257 |
| Acute renal failure | 2 (0.9) | 2 (1.0) | >0.999 |
| Urinary tract infection | 5 (2.1) | 9 (4.7) | 0.142 |
| Cardiac arrest | 5 (2.1) | 1 (0.5) | 0.229a |
| Bleeding requiring transfusion | 44 (18.7) | 27 (14.0) | 0.190 |
| Transfusion day 0 | 34 (14.5) | 23 (11.9) | |
| Transfusion ≥day 1 | 10 (4.3) | 4 (2.0) | |
| DVT/thrombophlebitis | 7 (3.0) | 5 (2.6) | 0.809 |
| Sepsis/septic shock | 23 (9.8) | 18 (9.3) | 0.872 |
| Pancreatic fistula | |||
| None | 189(81.1) | 152 (79.2) | 0.075 |
| Without intervention | 24 (10.3) | 31 (16.1) | |
| With intervention | 20 (8.6) | 9 (4.7) | |
| Delayed gastric emptying | 43 (18.6) | 28 (14.6) | 0.269 |
Fischer’s test
Abbreviations: DVT, deep vein thrombosis; PD, pancreaticoduoedenectomy; SSI, surgical site infection
On multivariable analysis, we found that the type of minimally invasive approach, whether laparoscopic or robotic, was not associated with overall complications (Table 3). The predictors of overall complication were higher body mass index (odds ratio [OR], 1.05; 95% confidence interval [CI], 1.02–1.09), vascular resection (OR, 2.10; 95% CI, 1.23–3.58), and operative time (OR, 1.002; 95% CI, 1.001–1.004).
Table 3.
Multivariable analysis determining predictors of overall complication
| Univariate OR | Univariate p value | Multivariable OR (95% CI) | |
|---|---|---|---|
| Gender | |||
| Male | Ref | ||
| Female | 0.71 | 0.073 | |
| Age (mean, SD years) | 1.01 | 0.370 | |
| Race | |||
| White | Ref | ||
| African American | 1.79 | 0.108 | |
| Others/unknown | 0.81 | 0.563 | |
| Body mass index (mean, SD kg/m2)a | 1.05 | 0.002 | 1.05 (1.01–1.08) |
| Obstructive jaundice | 0.78 | 0.209 | |
| Weight loss >10% | 1.07 | 0.832 | |
| ASA class | |||
| Class I | Ref | ||
| Class II | 0.34 | 0.367 | |
| Class III | 0.33 | 0.338 | |
| Class IV | 1.67 | 0.698 | |
| Diabetes mellitus | 1.36 | 0.184 | |
| Hypertension | 1.54 | 0.027 | |
| Dyspnea | 1.92 | 0.199 | |
| Diagnosis group | |||
| ≤5 cm, benign | Ref | ||
| >5 cm, benign | 0.65 | 0.517 | |
| T0–T2, malignant | 0.93 | 0.821 | |
| T3–T4, malignant | 0.75 | 0.331 | |
| Pancreatitis | 1.34 | 0.592 | |
| Neoadjuvant chemotherapy | 0.92 | 0.745 | |
| Neoadjuvant radiotherapy | 1.01 | 0.981 | |
| Surgery type | |||
| Whipple | Ref | 0.621 | |
| PPPD | 0.89 | ||
| Vascular resection | 2.35 | 0.001 | 2.28 (1.33–3.90) |
| Multivisceral resection | 1.90 | 0.071 | |
| Intraoperative drains | 1.49 | 0.398 | |
| Approach | |||
| Laparoscopic | Ref | ||
| Robotic | 1.27 | 0.218 | |
| Operative timea | 1.003 | 0.001 | 1.002 (1.001–1.004) |
Continuous variable
Abbreviations: ASA, American Society of Anesthesiologists; CI, confidence interval; OR, odds ratio; PPPD, pylorus-preserving pancreaticoduoedenectomy; SD, standard deviation
Conversion of MIPD: Predictors
The overall conversion rate for MIPD was 19.4% (Table 4). The independent predictors of conversion were dyspnea (OR, 4.56; 95% CI, 1.63–12.74), PPPD (OR, 2.42; 95% CI, 1.33–4.39), multivisceral resection (OR, 2.86; 95% CI, 1.32–6.23), and vascular resection (OR, 5.30; 95% CI, 2.97–9.45). After adjusting for these factors, robotic surgery was independently associated with a lower odds of conversion (OR, 0.46; 95% CI, 0.26–0.81).
Table 4.
Multivariable analysis determining predictors of conversion of minimally invasive pancreaticoduodenectomy
| Nonconverted n (%) | Converted n (%) | Univariate p value | Multivariable OR (95% CI) | |
|---|---|---|---|---|
| Total patients | 345 (80.6) | 83 (19.4) | ||
| Gender | ||||
| Female | 166 (48.1) | 32 (38.6) | 0.117 | |
| Male | 179 (51.9) | 51 (61.4) | ||
| Age (mean, SD years) | 63.3 (12.1) | 64.0 (9.9) | 0.641 | |
| Race | 0.810 | |||
| White | 285 (82.6) | 71 (85.5) | ||
| African American | 31 (9.0) | 6 (7.2) | ||
| Others/unknown | 29 (8.4) | 6 (7.2) | ||
| Body mass index (mean, SD kg/m2) | 27.5 (6.0) | 28.4 (6.3) | 0.231 | |
| Obstructive jaundice | 140 (40.6) | 37 (44.6) | 0.507 | |
| Weight loss | 0.565 | |||
| ≤10% loss | 307 (89.0) | 72 (86.7) | ||
| >10% loss | 38 (11.0) | 11 (13.3) | ||
| ASA class | 0.425 | |||
| Class I/II | 85 (24.6) | 17 (20.5) | ||
| Class III/IV | 260 (75.4) | 66 (79.5) | ||
| Diabetes mellitus | 78 (22.6) | 23 (27.7) | 0.326 | |
| Hypertension | 168 (48.7) | 48 (57.8) | 0.135 | |
| Dyspnea | 9 (2.6) | 9 (10.8) | 0.003a | 4.56 (1.63–12.74) |
| Diagnosis group | 0.707 | |||
| ≤5 cm, benign | 47 (13.6) | 10 (12.0) | ||
| >5 cm, benign | 10 (2.9) | 1 (1.2) | ||
| T0–T2, malignant | 74 (21.4) | 20 (24.1) | ||
| T3–T4, malignant | 197 (57.1) | 50 (60.2) | ||
| Pancreatitis | 17 (4.9) | 2 (2.4) | ||
| Neoadjuvant chemotherapy | 68 (19.7) | 12 (14.5) | 0.270 | |
| Neoadjuvant radiotherapy | 22 (6.4) | 5 (6.0) | 0.906 | |
| Surgery type | 0.045 | |||
| Whipple | 269 (78.0) | 56 (67.5) | Ref | |
| PPPD | 76 (22.0) | 27 (32.5) | 2.42 (1.33–4.39) | |
| Multivisceral resection | 22 (6.4) | 16 (19.3) | <0.001 | 2.86 (1.32–6.23) |
| Vascular resection | 43 (12.5) | 36 (43.4) | <0.001 | 5.30 (2.97–9.45) |
| Intraoperative drains | 331 (95.9) | 78 (94.0) | 0.435 | |
| Approach | <0.001 | |||
| Laparoscopic | 174 (50.4) | 61 (73.5) | Ref | |
| Robotic | 171 (49.6) | 22 (26.5) | 0.46 (0.26–0.81) |
Fischer’s test
Abbreviations: ASA, American Society of Anesthesiologists; CI, confidence interval; OR, odds ratio; PPPD, pylorus-preserving pancreaticoduoedenectomy; SD, standard deviation
Discussion
In this large study from a national cohort of patients, we found that robotic surgery was associated with a similar 30-day overall complication rate in an intention-to-treat comparison to LPD. Furthermore, we found that RPD was associated with a lower conversion rate. The predictors of conversion were dyspnea, PPPD, laparoscopic approach, and multivisceral and vascular resection. This report provides evidence that both approaches seem to have similar 30-day outcomes, with RPD having the advantage of a lower conversion rate.
Robotic surgery is thought to be superior to laparoscopy in different disciplines, including urological,[22] gynecological,[23] colorectal,[24] gastric,[25] and distal pancreatic operations,[26] but none have compared RPD to LPD in a large study,[19] as few institutions perform this complex operation using minimally invasive techniques, and the majority have adopted one approach versus the other, preventing single institutional comparison. The robotic platform provides a magnified three-dimensional image, 7 degrees of freedom, and eliminates hand tremor and the fulcrum effect of rigid laparoscopic instruments—allowing for precise suturing, easier tissue handling, better control of large blood vessels, and the ability to work at angles not possible with the laparoscope. Such advantages are important, especially in a complex operation such as PD due to the need to perform intracorporal anastomoses such as the pancreaticojejunostomy and hepaticojejunostomy. In this report, we have shown that there was no difference between the approaches in adjusted overall complications; however we could not determine the severity of complications as described by the Clavien-Dindo classification as the NSQIP database lacks this variable. The robotic platform was superior to laparoscopy with respect to conversion, even after adjusting for important factors associated with conversion like concomitant vascular and multivisceral resection.
Identifying risk factors for conversion may help the surgeon in better selecting patients, reducing conversion, and in better counseling patients on the risks of such an event. In this study we identified dyspnea, PPPD, laparoscopic approach, and multivisceral and vascular resection as important predictors of conversion. The advantage of using a national database is the ability to determine the adjusted odds of conversion for the different risk factors due to the presence of a relatively large number of conversions; however, we could not identify the exact causes for conversion in this dataset, such as bleeding, as that level of detail is not captured in NSQIP.[27] Several factors may be involved in conversion. Vascular and multivisceral resections can pose challenges when completing the operation in a minimally invasive fashion and are usually associated with locally advanced disease, leading to a difficult dissection. Having pulmonary disease has been previously associated with a higher conversion in other minimally invasive operations and it may correlate with poor pulmonary reserve, leading to an inability to tolerate pneumoperitoneum.[28, 29] Interestingly, PPPD was associated with a higher conversion rate, which may be due to the need for a more challenging gastrojejunal anastomosis. The use of the robot may facilitate the performance of the complex reconstructions needed in PD and the control of bleeding due to improved ergonomics, dexterity, and better visualization in comparison to laparoscopy, explaining the lower rate of conversion.
This study was limited by its retrospective design and its relatively small sample size, preventing a more robust analysis such as propensity matching, which would allow us to adjust for all known and clinically important confounders and subsequently let us compare all outcomes between both groups. Instead we chose two important outcomes—30-day overall complication and conversion rates— and determined their independent association with the robotic or laparoscopic surgical approach using logistic regression and adjusting for other important factors. Thus, caution should be taken when comparing both approaches with regard to the other unadjusted outcomes, as the groups were imbalanced at baseline. In addition, the NSQIP database lacks information on surgeon and hospital volumes of pancreatic surgery in general, and MIPD specifically, and therefore we could not determine where the surgeons performing these operations were in their learning curves. This is important, as MIPD is associated with a steep learning curve and requires performing at least 80 cases to reduce operative time and 20 cases to reduce conversion.[30] Finally, we could not assess the oncological outcomes between both operations as the NSQIP database does not provide pathological variables or survival data; therefore such an analysis would be best performed from other cancer-targeted prospective databases.
Conclusions
RPD was associated with a lower rate of conversion than LPD and a similar 30-day overall complication rate. This report suggests that both minimally invasive approaches have similar outcomes with regard to postoperative complications. Prospective evaluation is needed to corroborate these findings. Adopting one approach versus the other should be based on objective data, and more importantly, on the experience of the surgeon with the minimally invasive platform used.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001105. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. They would like to thank Dave Primm for his help in editing this manuscript and Helen Mayo from the UT Southwestern Health Sciences Digital Library and Learning Center for assistance with literature searches. RMM is the Alvin Baldwin, Jr. Chair in surgery. MRP is the Dedman Family Scholar in clinical care. SCW is a UT Southwestern Disease-Oriented Clinical Scholar.
Footnotes
Authors contribution: I. Nassour and R. Minter: study design, data analysis and interpretation, writing initial draft, revising and approving final draft; S. Wang, P. Polanco, M. Augustine, J. Mansour, M. Porembka, A. Yopp M. Choti: data interpretation, revising and approving final draft
The authors have no conflicts of interest to declare.
References
- 1.Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10–15. doi: 10.1097/01.sla.0000217673.04165.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dokmak S, Ftériche FS, Aussilhou B, Bensafta Y, Lévy P, Ruszniewski P, Belghiti J, Sauvanet A. Laparoscopic pancreaticoduodenectomy should not be routine for resection of periampullary tumors. J Am Coll Surg. 2015;220:831–838. doi: 10.1016/j.jamcollsurg.2014.12.052. [DOI] [PubMed] [Google Scholar]
- 3.Song KB, Kim SC, Hwang DW, Lee JH, Lee DJ, Lee JW, Park KM, Lee YJ. Matched case-control analysis comparing laparoscopic and open pylorus-preserving pancreaticoduodenectomy in patients with periampullary tumors. Ann Surg. 2015;262:146–155. doi: 10.1097/SLA.0000000000001079. [DOI] [PubMed] [Google Scholar]
- 4.Tran TB, Dua MM, Worhunsky DJ, Poultsides GA, Norton JA, Visser BC. The first decade of laparoscopic pancreaticoduodenectomy in the United States: costs and outcomes using the nationwide inpatient sample. Surg Endosc. 2016;30:1778–1783. doi: 10.1007/s00464-015-4444-y. [DOI] [PubMed] [Google Scholar]
- 5.Adam MA, Choudhury K, Dinan MA, Reed SD, Scheri RP, Blazer DG, 3rd, Roman SA, Sosa JA. Minimally invasive versus open pancreaticoduodenectomy for cancer: practice patterns and short-term outcomes among 7061 patients. Ann Surg. 2015;262:372–377. doi: 10.1097/SLA.0000000000001055. [DOI] [PubMed] [Google Scholar]
- 6.Croome KP, Farnell MB, Que FG, Reid-Lombardo KM, Truty MJ, Nagorney DM, Kendrick ML. Total laparoscopic pancreaticoduodenectomy for pancreatic ductal adenocarcinoma. Ann Surg. 2014;260:633–640. doi: 10.1097/SLA.0000000000000937. [DOI] [PubMed] [Google Scholar]
- 7.Sharpe SM, Talamonti MS, Wang CE, Prinz RA, Roggin KK, Bentrem DJ, Winchester DJ, Marsh RD, Stocker SJ, Baker MS. Early national experience with laparoscopic pancreaticoduodenectomy for ductal adenocarcinoma: a comparison of laparoscopic pancreaticoduodenectomy and open pancreaticoduodenectomy from the National Cancer Data Base. J Am Coll Surg. 2015;221:175–184. doi: 10.1016/j.jamcollsurg.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 8.Baker EH, Ross SW, Seshadri R, Swan RZ, Iannitti DA, Vrochides D, Martinie JB. Robotic pancreaticoduodenectomy: comparison of complications and cost to the open approach. Int J Med Robot. 2016;12:554–560. doi: 10.1002/rcs.1688. [DOI] [PubMed] [Google Scholar]
- 9.Stauffer JA, Coppola A, Villacreses D, Mody K, Johnson E, Li Z, Asbun HJ. Laparoscopic versus open pancreaticoduodenectomy for pancreatic adenocarcinoma: long-term results at a single institution. Surg Endosc. 2016;31:1–9. doi: 10.1007/s00464-016-5222-1. [DOI] [PubMed] [Google Scholar]
- 10.Zureikat AH, Postlewait LM, Liu Y, Gillespie TW, Weber SM, Abbott DE, Ahmad SA, Maithel SK, Hogg ME, Zenati M, Cho CS, Salem A, Xia B, Steve J, Nguyen TK, Keshava HB, Chalikonda S, Walsh RM, Talamonti MS, Stocker SJ, Bentrem DJ, Lumpkin S, Kim HJ, Zeh HJ, 3rd, Kooby DA. A multi-institutional comparison of perioperative outcomes of robotic and open pancreaticoduodenectomy. Ann Surg. 2016;264:640–649. doi: 10.1097/SLA.0000000000001869. [DOI] [PubMed] [Google Scholar]
- 11.Tee MC, Croome KP, Shubert CR, Farnell MB, Truty MJ, Que FG, Reid-Lombardo KM, Smoot RL, Nagorney DM, Kendrick ML. Laparoscopic pancreatoduodenectomy does not completely mitigate increased perioperative risks in elderly patients. HPB (Oxford) 2015;17:909–918. doi: 10.1111/hpb.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McMillan MT, Zureikat AH, Hogg ME, Kowalsky SJ, Zeh HJ, Sprys MH, Vollmer CM., Jr A propensity score–matched analysis of robotic vs open pancreatoduodenectomy on incidence of pancreatic fistula. JAMA Surg. 2017 Apr 1;152(4):327–335. doi: 10.1001/jamasurg.2016.4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asbun HJ, Stauffer JA. Laparoscopic vs open pancreaticoduodenectomy: overall outcomes and severity of complications using the Accordion Severity Grading System. J Am Coll Surg. 2012;215:810–819. doi: 10.1016/j.jamcollsurg.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Nassour I, Wang SC, Christie A, Augustine MM, Porembka MR, Yopp AC, Choti MA, Mansour JC, Xie XJ, Polanco PM, Minter RM. Minimally invasive versus open pancreaticoduodenectomy: a propensity-matched study from a national cohort of patients. Ann Surg. 2017 May; doi: 10.1097/SLA.0000000000002259. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H, Wu X, Zhu F, Shen M, Tian R, Shi C, Wang X, Xiao G, Guo X, Wang M, Qin R. Systematic review and meta-analysis of minimally invasive versus open approach for pancreaticoduodenectomy. Surg Endosc. 2016;30:5173–5184. doi: 10.1007/s00464-016-4864-3. [DOI] [PubMed] [Google Scholar]
- 16.Wang M, Cai H, Meng L, Cai Y, Wang X, Li Y, Peng B. Minimally invasive pancreaticoduodenectomy: a comprehensive review. Int J Surg. 2016;35:139–146. doi: 10.1016/j.ijsu.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 17.Doula C, Kostakis ID, Damaskos C, Machairas N, Vardakostas DV, Feretis T, Felekouras E. Comparison between minimally invasive and open pancreaticoduodenectomy: a systematic review. Surg Laparosc Endosc Percutan Tech. 2016;26:6–16. doi: 10.1097/SLE.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 18.Correa-Gallego C, Dinkelspiel HE, Sulimanoff I, Fisher S, Viñuela EF, Kingham TP, Fong Y, DeMatteo RP, D’Angelica MI, Jarnagin WR, Allen PJ. Minimally-invasive vs open pancreaticoduodenectomy: systematic review and meta-analysis. J Am Coll Surg. 2014;218:129–139. doi: 10.1016/j.jamcollsurg.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Liu R, Zhang T, Zhao ZM, Tan XL, Zhao GD, Zhang X, Xu Y. The surgical outcomes of robot-assisted laparoscopic pancreaticoduodenectomy versus laparoscopic pancreaticoduodenectomy for periampullary neoplasms: a comparative study of a single center. Surg Endosc. 2017;31:2380–2386. doi: 10.1007/s00464-016-5238-6. [DOI] [PubMed] [Google Scholar]
- 20.Wright GP, Zureikat AH. Development of minimally invasive pancreatic surgery: an evidence-based systematic review of laparoscopic versus robotic approaches. J Gastrointest Surg. 2016;20:1658–1665. doi: 10.1007/s11605-016-3204-1. [DOI] [PubMed] [Google Scholar]
- 21.American College of Surgeons. User Guide for the 2015 ACS NSQIP Procedure Targeted Participant Use Data File (PUF) https://www.facs.org/~/media/files/quality%20programs/nsqip/pt_nsqip_puf_user_guide_2015.ashx. Accessed December 12, 2016.
- 22.Leow JJ, Heah NH, Chang SL, Chong YL, Png KS. Outcomes of robotic versus laparoscopic partial nephrectomy: an updated meta-analysis of 4,919 Patients. J Urol. 2016;196:1371–1377. doi: 10.1016/j.juro.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 23.Xie W, Cao D, Yang J, Shen K, Zhao L. Robot-assisted surgery versus conventional laparoscopic surgery for endometrial cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2016;142:2173–2183. doi: 10.1007/s00432-016-2180-x. [DOI] [PubMed] [Google Scholar]
- 24.Kim CW, Kim CH, Baik SH. Outcomes of robotic-assisted colorectal surgery compared with laparoscopic and open surgery: a systematic review. J Gastrointest Surg. 2014;18:816–830. doi: 10.1007/s11605-014-2469-5. [DOI] [PubMed] [Google Scholar]
- 25.Park JM, Kim HI, Han SU, Yang HK, Kim YW, Lee HJ, An JY, Kim MC, Park S, Song KY, Oh SJ, Kong SH, Suh BJ, Yang DH, Ha TK, Hyung WJ, Ryu KW. Who may benefit from robotic gastrectomy? A subgroup analysis of multicenter prospective comparative study data on robotic versus laparoscopic gastrectomy. Eur J Surg Oncol. 2016;42:1944–1949. doi: 10.1016/j.ejso.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 26.Daouadi M, Zureikat AH, Zenati MS, Choudry H, Tsung A, Bartlett DL, Hughes SJ, Lee KK, Moser AJ, Zeh HJ. Robot-assisted minimally invasive distal pancreatectomy is superior to the laparoscopic technique. Ann Surg. 2013;257:128–132. doi: 10.1097/SLA.0b013e31825fff08. [DOI] [PubMed] [Google Scholar]
- 27.Zureikat AH, Borrebach J, Pitt HA, Mcgill D, Hogg ME, Thompson V, Bentrem DJ, Hall BL, Zeh HJ. Minimally invasive hepatopancreatobiliary surgery in North America: an ACS-NSQIP analysis of predictors of conversion for laparoscopic and robotic pancreatectomy and hepatectomy. HPB (Oxford) 2017;19(7):595–602. doi: 10.1016/j.hpb.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Sippey M, Grzybowski M, Manwaring ML, Kasten KR, Chapman WH, Pofahl WE, Pories WJ, Spaniolas K. Acute cholecystitis: risk factors for conversion to an open procedure. J Surg Res. 2015;199:357–361. doi: 10.1016/j.jss.2015.05.040. [DOI] [PubMed] [Google Scholar]
- 29.Bhama AR, Charlton ME, Schmitt MB, Cromwell JW, Byrn JC. Factors associated with conversion from laparoscopic to open colectomy using the National Surgical Quality Improvement Program (NSQIP) database. Colorectal Dis. 2015;17:257–264. doi: 10.1111/codi.12800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boone BA, Zenati M, Hogg ME, Steve J, Moser AJ, Bartlett DL, Zeh HJ, Zureikat AH. Assessment of quality outcomes for robotic pancreaticoduodenectomy: identification of the learning curve. JAMA Surg. 2015;150:416–422. doi: 10.1001/jamasurg.2015.17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


