Abstract
Objectives:
The study aimed to test the effect of intraoperative intravenous (IV) lidocaine on the incidence of postextubation laryngospasm in adult patients.
Methods:
The prospective randomized clinical trial was conducted at tertiary care hospital in Riyadh, between January and December 2012. Seventy-two patients undergoing laparoscopic cholecystectomy were randomly assigned to receive either placebo (n = 36) or IV lidocaine (n = 36), 1 mg/kg bolus after desflurane was discontinued. Laryngospasm was graded from 0 to 3 based on the absence or presence of signs and the severity of postextubation laryngospasm.
Results:
The study was terminated early by the data monitoring committee because of safety concerns due to an increased incidence of postextubation laryngospasm. Patient demographics were similar for both groups. The incidence of postextubation laryngospasm was 19.5% in the placebo group and 0% in the treatment (lidocaine) group; this difference was statistically significant (P = 0.017; 95% confidence interval, 4.6% to 36.0%).
Conclusions:
The cause of laryngospasm in our study was most likely the rapid increase in the concentration of inspired desflurane, which might have caused airway irritation. Therefore, we believe that pretreating patients at risk of developing laryngospasm with IV lidocaine could be effective.
Keywords: Desflurane, intravenous anesthesia, laryngismus, lidocaine
Introduction
In anesthesiology, one of the common complications of airway management is laryngospasm. The etiology of laryngospasm is unknown, but it may be due to an insufficient depth of anesthesia during tracheal intubation, a light plane of anesthesia during tracheal extubation, pain; or the presence of an airway irritant (such as a laryngoscope blade), an irritating volatile agent, a suction catheter, surgical debris, mucus, blood, or another foreign body.[1,2,3] Laryngospasm occurs in patients of both sexes and all ages. The incidence of laryngospasm reported to the Australian Incident Monitoring Study (AIMS) was 5%, and 22% of these cases occurred without an attributable cause.[4]
At present, there is no proven prophylactic for laryngospasm, and the available treatments for laryngospasm are used postoccurrence. Identifying and eliminating the factors that lead to laryngospasm represent the most effective means by which to reduce laryngospasm incidence.[5,6]
Intravenous (IV) lidocaine interrupts nerve conduction by blocking sodium channels.[7] A recent meta-analysis showed that IV lidocaine was able to prevent laryngospasm in children.[8] However, the literature lacks studies investigating the effects of IV lidocaine in adults relative to the number of studies of its effects in children.
This trial was aimed to evaluate the effect of IV lidocaine on the incidence of postextubation laryngospasm in adult patients.
Methods
After Clinical Trials registration (NCT01445847) was obtained and the Institutional Review Board approved the trial (IRB Project No. E.11-491), patients scheduled for laparoscopic cholecystectomy at Tertiary Care Hospital, Riyadh, between January 2012 and December 2012 were approached for enrollment on the same or the preceding day. Written informed consent was obtained from the patients who agreed to participate in the study. Patients 18–60 years of age with American Society of Anesthesiologists physical status I or II were deemed eligible for the study. The following exclusion criteria were applied: allergy to local anesthetics, history of upper respiratory tract infection within the previous 2 weeks, persistent type of hyperreactive airway or asthma, gastroesophageal reflux disease, moderate to heavy smoking (smoking of at least 11 cigarettes per day or daily water pipe smoking), intubation difficulty requiring more than one intubation attempt, and current use of sedatives or narcotics (i.e., psychiatric or pain control medications).
Using random allocation software and a 1:1 ratio with an equal block size of 10 per block in numeric sequential sequence, the subjects were randomized into 1 of 2 study drug arms: placebo (normal saline) or lidocaine HCl (Hospira, Inc., Lake Forest, IL 60045 USA). The anesthesiologist was given a concealed 10-cc syringe containing 10 mg/ml lidocaine or normal saline; the syringe was prepared by the assigned technician who generated the random allocation sequence and who was responsible for assigning participants to each intervention. Patients were randomized immediately after induction of anesthesia. Before discontinuing desflurane, a bolus of lidocaine or placebo was administered to each patient at a dose of 1 mg/kg (i.e., a volume of 0.1 ml/kg), with a maximum dose of 100 mg (10 ml) (i.e., patients weighing over 100 kg received a fixed dose of 100 mg). Because of body composition differences between nonobese and obese patients, this procedure was followed to prevent the prospect of overdose and to maintain blinding of the study arms. The syringe design allowed the addition of 0–0.1 ml (0–1 mg) of the administered study drug. We elected to discontinue the use of desflurane upon the administration of the study drugs based on the assumption that a 5-min period before extubation was clinically applicable (i.e., the anticipated awake status following general anesthesia); no study has evaluated the effect of IV lidocaine administered more than 5 min before extubation. The patients and research team remained blinded to the drug administration until all of the data were analyzed.
General anesthesia was conducted to all patients. Premedication was not prescribed to any study subject. An IV line was secured with maintenance fluid during the fasting period. Standard monitors, including a neuromuscular monitor, were used for all patients. Patients were preoxygenated with 100% oxygen for 3–5 min. Then, patients were intravenously induced with 1% propofol (2 mg/kg), 2.5 mcg/kg fentanyl, and 0.15 mg/kg cisatracurium besilate. After manual ventilation with oxygen-desflurane for 3 min, a properly sized endotracheal tube (ETT, size 7.5 for males and size 7 for females) was placed using the appropriate Macintosh laryngoscope blade; neither instrument was lubricated with lidocaine gel or spray. The ETT balloon was filled with air with a water pressure of 25 cm H2O using a manometer, and the ETT position was checked. Anesthesia was maintained with 50% oxygen, 50% air, 1 minimum alveolar concentration (MAC) desflurane, and 25–50 mcg of fentanyl as necessary. No additional muscle relaxants were administered. The patients were mechanically ventilated, and the gas flow was set on autocontrol. An orogastric tube (OGT) was inserted. A temperature probe was placed orally, and a warmer device was applied to maintain the body temperature of the patient above 36°C throughout the procedure. One gram of paracetamol was infused. The surgeons injected bupivacaine subcutaneously at the surgical site.
When the surgeons began closing the wounds, the patients were allowed to breathe spontaneously with 100% oxygen. Inspired desflurane was increased to 8% at a flow rate of 6 L/min to maintain expired desflurane at 6% (i.e., MAC 1). At the time of the last surgical suture, 2.5 mg of neostigmine and 0.4 mg of glycopyrrolate were administered intravenously. Next, the OGT was removed, and the oral cavity of the patient was suctioned gently with a disposable suction catheter (size 14) using a finger control valve. The time interval between injecting the reversal drugs and discontinuing desflurane was 5–7 min. When the patient was cleared by the surgeons and the nurses, desflurane was discontinued, and the study drug (1 mg/kg actual body weight) was intravenously injected, after which the patients were placed in a semi-Fowler's position. The oral cavity was gently suctioned for a second time, and the ETT cuff was slowly deflated. Absolutely no stimulation was allowed until the patient awoke spontaneously. Tracheal extubation was performed when the following criteria were met: returned airway reflexes; a MAC of 0.2–0.3;[9] an ability to take an adequate tidal volume (>4 ml/kg predicted body weight); a regular breathing pattern, with >8 and <25 breaths/min; a train-of-four ratio at the ulnar nerve site >0.9, and an ability to open their eyes and/or show significant movement (i.e., sustained 5-s head lift or hand grasp and/or the ability to follow commands).
The extubation period was defined as the time interval between discontinuation of the inhalational agent and tracheal extubation and was documented as were the vital signs of the patients at baseline and the time of desflurane discontinuation (minimum and maximum heart rate (HR) during the extubation period as well as at the time of tracheal extubation were also documented for reference). Face masks were gently held onto the face of each patient, and laryngospasm events were scored by the attending consultant, a professor, and the senior staff assigned to each case using the following four-point scale:[10]
0 = Successful tracheal extubation without signs of compromised airway
1 = Partial occlusion of cords, stridor during inspiration (decreased tidal volume with stable pulse oximeter oxygen saturation [SpO2 >95%])
2 = Total occlusion of cords (respiratory silence with ventilatory obstruction, which can be characterized by inspiratory efforts of the accessory muscles and paradoxical thoracic movements, and SpO2> 85%)
3 = Cyanosis (associated with desaturation SpO2 < 85% and bradycardia of a severe type).
Postoperatively, all patients were transported to the postanesthesia care unit and monitored per protocol.
Interim analysis
The data monitoring committee (DMC) blindly and regularly monitored the study results. The DMC was composed of three senior professors and associate professor including head of the department, head of scientific research, and program director. The proposed cut-points for interim analyses were decided to be after every 100 participants. The DMC weighed whether the study should proceed and if so, whether the study protocol should be improved after each interim analysis. It was decided that the study would be discontinued if the incidence of postextubation laryngospasm was found at any of the interim analyses points or was higher than expected. However, the DMC also had the authority to terminate the study due to safety concerns, outstanding benefit, or futility.
Outcome measurements
The outcome of interest was the incidence of laryngospasm postextubation up to 5 min.
Statistical analysis
We used the incidence level reported by the AIMS study[4] (i.e., 5%) to calculate the sample size for this study. We set the null hypothesis as follows: (percentage of laryngospasm incidence in the placebo group [μ1] – percentage of laryngospasm incidence in the lidocaine group [μ2] = 0); an alternative hypothesis (μ1> μ 2) was analyzed (i.e., with 5%, assuming lidocaine percentage = 0) by comparing two groups. A sample size of 380 patients (190 per group) was deemed adequate to detect a 5% difference in the incidence of postextubation laryngospasm with 80% power assuming a 5% total reduction in the incidence of postextubation laryngospasm. A P ≤ 0.05 was considered statistically significant. Expanding the enrollment was considered to include 5% of the calculated sample size for potential withdrawal and incomplete data.
Statistical analyses were performed using MedCalc Statistical Software version 12.1.1 (MedCalc Software bvba, Ostend, Belgium). Normality of distribution was evaluated using the Kolmogorov–Smirnov test to determine the appropriate comparison tests. The mean, standard deviation, median, and 25th to 75th interquartile range were used for the descriptive analyses of normally distributed variables and the non-normally distributed variables, respectively. Comparison of proportions was used to evaluate the incidence percentage difference between the two groups with per protocol and intention-to-treat approaches. A two-tailed t-test was used to evaluate the mean difference between the two groups for normally distributed data, whereas the Mann–Whitney U-test was used for the non-normally distributed data. Categorical variables were compared using the Chi-squared test or Fisher's exact test, as appropriate. A P = 0.05 was considered statistically significant.
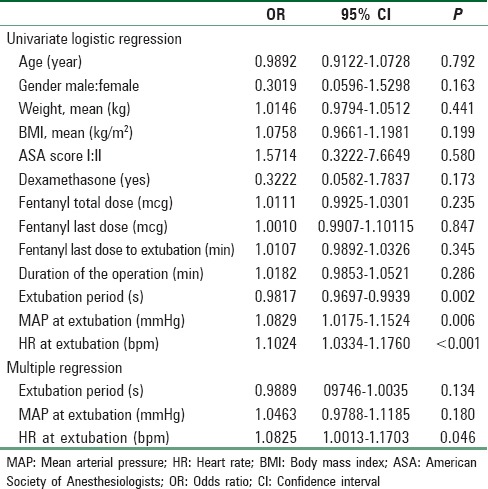
Due to the study termination, additional statistical tests were added to determine the contributory factors using univariate logistic regression although it was not the aim of the study. All parameters with statistical significant (i.e., P < 0.05) in univariate analyses were included in a multivariate logistic regression model.
An inter-rater reliability analysis using the weighted Cohen's Kappa statistic was performed to determine the consistency among the raters. The coding system was based on the presence or absence of postextubation laryngospasm, and there were three raters: attending consultant, senior professor, and assistant or associate consultant.
Results
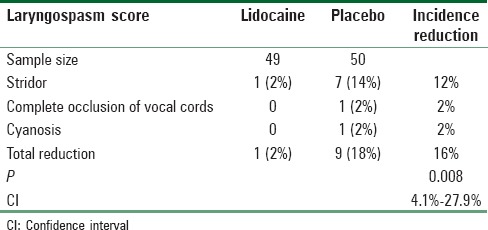
An increase in the number of postextubation laryngospasm events resulted in the early termination of this trial by the DMC. The decision to terminate the study was based on possible causes, which was announced during an academic morning meeting. One hundred and thirty-four patients were assessed for eligibility, and 72 patients completed the study. A CONSORT trial flow diagram is shown in Figure 1. The two groups were randomized, and there were no statistically significant differences in demographic or clinical characteristics between the two groups [Table 1].
Figure 1.
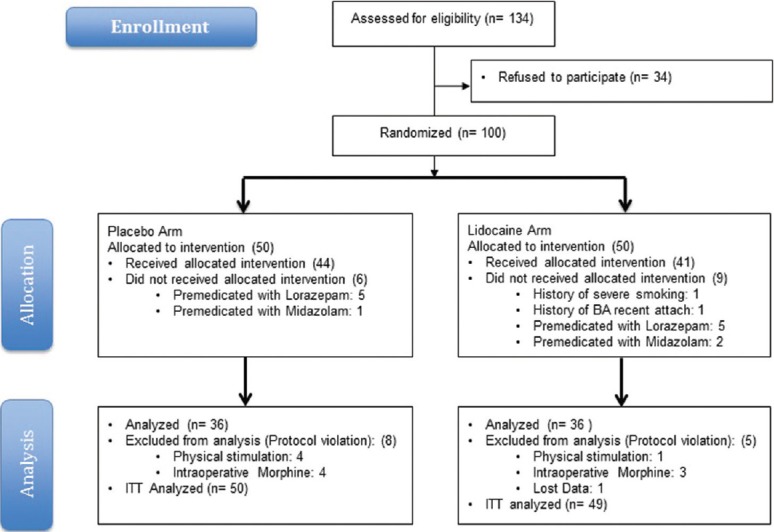
Consort flow chart
Table 1.
Demographic and clinical characteristics of the study population
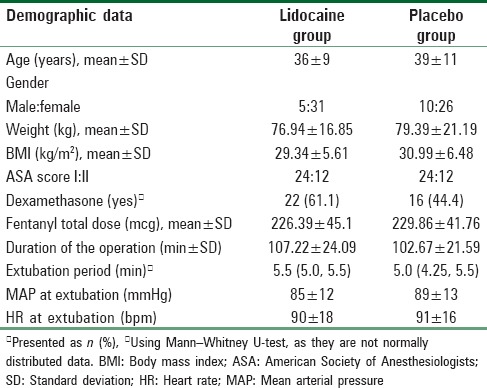
The incidence of postextubation laryngospasm per protocol approach was 19.5% in the placebo group and 0% in the lidocaine group. There was a 19.5% reduction in the incidence of laryngospasm after administering IV lidocaine (P = 0.017; 95% confidence interval [CI], 4.6% to 36.0%); this difference was statistically significant according to the comparison of proportions [Table 2]. However, the incidence of postextubation laryngospasm with intention-to-treat approach was 18% in the placebo group and 2% in the lidocaine group. There was a 16% reduction in the incidence of laryngospasm after administering IV lidocaine (P = 0.008; 95% CI, 4.1% to 27.9%); this difference was statistically significant according to the comparison of proportions [Table 3].
Table 2.
The incidence of laryngospasm per protocol approach
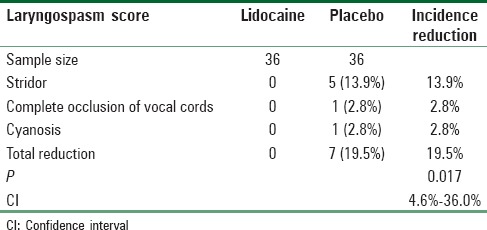
Table 3.
The incidence of laryngospasm with intention-to-treat approach
The possible contributory factors of increased incidence of laryngospasm postextubation were studied in details. It showed statistical differences between patients who suffer from postextubation laryngospasm and patient who did not suffer from it in terms of mean HR, mean arterial pressure, and extubation period [Table 4], similar findings were showed with univariate logistic regression model. However, only mean HR showed statistically significant with multivariate logistic regression model [Table 5].
Table 4.
The possible contributory factors in relation to incidence of laryngospasm
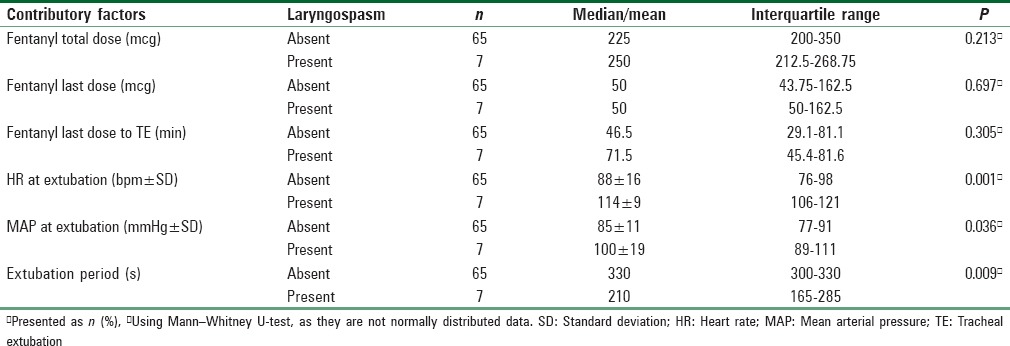
Table 5.
Logistic regression analysis of contributory factors for laryngospasm
The estimate of weighted Cohen's Kappa averaged across coder pairs was 0.86 (P < 0.001; 95% CI, 0.68–1; coder pair kappa estimates = 0.94 [coders 1 and 2], 0.85 [coders 2 and 3], and 0.80 [coders 1 and 3]), indicating almost perfect inter-rater agreement. Coder 1: attending consultant, Coder 2: senior professor, and Coder 3: assistant or associate consultant.
Discussion
The incidence of laryngospasm was unexpectedly high (7/72 events), which required a halt to the recruitment of subjects on April 26, 2012 and an investigation. The cause of the high rate of laryngospasm was not known at that time of study recruitment. Five of seven patients had a partial postoperative laryngospasm, which was easily detected and managed by applying continuous positive airway pressure. One of the seven patient events was a complete postoperative laryngospasm, which was managed by intermittent positive pressure ventilation with jaw trust maneuver, and 1 out of 7 patient events resulted in cyanosis, which required reintubation. It was a wise decision to suspend recruitment and begin investigation after the last incidence of postoperative laryngospasm. It was necessary to disclose limited data to the DMC to guide the DMC in this decision. Data disclosure occurred in different stages: disclosure of total incidences, division of the incidences into two groups blindly, disclosure of the actual groups, and the release of the rest of study data.
The most likely cause of the high incidence of laryngospasm was iatrogenic volatile irritation of the airway. Our investigation revealed that the vital signs of the subjects were stable throughout the perioperative period until the point at which the assigned anesthesiologists attempted to return patients to spontaneous breathing while desflurane was still at MAC 1; this procedure was followed to maintain an adequate plan of anesthesia as the BIS index was not used during this trial. To maintain a MAC 1 of desflurane with spontaneous breathing, the inspired concentration of desflurane was increased to 8% with a flow rate of 6 l/min. This flow rate was chosen to overcome circuit resistance, prevent rebreathing, and unify the process throughout the extubation period (i.e., washout).[11] Therefore, it was necessary to increase the desflurane concentration by 1%–2% above the initial setting due to the high-flow rate.[12] Thus, the accelerated HR was most likely due to β-adrenergic activation occurring due to the stimulation of rapidly adapting upper airway receptors caused by inspired desflurane, which led to significant tachycardia and hypertension.[13] Although a meta-analysis[14] concluded otherwise, an increase in the concentration of inspired desflurane could cause airway irritation and provoke coughing, bronchospasm, bronchial secretion, and laryngospasm.[13,15] However, subclinical airway irritation due to environmental pollution and dusty weather cannot be ruled out without proper investigation which may enhance the irritation effect of desflurane in our population. In this trial, the significantly increased HR (the mean HR was 114 bpm) during the extubation process was obvious in patients who developed postextubation laryngospasm, which supports the possibility of causality between the increased HR and the increased incidence of postextubation laryngospasm. Maintaining the concentration of inspired desflurane at 6% and deprioritizing the maintenance of MAC 1 during the extubation process may have influenced the results. However, the DMC terminated the study for safety concerns because the incidence of laryngospasm would not decrease upon the next interim analysis, the incidence was significantly different between the two groups, and the anticipation of postoperative laryngospasm would be extremely difficult considering that the HR was not evidently a marker. In addition, a switch to sevoflurane would require conducting a discrete trial because the methodology would require a significant change due to a significant difference between desflurane and sevoflurane in the extubation period and the fact that no study has evaluated the effect of IV lidocaine administered more than 5 min before extubation.[2,10,16,17,18] Therefore, the appropriate time for administering IV lidocaine would need to be altered.
Based on our analyses, premature tracheal extubation is another factor that may have increased the incidence of postextubation laryngospasm.[19] The extubation period was the time required to transfer the patient from the deep plane of anesthesia to the awake plane. In this trial, smooth emergence was observed after 330 s for 40 patients who showed signs of wakefulness (i. e., open eyes) and after 210 s for patients who developed postextubation laryngospasm. However, this interpretation is not supported by the results of a meta-analysis, as these extubation periods are within the range reported in most previous studies.[14,20,21] Thus, sedation effect of lidocaine may contribute in delay of recovery rather than extubation in light plan of anesthesia. In another hand, dexamethasone did not influence the result of incidence of postextubation laryngospasm. In addition, 40% among who developed postextubation laryngospasm received IV dexamethasone. It was used prophylactically with low dose (i.e., 0.1 mg/kg) for postoperative nausea and vomiting. Similar to the studies included in the meta-analysis,[8] close observation of the subjects for postextubation laryngospasm may have contributed to the increase in laryngospasm incidence, as the actual incidence of postextubation laryngospasm has been reported to be between 0.75% and 5%.[4]
One milligram/kilogram IV lidocaine did not change the hemodynamic status of the patients. A similar finding was reported by Sanikop et al. who used 1.5 mg/kg of IV lidocaine.[2] In addition, 14 patients (7 per group) had an accelerated HR during the extubation period. The seven patients in the placebo group had a postextubation laryngospasm, whereas none of the patients in the lidocaine group had a laryngospasm. Although an association between laryngospasm and tachycardia has not yet been established, we found a strong association between these two parameters, as did Sanikop et al.[2] Therefore, we postulate that IV lidocaine primarily prevented postextubation laryngospasm caused by the irritability induced by the high concentration of desflurane[22] by holding superior laryngeal nerve sodium channels in specific functional states (i.e., open or inactive). Determining criteria for identifying patients at risk for developing postextubation laryngospasm who would benefit from lidocaine pretreatment is warranted. Although the beneficial of IV lidocaine was demonstrated, the termination of the study was due to the high incidence of laryngospasm. It would be difficult to predict the results of next interim analyses on whether the harm or the beneficial would maintain or not. Although the early termination led to overestimated results, it would not result in misguided decisions due to the methodology quality in terms of blinding and concealment randomization, power of the study, and beneficial of IV lidocaine was supported by meta-analyses.
Even with objective correlations, the postextubation laryngospasm scoring system was a subjective measurement, which is a limitation of our study.
Conclusions
In conclusion, termination of the study may lead to overestimated results due to its impact on the sample size. However, this study contributes to our knowledge of the value of administering IV lidocaine to adult patients to prevent postextubation laryngospasm. Furthermore, our results have potential clinical implications, which require further investigation and confirmation by other studies. The cause of laryngospasm in our study was most likely the rapid increase in the concentration of inspired desflurane, which might have caused airway irritation. Therefore, we believe that pretreating patients at risk of developing laryngospasm with IV lidocaine could be effective. We believe that focusing on maintaining the concentration of inspired desflurane rather than expired desflurane may decrease the incidence of postextubation laryngospasm.
Financial support and sponsorship
The study was supported by the College of Medicine Research Center, Deanship of Scientific Research, King Saud University.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank Dr. Eldawlatly who served as the scientific advisor and critically reviewed the study proposal.
References
- 1.Mevorach DL. The management and treatment of recurrent postoperative laryngospasm. Anesth Analg. 1996;83:1110–1. doi: 10.1097/00000539-199611000-00037. [DOI] [PubMed] [Google Scholar]
- 2.Sanikop C, Bhat S. Efficacy of intravenous lidocaine in prevention of post extubation laryngospasm in children undergoing cleft palate surgeries. Indian J Anaesth. 2010;54:132–6. doi: 10.4103/0019-5049.63654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hobaika AB, Lorentz MN. Laryngospasm. Rev Bras Anestesiol. 2009;59:487–95. doi: 10.1590/s0034-70942009000400012. [DOI] [PubMed] [Google Scholar]
- 4.Visvanathan T, Kluger MT, Webb RK, Westhorpe RN. Crisis management during anaesthesia: Laryngospasm. Qual Saf Health Care. 2005;14:e3. doi: 10.1136/qshc.2002.004275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller KA, Harkin CP, Bailey PL. Postoperative tracheal extubation. Anesth Analg. 1995;80:149–72. doi: 10.1097/00000539-199501000-00025. [DOI] [PubMed] [Google Scholar]
- 6.Difficult Airway Society Extubation Guidelines Group. Popat M, Mitchell V, Dravid R, Patel A, Swampillai C, et al. Difficult airway society guidelines for the management of tracheal extubation. Anaesthesia. 2012;67:318–40. doi: 10.1111/j.1365-2044.2012.07075.x. [DOI] [PubMed] [Google Scholar]
- 7.Herroeder S, Pecher S, Schönherr ME, Kaulitz G, Hahnenkamp K, Friess H, et al. Systemic lidocaine shortens length of hospital stay after colorectal surgery: A double-blinded, randomized, placebo-controlled trial. Ann Surg. 2007;246:192–200. doi: 10.1097/SLA.0b013e31805dac11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mihara T, Uchimoto K, Morita S, Goto T. The efficacy of lidocaine to prevent laryngospasm in children: A systematic review and meta-analysis. Anaesthesia. 2014;69:1388–96. doi: 10.1111/anae.12788. [DOI] [PubMed] [Google Scholar]
- 9.Chortkoff BS, Eger EI, 2nd, Crankshaw DP, Gonsowski CT, Dutton RC, Ionescu P. Concentrations of desflurane and propofol that suppress response to command in humans. Anesth Analg. 1995;81:737–43. doi: 10.1097/00000539-199510000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Leicht P, Wisborg T, Chraemmer-Jørgensen B. Does intravenous lidocaine prevent laryngospasm after extubation in children? Anesth Analg. 1985;64:1193–6. [PubMed] [Google Scholar]
- 11.Kaul TK, Mittal G. Mapleson's breathing systems. Indian J Anaesth. 2013;57:507–15. doi: 10.4103/0019-5049.120148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hönemann C, Hagemann O, Doll D. Inhalational anaesthesia with low fresh gas flow. Indian J Anaesth. 2013;57:345–50. doi: 10.4103/0019-5049.118569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kapoor MC, Vakamudi M. Desflurane – Revisited. J Anaesthesiol Clin Pharmacol. 2012;28:92–100. doi: 10.4103/0970-9185.92455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevanovic A, Rossaint R, Fritz HG, Froeba G, Heine J, Puehringer FK, et al. Airway reactions and emergence times in general laryngeal mask airway anaesthesia: A meta-analysis. Eur J Anaesthesiol. 2015;32:106–16. doi: 10.1097/EJA.0000000000000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKay RE, Bostrom A, Balea MC, McKay WR. Airway responses during desflurane versus sevoflurane administration via a laryngeal mask airway in smokers. Anesth Analg. 2006;103:1147–54. doi: 10.1213/01.ane.0000237293.39466.65. [DOI] [PubMed] [Google Scholar]
- 16.He J, Zhang Y, Xue R, Lv J, Ding X, Zhang Z. Effect of desflurane versus sevoflurane in pediatric anesthesia: A meta-analysis. J Pharm Pharm Sci. 2015;18:199–206. doi: 10.18433/j31882. [DOI] [PubMed] [Google Scholar]
- 17.Baraka A. Intravenous lidocaine controls extubation laryngospasm in children. Anesth Analg. 1978;57:506–7. doi: 10.1213/00000539-197807000-00028. [DOI] [PubMed] [Google Scholar]
- 18.Erb TO, von Ungern-Sternberg BS, Keller K, Frei FJ. The effect of intravenous lidocaine on laryngeal and respiratory reflex responses in anaesthetised children. Anaesthesia. 2013;68:13–20. doi: 10.1111/j.1365-2044.2012.07295.x. [DOI] [PubMed] [Google Scholar]
- 19.Koç C, Kocaman F, Aygenç E, Ozdem C, Cekiç A. The use of preoperative lidocaine to prevent stridor and laryngospasm after tonsillectomy and adenoidectomy. Otolaryngol Head Neck Surg. 1998;118:880–2. doi: 10.1016/S0194-5998(98)70290-6. [DOI] [PubMed] [Google Scholar]
- 20.Chen G, Zhou Y, Shi Q, Zhou H. Comparison of early recovery and cognitive function after desflurane and sevoflurane anaesthesia in elderly patients: A meta-analysis of randomized controlled trials. J Int Med Res. 2015;43:619–28. doi: 10.1177/0300060515591064. [DOI] [PubMed] [Google Scholar]
- 21.Wachtel RE, Dexter F, Epstein RH, Ledolter J. Meta-analysis of desflurane and propofol average times and variability in times to extubation and following commands. Can J Anaesth. 2011;58:714–24. doi: 10.1007/s12630-011-9519-1. [DOI] [PubMed] [Google Scholar]
- 22.Brochu RM, Dick IE, Tarpley JW, McGowan E, Gunner D, Herrington J, et al. Block of peripheral nerve sodium channels selectively inhibits features of neuropathic pain in rats. Mol Pharmacol. 2006;69:823–32. doi: 10.1124/mol.105.018127. [DOI] [PubMed] [Google Scholar]


