Abstract
Background:
Patients’ surgical experiences are influenced by their perception of pain management. Duloxetine (Dulox) and dexamethasone (Dex) are used in multimodal analgesia to reduce opioid use and side effects. Dulox is a selective serotonin and norepinephrine reuptake inhibitor and has efficacy in chronic pain conditions. Dex enhances postoperative (PO) analgesia and reduces PO nausea and vomiting (PONV).
Methods:
Seventy-five female patients were randomly allocated into one of three equal groups. GI received Dulox 60 mg orally and 100 ml 0.9% sodium chloride (normal saline [NS]) intravenous infusion (IVI) over 15 min, GII: received as GI except Dex 0.1 mg/kg was mixed with NS and GIII received identical placebo for Dulox capsule and Dex IVI, 2 h preoperatively. Patients’ vitals, visual analog scale (VAS), and sedation score were assessed at 30 min, 1 h, 2 h, 6 h, and 12 h postoperatively. Total pethidine requirements, plasma cortisol, PONV, and patients satisfaction were recorded.
Results:
PO time for 1st rescue analgesic was significantly high in GI and GII compared to GIII and in GII compared to GI. There was a significant less VAS score, heart rate, mean arterial pressure, and a high sedation score in GI and GII compared to GIII at 30 min, 1, 2, and 6 h postoperatively. Total pethidine requirements were significantly less in GI and GII compared to GIII 12 h postoperatively. There was a significant reduction in the 2 h PO serum cortisol (μg/dl) and a significant increase in the PO patients satisfaction score in GI and GII compared to GIII. PONV was decreased significantly in GII compared to GI and GIII.
Conclusion:
The use of oral Dulox 60 mg combined with Dex 0.1 mg/kg IVI is more effective than oral Dulox 60 mg alone, 2 h preoperatively, for improving PO pain by reducing the requirements for rescue analgesia and PONV.
Keywords: Dexamethasone, duloxetine, postoperative pain, side effects
Introduction
Central sensitization develops after the surgical incision with a remarkable postoperative (PO) pain. Analgesic treatment results in preventing the establishment of altered central processing leading to a reduction in PO and chronic pain development.[1] The best use of analgesic adjuncts aiming of decreasing opioid consumption and opioid-related side effects necessitates getting acquainted with their dose-response relationships when combined with opioids to optimize the analgesic efficacy with less side effects from the combinations.[2]
Duloxetine (Dulox) is a selective serotonin-norepinephrine reuptake inhibitor and is prescribed for the treatment of generalized anxiety disorder and major depression.[3] Dulox, which blocks persistent late Nav1.7 Na+ currents present in both central nervous system and peripheral nervous system, is useful in the treatment of pain caused by diabetic neuropathy and fibromyalgia[4] and may prevent transient PO anxiety that is common in female patients.[5] The oral bioavailability of Dulox (60 mg dose) averaged 50% with maximum plasma concentration occurring approximately 6 h after the dose and its elimination half-life is 12 h.[6]
Dulox is affected by smoking Tobacco. Tobacco smoke contains polycyclic aromatic hydrocarbons (PAHs) that induce certain hepatic enzymes CYP1A1, CYP1A2, and possibly CYP2E1 significantly lower serum concentrations of Dulox (approximately 50%) than nonsmokers. Individuals who smoke while taking Dulox require higher dosages to achieve therapeutic levels.[7]
Glucocorticoids are characterized by their analgesic, anti-inflammatory, modulating stress response to surgical trauma, and antiemetic effects.[8] Glucocorticoids decrease prostaglandin formation by minimizing activity of phospholipase A2 and blocking the expression of cyclooxygenase (COX)-2 mRNA with a trifle impact on COX-1[9] and they inhibit mediators of inflammatory hyperalgesia such as tumor necrosis factor, interleukin 1, and interleukin 6.[10] Preoperative intravenous (IV) dexamethasone (Dex) (8 mg) reduced pain, fatigue, nausea and vomiting, and duration of convalescence in patients undergoing noncomplicated laparoscopic cholecystectomy when compared with placebo.[11] A single dose of Dex (4 mg IV) reduced the convalescence time from surgical procedures and decreased PO pain score.[12] A single dose of preoperative methylprednisolone (30 mg/kg) was not associated with any side effects in a meta-analysis with more than 1900 patients included in this study.[13]
The primary aim of this study was to compare the analgesic efficacy of Dulox alone, with Dulox and Dex combination in reducing PO pain measured by total pethidine requirements 12 h after gynecological surgeries. The secondary aim was to compare the effects of Dulox to Dulox plus Dex to record the patients’ vitals (heart rate [HR], mean arterial pressure [MAP]), arterial SpO2, sedation score, visual analog scale (VAS), the first analgesic requirement time, patients satisfaction, plasma cortisol, and adverse effects (e.g., PO nausea and vomiting [PONV], pruritus).
Methods
After approval by the institute ethics committee and registration with the ClinicalTrials.gov (NCT03250494), this study was conducted in Ain-Shams university hospitals, from December 2014 to January 2016, on 75 female patients aged between 25 and 35 years old of the American Society of Anesthesiologists (ASA) physical status I and II scheduled for elective laparoscopic gynecological surgeries for infertility to determine whether there are any defects such as scar tissue, endometriosis, fibroid tumors and other abnormalities of the uterus, fallopian tubes and ovaries under general anesthesia. A written informed consent was obtained from all patients.
Patients were not admitted to the study if any of the following criteria were present: (1) patient's refusal, (2) duration of surgery more than 90 min, (3) allergy to any drugs of the study, (4) smokers, history of drug or alcohol abuse, (5) treatment with antidepressants, (6) history of diabetes or epilepsy, (7) history of chronic pain or daily intake of analgesics within 24 h before surgery, (8) treatment with systemic glucocorticoids within 4 weeks before surgery, and (9) impaired kidney or liver functions.
This study was designed to be a randomized, placebo-controlled, double-blinded parallel study. Patients were randomly allocated into 3 equal groups, group (I) (GI) (n = 25) each patient received Dulox capsule (60 mg) orally with sips of water 2 h preoperatively and 100 ml 0.9% sodium chloride solution (normal saline [NS]) IV infusion (IVI) over 15 min (Placebo), group (II) (GII) (n = 25) each patient received combined Dulox capsule (60 mg) orally with sips of water and Dex 0.1 mg/kg diluted in 100 ml 0.9% NS IVI over 15 min, 2 h preoperatively and group (III) (GIII) (control group) (n = 25) each patient received a placebo capsule identical to Dulox capsule and 100 ml 0.9% NS IVI over 15 min, as a placebo for Dex 2 h preoperatively. Dulox was presented as Cymbalta® capsules manufactured by Lilly del Caribe Inc. and Dex was presented as Dex Sodium Phosphate ampoules 8 mg in 2 ml. Randomization was done using computer-generated number table of random numbers in a 1:1 ratio and conducted using sequentially numbered, opaque, and sealed envelope. Active Dulox capsules were indistinguishable from placebo capsules and placebo capsules contained starch. The study drugs were prepared by the hospital pharmacy and follow-up of patients was conducted by the anesthesia residents not involved in any other part of the study.
During the preoperative anesthetic evaluation, patients were familiarized with 10 cm marked VAS for PO assessment of pain, where 0 cm defines no pain, and 10 cm defines the maximum intolerable pain. Patients were also assured that they would receive intramuscular injection of pethidine 0.5 mg/kg (to avoid respiratory depression that could result in hypoxia and respiratory arrest and to guarantee sustained prolonged release) once they experienced pain postoperatively (patients with VAS >3) in the gynecological ward. The gynecological ward nurses were familiar with sedation score, VAS, the first analgesic requirement time and adverse effects (e.g., PONV, pruritus) and recorded the scores and side effects on nursing records.
The general anesthesia technique was standardized for all the patients as well as monitors including 5 lead ECG, noninvasive blood pressure (NIBP) monitor, pulse oximetry and capnography after intubation using Datascope monitors. Neuromuscular function was monitored using a peripheral nerve stimulator. After establishing an IV line, induction of general anesthesia with fentanyl (2 μg/kg) and sleeping dose of propofol followed by rocuronium (0.6 mg/kg) to facilitate orotracheal intubation was done. Anesthesia was maintained with oxygen 50% in air and sevoflurane 1%–2%. Ranitidine (50 mg/ampoule) was given diluted in 10 ml 0.9% sodium chloride solution (NS) slowly (IV) over 10 min as a gastroprotective regimen. At the end of the surgery, the residual neuromuscular paralysis was antagonized with neostigmine (0.05 mg/kg) and atropine (0.01 mg/kg). After satisfactory recovery, patients were extubated and transferred to the postanesthesia care unit (PACU) where they were monitored with ECG, NIBP, and pulse oximetry.
Assessment of patients’ vitals (HR, MAP), arterial SpO2, sedation score, VAS, the first analgesic requirement time, and adverse effects (e.g., PONV, pruritus) were done at 30 min, 1 h, 2 h, 6 h, and 12 h postoperatively.
PO pain was evaluated at rest based on visual analogue scale, first time to ask for rescue analgesia and total pethidine requirements in 12 hours (mg) postoperatively were recorded. Assessment of sedation was according to sedation score (Ramsay sedation scale)[14] [Table 1].
Table 1.
Ramsay sedation scale[14]
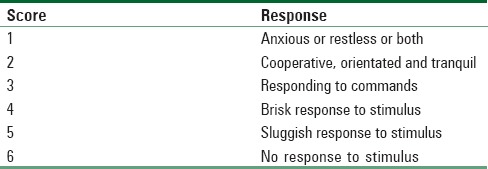
Hypotension was considered if there was 20% decrease below the baseline for MAP and was treated with IV bolus of ephedrine (3–6 mg). Bradycardia was considered if the heart rate <55 beats/min and was treated with IV atropine (0.01 mg/kg). Respiratory depression was defined as a respiratory rate <10 breaths/min or peripheral oxygen saturation <95% and was treated with oxygen through a transparent face mask and the intermittent doses of IV naloxone (0.4 mg). IV granisetron (1 mg) was given in case of vomiting or after 2 successive episodes of nausea. Pruritus was evaluated with a 4-point scale (0 = absent, 1 = mild, 2 = moderate, 3 = severe or requiring treatment), and patients with severe pruritus were treated with IV clemastine (TavegylR) (2 mg / ampoule) (produced by: NOVARTIS PHARMA S.A.E- Cairo Under license from Novartis Consumer Health, Schweiz AG, Bern Switzerland).
Patients satisfaction was done by asking the patient to answer the question, “How would you rate your experience after the surgery?” using a 7-point Likert verbal rating scale[15] [Figure 1] and acceptable satisfaction score of the patient being 5–7.
Figure 1.

A 7-point Likert-like verbal rating scale[15] for assessment of patients satisfaction
Hormonal stress response was assessed through recording plasma cortisol (μg/dl) 2 h postoperatively. Serum cortisol was measured by a Fluorescence Polarization Immunoassay Technology by the Abbott AXSYM system with the following reference ranges (morning serum cortisol 4.2–38.4 μg/dl) and evening serum cortisol 1.7–16.6 μg/dl).
Analysis of data
Using PASS 11 for sample size calculation, (NCSS, LLC. Kaysville, Utah, USA) in a one-way ANOVA study, it was calculated that a sample size of 22 patients per group will achieve 80% power to detect a mean difference of 50 mg in total pethidine consumption with a standard deviation (SD) of 25 between the three groups using a F-test with a 0.05 significance level. Twenty-five patients per group were intended to be included to replace any dropouts.[16,17]
The collected data were coded, tabulated, and statistically analyzed using IBM Statistical Package for Social Sciences statistics software version 22.0, IBM Corp., Chicago, USA, 2013.
Descriptive statistics were done for quantitative data as minimum and maximum of the range as well as mean ± SD for quantitative normally distributed data, median and 1st and 3rd interquartile range for quantitative non-normally distributed data, while it was done for qualitative data as number and percentage.
Inferential analyses were done for quantitative variables using ANOVA test for more than two independent groups with normally distributed data with post hoc Bonferroni test and Kruskal–Wallis Test for more than two independent groups with non-normally distributed data with post hoc Dunn's test. In qualitative data, inferential analyses for independent variables were done using Fisher's exact test for variables with small expected numbers. The level of significance was taken at P < 0.050 as significant, otherwise as nonsignificant.
Results
Ninety patients were examined for eligibility, out of which 75 patients completed the study and were randomized (25 patients for each group), and their data were included in the final analysis. Fifteen patients were excluded from this study on account of patients did not meet inclusion criteria (9 patients) and patient's refusal (6 patients) [Figure 2].
Figure 2.
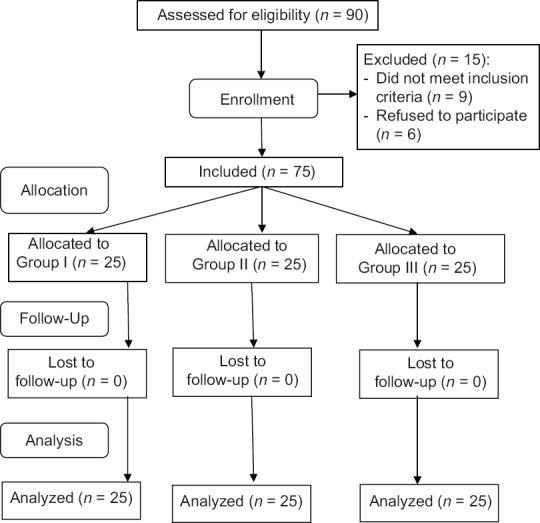
Flowchart of patients (study design)
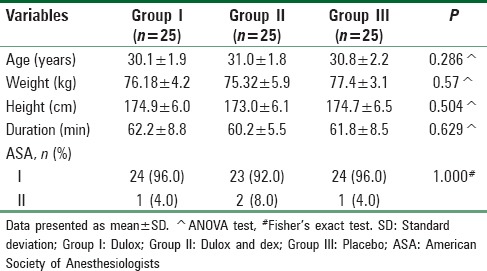
Results of the current study did not show any significant differences in the demographic data of the groups of patients as regard age, body weight, height, ASA physical status, and the duration of surgery in minutes as shown in Table 2.
Table 2.
The demographic data
There was a significant reduction in the mean HR in GI and GII compared to GIII at 30 min, 1 h, 2 h, and 6 h postoperatively as shown in (P < 0.001) [Figure 3].
Figure 3.
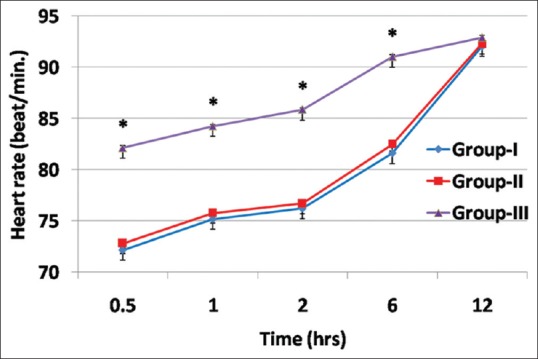
Comparison between study groups regarding heart rate
There was a significant reduction in the MAP in the GI and GII compared to GIII at 30 min, 1 h, 2 h, and 6 h postoperatively as shown in (P < 0.001) [Figure 4].
Figure 4.
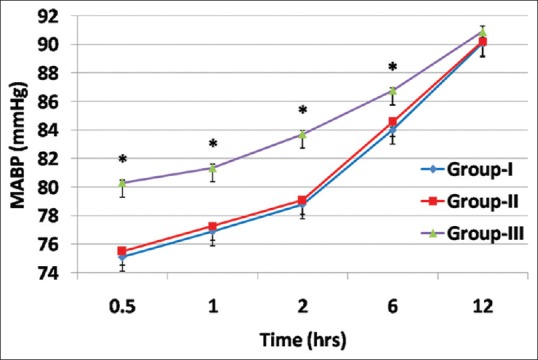
Comparison between study groups regarding mean arterial pressure
No significant changes were noted in the SpO2 between the studied groups throughout the study period (P > 0.05).
There were significant lower recorded values in visual analog scale in GI and GII in comparison to G III at 30 min, 1 h, 2 h, and 6 h postoperatively (P < 0.001) [Figure 5].
Figure 5.
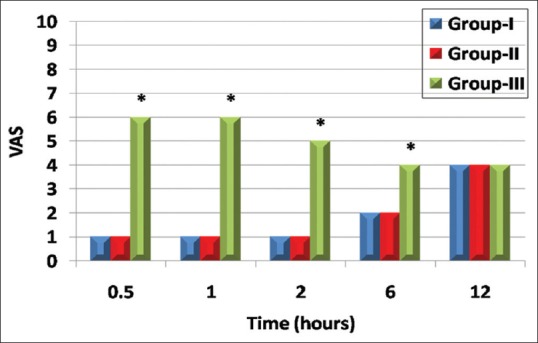
Comparison between study groups regarding visual analog scale score
Sedation score was significantly higher in GI and GII than in GIII at 30 min, 1 h, 2 h, and 6 h postoperatively (P < 0.001) [Figure 6].
Figure 6.
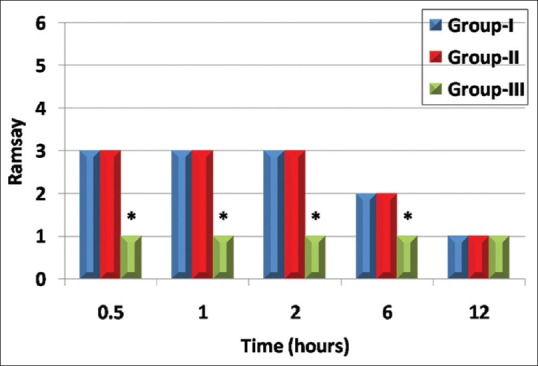
Comparison between study groups regarding Ramsay sedation score
Serum cortisol levels showed nonsignificant difference among the study groups 2 h preoperatively (P = 0.904), then showed significant decrease postoperatively in comparison to preoperative values in GI and GII (P < 0.001) and a significant decrease between GI and GII compared to GIII postoperatively (P < 0.001).[Table 3].
Table 3.
Comparison between the study groups regarding serum cortisol level (μg/dl)

PO significant differences between GI and GII in comparison to GIII as regard the time for 1st rescue analgesic (minutes) (P < 0.001) were present, and it was significantly more in GII compared to GI (P < 0.001) [Table 4]. The total pethidine requirements (mg) 12 h postoperatively were significantly less in GI and GII compared to GIII (P < 0.001) and nonsignificantly less in GII compared to GI. The patients satisfaction score was significantly more in GI and GII compared with GIII (P < 0.001) with no statistical difference between GI and GII [Table 4].
Table 4.
Comparison between the study groups as regards postoperative data

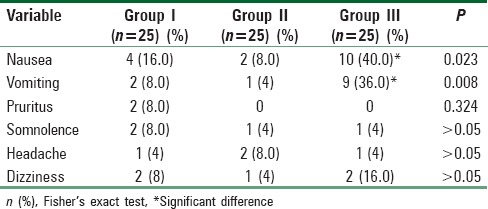
Regarding side effects, all cases of the three groups were hemodynamically stable, no patient developed hypoxia, and there were no reported intraoperative complications interfering with the course of surgery or interrupting the surgeons [Table 5].
Table 5.
Comparison between study groups regarding postoperative side effects
Discussion
The present study demonstrated that the postoperative time for 1st rescue analgesic was significantly high in GI and GII compared to GIII and in GII compared to GI. There was a significant less VAS score, HR, MAP and a high sedation score in GI and GII compared to GIII at 30 min, 1, 2, and 6 h postoperatively. Total pethidine requirements were significantly less in GI and GII compared to GIII 12 h postoperatively. There was a significant reduction in the 2 h PO serum cortisol (μg/dl) and significant increase in the PO patients satisfaction score in GI and GII compared to GIII. PONV was decreased significantly in GII compared to GI and GIII.
As regard PO side effects in this study, all cases of the three groups were hemodynamically stable, no patients developed hypoxia and this study showed there was not any significant difference between GI, GII, and GIII as regard somnolence, headache, and dizziness at any time postoperatively (P > 0.05). The most common side effects experienced by subjects in this study were PONV [Table 5]. GII developed nonsignificant PO nausea and vomiting less than group (I). All affected patients responded to granisetron (1 mg IV). Only two patients in GI (P > 0.05) developed pruritus, and all affected patients responded to IV clemastine (TavegylR) (2 mg/ampoule). The leaflet of the consumer medicine information of CYMBALTA® used in this clinical trial documented that pruritus is uncommon side effect which supports the results of this study.
Opioid-related side effects, pain, and emotional factors may be factors responsible for poor quality of postsurgical recovery in women.[18,19] The ASA recommended the use of different pharmacological modalities for PO pain management in spite of variation due to the patient, the setting and the surgical procedure.[20]
The dose of dulox was based on the previous study conducted by Ho et al.,[21] administering preoperative Dulox 60 mg that was efficacious in decreasing morphine requirements 48 h after knee replacement surgery and the same dose of Dulox was used for chronic neuropathic pain.[22]
Because of Dex painful perineal burning sensation in 50%–70% of patients when injected IV, Dex was administered diluted in 100 ml 0.9% sodium chloride solution (NS) IVI over 15 min, 2 h preoperatively.[23] De Oliveira et al. reported that administration of Dex (at doses >0.1 mg/kg) IV before surgery is an effective adjunct in multimodal strategies to reduce PO pain and opioid requirements after surgery.[24] Wang et al. evaluated the effect of timing of Dex administration on its efficacy as a prophylactic antiemetic on PONV and found that Dex when given before induction of anesthesia, was more better than when given at the end of anesthesia.[25]
Study results were supported with the findings of Saoud and Elkabarity,[26] Nasr[27] and Castro-Alves et al.,[5] who found that Dulox 60 mg orally 2 h before surgery improved PO convalescence and significantly decreased PO opioid consumption.
Concomitant with our results, Karst et al.,[28] Aminmansour et al.[29] and Kardash et al.,[30] concluded that the administration of a single dose of 40 mg IV Dex intraoperatively[28] or before surgery[29,30] significantly decreased opioid requirements after surgery and reduced PONV.[30]
Our results coincide with the study of Jno-Baptiste et al., who evaluated the effect of Dex (0.5 mg/kg and not >40 mg) 5 min after starting of anesthesia and before surgical incision on PO opioid consumption on 40 patients underwent gynecological surgery and found that, it was safe and decreased PO opioid requirements and increased the satisfaction of patients.[31]
Consistent with our study, Movafegh et al. reported the effectiveness of the intake of the IV Dex (0.1 mg/kg) before the intrathecal meperidine injection (15 mg) in the enhancement of analgesia and decreasing PONV.[32]
Agrawal et al. studied 60 patients underwent orthopedic surgeries under spinal anesthesia to find that preoperative intake of one dose of 300 mg pregabalin along with 16 mg Dex provided analgesic benefits superior to that of pregabalin alone, by reducing the requirement of rescue analgesia with nonsignificant reduction of 24 h PONV.[33]
These results were supported by the studies of Worni et al.,[34] Fukami et al.,[35] Mathiesen et al.[36] and Mohtadi et al.,[37] who reported that the intake of a single 8 mg Dex before surgery significantly decreased the PO pain score, the PO opioid requirements and significantly reduced the incidence of PONV.[34,35,36]
In concordant with our study, Waldron et al., compared forty-five studies involving 5796 patients receiving Dex 1.25–20 mg in adult patients undergoing general anesthesia and recorded pain outcomes. They concluded that a single IV perioperative dose of Dex had small but statistically significant analgesic benefits.[38]
Partially consistent with our study, Asad and Khan, found that Dex significantly reduced intraoperative rescue analgesia and nonsignificantly decreased PO rescue analgesia explained by the timing of intake of Dex which was before induction of anesthesia.[39]
In contrast to our study, Coloma et al. designed a placebo-controlled study on eighty patients to assess that a single dose of Dex 4 mg would facilitate discharge after outpatient anorectal surgery. It showed the nonsignificant difference in PO VAS score. It may be explained by lower dose of Dex than our study, also, nonsignificant difference in PO nausea compared with placebo, explained by timing of administration of Dex which was just before induction of anesthesia.[12]
Furthermore, in contrast to our study, the study conducted by Mathiesen et al., reported that Dex did not decrease morphine consumption and pain score compared with paracetamol alone for 160 patients undergoing abdominal hysterectomy. Dex and the use of ondansetron decreased PONV.[40]
Our results correlated with the study of Akural et al., who found that the median decreases in the cortisol concentration on the first PO day relative to the preoperative baseline were greater in the preemptive group than in the control group.[41]
This study proved that the use of a single oral Dulox 60 mg, 2 h preoperatively, prevented transient PO anxiety that is common in female patients[5] and there was not any significant difference between GI, GII and GIII as regard somnolence.
Gahimer et al. found that most people taking Dulox will have at least one adverse effect. These were mostly minor and were commonly nausea, headache, dizziness, constipation, and diarrhea.[42]
Our study presented several limitations
First, we only studied patients undergoing laparoscopic gynecological surgeries, and we should generalize our finding to different surgical procedures. The second limitation was that, we did not evaluate dose-response effects of Dulox on the results of the study and restricted to use the same dose as previous studies. The third limitation was to enlarge the sample size to achieve significant differences of side effects. The fourth limitation was that time to hospital discharge, an important outcome due to its economic implications and affected by the presence of PO pain should be taken in consideration in further randomized clinical trials.
The research team suggested that these results of this work justified the conduct of a larger size, double blinded randomized controlled trial including the use of Dulox- Dex combination as a component of a multimodal analgesic system, evaluating different doses of Dulox and Dex to provide an effective PO analgesic regimen.
Conclusion
Administration of a single dose of oral Dulox 60 mg 2 h preoperatively along with Dex 0.1 mg/kg IV just before induction of anesthesia provided analgesic benefits superior to that of Dulox 60 mg oral alone, by reducing the requirement of rescue analgesia. The combination significantly reduced the side effects in the PO 12 h.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Desborough JP. The stress response to trauma and surgery. Br J Anaesth. 2000;85:109–17. doi: 10.1093/bja/85.1.109. [DOI] [PubMed] [Google Scholar]
- 2.Habib AS, Gan TJ. Role of analgesic adjuncts in postoperative pain management. Anesthesiol Clin North America. 2005;23:85–107. doi: 10.1016/j.atc.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Carter NJ, McCormack PL. Duloxetine: A review of its use in the treatment of generalized anxiety disorder. CNS Drugs. 2009;23:523–41. doi: 10.2165/00023210-200923060-00006. [DOI] [PubMed] [Google Scholar]
- 4.Wang SY, Calderon J, Kuo Wang G. Block of neuronal Na+ channels by antidepressant duloxetine in a state-dependent manner. Anesthesiology. 2010;113:655–65. doi: 10.1097/ALN.0b013e3181e89a93. [DOI] [PubMed] [Google Scholar]
- 5.Castro-Alves LJ, Oliveira de Medeiros AC, Neves SP, Carneiro de Albuquerque CL, Modolo NS, De Azevedo VL, et al. Perioperative duloxetine to improve postoperative recovery after abdominal hysterectomy: A Prospective, randomized, double-blinded, placebo-controlled study. Anesth Analg. 2016;122:98–104. doi: 10.1213/ANE.0000000000000971. [DOI] [PubMed] [Google Scholar]
- 6.Bymaster FP, Lee TC, Knadler MP, Detke MJ, Iyengar S. The dual transporter inhibitor duloxetine: A review of its preclinical pharmacology, pharmacokinetic profile, and clinical results in depression. Curr Pharm Des. 2005;11:1475–93. doi: 10.2174/1381612053764805. [DOI] [PubMed] [Google Scholar]
- 7.Fric M, Pfuhlmann B, Laux G, Riederer P, Distler G, Artmann S, et al. The influence of smoking on the serum level of duloxetine. Pharmacopsychiatry. 2008;41:151–5. doi: 10.1055/s-2008-1073173. [DOI] [PubMed] [Google Scholar]
- 8.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 9.Schleimer RP. An overview of glucocorticoid anti-inflammatory actions. Eur J Clin Pharmacol. 1993;45(Suppl 1):S3–7. doi: 10.1007/BF01844196. [DOI] [PubMed] [Google Scholar]
- 10.Ferreira SH, Cunha FQ, Lorenzetti BB, Michelin MA, Perretti M, Flower RJ, et al. Role of lipocortin-1 in the anti-hyperalgesic actions of dexamethasone. Br J Pharmacol. 1997;121:883–8. doi: 10.1038/sj.bjp.0701211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bisgaard T, Klarskov B, Kehlet H, Rosenberg J. Preoperative dexamethasone improves surgical outcome after laparoscopic cholecystectomy: A randomized double-blind placebo-controlled trial. Ann Surg. 2003;238:651–60. doi: 10.1097/01.sla.0000094390.82352.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coloma M, Duffy LL, White PF, Kendall Tongier W, Huber PJ., Jr Dexamethasone facilitates discharge after outpatient anorectal surgery. Anesth Analg. 2001;92:85–8. doi: 10.1097/00000539-200101000-00017. [DOI] [PubMed] [Google Scholar]
- 13.Sauerland S, Nagelschmidt M, Mallmann P, Neugebauer EA. Risks and benefits of preoperative high dose methylprednisolone in surgical patients: A systematic review. Drug Saf. 2000;23:449–61. doi: 10.2165/00002018-200023050-00007. [DOI] [PubMed] [Google Scholar]
- 14.Liu LL, Gropper MA. Postoperative analgesia and sedation in the adult Intensive Care Unit: A guide to drug selection. Drugs. 2003;63:755–67. doi: 10.2165/00003495-200363080-00003. [DOI] [PubMed] [Google Scholar]
- 15.Streiner DL, Norman GR. Oxford, England, UK: Oxford iversity Press; 1995. Health measurement scales: A practical guide to their development and use; pp. 28–53. [Google Scholar]
- 16.Hintze J. PASS 11. Kaysville, Utah, USA: NCSS, LLC; 2011. [Google Scholar]
- 17.Machin D, Campbell M, Fayers P, Pinol A. Sample Size Tables for Clinical Studies. 2nd ed. Oxford, England, UK: Blackwell Science Ltd; 1997. [Google Scholar]
- 18.De Oliveira GS, Bialek J, Fitzgerald P, Kim JY, McCarthy RJ. Systemic magnesium to improve quality of post-surgical recovery in outpatient segmental mastectomy: A randomized, double-blind, placebo-controlled trial. Magnes Res. 2013;26:156–64. doi: 10.1684/mrh.2014.0349. [DOI] [PubMed] [Google Scholar]
- 19.De Oliveira GS, Jr, Fitzgerald PC, Marcus RJ, Ahmad S, McCarthy RJ. A dose-ranging study of the effect of transversus abdominis block on postoperative quality of recovery and analgesia after outpatient laparoscopy. Anesth Analg. 2011;113:1218–25. doi: 10.1213/ANE.0b013e3182303a1a. [DOI] [PubMed] [Google Scholar]
- 20.Chou R, Gordon DB, de Leon-Casasola OA, Rosenberg JM, Bickler S, Brennan T, et al. Management of postoperative pain: A clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on regional anesthesia, executive committee, and administrative council. J Pain. 2016;17:131–57. doi: 10.1016/j.jpain.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Ho KY, Tay W, Yeo MC, Liu H, Yeo SJ, Chia SL, et al. Duloxetine reduces morphine requirements after knee replacement surgery. Br J Anaesth. 2010;105:371–6. doi: 10.1093/bja/aeq158. [DOI] [PubMed] [Google Scholar]
- 22.Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. 2014;1:CD007115. doi: 10.1002/14651858.CD007115.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neff SP, Stapelberg F, Warmington A. Excruciating perineal pain after intravenous dexamethasone. Anaesth Intensive Care. 2002;30:370–1. doi: 10.1177/0310057X0203000319. [DOI] [PubMed] [Google Scholar]
- 24.De Oliveira GS, Jr, Almeida MD, Benzon HT, McCarthy RJ. Perioperative single dose systemic dexamethasone for postoperative pain: A meta-analysis of randomized controlled trials. Anesthesiology. 2011;115:575–88. doi: 10.1097/ALN.0b013e31822a24c2. [DOI] [PubMed] [Google Scholar]
- 25.Wang JJ, Ho ST, Tzeng JI, Tang CS. The effect of timing of dexamethasone administration on its efficacy as a prophylactic antiemetic for postoperative nausea and vomiting. Anesth Analg. 2000;91:136–9. doi: 10.1097/00000539-200007000-00025. [DOI] [PubMed] [Google Scholar]
- 26.Saoud AF, Elkabarity R. Effect of perioperative duloxetine on postoperative pain relief following anterior cervical microdiscectomy and fusion. A pilot study. World Spinal Column J. 2013;2:57–66. [Google Scholar]
- 27.Nasr DA. Efficacy of perioperative duloxetine on acute and chronic post-mastectomy pain. Ain Shams J Anesthesiol. 2014;7:129–33. [Google Scholar]
- 28.Karst M, Kegel T, Lukas A, Lüdemann W, Hussein S, Piepenbrock S, et al. Effect of celecoxib and dexamethasone on postoperative pain after lumbar disc surgery. Neurosurgery. 2003;53:331–6. doi: 10.1227/01.neu.0000073530.81765.6b. [DOI] [PubMed] [Google Scholar]
- 29.Aminmansour B, Khalili HA, Ahmadi J, Nourian M. Effect of high-dose intravenous dexamethasone on postlumbar discectomy pain. Spine (Phila Pa 1976) 2006;31:2415–7. doi: 10.1097/01.brs.0000238668.49035.19. [DOI] [PubMed] [Google Scholar]
- 30.Kardash KJ, Sarrazin F, Tessler MJ, Velly AM. Single-dose dexamethasone reduces dynamic pain after total hip arthroplasty. Anesth Analg. 2008;106:1253–7. doi: 10.1213/ANE.0b013e318164f319. [DOI] [PubMed] [Google Scholar]
- 31.Jno-Baptiste B, Scarlett MD, Harding HE. The effect of dexamethasone on postoperative opioid requirement in patients who underwent gynecology surgery at the university hospital in Jamaica. J Anesth Clin Res. 2014;5:470. [Google Scholar]
- 32.Movafegh A, Soroush AR, Navi A, Sadeghi M, Esfehani F, Akbarian-Tefaghi N, et al. The effect of intravenous administration of dexamethasone on postoperative pain, nausea, and vomiting after intrathecal injection of meperidine. Anesth Analg. 2007;104:987–9. doi: 10.1213/01.ane.0000257926.07491.55. [DOI] [PubMed] [Google Scholar]
- 33.Agrawal R, Mehta M, Patel J. An observational study to compare the effect of pregabalin with pregabalin and dexamethasone for post-operative analgesia in orthopedic surgeries under spinal anesthesia. IAIM. 2016;3:146–55. [Google Scholar]
- 34.Worni M, Schudel HH, Seifert E, Inglin R, Hagemann M, Vorburger SA, et al. Randomized controlled trial on single dose steroid before thyroidectomy for benign disease to improve postoperative nausea, pain, and vocal function. Ann Surg. 2008;248:1060–6. doi: 10.1097/SLA.0b013e31818c709a. [DOI] [PubMed] [Google Scholar]
- 35.Fukami Y, Terasaki M, Okamoto Y, Sakaguchi K, Murata T, Ohkubo M, et al. Efficacy of preoperative dexamethasone in patients with laparoscopic cholecystectomy: A prospective randomized double-blind study. J Hepatobiliary Pancreat Surg. 2009;16:367–71. doi: 10.1007/s00534-009-0079-5. [DOI] [PubMed] [Google Scholar]
- 36.Mathiesen O, Jørgensen DG, Hilsted KL, Trolle W, Stjernholm P, Christiansen H, et al. Pregabalin and dexamethasone improves post-operative pain treatment after tonsillectomy. Acta Anaesthesiol Scand. 2011;55:297–305. doi: 10.1111/j.1399-6576.2010.02389.x. [DOI] [PubMed] [Google Scholar]
- 37.Mohtadi A, Nesioonpour S, Salari A, Akhondzadeh R, Masood Rad B, Aslani SM, et al. The effect of single-dose administration of dexamethasone on postoperative pain in patients undergoing laparoscopic cholecystectomy. Anesth Pain Med. 2014;4:e17872. doi: 10.5812/aapm.17872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waldron NH, Jones CA, Gan TJ, Allen TK, Habib AS. Impact of perioperative dexamethasone on postoperative analgesia and side-effects: Systematic review and meta-analysis. Br J Anaesth. 2013;110:191–200. doi: 10.1093/bja/aes431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Asad MV, Khan FA. Effect of a single bolus of dexamethasone on intraoperative and postoperative pain in unilateral inguinal hernia surgery. J Anaesthesiol Clin Pharmacol. 2015;31:339–43. doi: 10.4103/0970-9185.161669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mathiesen O, Rasmussen ML, Dierking G, Lech K, Hilsted KL, Fomsgaard JS, et al. Pregabalin and dexamethasone in combination with paracetamol for postoperative pain control after abdominal hysterectomy. A randomized clinical trial. Acta Anaesthesiol Scand. 2009;53:227–35. doi: 10.1111/j.1399-6576.2008.01821.x. [DOI] [PubMed] [Google Scholar]
- 41.Akural EI, Salomäki TE, Tekay AH, Bloigu AH, Alahuhta SM. Pre-emptive effect of epidural sufentanil in abdominal hysterectomy. Br J Anaesth. 2002;88:803–8. doi: 10.1093/bja/88.6.803. [DOI] [PubMed] [Google Scholar]
- 42.Gahimer J, Wernicke J, Yalcin I, Ossanna MJ, Wulster-Radcliffe M, Viktrup L, et al. A retrospective pooled analysis of duloxetine safety in 23,983 subjects. Curr Med Res Opin. 2007;23:175–84. doi: 10.1185/030079906X162719. [DOI] [PubMed] [Google Scholar]


