Abstract
Objective
The purpose of this study was to compare the efficacy of continuous low pressure support (PSV) and T-piece as strategies for discontinuation of mechanical ventilation and extubation in a surgical ICU.
Patients and Methods
This was a prospective open label randomized control study in surgical ICU patients who were intubated, mechanically ventilated, and who met criteria for a spontaneous breathing trial. Eligible, enrolled patients were randomized to receive low-level pressure supportup to 7 cmH2O (PSV) or T-piece as the mode of their spontaneous breathing trial.
Results
A total of 520 patients were randomized (260 in PSV group and 260 in T-piece group). There were no differences between the groups in baseline characteristics except duration of MV before trial was longer in PSV group. There were also no differences in hemodynamic and respiratory measures between groups. The PSV had a significant higher number of SBT attempt before success and extubation. After extubation, the re-intubation within 48 hours had a lower trend in PSV group (PSV vs. T-piece: 10% vs. 14.6%; p=0.11). The pneumonia occurrence, hospital mortality, hospital and ICU length of stay were not significant different between groups. In multivariable analysis, PSV was associated with a lower risk of success at the first SBT (adjusted relative risk, RR 0.79 [95% confidence interval, CI, 0.70 - 0.88]; p<0.001], and a lower risk of re-intubation within 48 hours after extubation (adjusted RR 0.62 [95%CI 0.40 - 0.98]; p=0.04). There were no differences between groups in pneumonia after extubation and in hospital mortality rate.
Conclusion
Although PSV needs a higher number of SBT trial before success and extubation, the re-intubation within 48 hours is lower than T piece. However, there were no differences between the groups in term of pneumonia after extubation, hospital mortality as well as ICU and hospital length of stay.
Keywords: Spontaneous breathing trial, Weaning of mechanical ventilator, T piece method, Pressure support, Reintubation
1. INTRODUCTION
In one cross-sectional survey, about 80 - 90% of ICU patients had received Mechanical ventilation (MV) (1). Of these, about 20-40 percent had undergone the process of discontinuation of MV(1). In addition, the time spent during the weaning process accounts for 41% of total ventilator time (2). The most commonly utilized modes of weaning from mechanical ventilation in surgical intensive care unit are T-piece, pressure support, and synchronous intermittent mandatory ventilation (SIMV) (1, 3-6). Esteban et al performed a randomized controlled study to compare T-piece and low pressure support ventilation (7 cm H2O) as modes of a spontaneous breathing trial (SBT). They found that the percentage of patients who had a successful SBT was significantly higher in the pressure support group but, after a successful SBT and extubation, there was no difference between the groups in the rate of reintubation (7). However, the extubation outcomes were followed only after the first successful SBT. Outcomes after subsequent SBTs were excluded. In addition, the study population contained only 19 percent surgical patients. Therefore, the purpose of this study was to test the hypothesis that use of pressure support is non-inferior to T-piece in terms of the success of an SBT in critically ill post-operative patients. Secondary outcomes were the occurrence of post-extubation pneumonia, re-intubation within 48 hours after extubation, hospital mortality, and ICU and hospital length of stay.
2. MATERIALS AND METHODS
Participants and interventions
All post-operative patients who were 18 years of age or older, and intubated and mechanically ventilated (MV) for at least 12 hours in the general surgical intensive care unit (ICU) were considered for enrollment in this study.Additional inclusion criteria were: the hemodynamic, respiratory, and metabolic causes, which were the causes of ventilator use, were corrected, alert and able to follow commands (Glasgow Coma Score higher than 13) with low analgesic requirement and numeric rating scale of pain score less than 5, not receiving muscle relaxants, effective cough, hemodynamic stable (systolic blood pressure between 90-160 mmHg and heart rate less than 120 beat per minute, on very low dose of vasopressor/or inotropic agents with intention to discontinue in a short period, low levels of respiratory support, near normal and stable arterial blood gas profile, serum hemoglobin concentration greater than 8 g/dL, and ability to tolerate a three minute trial of low level pressure support (5-7 cm- H2O). Patients were excluded from the study if any of the following criteria were present: intractable or persistent hypotension and expected to die, ventilator dependence due to any causes of chronic respiratory diseases or uncontrolled chronic respiratory diseases, heart failure and/or myocardial infarction, uncorrected anemia, neuromuscular diseases or central nervous system defect, andinability to obtain consent. After ensuring that the patient met all inclusion criteria and did not have any exclusion criteria, written informed consent was obtained from the patient. The institutional review board and Ethics committee of our hospital approved the study protocol (SUR110606A13). The registration trial number was TCTR 20130921001 (www.clinicaltrials.in.th).
After patient consent, the patients were randomized to receive either ongoing low pressure support or T- piece as the mode for a SBT. The attending physicians dispensed the SBT modes according to a randomization sequence list containing in sealed, opaque, sequential numbered envelopes. The randomization sequence list was developed using a computer random number generator to select random sequences of permuted blocks of four. Based on 0.8 power to detect a significant difference (P=0.05, two sided) with 0.01 non-inferiority margin, 223 patients were required for each study group. To compensate for 15 percent unexpected patient loss to follow up or protocol violations, 257 patients were required per group. Therefore, this study planned to enroll 260 patients per group.
Due to the nature of the interventions they could not be blinded. The SBT modes were set up and maintained by trained ICU nurses. In the pressure support group, the ventilator was continued on the pressure support mode (inspiratory pressure 5- 7 cmH2O, PEEP 5 cm- H2O, FiO2 0.4, expiration triggered at 25% of peak inspiratory flow rate). In the T-piece group, the ventilator was stopped and the endotracheal tube was connected to a T–tube circuit with oxygen flow at 10-15 liter per minute. All patients underwent a SBT for up to 120 minutes. Success was defined as meeting all of these criteria at the end of the SBT: respiratory rate less than 30 breaths/minute, oxygen saturation greater than 90%, heart rate less than 120 beats per minute or less than 20% change from baseline without serious arrhythmias, systolic blood pressure between 90 and 160 mmHg, alert level of consciousness, and rapid shallow breathing index (RSBI: a ratio of respiratory rate and tidal volume in liter) less than 105; patients who had a successful SBT were considered for extubation (3). On the other hand, if the patient had any sign of respiratory insufficiency, unstable hemodynamic measures, decreased level of consciousness, agitation, diaphoresis, or anxiety during the SBT, fully-supported mechanical ventilation of pressure or volume assisted control ventilation mode was reinstituted. Causes of SBT failure were identified and corrected, if possible. Patients who did not succeed during their first SBT were evaluated daily and SBT trial until success and extubation. After extubation, the patients received supplemental oxygen 10-15 liters/min by face mask with reservoir. Non-invasive mechanical ventilation (NIV) was not allow after extubation.
Clinical monitoring and outcomes
All of the clinical measures and outcomes were collected by nurses, and physicians who were not involved in the study. Arterial blood gases (ABG) were measured at the time of enrollment and at the end of a successful SBT. In the case of SBT failure according to clinical criteria, ABG were not measured. The respiratory rate, heart rate, systolic and diastolic blood pressure, and oxygen saturation measured by pulse oximetry were recorded at the time of enrollment, during the SBT, and after extubation at 5, 30 and 60 minute. Tidal volume was recorded at the time of enrollment, after the 3 minute trial of PSV, and after the SBT. When the patient was receiving support from the mechanical ventilator, tidal volume was obtained from the ventilator data report. If the patient was breathing on a T-piece mode, tidal volume was obtained from a spirometer.
A successful extubation was defined as the patient who could be extubated without re-intubation within 48 hours after extubation. Causes of re-intubation were categorized into five groups: airway causes (post-extubation stridor and secretion problems), pulmonary causes (hypoxemia and hypercapnic respiratory failure), metabolic causes (metabolic acidosis or electrolyte imbalance), cardiac causes (new-onset of cardiac events such as unstable angina, myocardial infarction and severe arrhythmia), and neurological causes (decreased sensorium, new stroke, or muscle weakness not due to electrolyte imbalance). Pneumonia was defined as the new occurrence of pneumonia after recruitment. The diagnosis was defined by clinical suspicion, microbiological culture by endobronchial aspiration, and confirmation by the attending staff. Survival status was followed until each patient was hospital discharged. The length of ICU stay was defined as time from admission in ICU until transfer to a non-critical care unit and the length of hospital stay was defined as time from admission to either discharge from the hospital or death.
Statistical analysis
The outcomes of all enrolled patients after randomization regardless of number of SBT trialuntil extubation were analyzed in an intention to treat analysis (ITT). The outcomes of the group of patients who succeed the first spontaneous breathing trial and extubation were analyzed as per-protocol analysis (PP). All continuous variable data was reported as mean and standard deviation (SD) or median and inter-quartile range (IQR). Group differences were calculated using Student’s t test or Mann-Whitney U test, and Pearson’s chi-square. Multivariable regression models were developed to control for imbalance of confounding demographic. These results were reported as relative risk (RR) or co-efficient difference and 95% confidence interval (95% CI). Statistical significance was defined as a p-value less than 0.05.
3. RESULTS
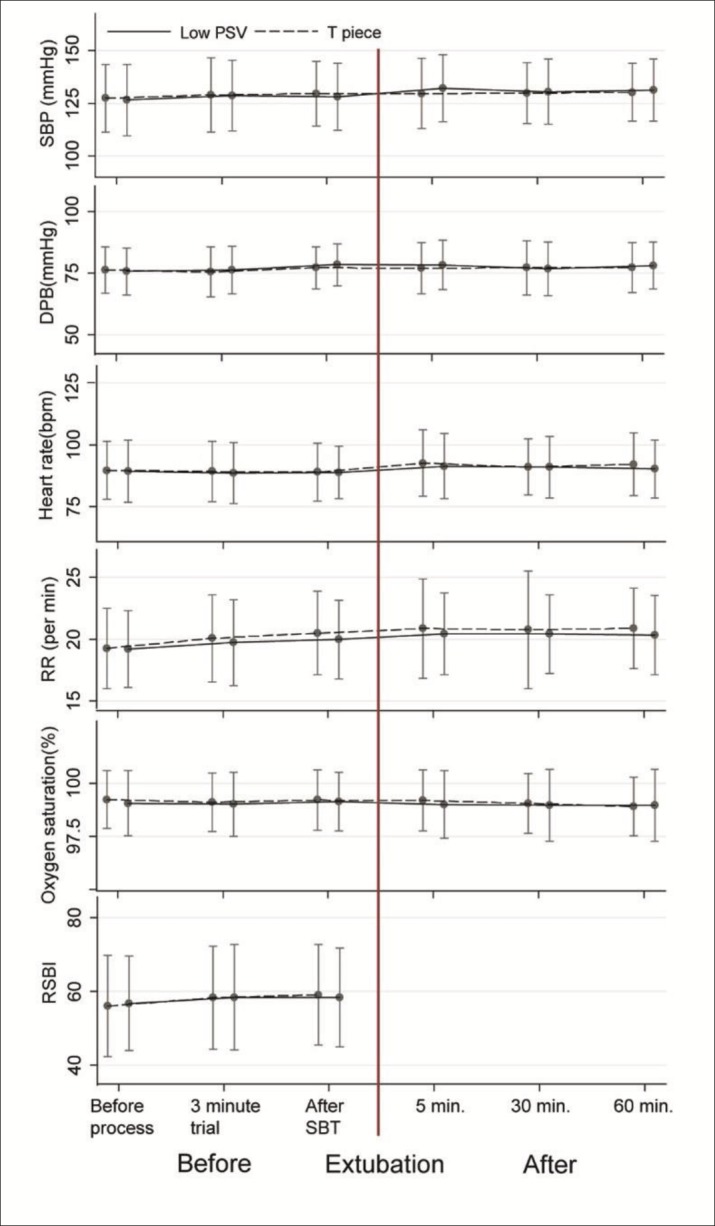
From 1st June 2011 to 30th November 2013, a total of 704 patients were admitted to the study ICU and were mechanically ventilated. Of these, 184 patients were excluded (Figure 1). The remaining 520 patients were randomized into 260 patients each arm. All patients remained in the study - there were not drop outs after randomization. There were no differences in demographic data or arterial blood gas results (at enrollment and at the end of SBT) between groups except for the duration of ventilator support before starting the SBT (Table 1). The PSV group had a longer period of mechanical ventilation before the SBT median (IQR) 31(18 - 60.5) hours vs. 26(15.5 - 46) hours in the T-piece group (p=0.023). There were no differences between the two groups in respiratory and hemodynamic measurements including systolic blood pressure, diastolic blood pressure, heart rate, respiratory rate, oxygen saturation, and RSBI at the time of enrollment, after the 3-minute trial of PSV, or after the last SBT but before extubation (Figure 2). In addition, there were no differences between the groups in blood pressure, heart rate, respiratory rate, or oxygen saturation at 5, 30, and 60 minutes after extubation (Figure 2).
Figure 1. Study flow. Abbreviation: SBT, Spontaneous breathing trial.
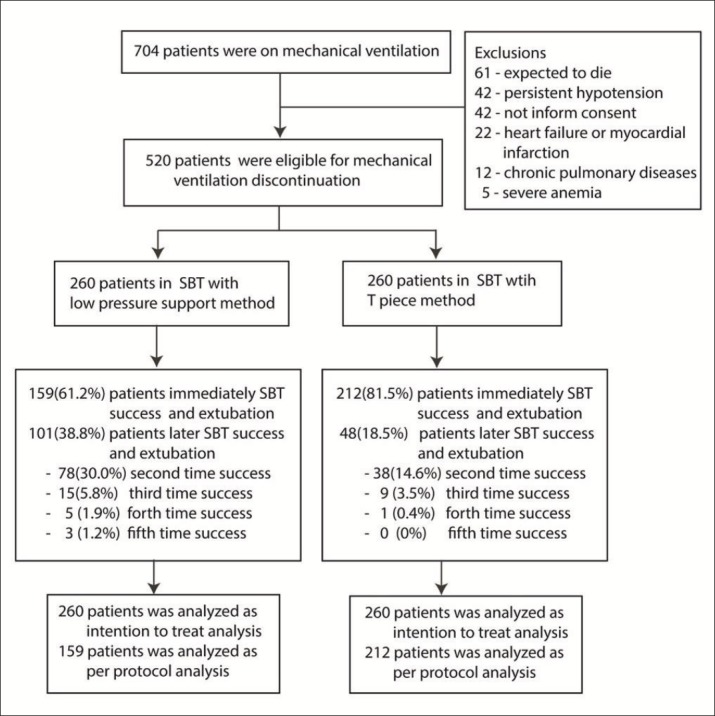
Table 1. Baseline characteristics. Abbreviations: ABG, arterial blood gas, IQR, interquartile range, SBT, spontaneous breathing trial.
| All (n=520) | T piece (n=260) | Low PSV (n=260) | P value | |
|---|---|---|---|---|
| Age, year, median (IQR) | 56(45 – 66) | 56(46 – 65) | 56(45 – 67) | 0.775 |
| BMI, kg/m2, median(IQR) | 21.0(19.3 – 22.9) | 21.2 (19.5 – 22.8) | 20.8 (18.8 – 23.1) | 0.419 |
| Male, n (%) | 295(56.73) | 142(54.62) | 153(58.85) | 0.330 |
| Duration of ventilator support before trial recruitment, hour, median(IQR) | 28(17 – 56) | 26(15.5 – 46) | 31(18 - 60.5) | 0.023 |
| 12 – 24 hours (%) | 88 (16.92) | 53 (20.38) | 35 (13.46) | 0.109 |
| 24 – 48 hours (%) | 141(27.12) | 68 (26.15) | 73 (28.08) | |
| > 48 hours (%) | 291(55.96) | 139 (53.46) | 152 (58.46) | |
| Primary surgical disorders, n (%) | ||||
| Gastrointestinal | 128(24.62) | 77(29.62) | 51(19.62) | 0.060 |
| Hepato-biliary-pancreas | 119(22.88) | 56(21.54) | 63(24.23) | |
| Vascular | 93(17.88) | 45(17.31) | 48(18.46) | |
| Soft tissue | 42(8.08) | 20(7.69) | 22(8.46) | |
| Chest (Non-cardiac) | 63(12.12) | 23(8.85) | 40(15.38) | |
| Others | 75(14.42) | 39(15.00) | 36(13.85) | |
| Comorbidity (%) | ||||
| Hypertension | 186 (35.77) | 90 (34.62) | 96 (36.92) | 0.583 |
| Asthma | 26 (5.00) | 14 (5.38) | 12 (4.62) | 0.687 |
| COPD | 41 (7.88) | 19 (7.31) | 22 (8.46) | 0.625 |
| Diabetes mellitus | 108 (20.77) | 49 (18.85) | 59 (22.69) | 0.280 |
| Chronic renal failure | 95 (18.27) | 44 (16.92) | 51 (19.62) | 0.427 |
| Malignancy | 112 (21.54) | 53 (20.38) | 59 (22.69) | 0.522 |
| HIV | 11 (2.12) | 5 (1.92) | 6 (2.31) | 0.761 |
| Severity, median (IQR) | ||||
| Charlson’s comorbidity index | 10(8 – 12) | 10(8 – 12) | 10(8 – 12) | 0.593 |
| APACHE II score | 11(9 – 13) | 11(9 – 13) | 11(9 – 13) | 0.316 |
| ABG at enrolling, median(IQR) | ||||
| pH | 7.40(7.37 – 7.42) | 7.40(7.37 – 7.42) | 7.40(7.38 – 7.42) | 0.419 |
| PaO2 | 177(113 – 219) | 179(115 – 216) | 176 (110 – 223) | 0.964 |
| PaCO2 | 38 (35 – 41) | 38 (35 – 41) | 38 (35 – 41) | 0.331 |
| HCO3 | 24 (22 – 28) | 24 (22 – 28) | 24 (22 – 28) | 0.622 |
| PF ratio | 443(282 – 547) | 446(288 -541) | 440(275 – 557) | 0.964 |
| ABG and respiratory parameter at the end of SBT, median(IQR) | ||||
| pH | 7.40(7.37 – 7.42) | 7.40(7.37 – 7.42) | 7.40(7.37 – 7.42) | 0.964 |
| PaO2 | 180(132 – 235) | 178 (140 – 229) | 189 (125 – 244) | 0.648 |
| PaCO2 | 38 (36 – 41) | 38 (36 – 41) | 38 (35 – 41) | 0.473 |
| HCO3 | 24(22 – 27) | 24(22 – 27) | 24(22 – 27.5) | 0.832 |
| PF ratio | 450(330 - 587) | 445(350 - 572) | 472(313 - 610) | 0.648 |
| Tidal volume, mL | 348(310 – 390) | 350(310 – 390) | 340(310 – 390) | 0.487 |
| Respiratory rate, per minute | 20(18 – 22) | 20(18 – 22) | 20(18 – 22) | 0.136 |
| Minute volume, L | 7.0 (6.1 - 8.1) | 7.2(6.2 – 8.1) | 6.8 (5.8 – 8.1) | 0.101 |
| RSBI | 57.9(49.3 – 66.7) | 58.1 (49.9 – 68.6) | 57.5(48.8 – 66.3) | 0.713 |
Figure 2. Respiratory and hemodynamic parameters before and after extubation between groups at the last successful extubation. Abbreviations: SBP, Systolic blood pressure; DBP, Diastolic blood pressure; RR, Respiratory rate; RSBI, Rapid shallow breathing index; SBT, Spontaneous breathing trial.
After the first extubation (regardless of how many SBTs the patient had), a total of 64/520 patients (12.3%) required reintubation within 48 hours (38/260 in T piece and 26/260 in low PSV, p=0.109). However, on the subgroup of first success or per protocol patient, there intubation within 48 hours in the T piece group was higher than in the PSV group (29/212 vs. 9/159, respectively; p=0.012). In addition, the reintubation rate was higher after later success of weaning trial respectively (1st trial, 10.2%; 2nd trial 12.1%; 3rd trial 33.3%, and 4th trial, 100%; Table 2). This finding occurred similarly in both groups. The most common causes of reintubation were airway (42%), pulmonary (30%), and metabolic causes (16%) respectively, and were not different between the groups (Table 2). There were no differences between the groups in incidence of pneumonia, hospital mortality, hospital length of stay, or ICU length of stay (Table 2).
Table 2. Treatment outcomes. Abbreviations: IQR, interquartile range Note: * European classification: Simple weaning (first trial success); Difficult weaning (second and third success); and Prolong weaning (more than third trial success).
| All (n=520) | T piece (n=260) | Low PSV (n=260) | P-value | |
|---|---|---|---|---|
| SBT success and extubation (%)* | ||||
| Number of trial, median(IQR) | 1(1 – 2) | 1(1 – 1) | 1(1 – 2) | <0.001 |
| First trial | 371(71.35) | 212(81.54) | 159(61.15) | <0.001 |
| Second trial | 116(22.31) | 38(14.62) | 78(30.00) | |
| Third trial | 24(4.62) | 9(3.46) | 15(5.77) | |
| Forth trial | 6(1.15) | 1(0.38) | 5(1.92) | |
| Fifth trial | 3(0.58) | 0(0.00) | 3(1.15) | |
| Re-intubation, n (%) | ||||
| Intention to treat | 64(12.31) | 38(14.62) | 26(10.00) | 0.109 |
| Per protocol (First trial) | 38/371(10.2) | 29/212(13.7) | 9/159 (5.7) | 0.012 |
| Second trial | 14/116(12.1) | 6/38 (15.8) | 8/78 (10.3) | |
| Third trial | 7/24 (29.2) | 3/9 (33.3) | 4/15 (26.7) | |
| Forth trial | 2/6 (33.3) | 0/1 (0) | 2/5 (40.0) | |
| Fifth trial | 3/3 (100) | 0/0 (0) | 3/3 (100) | |
| Causes of reintubation, n (%) | ||||
| Airway causes | 27/64(42.19) | 18/38(47.37) | 9/26(34.62) | 0.353 |
| Pulmonary causes | 19/64(29.69) | 10/38(26.32) | 9/26(34.62) | |
| Metabolic causes | 10/64(15.63) | 5/38(13.16) | 5/26(19.23) | |
| Cardiac causes | 5/64(7.81) | 4/38(10.53) | 1/26(3.85) | |
| Neurological causes | 3/64(4.69) | 1/38(2.63) | 2/26(7.69) | |
| Pneumonia, n (%) | ||||
| Intention to treat | 68(13.08) | 31(11.92) | 37(14.23) | 0.435 |
| Per protocol | 36(9.70) | 20(9.43) | 16(10.06) | 0.840 |
| Hospital mortality, n (%) | ||||
| Intention to treat | 17 (3.27) | 9 (3.46) | 8 (3.08) | 0.805 |
| Per protocol | 13 (3.50) | 8 (3.77) | 5(3.14) | 0.744 |
| Hospital stay in day, median (IQR) | ||||
| Intention to treat | 16(11-26.5) | 16(11-25) | 17.5(11-27) | 0.307 |
| Per protocol | 16(11-24) | 16(11-22) | 17(11-26) | 0.371 |
| ICU stay in day, median(IQR) | ||||
| Intention to treat | 4(2-6) | 4(2-6) | 4(2-7) | 0.326 |
| Per protocol | 3(2-6) | 3(2-5) | 3(2-6) | 0.502 |
In multivariable analyses, we adjusted the risk of outcomes for primary surgical disorder and duration of ventilator support before enrollment into the trial (Table 1 and 3). In these adjusted analyses, PSV was associated with lower success at the first SBT trial and extubation (adjusted RR 0.79, 95% CI 0.70 to 0.88; p<0.001). However, PSV was associated with a lower risk of reintubation in adjusted models after both the first SBT (adjusted RR 0.37, 95% CI 0.18 to 0.74; p=0.005) and after all SBTs (adjusted RR 0.62, 95% CI 0.40 to 0.98; p=0.040). In the adjusted models, there were no associations between the treatment groups and any of pneumonia, hospital mortality, or hospital and ICU length of stay (Table 3).
Table 3. Multivariable analysis of low pressure support compare with T piece. Adjusted by primary surgical disorders and ventilator duration before extubation.
| Multivariable analysis (95% CI) | ||||||
|---|---|---|---|---|---|---|
| All (Intention to treat analysis) | Success at first trial (Per protocol analysis) | |||||
| Adjusted RR | P value | Adjusted RR | P value | |||
| Success at first trial | 0.79 | (0.70 to 0.88) | <0.001 | - | ||
| Pneumonia | 1.09 | (0.71 to 1.67) | 0.686 | 1.06 | (0.58 to 1.98) | 0.832 |
| Reintubation | 0.62 | (0.40 to 0.98) | 0.040 | 0.37 | (0.18 to 0.74) | 0.005 |
| Hospital mortality | 0.85 | (0.33 to 2.17) | 0.739 | 0.80 | (0.26 to 2.42) | 0.697 |
| Adjusted coefficient | Adjusted coefficient | |||||
| Hospital stay | -1.75 | (-5.00 to 1.51) | 0.292 | -0.77 | (-4.07 to 2.53) | 0.646 |
| ICU stay | -0.11 | (-1.62 to 1.40) | 0.882 | -0.48 | (-2.19 to 1.24) | 0.583 |
4. DISCUSSION
This study was a randomized control trial that compared PSV to T-piece as modes of SBTs in surgical critically ill patients. Regarding the previous study, Esteban et al also compared these two modes (7), their study enrolled. The study enrolled the patients on mixed of medical and surgical patients and the surgical patients accounted for only 26.2 percent of the total. In addition, a total of 87 patients (18%) were excluded after randomization because of failure of the first SBT trial (22%, in T piece arm and 14%, in pressure support arm). These imbalances after randomization might result in attrition bias and dilution of the randomization (8). To prevent attrition bias, we followed all of the patients and did not exclude any after randomization. In addition, in our analysis, we examined outcomes after both the first SBT, and after all SBTs.
Although blinded intervention is an important issue in randomization, it would be impossible to blind the mode of the SBT. We attempted to design the study to prevent as much bias as possible (9). First, we screened patients strictly and ensured sequential enrollment of eligible patients. At the same day enrolling patients, the randomization of weaning methods were performed at the sequential assignment. The research nurse opened the label on each assigned patients without staff knowing the methods for prevention the knowing the assignment on prior patient to prevent the selection bias. Second, the main study results were objective outcomes including success of first trial, reintubation, pneumonia occurrence, mortality, and length of stay. In addition, the independence assessors, who did not involve in the study, recorded the outcomes in this study. Third, we performed the statistical analysis before the intervention was decoded. This study outcome was a combination of successful completion of the SBT and successful extubation. All of the success of SBT trial were extubated. Although Mc Convill JF and Kress JP. showed the step approach using separated criteria between the readiness to undergo SBT at first step and then the criteria for adequate factor for airway assessment and mentation on the later step (10), all the patients enrolled before intervention assignment in this study was fulfil factors of adequate airway assessment, coughing and secretion clearance ability, and mentation in this study. The others were excluded until obtained the criteria. However, although the airway assessment was carefully evaluated at enrolled process, the major cause of reintubation in this study was airway problem (42.19%) (Table 2).
Although the non-invasive ventilation (NIV) has an important role as the supportive therapy on the area of weaning, reduction in reintubation rates post-extubation on ICU, and reduction in respiratory failure after major surgery (11-15), in systematic review and meta-analysis, this method could be reduced the risk of reintubation only when used post-surgery but not affect the risk of reintubation for the weaning patient (11).
Regarding the SBT success at first trial, Esteban et al reported the percent of SBT success and extubation using PSV was significant higher than T piece (7). This is contrast to our results, although there were no difference between groups in term of blood gas, respiratory parameter, and hemodynamics (Table 1 and Figure 1), the first trial success and extubation was significant lower than T piece. However, the prolong ventilator use could be an important confounder and impact the patient outcome in our study (16). Regarding these concerns, we performed the multivariable regression analysis and found the effect also persist (Table 3). However, the overall incidence of reintubation within 48 hour in our study was 12.3% which comparable to the previous report as 10-19% depending on patient classification and weaning method (7, 16-19). Esteban et al reported the reintubation after extubation between PSV and T piece was comparable after they excluded first SBT trial failure patients. However, these results were contrast to our study. Although the results of reintubation within 48 hours rate in low PSV was tendency lower than T piece on ITT, low PSV was significant lower reintubation rate on PP after first trial failure patients were excluded. PSV were significant lower risk of reintubation than T piece on both of ITT and PP after adjusted by primary surgical disorders and ventilator duration before extubation (Table 3). In addition, the reintubation rate also associated with the number of trial success in our study (Table 2). Although the accurate explanation of these results were unknown, the possible reasons explained these heterogeneity as follows. First, all of the study population in this study was surgical patients, the previous study population was mixed medical and surgical patients. The surgical patient accounted for less than 30% in Esteban et al study (7). Second, there were difference method on RSBI measurement at the end of SBT. While RSBI in PSV group was measured by MV, the T piece group was manually measure tidal volume by spirometer. Although the RSBI measurement during continuous positive airway pressure may lead to the lower value of RSBI when comparison with spontaneous breathing which might lead to the premature discontinuation of MV(20, 21). However, in the contrary, our results on the first SBT success in PSV group was significant lower than T piece group. The concerning of premature discontinuation of MV had a fewer effects in our study. The weaning of MV parameters not only concern on RSBI, but also concern on the other clinical parameters such as hemodynamic, consciousness, and other respiratory parameters. Third, although the history of comorbidity disorders and Charlson’s comorbidity index was comparable between groups, the reintubation by cardiac causes was prominent in T piece group (PSV 3.8% vs. T piece 10.5%). Currently, echocardiography might support to prevent the failure of extubation from cardiac causes (22). The investigation was not performed at the end of SBT in this study.
The strength of this study was the randomized control trial compare between T piece and PSV in surgical patients. The study demonstrated both ITT and PP results. However, there were some inevitable limitation of this study. First, the reintubation or the patient remain extubation after 48 hours after SBT trial was the composite outcome between of SBT trial success and patent of airway. Second, although we carefully randomized the intervention assignment, there were unbalance between arm of some patient characters such as primary surgical disorder and duration of ventilation before SBT. Third, the interventions was opened label because they could not be blinded as the nature of study. Finally, regarding the European classification of weaning process (23), most of our study patients were categorized in simple weaning (71%) and difficult weaning (27%). The prolonged weaning patient was accounted for only about 2 % (Table 2). The implication of result should be cautious especially in the prolonged weaning patients.
5. CONCLUSIONS
Although the success of the first SBT in T piece method was higher than PSV, the reintubation within 48 hours after extubationof low PSV was significant lower than T piece in adjusted model. However, there were no difference in pneumonia after extubation, hospital mortality as well as ICU and hospital length of stay.
Acknowledgement
I would like to thanks all of the ICU nurses to facilitate and support during data collection. Faculty of Medicine, Chiang Mai University granted for this study.
Conflict of interests
All the authors had no conflict of interest in this study.
REFERENCES
- 1.Chittawatanarat K, Jaikriengkrai K, Permpikul C. Thai Society of Critical Care Study group. Survey of respiratory support for intensive care patients in 10 Tertiary hospital of Thailand. J Med Assoc Thai. 2014;97(1 Suppl):S8–S14. [PubMed] [Google Scholar]
- 2.Esteban A, Alia I, Ibanez J, Benito S, Tobin MJ. Modes of mechanical ventilation and weaning. A national survey of Spanish hospitals. The Spanish Lung Failure Collaborative Group. Chest. 1994;106(4):1188–93. doi: 10.1378/chest.106.4.1188. [DOI] [PubMed] [Google Scholar]
- 3.Chittawatanarat K, Thongchai C. Spontaneous breathing trial with low pressure support protocol for weaning respirator in surgical ICU. J Med Assoc Thai. 2009;92(10):1306–12. [PubMed] [Google Scholar]
- 4.Frutos-Vivar F, Esteban A. Weaning from mechanical ventilation: why are we still looking for alternative methods? Med Intensiva. 2013;37(9):605–17. doi: 10.1016/j.medin.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Schadler D, Engel C, Elke G, Pulletz S, Haake N, Frerichs I, et al. Automatic control of pressure support for ventilator weaning in surgical intensive care patients. Am J Respir Crit Care Med. 2012;185(6):637–44. doi: 10.1164/rccm.201106-1127OC. [DOI] [PubMed] [Google Scholar]
- 6.Branson RD. Modes to facilitate ventilator weaning. Resp care. 2012;57(10):1635–48. doi: 10.4187/respcare.02081. [DOI] [PubMed] [Google Scholar]
- 7.Esteban A, Alia I, Gordo F, Fernandez R, Solsona JF, Vallverdu I, et al. Extubation outcome after spontaneous breathing trials with T-tube or pressure support ventilation. The Spanish Lung Failure Collaborative Group. Am J Respir Crit Care Med. 1997;156(2 Pt 1):459–65. doi: 10.1164/ajrccm.156.2.9610109. [DOI] [PubMed] [Google Scholar]
- 8.Bell ML, Kenward MG, Fairclough DL, Horton NJ. Differential dropout and bias in randomised controlled trials: when it matters and when it may not. BMJ. 2013;346:e8668. doi: 10.1136/bmj.e8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kahan BC, Cro S, Dore CJ, Bratton DJ, Rehal S, Maskell NA, et al. Reducing bias in open-label trials where blinded outcome assessment is not feasible: strategies from two randomised trials. Trials. 2014;15:456. doi: 10.1186/1745-6215-15-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McConville JF, Kress JP. Weaning patients from the ventilator. New Eng J Med. 2012;367(23):2233–9. doi: 10.1056/NEJMra1203367. [DOI] [PubMed] [Google Scholar]
- 11.Glossop AJ, Shephard N, Bryden DC, Mills GH. Non-invasive ventilation for weaning, avoiding reintubation after extubation and in the postoperative period: a meta-analysis. Br J Anaesth. 2012;109(3):305–14. doi: 10.1093/bja/aes270. [DOI] [PubMed] [Google Scholar]
- 12.Ornico SR, Lobo SM, Sanches HS, Deberaldini M, Tofoli LT, Vidal AM, et al. Noninvasive ventilation immediately after extubation improves weaning outcome after acute respiratory failure: a randomized controlled trial. Critical Care. 2013;17(2):R39. doi: 10.1186/cc12549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrer M. Non-invasive ventilation in the weaning process. Minerva Anestesiol. 2008;74(6):311–4. [PubMed] [Google Scholar]
- 14.Zarbock A, Mueller E, Netzer S, Gabriel A, Feindt P, Kindgen-Milles D. Prophylactic nasal continuous positive airway pressure following cardiac surgery protects from postoperative pulmonary complications: a prospective, randomized, controlled trial in 500 patients. Chest. 2009;135(5):1252–9. doi: 10.1378/chest.08-1602. [DOI] [PubMed] [Google Scholar]
- 15.Chiumello D, Chevallard G, Gregoretti C. Non-invasive ventilation in postoperative patients: a systematic review. Intensive Care Med. 2011;37(6):918–29. doi: 10.1007/s00134-011-2210-8. [DOI] [PubMed] [Google Scholar]
- 16.Penuelas O, Frutos-Vivar F, Fernandez C, Anzueto A, Epstein SK, Apezteguia C, et al. Characteristics and outcomes of ventilated patients according to time to liberation from mechanical ventilation. Am J Respir Crit Care Med. 2011;184(4):430–7. doi: 10.1164/rccm.201011-1887OC. [DOI] [PubMed] [Google Scholar]
- 17.Frutos-Vivar F, Esteban A, Apezteguia C, Gonzalez M, Arabi Y, Restrepo MI, et al. Outcome of reintubated patients after scheduled extubation. J Crit Care. 2011;26(5):502–9. doi: 10.1016/j.jcrc.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 18.Thille AW, Harrois A, Schortgen F, Brun-Buisson C, Brochard L. Outcomes of extubation failure in medical intensive care unit patients. Crit Care Med. 2011;39(12):2612–8. doi: 10.1097/CCM.0b013e3182282a5a. [DOI] [PubMed] [Google Scholar]
- 19.Thille AW, Cortes-Puch I, Esteban A. Weaning from the ventilator and extubation in ICU. Curr Opin Crit Care. 2013;19(1):57–64. doi: 10.1097/MCC.0b013e32835c5095. [DOI] [PubMed] [Google Scholar]
- 20.El-Khatib MF, Jamaleddine GW, Khoury AR, Obeid MY. Effect of continuous positive airway pressure on the rapid shallow breathing index in patients following cardiac surgery. Chest. 2002;121(2):475–9. doi: 10.1378/chest.121.2.475. [DOI] [PubMed] [Google Scholar]
- 21.Patel KN, Ganatra KD, Bates JH, Young MP. Variation in the rapid shallow breathing index associated with common measurement techniques and conditions. Resp Care. 2009;54(11):1462–6. [PubMed] [Google Scholar]
- 22.Penuelas O, Thille AW, Esteban A. Discontinuation of ventilatory support: new solutions to old dilemmas. Curr Opin Crit Care. 2015;21(1):74–81. doi: 10.1097/MCC.0000000000000169. [DOI] [PubMed] [Google Scholar]
- 23.Boles JM, Bion J, Connors A, Herridge M, Marsh B, Melot C, et al. Weaning from mechanical ventilation. Eur Resp J. 2007;29(5):1033–56. doi: 10.1183/09031936.00010206. [DOI] [PubMed] [Google Scholar]


