Abstract
Introduction
Impaired diabetic wound healing is an important issue in diabetic complications. The present study aims to evaluate the protective effect on glycemic control against impaired diabetic wound healing using a diabetic rat model. We investigated the wound healing process and effect on the impaired wound repair by glycemic control in the Spontaneously Diabetic Torii (SDT) fatty rat, which is a new animal model of obese type 2 diabetes and may be a good model for study impaired wound healing.
Material and methods
Male SDT fatty rats at 15 weeks of age were administered orally with sodium glucose co-transporter (SGLT) 2 inhibitor for 3 weeks. Wounds were induced at 2 weeks after SGLT 2 inhibitor treatment, and the wound areas were periodically examined in morphological and histological analyses.
Results
The SDT fatty rats showed a delayed wound healing as compared with the normal rats, but a glycemic control improved the impaired wound healing. In histological analysis in the skin of SDT fatty rats showed severe infiltration of inflammatory cell, hemorrhage and many bacterial masses in the remaining and slight fibrosis of crust on skin tissue. Thought that this results skin performance to be a delay of crust formation and regeneration of epithelium; however, these findings were ameliorated in the SGLT 2 inhibitor treated group.
Conclusion
Glycemic control is effective for treatment in diabetic wounds and the SDT fatty rat may be useful to investigate pathophysiological changes in impaired diabetic wound healing.
Keywords: blood glucose, diabetic complication, rat, sodium-glucose transporter 2, wound healing
1. INTRODUCTION
Diabetes mellitus is one of the most common chronic diseases all over the world, and continues to increase in numbers: in 2011, 366 million people worldwide suffered from diabetes, it is estimated to increase to 552 million in 2030 (1). The growing population of diabetic patients has resulted in a rapid increase in the number of patients who have diabetic complications; microangiopathy, such as nephropathy, retinopathy, and neuropathy, and macroangiopathy, such as ischemic heart disease, stroke, and peripheral vascular disease which leads to diabetic foot ulcers and the amputation (2-4). In addition to adverse effects on the quality of life in the patients, the increasing number of patients contributes importantly to rising medical costs (5, 6).
Approximately 4-10% of diabetic patients suffer from diabetic foot ulcers and the patients often require surgery, potentially causing chronic wounds, and the impaired wound repair is connected to increases of morbidity and mortality (7). Also, the formation of wounds is connected to neuropathy with loss of sensitivity, decreased blood flow, and impaired ability to high infection (8). The pathogenesis of a diabetic wound are complicated, and the wound repair has a dynamic process involving many factors, and cell types, such as soluble mediators, blood cells, fibroblasts, endothelial cells, and extracellular matrix (9, 10).
New animal models of impaired wound repair with diabetes mellitus are required to elucidate the pathogenesis of diabetic wound and develop the new therapies and the new drugs. The Spontaneously Diabetic Torii (SDT) fatty rat, established by introducing the fa allele of the Zucker fatty rat into the SDT genome, is a new model of obese type 2 Diabetes. The SDT fatty rat showed overt obesity, and hyperglycemia and dyslipidemia were observed at a young age. With the early incidence of diabetes, the diabetic complications were seen at younger ages as compared with those in other type 2 diabetic models (4, 11, 12). In the study of diabetic peripheral neuropathy, the SDT fatty rat showed a delay of motor nerve conduction velocity and a decrease of the number of sural nerve fibers (13). In this study, we investigated the characteristics of impaired wound healing in the SDT fatty rats and the effects of glycemic control on the impaired wound healing.
2. MATERIALS AND METHODS
Animals and treatment
Male SDT fatty rats (CLEA Japan, Tokyo, Japan) at 15 weeks of age were used for the study. Age-matched male Sprague-Dawley (SD) rats (CLEA Japan, Tokyo, Japan) were used as control animals. All animal procedures and protocol complied with the guidelines for animal experimentation set by the Ethics Committee for Animal Use at Tokyo University of Agriculture or Niigata University.
The rats were maintained at 23 ± 3°C on a 12h/12h light-dark cycle with ad libitum access to a standard diet (CRF-1; Oriental Yeast, Tokyo, Japan) and water. The rats were administered orally with either vehicle (0.5% methyl cellulose, control group) or sodium glucose co-transporter inhibitor canagliflozin 10 mg/kg (SGLT2-I group) suspended in a vehicle once daily for 3 weeks.
Measurement of biological parameters
At the starting point of the experiment (Day -14), Day 0, and Day 7, body weights were measured and blood samples were collected from the tail vein to determine the blood chemical parameters, such as glucose, triglyceride (TG), total cholesterol (TC), and insulin levels. Moreover, glycated hemoglobin (HbA1c) level was measured on Day 7. These biochemical parameters were measured using commercial kits as in our previous study (14).
Morphological and histological analyses
Wounds were induced at 2 weeks after the canagliflozin-treatment. The skin on the back of anesthetized and took the analgesic rats were shaven and disinfected. Two circular wounds (20 mm in diameter) were made on the skin of left and right sides on the back (Day 0) (9). The wound area was determined by the average value of areas on both sides and was measured on Day 5 and Day 11. The relative wound size was expressed according to the following formula;
% wound area = wound area on Day 5 or 11/wound area on Day 0.
Necropsy was performed at 18 weeks of age. The skin was fixed at 10% neutral buffered formalin. After resection, tissues were paraffin-embedded using standard techniques and thin-sectioned (3 to 5 μm). Sections were stained with hematoxylin and eosin (HE).
Statistical analysis
Data analysis was expressed as the mean ± standard deviation. Statistical analyses of differences between mean values were performed using an F-test, followed by a Student’s t-test or Aspin-Welch’s t-test. Differences were defined as significant at p < 0.05.
3. RESULTS
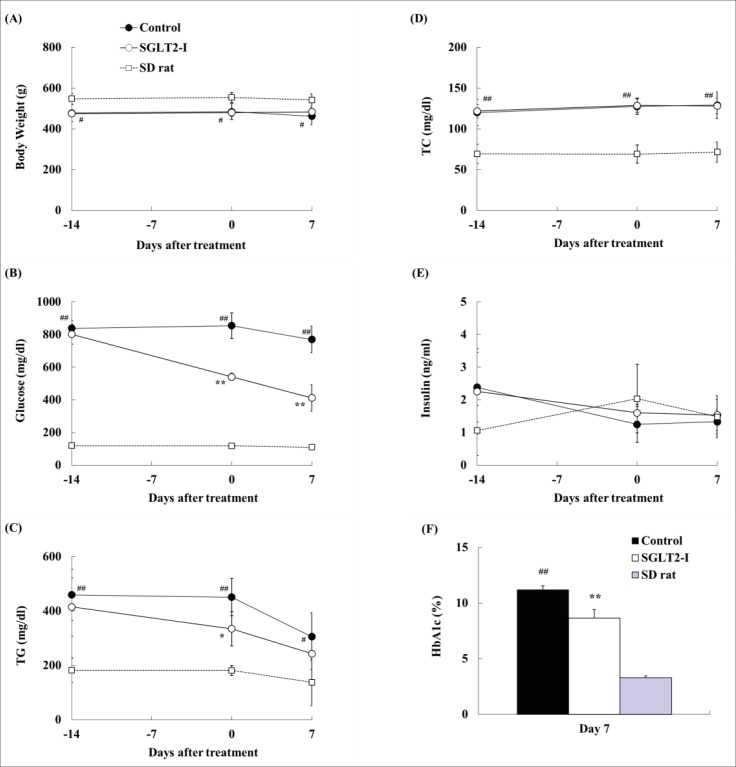
In biological analysis, body weight, plasma glucose, TG, TC, and insulin levels, and HbA1c level were measured (Figure 1). Plasma glucose levels in the SGLT2-I group significantly decreased at Days 0 and 7, as compared with those in the control group (Figure 1B). Moreover, HbA1c level in the SGLT2-I group is lower than that in the control group (control, 11.2 ± 0.4% vs. SGLT2-I, 8.6 ± 0.8%) (Figure 1F). Plasma TG levels in the SGLT2-I group decreased at Day 0, as compared with that in the control group (Figure 1C). Body weight and plasma TC and insulin levels in the SGLT2-I group were comparable to those in the control group (Figures 1A, 1D, and 1E).
Figure 1. Changes in body weight (A), and serum glucose (B), TG (C), TC (D), insulin (E) and HbA1c (F) levels in SDT fatty rats with canagliflozin (SGLT2-I)-treatment. Data are represented as means ± standard deviation or + standard deviation (n=4-6). *p<0.05, ** p<0.01; significant difference compared with the control group. #p<0.05, ## p<0.01; significant difference compared to the SD group.
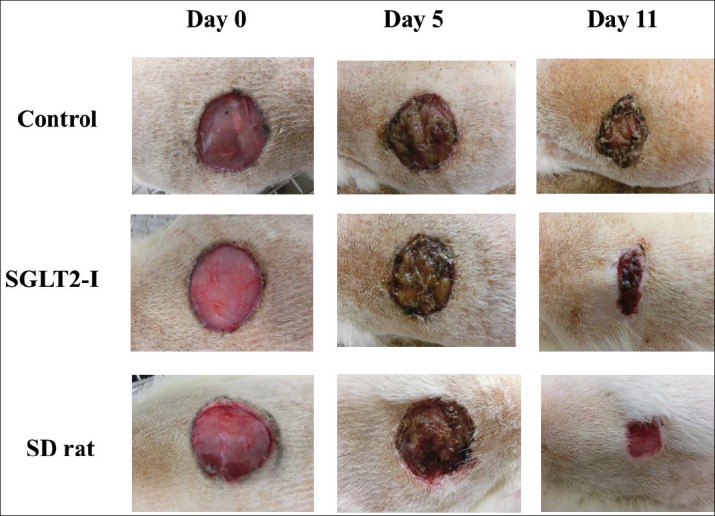
Changes in % wound area (wound-size) were shown in Figure 2. The representative photograph in each group was shown in Figure 3. The wound-size in SDT fatty rats significantly increased as compared with those in SD rats at Days 5 and 11 (Day 5; SDT fatty rat 95.7 ± 10.0% vs. SD rat 82.5 ± 6.7%, Day 11; SDT fatty rat 55.5 ± 16.6% vs. SD rat 32.1 ± 3.1%). The wound-size in SDT fatty rats by SGLT2-I treatment significantly decreased, as compared with that in the control group at Day 5 (control group 95.7 ± 10.0% vs. SGLT2-I group 79.4 ± 5.5%). Moreover, the wound-size of the SGLT2-I group showed a tendency to decrease at Day 11 (control group 55.5 ± 16.6% vs. SGLT2-I group 37.5 ± 10.0%).
Figure 2. Wound-size changes in SDT fatty rats with canagliflozin (SGLT2-I)-treatment. Data are represented as means + standard deviation (n=4-6). *p<0.05; significant difference compared with the control group. #p<0.05; significant difference compared with the SD group.
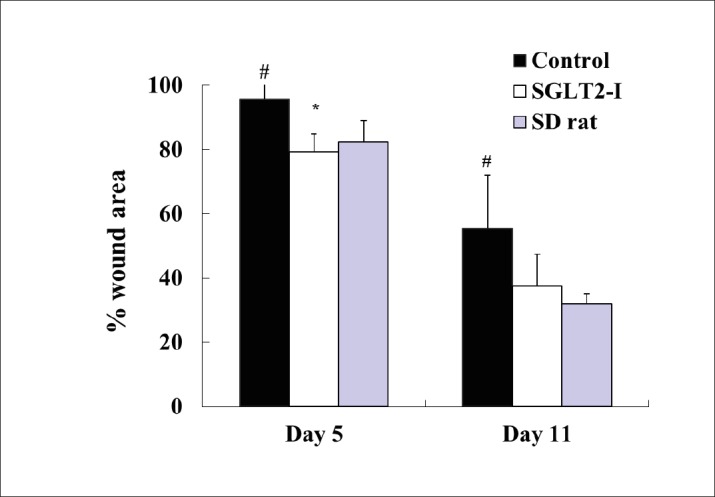
Figure 3. Wound-size changes in SDT fatty rats with canagliflozin (SGLT2-I)-treatment. Representative photo in each group.
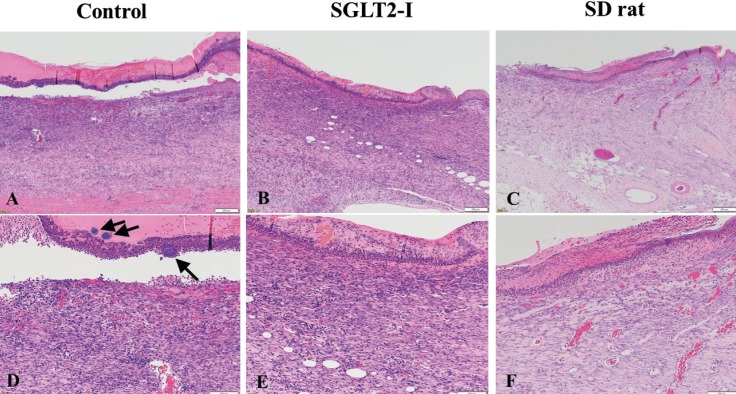
On histopathology (Figure 4), prominent fibrosis, slight inflammatory cell infiltration, and neovascularization were observed in normal SD rats. However, severe infiltration of inflammatory cell and hemorrhage and many bacterial masses in the crust on skin tissue were seen and slight fibrosis that were noted in SDT fatty rats. In addition, cystic formation in subcutaneous tissue was diffusely observed in SDT fatty rats. These findings in SDT fatty rats were ameliorated and/or slightly observed in the SGLT2-I treated group.
Figure 4. Histopathological analysis of skin identified by HE staining. (A) and (D) Control group; (B) and (E) SGLT2-I group; (C) and (F) SD group. (D) Into (F) are higher magnification of each photo. Bar = 200μm in photos (A) to (C) and Bar = 100μm in photos (D) to (F). (D) Many bacterial masses remaining in the crust on skin tissue (Arrow).
4. DISCUSSION
In this study, we investigated the characteristics of skin repair in male SDT fatty rats, using the SGLT 2 inhibitor canagliflozin. Canagliflozin is a novel anti-diabetic drug and the drug show a glucose lowering effect by inhibiting glucose re-absorption in the proximal renal tubules (15, 16). The SDT fatty is a new diabetic model (17), and the plasma glucose, TG, and TC levels increased as compared with those levels in SD rat, as shown in previous studies (4, 11, 18).
The normal wound healing mechanism is composed of several steps, homeostasis, inflammation, granulation, tissue formation, and tissue remodeling. Moreover, the delayed healing is caused by defective regulation of the complex molecular and biological factors involved in proper healing (19, 20, 21). These factors reportedly include impaired keratinocyte, fibroblast, macrophage function, aberrant angiogenic response, and growth factor production (8). It is also reported that impaired wound healing is caused by the presence of advanced glycosylation end (AGE) products or increase of reactive oxygen species (ROS) production (22, 23). In this study, the SDT fatty rats by canagliflozin-treatment sustained a good glycemic control during the experimental period, and the glycemic controlled led to the recovery effect on the delayed wound healing in SDT fatty rats. This pharmacological effect is considered to be induced by the decreases of AGE or ROS production by glycemic control.
The diabetic complications, including microangiopathy and macroangiopathy are caused by the uncontrolled diabetes mellitus for long-term (24). On the other hand, impairment of wound healing is caused by the short-term controlled. In this study, also, the impaired wound healing in SDT fatty rats is improved by glycemic control for short-term, 2-3 weeks. In histological analyses in the skin, prominent fibrosis, slight inflammatory cell infiltration, and neovascularization were observed in normal SD rats; however, a delay of crust formation and regeneration of the epithelium and many bacterial masses in the crust on skin tissue were noted in SDT fatty rats. These changes in SDT fatty rats showed a delayed wound healing in diabetes. In addition, these findings in SDT fatty rats were ameliorated and progressed of wound healing by the glycemic control with the SGLT2-I treatment.
It is reported that tight glycemic control is a key factor in wound healing in other experimental diabetic models (25). In the Goto-Kakizaki rat, a non-obese type 2 diabetic model, a glycemic control with metformin-treatment improved the peri-implant wound healing (26). Also, in the streptozotocin-induced diabetic rats, the glycemic control showed a therapeutic potential in treatment of diabetic wounds (27).
5. CONCLUSION
Glycemic control is effective for treatment in diabetic wounds and thought that the SDT fatty rat will be useful to investigate the pathophysiological changes in impaired diabetic wound healing.
Competing interest
No competing interest exists.
Conflict of interest
The authors do not have any conflict of interest.
Authors contributions
KM, TO, and TY designed the study, and wrote the initial draft of the manuscript. SHY, HY, MS, and FF contributed to analysis and interpretation of data, and assisted in the preparation of the manuscript.
REFERENCES
- 1.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94(3):311–21. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Hall JE, Kuo JJ, da Silva AA, de Paula RB, Liu J, Tallam L. Obesity-associated hypertension and kidney disease. Curr Opin Nephrol Hypertens. 2003;12(2):195–200. doi: 10.1097/00041552-200303000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Ritz E, Stefanski A. Diabetic nephropathy in type II diabetes. Am J Kidney Dis. 1996;27(2):167–94. doi: 10.1016/s0272-6386(96)90538-7. [DOI] [PubMed] [Google Scholar]
- 4.Katsuda Y, Ohta T, Miyajima K, Kemmochi Y, Sasase T, Tong B, et al. Diabetic complications in obese type 2 diabetic rat models. Exp Anim. 2014;63(2):121–32. doi: 10.1538/expanim.63.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calcutt NA, Cooper ME, Kern TS, Schmidt AM. Th erapies for hyperglycaemia-induced diabetic complications: from animal models to clinical trials. Nat Rev Drug Discov. 2009;8(5):417–29. doi: 10.1038/nrd2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeFronzo RA, Abdul-Ghani M. Type 2 diabetes can be prevented with early pharmacological intervention. Diabetes Care. 2011;34(Suppl 2):S202–209. doi: 10.2337/dc11-s221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apelqvist J, Bakker K, van Houtum WH, Nabuurs-Franssen MH, Schaper NC. International consensus and practical guidelines on the management and the prevention of the diabetic foot. International Working Group on the Diabetic Foot. Diabetes Metab Res Rev. 2000;16(Suppl 1):S84–92. doi: 10.1002/1520-7560(200009/10)16:1+<::aid-dmrr113>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 8.Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117(5):1219–22. doi: 10.1172/JCI32169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slavkovsky R, Kohlerova R, Tkacova V, Jiroutova A, Tahmazoglu B, Velebny V, et al. Zucker diabetic fatty rat: a new model of impaired cutaneous wound repair with type II diabetes mellitus and obesity. Wound Repair Regen. 2011;19(4):515–25. doi: 10.1111/j.1524-475X.2011.00703.x. [DOI] [PubMed] [Google Scholar]
- 10.Dhall S, Do DC, Garcia M, Kim J, Mirebrahim SH, Lyubovitsky J, et al. Generating and reversing chronic wounds in diabetic mice by manipulating wound redox parameters. J Diabetes Res. 2014. pp. 562–625. [DOI] [PMC free article] [PubMed]
- 11.Matsui K, Ohta T, Oda T, Sasase T, Ueda N, Miyajima K, et al. Diabetes-associated complications in Spontaneously Diabetic Torii fatty rats. Exp Anim. 2008;57(2):111–21. doi: 10.1538/expanim.57.111. [DOI] [PubMed] [Google Scholar]
- 12.Ohta T, Katsuda Y, Miyajima K, Sasase T, Kimura S, Tong B, et al. Gender differences in metabolic disorders and related diseases in Spontaneously Diabetic Torii-Lepr(fa) rats. J Diabetes Res. 2014;2014:841957. doi: 10.1155/2014/841957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamaguchi T, Sasase T, Mera Y, Tomimoto D, Tadaki H, Kemmochi Y, et al. Diabetic peripheral neuropathy in Spontaneously Diabetic Torii-Lepr(fa) (SDT fatty) rats. J Vet Med Sci. 2012;74(12):1669–73. doi: 10.1292/jvms.12-0149. [DOI] [PubMed] [Google Scholar]
- 14.Sakata S, Mera Y, Kuroki Y, Nashida R, Kakutani M, Ohta T. Combination therapy of an intestine-specific inhibitor of microsomal triglyceride transfer protein and peroxisome proliferator-activated receptor γ agonist in diabetic rat. J Diabetes Res. 2014. p. 890639. [DOI] [PMC free article] [PubMed]
- 15.Nomura S, Sakamaki S, Hongu M, Kawanishi E, Koga Y, Sakamoto T, et al. Discovery of canagliflozin, a novel C-glucoside with thiophene ring, as sodium-dependent glucose cotransporter 2 inhibitor for the treatment of type 2 diabetes mellitus. J Med Chem. 2010;53(17):6355–60. doi: 10.1021/jm100332n. [DOI] [PubMed] [Google Scholar]
- 16.Sha S, Devineni D, Ghosh A, Polidori D, Chien S, Wexler D, et al. Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects. Diabetes Obes Metab. 2013;13(7):669–72. doi: 10.1111/j.1463-1326.2011.01406.x. [DOI] [PubMed] [Google Scholar]
- 17.Masuyama T, Katsuda Y, Shinohara M. A novel model of obesity-related diabetes: introgression of the Lepr(fa) allele of the Zucker fatty rat into nonobese Spontaneously Diabetic Torii (SDT) rats. Exp Anim. 2005;54(1):13–20. doi: 10.1538/expanim.54.13. [DOI] [PubMed] [Google Scholar]
- 18.Ishii Y, Ohta T, Sasase T, Morinaga H, Ueda N, Hata T, et al. Pathophysiological analysis of female Spontaneously Diabetic Torii fatty rats. Exp Anim. 2010;59(1):73–84. doi: 10.1538/expanim.59.73. [DOI] [PubMed] [Google Scholar]
- 19.Maruyama K, Asai J, Ii M, Thorne T, Losordo DW, D’Amore PA. Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am J Pathol. 2007;170(4):1178–91. doi: 10.2353/ajpath.2007.060018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galiano RD, Tepper OM, Pelo CR, Bhatt KA, Callaghan M, Bastidas N, et al. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am J Pathol. 2004;164(6):1935–47. doi: 10.1016/S0002-9440(10)63754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lobmann R, Ambrosch A, Schultz G, Waldmann K, Schiweck S, Lehnert H. Expression of matrix-metalloproteinases and their inhibitors in the wounds of diabetic and non-diabetic patients. Diabetologia. 2002;45(7):1011–6. doi: 10.1007/s00125-002-0868-8. [DOI] [PubMed] [Google Scholar]
- 22.Ahmed N. Advanced glycation endproducts-role in pathology of diabetic complications. Diabetes Res Clin Pract. 2005;67(1):3–21. doi: 10.1016/j.diabres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Petreaca ML, Do D, Dhall S, McLelland D, Serafino A, Lyubovitsky J, et al. Deletion of a tumor necrosis superfamily gene in mice leads to impaired healing that mimics chronic wounds in humans. Wound Repair Regen. 2012;20(3):353–66. doi: 10.1111/j.1524-475X.2012.00785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rossini AA. Why control blood glucose levels? Arch Surg. 1976;111(3):229–33. doi: 10.1001/archsurg.1976.01360210023004. [DOI] [PubMed] [Google Scholar]
- 25.O’Sullivan JB, Hanson R, Chan F, Bouchier-Hayes DJ. Tight glycaemic control is a key factor in wound healing enhancement strategies in an experimental diabetes mellitus model. Ir J Med Sci. 2011;180(1):229–36. doi: 10.1007/s11845-010-0630-z. [DOI] [PubMed] [Google Scholar]
- 26.Inouye KA, Bisch FC, Elsalanty ME, Zakhary I, Khashaba RM, Borke JL. Effect of metformin on periimplant wound healing in a rat model of type 2 diabetes. Implant Dent. 2014;23(3):319–27. doi: 10.1097/ID.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 27.Ahmad M, Ansari MN, Alam A, Khan TH. Oral dose of citrus peel extracts promotes wound repair in diabetic rats. Pak J Biol Sci. 2013;16(20):1086–94. doi: 10.3923/pjbs.2013.1086.1094. [DOI] [PubMed] [Google Scholar]