Abstract
Introduction
Cervical cancer is a malignancy originating in the transformation zone of the cervix, most commonly in the squamous cells. It is the fourth most common cancer in women worldwide, and the third most common cause of female cancer death. Genital human papilloma viruses (HPV) are sexually transmitted and approximately 630 milion people worldwide are infected. More than 200 genotypes, subtypes and variants have been reported, 13-15 being oncogenic type, which could be responsible for cervical intraepithelial lesions (CIN) or cancer.
Aim
Aim of this study was to evaluate the prevalence of this infection and to identify specific types of human papiloma virus in cervical intraepithelial lesions and cervical cancer in Macedonian women.
Material and methods
The study was conducted at the University Clinic for Obstetrics and Gynecology, Skopje, Macedonia, in a period of four years. The study was performed on a cohort of 1895, 18 - 73 year old patients who during primary examination had already abnormal PAP smear test. Cervical cells were collected in the lithotomy gynecological position of the patient, using endocervical cytobrush and cotton-tipped swab, and both were placed in sterile test tube with phosphate buffered saline. Samples were stored at temperature of 2 - 8 °C and Human Pappiloma Virus (HPV) genotyping was analyzed within 7 days by multiple Polymerase Chain Reaction (PCR) methods.
Results
The mean age of enrolled women was 40,8 years±10.36 SD(minimum of 18 and maximum 73 years. Among the patients, the presence of HPV by using PCR was detected in 40,68 % (769 patients) and was highly associated with cervical abnormalities. The prevalence of HPV was highest (82,1%) in women aged 20-years or less and it decreased with age and was lowest (19,9%) among patients older than 50 years. The prevalence of oncogenic types of the virus was higher if the cytologic diagnosis is CIN 3/Carcinoma in situ (CIS). In these patients detection of high risk HPV was in 79,1% females with CIN 3 and 97,5 % in females with CIS. The lowest prevalence was detected in patients with atypical squamous cells of undetermined significance (ASCUS) (23,9%) and CIN 1-25 (6%). Results of HPV typing show that genotypes were found either single or multiple in both single and multiple infections. We have seen that HPV 16, 18 and 31 were the most common types detected among the patients from Macedonia. HPV 16 was present even in 52,1 % of women with CIS and in 41,2% in women with CIN 3. HPV type 31 ranked second in patients wit CIN1, CIN2, CIN3 but HPV 18 ranked second in patients with CIS with (12,8%). Surprisingly, patients with mixed infection had more low grade intraepithelial squamous lesions (LSIL) and high grade squamous intraepithelial lesions (HSIL) then CIS.
Conclusion
Among Macedonian women, HPV 16, 31 and 18 were HPV types strongly associated with intraepithelial cervical lesions and cervical cancers. The prevalence of high risk HPV was highest in youngest women, but the risk was highest among patients with invasive cervical cancer (ICC). Surprisingly, patients with mixed infection had more LSIL and HSIL then CIS.
Keywords: HPV genotypes, PCR, intraepithelial lesions, cervical cancer, age
1. INTRODUCTION
Cervical cancer is a malignancy originating in the transformation zone of the cervix, most commonly in the squamous cells (1). It is the fourth most common cancer in women worldwide, and the third most common cause of female cancer death, with aproximately 200.000 cervical cancer related deaths in 2010, including 46.000 women aged 15-49 in developing countries (2, 3). Most affected women are those aged 30-45 years, who have never had a PAP smear neither had participated regularly in citology screening program (4). Around 80% of new cases occur in non developed countries (2). In developed countries there is decreased incidence of squamous cell carcinoma due to screening capacity and availability of human papilloma virus vaccine, but there is increased incidence of adenocarcinoma. In Macedonia, breast cancer is the leading cause for malignant neoplasms among women in 2012, with an incidence rate of 111.7 per 100 000. Cervical cancer ranks as the 6th leading cause of female cancer in Macedonia in 2012, with 171 new cases and incidence rate of 16,6 infected women per 100.000 (5).
Genital human papilloma viruses are commonly sexually transmitted. Around 630 milion people worldwide are infected with the virus, and 75-89% of sexually active individuals will become infected at some point of their life (6). More than 200 genotypes, subtypes and variants have been reported, 13-15 being oncogenic, which could be responsible for cervical intraepithelial lesions or cancer (7). HPV is detected in almost 100% of cervical cancer cells, while it is present in only 10,4 % in healthy women (8). Low grade and high grade intraepithelial lesions of the uterine cervix as well as cervical cancer are associated with infection of HPV, mostly with 16 and 18 genotypes. In over 50 % of diagnosed cervical cancer cells we can detect HPV type 16 (8). Many studies have shown that commonly found HPVs in cells of cervical cancer are types 16, 18, 45 and 31 (8).
Evidence in the existing literature shows that vaccination against HPV 16 and 18 could reduce almost 75% of invasive cervical cancer worldwide (9).
Aim of this study was to detect the prevalence of the HPV infection in cervical intraepithelial lesions and to identify specific types of HPV among Macedonian women. The knowledge of distribution of different genotypes of HPV in developed and less developed countries could help us in further development of vaccination and reduction of high grade intraepithelial cervical lesions and cases of invasive cancer (10, 11).
2. OBJECTIVE
The study was conducted at University Clinic of obstetrics and gynecology, Skopje, Macedonia, in a period of 4 years (January 2012 - December 2015). The study included a cohort of 1895, 18 - 73 year old patients, who during primary examination had already had abnormal PAP smear tests, that was processed and read by the patologist at our clinic, using standard conventions according to ASCCP guidelines and cyto-pathological results using Betethesda clasification system (12). Based on pathology and medical records findings, results were classified as PAP smear test containing atypical squamous cells of undetermined significance (ASCUS), atypical squamous cells, cannot exclude high grade squamous intraepithelial lesion (ASC- H), low grade intraepithelial lesion (LSIL), high grade intraepithelial lesion (HSIL), or cervical intraepithelial cancer. We excluded the pregnant patients, those with inflammatory disease or atrophic alterations. Each patient got detailed explanation of the procedure and informed consent approved by The Macedonian Ethics Committee. It was signed by each patient before the examination and further procedures.
Cervical cells were collected in the lithotomy gynecological position of the patient, using endocervical cytobrush and cotton-tipped swab, and both were placed in sterile test tube with phosphate buffered saline. Samples were stored at temperature of 2 - 8 °C and HPV genotyping analyzed within 7 days by multiple PCR methods.
First step in HPV testing was isolation of DNA from collected cells (exfoliated cervical cells in phosphate buffered saline medium). The cervical cells were digested with an appropriate buffer containing Proteinase K at a temperature of 60 oC. DNA extraction employs glass fibers, fixed in column that specifically bands DNA in the presence of Chotropic salt. Genomic DNA was diluted by a low salt solution; a negative control was included for every DNA isolation.
HPV was detected by multiplex PCR technique with high sensitivity and specificity, by applying DPO (Dual Priming Oligonucleotide) technology. Positive and negative controls were included for each amplification and the internal control used was DNA plasmid.
HPV screening can simultaneously detect virus genotype and screen for 16 high-risk types (26, 31, 33, 35, 39, 45, 51, 52,53, 56, 58, 59, 66, 68, 73, 82) and 4 low-risk types (HPV 6, 11, 42 and 44). Aliquots of the PCR were run on a 1,5% agarose gel and analyzed under UV light following ethidium bromide standing.
The positive samples were genotyped with essay based on the reverse hybridization principle for identification of 37 different genotypes of the HPV. (HR HPV-16, 18, 26, 31, 33, 35, 39, 45, 51, 52,53, 56, 58, 59, 66, 68, 73, 82 and LR HPV-6, 11, 40, 42, 43, 44, 54, 61, 62, 67, 71, 72, 74, 81, 84, 91).
The essay uses specific primers (for each HPV type) for amplification of regions E6-E7 from 37 HPV types. During PCR, the amplification of control gene (GADPH or β –globine) also occurs, acting as an indicator of the absence of PCR inhibitors in the amplified DNA sample. At the stage of hybridization there is specific binding of DNA fragments amplified during the PCR reaction to a series of probas deposited on nylon membrane.
Data analysis was performed using statistical analysis software package SPSS (version 15.0, SPSS Inc. Chicago, Il, USA) The subjects of the study were analysed according to their age, diagnosed cervical intraepithelial lesions (cytologic diagnosis) and the presence of HPV type(s). Differences between the groups were tested by using Chi square or Fisher exact test. Differences were considered statistically significant if the level of significance was p<0,05.
3. RESULTS
Cervical samples adequate for cytologic diagnosis and HPV genotyping were available for 1895 women. All of them had cytologic examination and were diagnosed with intraepithelial cervical lesions of ASCUS, low grade, high grade or cervical cancer. The mean age of enrolled women was 40,8 years±10.36 SD, (minimum 18 and maximum 73 years). In the cohort, the presence of HPV was detected at 40,68 % (769 patients) and was highly associated with cervical abnormalities. The patients were divided in 5 aged groups, shown in Table 1.
Table 1. The distribution of HPV infection among women with intraepithelial lesions by age groups.
| Age group (years) | Negative | Positive | Total | ||
|---|---|---|---|---|---|
| n | % | n | % | N | |
| < 20 | 5 | 17.9 | 23 | 82.1 | 28 |
| 20 – 30 | 179 | 45.7 | 213 | 54.3 | 392 |
| 30 – 40 | 336 | 51.4 | 318 | 48.6 | 654 |
| 40 – 50 | 393 | 70.8 | 162 | 29.2 | 555 |
| > 50 | 213 | 80.1 | 53 | 19.9 | 266 |
| Total | 1126 | 59,3 | 769 | 40,7 | 1895 |
As we can see from the results presented in Table 1, the prevalence of HPV was highest in women younger than 20-years and the prevalence of HPV infection decreased with age and was lowest among patients older than 50 years. 82,1% of the patients younger than 20 years were positive of HPV, but only 19,9% of patients older than 50 years. Mean age of patients is presented in Table 2, showing that mean age was higher if the cytologic diagnosis was higher. Cervical carcinoma was associated with the highest mean age (44.4 ±11.7 years) of the patients.
Table 2. Mean age at the patients with cervical abnormalities of our group.
| ASCUS | CIN 1 | CIN 2 | CIN 3 | CIS | |
|---|---|---|---|---|---|
| Mean age (years) | 43.3 ±10.8 | 39.0 ±9.3 | 37.5 ±10.2 | 39.8 ±9.8 | 44.4 ±11.7 |
| Maximum age | 67 | 59 | 74 | 68 | 66 |
| Minimum age | 25 | 18 | 21 | 23 | 26 |
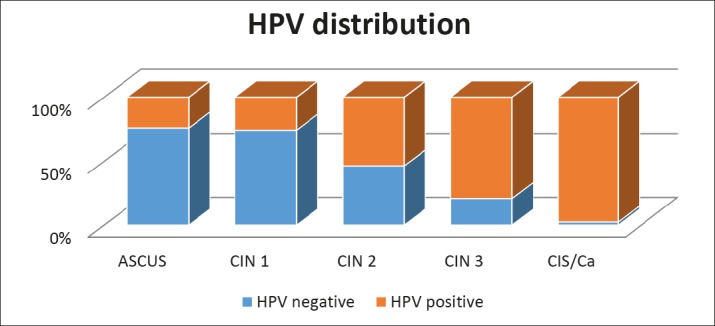
The prevalence of HPV infection among women according to the cytologic diagnosis is presented in Table 3 and Graph 1. Not surprisingly, the prevalence of oncogenic types is higher if the cytologic diagnosis is higher too, such as CIN 3/CIS. In these patients, high risk HPV was detected in 79,1% of the females with CIN 3 and even in extremely high 97,5 % in females with CIS.The lowest prevalence was detected in patients with ASCUS- 23,9% and CIN 1-25,6%.
Table 3. The distribution of HPV infection among women with intraepithelial lesions according to cytologic diagnosis.
| Cytological diagnosis | Negative | Positive | Total | ||
|---|---|---|---|---|---|
| n | % | n | % | N | |
| ASCUS | 235 | 76,1 | 74 | 23,9 | 309 |
| CIN 1 | 630 | 74,4 | 217 | 25,6 | 847 |
| CIN 2 | 221 | 46,4 | 255 | 53,6 | 476 |
| CIN 3 | 38 | 20,9 | 144 | 79,1 | 182 |
| CIS | 2 | 2,5 | 79 | 97,5 | 81 |
| Total | 1126 | Procent59,42 | 769 | Procent40,68 | 1895 |
Graph 1. The distribution of HPV infection among women with intraepithelial lesions according to cytologic diagnosis.
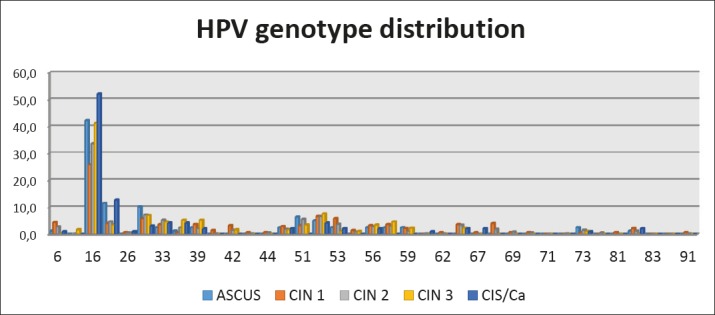
Acording to the results of HPV typing, DNA of high risk HPV genotypes was found in either single or multiple infections. We have seen that HPV 16, 18 and 31 were the most common types detected in the patients from Macedonia (Graph 2). HPV 16 was detected in 42,3 % of all patients, in 52,1 % of women with CIS and 41,2% women with CIN 3. HPV 16 is present in 45,3% of the patients with mixed infection. HPV type 31 ranked second in patients with CIN1, CIN2, CIN3, while HPV 18 was on second position among patients with CIS, presented in 12,8%. Concerning other HPV genotypes, HPV type 33,35 and 52 or mixed infection HPV 16/18 and HPV 16/31 were also present. Most of the patients were infected with high risk HPV, only few of them with mixed low and high risk, and none of them with low risk HPV 6 or 11. Surprisingly, patients with mixed infection had more LSIL and HSIL then CIS.
Graph 2. Percent (%) of HPV genotypes according to cytologic diagnosis.
4. DISCUSSION
In reduction of cervical cancer related morbidity and mortality requires development and expansion of screening programs for cervical pathology. This was launched in 2012 by the Ministry of Health of Republic of Macedonia, stem gynecologists, Institute for Public Health and the Centers for Public Health, which primarily resulted with decline in the incidence and mortality rates of cervical cancer among women in the Republic of Macedonia. Evaluation of the prevalence and identification of the specific types of human papiloma virus in cervical intraepithelial lesions and cervical cancer are important stages in the process of designing a new polyvalent vaccines in future, with a potential to prevent certain number of new cases. It could also help in vaccine development efforts that protect against the types which are not presented in the nowadays polivalent vaccines and against other HPV types as well (13).
Allthougt ASCUS,LSIL, HSIL and ICC were each associated with detection of any HPV (low risk, high risk and unclasified types), the strongest assosciations were observed between HSIL/cancer and high risk HPV types, as has been seen in previous studies (14-19). Our results showed that the prevalence of HPV infection among females with cervical intraepithellial lesions was 46,7% and in women with ICC was even 97,5%. Various studies have shown that prevalence of HPV infection may be from 17,9% in presence of ASCUS and more then 80% in the presence of HSIL (8).
Incidence of cervical cancer by age group in Macedonia, in 2012, was greatest in older women, 27 patients in the 50-54 years old group, and 25 in patients in the 55-59 years group (5). Mean age was highest (44.4 ±11.7 years) in females with ICC, but HPV prevalence was greatest in the youngest age group (<20 years). 82,1% of the patients were infected with high risk HPV. Among the population where women do not receive routine PAP screening the prevalence was highest in patients aged 55 years or older, as found in 2 population based studies of women in Costa Rica and Mexico, who reported an increased prevalence among older women (14, 20). Whereas in a population where women recive routine PAP screening, the prevalence is higest among younger women, most likely reflects the acquisition of HPV near the onset of sexual activity (16, 21-23).
Our study shows that among Macedonian females visiting our tertiary care facility (serving round 1,5 million people), HPV 16, 31 and 18 were the types most strongly associated with intraepithelial cervical lesions and cervical cancers. Many previous studies have shown that HPV 16 is the most common genotype isolated worldwide, but diferent types are specific for particular geographical regions (14, 20-22, 25-27). Our study detected HPV 16 in 52,1.% of the females with ICC, similar to findings of a Canadian study where HPV 16 was detected in 52% of patients with ICC. A meta-analysis of studies of 6978 women has found that the prevalence of HPV 16 on a global scale ranges from 51,8% (95%CI 50,1-53,5) in Europe to 33,3% (95%CI, 20,4-48,4) in Oceania (28). Another meta analysis conducted in Japan has detected a rate of high risk HPV as high as 72,4% (28). In Tanzanian women HPV was detected even in 73% of females with ICC (29). In our population, the second HPV genotype was HPV 31 for LSIL and HSIL which was published also in the studies of Dutch, Mexican and British population, but still HPV 18 ranked second for ICC (20, 22, 24). On the other hand, HPV 53 was detected after HPV 16 among Brazilian women and women in United States, HPV 58, among Costa Rican, West African, Japanese and Chinese women (20-22, 24, 31). Therefore, a conclusion is that the different HPV types might predominate in a specific population, probably as a result of the chance introduction of a specific virus into that population, or varying abilities of certain HPV types to sustain as an endemic infection in a particular geographic area. Also it has been published that the different distribution of HPV genotypes could be a result of different immune response of that population (32).
The literature has also reported that multiple HPV infections are related with an increased risk of HSIL/cancer (10, 33, 34). It is unclear if this is a result of deficient imune response to HPV, lower level of imunoglobulin G (IgG) and IgA in the infected tissue, or if it is synergistically enhanced by existing infection with another HPV genotype (9, 35-38). On the other hand, our results show some interesting differences with global data. Findings of a mixed infection had shown that it is more frequent among patients with LSIL and HSIL than among patients with ICC. Our results have also shown that HPV 16 was present in most multiple infections. HPV 16/18 and HPV 16/31 were the most common combination of mixed infection.
Findings of this study contribute to HPV knowledge in our country that could be usefull in future, primarily in the development of screening strategies and new vaccines. One of the limitations of our study was that we did not include women with normal cytology.
5. CONCLUSION
Human papilloma viruses 16, 18 and 31 are the most commonly detected types among Macedonian women and at the same time they are the most strongly associated with intraepithelial lesions and cases of cervical cancer. The prevalence was highest among younger women and the age of the patients was higher in the group of women with invasive cervical carcinoma. Patients with mixed infections most commonly had low grade squamous intraepithelial lesions and high grade squamous intraepithelial lesions than carcinoma in situ.
Authors’ contributions
Irena Aleksioska Papestiev: Designed the text for the first time, collected data and put everything together in its final form. esna Chibisheva: Collected and analyzed the collected data. Helped with text editing. Megi Micevska: Did all the laboratory work, collected and interpreted the results. Goran Dimitrov: He approved the final version of the completed article.
Conflict of interest
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Petignat P, Roy M. Diagnosis and management of cervical cancer. BMJ. 2007 Oct 13;335(7623):765–8. doi: 10.1136/bmj.39337.615197.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbera L, Thomas G. Management of early and locally advanced cervical cancer. Semin Oncol. 2009 Apr;36(2):155–9. doi: 10.1053/j.seminoncol.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Forouzandar MH, Foreman KJ, Delossantos AM, et al. Brest and cervical cancer in 187 countries between 1980 and 2010: a systematic analysis. Lancet. 2011 Oct 22;378(9801):1461–84. doi: 10.1016/S0140-6736(11)61351-2. [DOI] [PubMed] [Google Scholar]
- 4.Scottish Intercollegiate Guidelines Network (SIGN) SIGN; 2008. Jan, Management of cervical cancer. [Google Scholar]
- 5.Ferlay J, Soerjomataram I, Ervik M, et al. Lyon, France: International Agency for Research on Cancer; 2013. GLOBOCAN 2012 v1.2, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11 [Internet] [Google Scholar]
- 6.Medeiros LR, Hilgert JB, Zanini RR, et al. Vertical transmition of human papilloma virus: a systematic quantitive review. Cad Saude Publica. 2005;21(4):1006–15. doi: 10.1590/s0102-311x2005000400003. [DOI] [PubMed] [Google Scholar]
- 7.Bouvard V, Baan R, Straif K, et al. WHO International Agency for Research on Cancer Monograph Working group. A review of human carcinogens-Part B: biological agents. Lancet Oncol. 2009;10:321–2. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 8.Bosh FX, Burchell AN, Schiffman M, et al. Epidemiology and natural history of human papilloma virus infections and type specific implications in cervical neoplasia. Vaccine. 2008;26(Supll10):K1–16. doi: 10.1016/j.vaccine.2008.05.064. [DOI] [PubMed] [Google Scholar]
- 9.Munoz N, Bosh FX, Casteilsauge X, et al. Against which human papilloma virus tzpes shall we vaccinate and screen? The international pperspective. Int J Cancer. 2004;111:278–85. doi: 10.1002/ijc.20244. [DOI] [PubMed] [Google Scholar]
- 10.Coutlee F, Ratnam S, Ramanakumar AV, et al. Distribution of human papillomavirus genotypes in cervical intraepithelial neoplasia and invasive cervical cancer in Canada. J Med Virol. 2011;83:1034–41. doi: 10.1002/jmv.22081. [DOI] [PubMed] [Google Scholar]
- 11.Insinga RP, Liaw KL, Johnson LG, Madeleine MM. A systematic review of the prevalence and attribution of human papillomavirus types among cervical, vaginal, and vulvar precancers and cancers in the Unites States. Cancer Epidemiol Biomarkers Prev. 2008;17:1611–22. doi: 10.1158/1055-9965.EPI-07-2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Apgar BS, Zoschnick L, Wright TC. The 2001 Bethesda system terminology. Am Fam Physician. 1998;68:10. [PubMed] [Google Scholar]
- 13.Cuzick J, Arbyn M, Sankaranarayanan R, et al. Overview of human papillomavirus-based and other novel options for cervical cancer screening in developed and developing countries. Vaccine. 2008;26(Suppl 10):K29–41. doi: 10.1016/j.vaccine.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 14.Herrero R, Hildesheim A, Bratti C, et al. Population-based study of human papillomavirus infection and cervical neoplasia in rural Costa Rica. J Natl Cancer Inst. 2000;92:464–74. doi: 10.1093/jnci/92.6.464. [DOI] [PubMed] [Google Scholar]
- 15.Chan PK, Li WH, Chan MY, et al. High prevalence of human papillomavirus type 58 in Chinese women with cervical cancer and precancerous lesions. J Med Virol. 1999;59:232–8. [PubMed] [Google Scholar]
- 16.De Roda, Husman AM, Walboomers JM, et al. HPV prevalence in cytomorphologically normal cervical scrapes of pregnant 808 XI ET AL. women as determined by PCR: the age-related pattern. J Med Virol. 1995;46:97–102. doi: 10.1002/jmv.1890460203. [DOI] [PubMed] [Google Scholar]
- 17.Moscicki AB, Shiboski S, Broering J, et al. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J Pediatr. 1998;132:277–84. doi: 10.1016/s0022-3476(98)70445-7. [DOI] [PubMed] [Google Scholar]
- 18.Liaw KL, Glass AG, Manos MM, et al. Detection of human papillomavirus DNA in cytologically normal women and subsequent cervical squamous intraepithelial lesions. J Natl Cancer Inst. 1999;91:954–60. doi: 10.1093/jnci/91.11.954. [DOI] [PubMed] [Google Scholar]
- 19.Schiff M, Becker TM, Masuk M, et al. Risk factors for cervical intraepithelial neoplasia in Southwestern American Indian women. Am J Epidemiol. 2000;152:716–26. doi: 10.1093/aje/152.8.716. [DOI] [PubMed] [Google Scholar]
- 20.Lazcano-Ponce E, Herrero R, Munoz N, et al. Epidemiology of HPV infection among Mexican women with normal cervical cytology. Int J Cancer. 2001;91:412–20. doi: 10.1002/1097-0215(20010201)91:3<412::aid-ijc1071>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 21.Peyton CL, Gravitt PE, Hunt WC, et al. Determinants of genital human papillomavirus detection in a US population. Infect Dis. 2001;183:1554–64. doi: 10.1086/320696. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs MV, Walboomers JM, Snijders PJ, et al. Distribution of 37 mucosotropic HPV types in women with cytologically normal cervical smears: the age-related patterns for high-risk and low-risk types. Int J Cancer. 2000;87:221–7. [PubMed] [Google Scholar]
- 23.Melkert PW, Hopman E, van den Brule AJ, et al. Prevalence of HPV in cytomorphologically normal cervical smears, as determined by the polymerase chain reaction, is age dependent. Int J Cancer. 1993;53:919–23. doi: 10.1002/ijc.2910530609. [DOI] [PubMed] [Google Scholar]
- 24.Cuzick J, Beverley E, Ho L, et al. HPV testing in primary screening of older women. Br J Cancer. 1999;81:554–8. doi: 10.1038/sj.bjc.6690730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sasagawa T, Basha W, Yamazaki H, Inoue M. High-risk and multiple human papillomavirus infections associated with cervical abnormalities in Japanese women. Cancer Epidemiol Biomarker Prev. 2001;10:45–52. [PubMed] [Google Scholar]
- 26.Chan PK, Chang AR, Cheung JL, et al. Determinants of cervical human papillomavirus infection: differences between high-and low-oncogenic risk types. J Infect Dis. 2002;185:28–35. doi: 10.1086/338010. [DOI] [PubMed] [Google Scholar]
- 27.Giuliano AR, Papenfuss M, Abrahamsen M, et al. Human papillomavirus infection at the United States-Mexico border: implications for cervical cancer prevention and control. Cancer Epidemiol Biomarkers Prev. 2001;10:1129–36. [PubMed] [Google Scholar]
- 28.Hosaka M, Fujita H, Hanley SJ, et al. Incidence risk of cervical intraepithelial neoplasia 3 or more severe lesions is a function of human papillomavirus genotypes and severity of cytological and histological abnormalities in adult Japanese women. Int J Cancer. 2013 Jan 15;132(2):327–34. doi: 10.1002/ijc.27680. [DOI] [PubMed] [Google Scholar]
- 29.Vidal Adriana C, Murphy Susan K, Hernandez Brenda Y, et al. Distribution of HPV genotypes in cervical intraepithelial lesions and cervical cancer in Tanzanian women. Infect Agent Cancer. 2011;6:20. doi: 10.1186/1750-9378-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gudleviciene Z, Sepetiene A, Didziapetriene J, et al. Prevalence of human papillomavirus types in cervical intraepithelial lesions. Medicina (Kaunas) 2010;46(9):616–23. [PubMed] [Google Scholar]
- 31.Franco EL, Villa LL, Sobrinho JP, et al. Epidemiology of acquisition and clearance of cervical human papillomavirus infection in women from a high-risk area for cervical cancer. J Infect Dis. 1999;180:1415–23. doi: 10.1086/315086. [DOI] [PubMed] [Google Scholar]
- 32.Apple RJ, Erlich HA, Klitz W, et al. HLA DR-DQ associations with cervical carcinoma show papillomavirus-type specificity. Nat Genet. 1994;6:157–62. doi: 10.1038/ng0294-157. [DOI] [PubMed] [Google Scholar]
- 33.Adam E, Kaufman RH, Berkova Z, et al. Is human papillomavirus testing an effective triage method for detection of highgrade (grade 2 or 3) cervical intraepithelial neoplasia? Am J Obstet Gynecol. 1998;178:1235–44. doi: 10.1016/s0002-9378(98)70328-x. [DOI] [PubMed] [Google Scholar]
- 34.McLehose RF, Harpster A, Lanier AP, et al. Risk factors for cervical intraepithelial neoplasm in Alaska Native women: a pilot study. Alaska Med. 1999;41:76–85. [PubMed] [Google Scholar]
- 35.Bayo S, Bosch FX, de Sanjose S, et al. Risk factors of invasive cervical cancer in Mali. Int J Epidemiol. 2002;31:202–9. doi: 10.1093/ije/31.1.202. [DOI] [PubMed] [Google Scholar]
- 36.Kay P, Soeters R, Nevin J, et al. High prevalence of HPV16 in South African women with cancer of the cervix and cervical intraepithelial neoplasia. J Med Virol. 2003;71:265–73. doi: 10.1002/jmv.10479. [DOI] [PubMed] [Google Scholar]
- 37.Liaw KL, Hidelsheim A, Burk RD, et al. A prospective study of human papillomavirus (HPV) type 16 DNA detection by polymerase chain reaction and its association with acquisition and persistence of other HPV types. J Infect Dis. 2001;183:8–15. doi: 10.1086/317638. [DOI] [PubMed] [Google Scholar]
- 38.Castellsague X, Menendez C, Loscertales MP, et al. Human papillomavirus genotypes in rural Mozambique. Lancet. 2001;358:1429–30. doi: 10.1016/S0140-6736(01)06523-0. [DOI] [PubMed] [Google Scholar]


