Abstract
Background
The uteruses of most dairy cattle are easily infected by bacteria, especially gram-negative bacteria, following parturition. Macrophages are important cells of the immune system and play a critical role in the inflammatory response. In addition, cortisol levels become significantly increased due to the stress of parturition in dairy cattle, and cortisol is among the most widely used and effective therapies for many inflammatory diseases. In this study, we assessed the anti-inflammatory effects and potential molecular mechanisms of cortisol using a Lipopolysaccharide (LPS)-induced RAW264.7 macrophage cell line.
Results
Cortisol significantly suppressed the production of prostaglandin E2 (PGE2) and decreased the gene and protein expression of inducible NO synthase (iNOS) and cyclooxygenase-2 (COX-2) in a dose-dependent manner. Moreover, cortisol inhibited the mRNA expression of pro-inflammatory cytokines including tumor necrosis factor alpha (TNFα), interleukin-1β (IL-1β), and interleukin-6 (IL-6) and decreased IL-1β secretion in an LPS-treated RAW264.7 macrophage cell line. Moreover, we found that cortisol suppressed nuclear factor-kappa B (NF-κB) signaling in RAW264.7 macrophages stimulated with LPS. This suppression was mediated by the inhibition of IκBα degradation and NF-κB p65 phosphorylation. In addition, cortisol also suppressed the phosphorylation of mitogen-activated protein kinases (MAPK) such as extracellular signal-regulated kinase (ERK1/2), p38 MAPK, and c-Jun N-terminal kinase/stress-activated protein kinase (JNK).
Conclusions
These results suggest that high cortisol levels can attenuate LPS-induced inflammatory responses in the RAW264.7 macrophage cell line by regulating the NF-κB and MAPK signaling pathways.
Keywords: Macrophage, Cortisol, Anti-inflammatory, LPS, NF-κB, MAPKs
Background
Postpartum uterine infection and inflammation are the primary causes of reproductive failure in dairy cows [1, 2]. Almost all cows are susceptible to bacterial infection at the openings of anatomic barriers including the vulva, vagina, and cervix. Lipopolysaccharide (LPS) is the most common pathogenic endotoxin component in the outer membrane of gram-negative bacteria and can disturb the balance between immunity and inflammatory responses [3]. Inflammation is a major risk factor for many diseases, and macrophages are important immune cells that act as the first line of defense against invading agents (bacteria, viruses, and fungi) [4, 5]. During inflammation, macrophages produce excessive amounts of inflammatory mediators such as prostaglandin E2 (PGE2), inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) and pro-inflammatory cytokines including interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-alpha (TNFα) [6]. Moreover, iNOS and COX-2 are believed to be the most important inflammatory mediators [7]. Overproduction of these mediators can be harmful to animal organs.
Nuclear factor kappa-B (NF-κB) and mitogen-activated protein kinase (MAPK) are important signaling molecules in the Toll-like receptor (TLR) pathway [8, 9]. NF-κB plays an important role in regulating the inflammatory responses by increasing the expression of inflammatory mediators and pro-inflammatory cytokines such as PGE2, iNOS, COX-2, IL-1β, IL-6 and TNFα [10]. Under unstimulated conditions, heterodimers of NF-κB components, mainly p50/p65, remain in the cytoplasm in an inactive form due to linkage to the inhibitor of κB (IκB) protein. However, when induced by LPS, NF-κB (p50/p65) is released through the phosphorylation and degradation of IκB. As a result, NF-κB p65, which is believed to play a central role in inflammation, enters the nucleus and encodes various cytokines and chemokines [11–13]. The MAPKs represent a specific class of serine/threonine kinases that respond to extracellular signals, including extracellular signal-regulated kinase 1/2 (ERK1/2), p38, and c-Jun NH2-terminal kinase (JNK). Similar to NF-κB, the MAPK signaling pathways are involved in LPS-induced iNOS and COX-2 expression in activated macrophages [14]. Even more importantly, MAPKs play essential roles in the activation of NF-κB [15]. Therefore, inhibition of the NF-κB and MAPK pathways may be a potential therapeutic approach to inflammatory injury.
Dairy cows have high levels of cortisol due to many kinds of stress during the perinatal period, such as pregnancy, labor, and lactation [16–18]. Furthermore, cortisol effectively protects immune cells from excessive inflammation [19]. However, neither the anti-inflammatory activity of cortisol on macrophages nor the mechanism of this process has been reported.
In this study, we demonstrated the anti-inflammatory properties of cortisol on LPS-induced inflammation injury in the RAW264.7 macrophage cell line. We further investigated the ability of cortisol to inhibit the activation of NF-κB and mitogen-activated protein kinases (MAPKs) to clarify the mechanism of its anti-inflammatory effects. This study may reveal a vital role for endogenous glucocorticoids and the underlying mechanism of glucocorticoid-mediated anti-inflammatory activity in the postpartum cow uterus, thus proposing a scientific basis for the prevention and treatment of endometritis in dairy cattle.
Methods
Reagents
Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), and other tissue culture reagents were purchased from Gibco BRL Co. (Grand Island, NY, USA). Cortisol (H0888) and LPS (Escherichia coli 0111:B4) were purchased from Sigma (St. Louis, MO, USA). The Cell-Counting Kit-8 (CCK-8) reagents were obtained from Dojindo Molecular Technologies, Inc. (Kumamoto, Japan). Enzyme-linked immunosorbent assay (ELISA) kits for PGE2, IL-1β, IL-6, and TNFα were purchased from R & D Systems, Inc. (Minneapolis, MN, USA). β-actin, iNOS, COX-2, ERK1/2, phospho-ERK1/2, p38, phospho-p38, JNK, phospho-JNK, NF-κB p65, phospho-NF-κB p65, IκBα and phospho-IκBα antibodies were purchased from Cell Signaling Technology (Boston, MA, USA).
Cell culture and viability assays
The RAW264.7 macrophage cell line was obtained from the American Type Culture Collection (ATCC, MD, US). The cells were cultured at 37 °C in DMEM supplemented with 2 mM glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin and 10% fetal bovine serum (FBS) in a 5% CO2 environment [20]. To evaluate cell viability, RAW264.7 cells (5 × 103 cells/well) were seeded in 96-well plates and incubated for 18 h before experimental interventions. The cells were then treated with several concentrations of cortisol for 24 h. Ten microliters of the CCK-8 solution was added to each well, and the plate was incubated at 37 °C for 2 h. The optical density was then read at 450 nm using a microplate reader (Tecan, Austria).
PGE2, IL-1β, IL-6, and TNFα assays
RAW 264.7 cells were seeded in 12-well plates (5 × 105 cells/mL) and incubated at 37 °C for 18 h. The cells were co-treated with cortisol (5, 15 and 30 ng/mL) and LPS (1 μg/mL) for 6, 12 and 24 h. Supernatant levels of PGE2, IL-1β, IL-6, and TNFα were measured by ELISA according to the manufacturer’s instructions.
RNA extraction and real-time quantitative reverse transcription PCR
RAW 264.7 macrophages were treated with 1 μg/mL LPS in the presence or absence of cortisol (0, 5, 15 and 30 ng/mL). After 6-, 12- and 24-h incubation periods, total RNA was isolated from RAW 264.7 macrophages according to the manufacturer’s instructions using Trizol reagent (Invitrogen, US). The extracted RNA was quantified using a Nanodrop 2000 spectrophotometer (Thermo, USA). The RNA (900 ng) was then converted to cDNA as previously described [21]. The PCR contained 10 μL SYBR Green PCR mix, 0.5 μL each primer, and 1 μL cDNA template in a final reaction volume of 20 μL (Takara, Japan). The real-time PCR cycling conditions were 95 °C for 2 min, 40 cycles of 95 °C for 10 s, 60 °C for 30 s, and 72 °C for 30 s using a CFX connect real-time PCR system (BIO-RAD, US). The rat β-actin primers were used as the endogenous control. Relative gene expression was calculated using the comparative Ct method (2-△△Ct) as previously described [22]. The primer sequences used in this study are presented in Table 1.
Table 1.
Primer sequences used for qRT-PCR amplification
| Gene | Forward primer | Reverse primer | Accession number | Product size(bp) |
|---|---|---|---|---|
| β-actin | TGCTGTCCCTGTATGCCTCT | TTTGATGTCACGCACGATTT | NM_031144.3 | 224 |
| IL-1β | ACCTGTGTCTTTCCCGTGG | TCATCTCGGAGCCTGTAGTG | NM_031512.2 | 159 |
| TNFα | GCCTCCCTCTCATCAGTTCTA | GGCAGCCTTGTCCCTTG | NM_012675.3 | 246 |
| IL-6 | AGTTGTGCAATGGCAATTCTGA | AGGACTCTGGCTTTGTCTTTCT | NM_012589.2 | 223 |
| iNOS | TTCCAGAATCCCTGGACAAG | TGGTCAAACTCTTGGGGTTC | NM_012611.3 | 180 |
| COX-2 | AGAAGGAAATGGCTGCAGAA | GCTCGGCTTCCAGTATTGAG | NM_017232.3 | 194 |
Western blot analysis
RAW264.7 macrophages were stimulated with LPS alone or together with cortisol as described above. The total proteins were extracted, and protein concentrations were determined using a bicinchoninic acid (BCA) protein assay kit (BioChain, US). Total proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Germany). The membranes were immunoblotted with primary antibodies specific for iNOS, COX-2, NF-κB p65, phospho-NF-κB p65, IκBα, phospho-IκBα, p-ERK1/2, ERK1/2, p-p38, p38, p-JNK, JNK, and β-actin at 4 °C overnight and then incubated with HRP-conjugated secondary antibodies (CST, US) at room temperature for 1 h. The blots were washed with PBS-T, and the proteins of interest were detected using a chemiluminescence (ECL) assay according to the manufacturer’s instructions.
Statistical analysis
Each experiment was repeated at least three times, and all data are expressed as means ± standard error of the mean (SEM) for the number of experiments. Statistically significant differences throughout this study were calculated by one-way ANOVA followed by Dunnett’s test (SPSS 17.0 software). A two-sided p-value less than 0.05 was considered statistically significant.
Results
Effect of cortisol on RAW264.7 macrophage viability
The effect of cortisol on the RAW264.7 macrophage cell line viability was assessed using a CCK-8 assay. As shown in Fig. 1, cortisol did not affect the viability of the RAW 264.7 cells at concentrations from 5 to 15 ng/mL, but it did alter cell growth at 20 to 60 ng/mL. Therefore, cortisol concentrations of 5, 15, and 30 ng/mL were selected for further investigation.
Fig. 1.
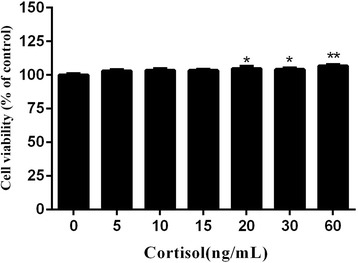
Effects of different concentrations of cortisol on RAW264.7 cell viability as measured by the CCK-8 assay. The data shown are means ± SEM (n = 6). *p < 0.05 and **p < 0.01 vs. control group
Cortisol modulation of extracellular PGE2, TNFα, IL-1β, and IL-6 production in LPS-induced RAW264.7 macrophages
To investigate the inhibitory effects of cortisol on the extracellular production of inflammatory mediators and pro-inflammatory cytokines including PGE2, TNFα, IL-1β, and IL-6 by LPS-induced RAW264.7 macrophages, cytokine-specific ELISAs were used to determine the levels of each molecule in RAW264.7 culture supernatants. As depicted in Fig. 2a, the PGE2 concentration in the culture medium of the LPS-treated group was significantly (p < 0.01) increased compared with the control group at 12 and 24 h. However, co-incubation with cortisol significantly (p < 0.05) suppressed this increased production in a dose-dependent manner. The expression levels of IL-1β, IL-6, and TNFα induced by LPS were significantly upregulated at the indicated time points (p < 0.01). However, cortisol significantly suppressed the extracellular levels of IL-1β when compared with the LPS treated group in a dose-dependent manner (Fig. 2b). The levels of TNFα and IL-6 were not affected by cortisol treatment (Fig. 2c and d).
Fig. 2.
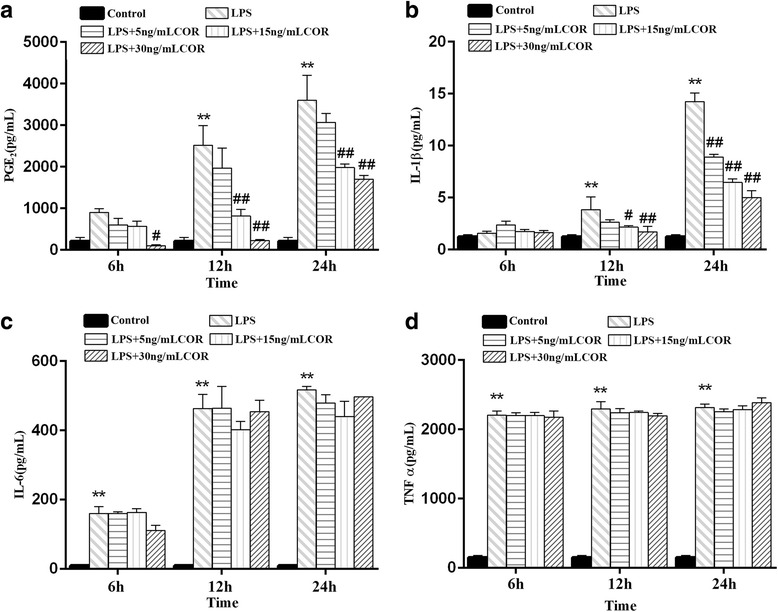
Effect of cortisol on PGE2 and cytokine production in LPS-stimulated RAW 264.7 macrophages. RAW264.7 cells were co-treated with cortisol (0, 5, 15 and 30 ng/mL) and LPS (1 μg/mL) for 0, 6, 12, and 24 h. Levels of PGE2 (a), IL-1β (b), IL-6 (c), and TNFα (d) in culture supernatants were measured by ELISA. The data presented are the means±SEM. ** p < 0.01 vs. the control group; # p < 0.05, ## p < 0.01 vs. the LPS group
Effects of cortisol on the protein and mRNA expression levels of iNOS and COX-2 in LPS-stimulated RAW264.7 macrophages
Since COX-2 and iNOS are enzymes for PGE2 and NO synthesis, we further investigated the inhibitory effects of cortisol treatment on COX-2 and iNOS expression using Western blotting and RT-PCR, respectively. As shown in Fig. 3a, the mRNA expression level of iNOS dramatically (p < 0.01) increased following stimulation of macrophages with LPS at 12 and 24 h. The mRNA expression levels of iNOS in the experimental groups were down-regulated by cortisol treatment at all concentrations (p < 0.05). Similarly, the COX-2 mRNA levels were significantly increased by stimulation of macrophages with LPS at 6, 12, and 24 h (Fig. 3b). In addition, COX-2 mRNA levels were also inhibited by cortisol in a dose-dependent manner.
Fig. 3.
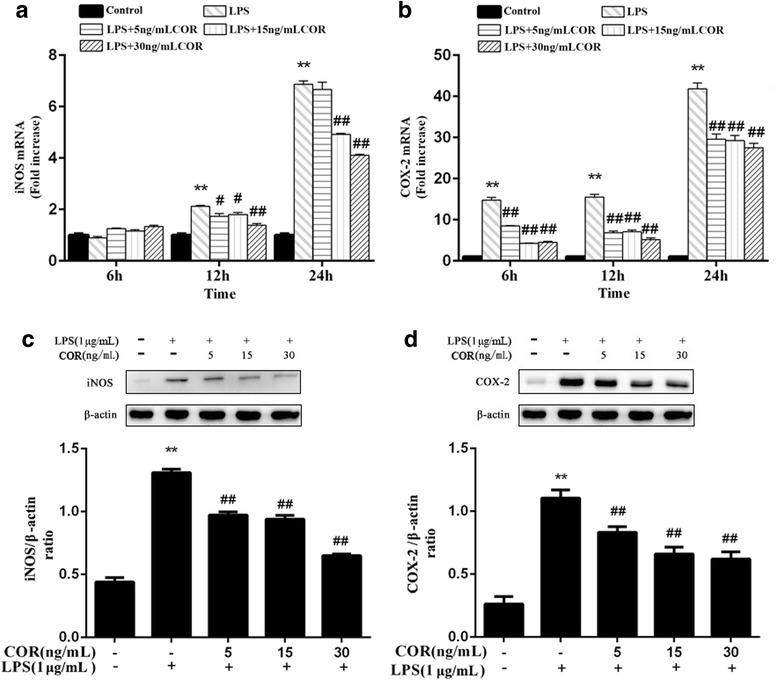
Effects of cortisol on the mRNA and protein expression levels of iNOS and COX-2 in LPS-stimulated RAW264.7 cells. a and b Cells were co-treated with cortisol (5,15 and 30 ng/mL) and LPS (1 μg/mL) for 0, 6, 12, and 24 h. RNA was isolated and analyzed by RT-PCR. c and d Cells were co-treated with cortisol (5, 15 and 30 ng/mL) and LPS (1 μg/mL) for 24 h. Total proteins were isolated and analyzed by Western blot. The data presented are the means±SEM. ** p < 0.01 vs. the control group; # p < 0.05, ## p < 0.01 vs. the LPS group
The protein expression levels of iNOS and COX-2 were significantly (p < 0.01) upregulated by stimulation of macrophages with LPS at 24 h. However, these effects were markedly (p < 0.01) inhibited by cortisol treatment in a dose-dependent manner (Fig. 3c and d).
Inhibitory effect of cortisol on LPS-induced TNFα, IL-1β and IL-6 mRNA expression
To determine the protective effect of cortisol on RAW264.7 macrophage inflammatory responses induced by LPS, we examined the mRNA expression levels of TNFα, IL-1β and IL-6 by RT-PCR. As shown in Fig. 4, the expression of TNFα, IL-1β and IL-6 induced by LPS was significantly upregulated at the indicated time points, whereas dose-dependent reductions in LPS-stimulated TNFα, IL-1β, and IL-6 mRNA expression levels were observed in macrophages after co-incubation with cortisol (p < 0.01).
Fig. 4.
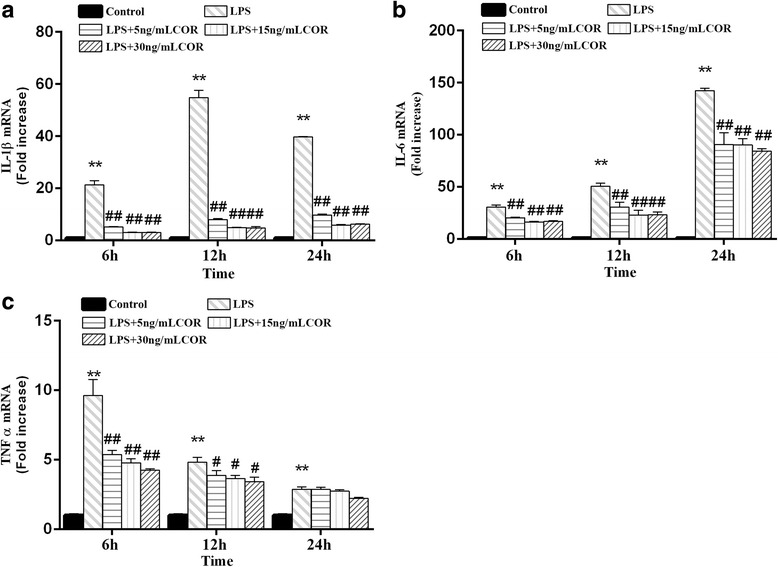
Effects of cortisol on IL-1β (a), IL-6 (b) and TNFα (c) mRNA expression in LPS-stimulated RAW264.7 cells. RAW264.7 cells were co-treated with cortisol (5, 15 and 30 ng/mL) and LPS (1 μg/mL) for 0, 6, 12, and 24 h. RNA was isolated and analyzed by RT-PCR. The data presented are the means±SEM. **p < 0.01 vs. the control group; # p < 0.05, ## p < 0.01 vs. the LPS group
Effects of cortisol on NF-κB activation in LPS-stimulated RAW264.7 macrophages
NF-κB is an important transcription factor that regulates the expression of most pro-inflammatory cytokines, as well as the levels of iNOS, COX-2, and PGE2. We investigated the critical proteins of this signaling pathway by Western blotting to determine the effect of cortisol on the NF-κB activity. As shown in Fig. 5, significant (p < 0.01) degradation of IκBα and increased expression of p-IκBα and p-p65 were observed in the cells following LPS exposure for 30 min, which indicated increased NF-κB activity. However, the degradation of IκBα and phosphorylation of IκBα and p65 were decreased after 45 min. Cortisol significantly inhibited the LPS-induced phosphorylation of p65, as well as phosphorylation and degradation of IκBα, in a dose-dependent manner. The data showed that the NF-κB activity in RAW264.7 macrophages induced by LPS was significantly (p < 0.01) inhibited by cortisol.
Fig. 5.
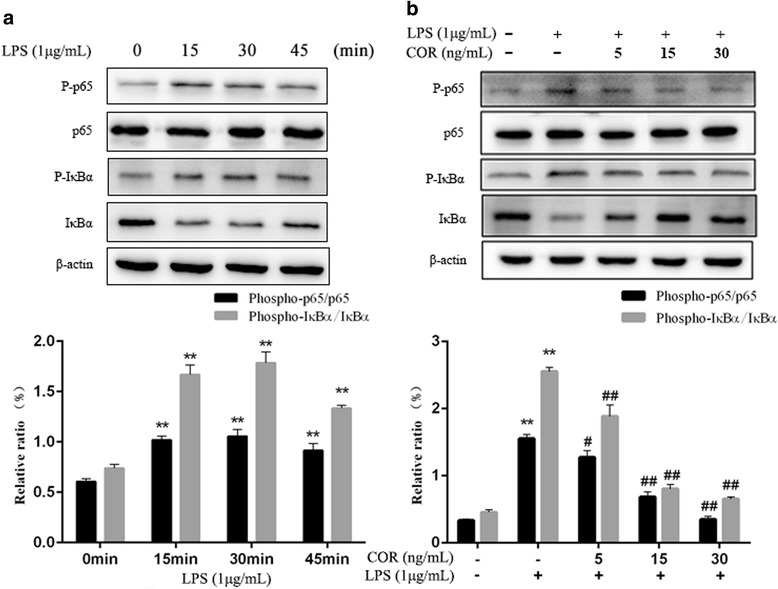
Inhibitory effects of cortisol on LPS-stimulated NF-κB p65 and IκBα phosphorylation in RAW 264.7 cells. a Cells were stimulated with LPS (1 μg/mL) alone for 0, 15, 30 and 45 min. b Cells were co-treated with cortisol (5, 15 and 30 ng/mL) and LPS (1 μg/mL) for 30 min. Total proteins were isolated and subjected to Western blotting. The data presented are the means±SEM. ** p < 0.01 vs. the control group; # p < 0.05, ## p < 0.01 vs. the LPS group
Effects of cortisol on the phosphorylation of MAPKs in LPS-stimulated RAW264.7 macrophages
MAPKs play important roles in the regulation of various physiological processes [23]. To determine the effect of cortisol on the MAPK pathway, we investigated the critical proteins of this signaling pathway by Western blot. The phosphorylation levels of ERK1/2, JNK, and p38 MAPK were significantly (p < 0.01) increased after the cells were treated with LPS for 30 min (Fig. 6a). However, the levels of phosphorylation were decreased after 45 min. Cortisol significantly (p < 0.01) inhibited the LPS-induced phosphorylation of ERK1/2, JNK, and p38 MAPK in a dose-dependent manner (Fig. 6b).
Fig. 6.
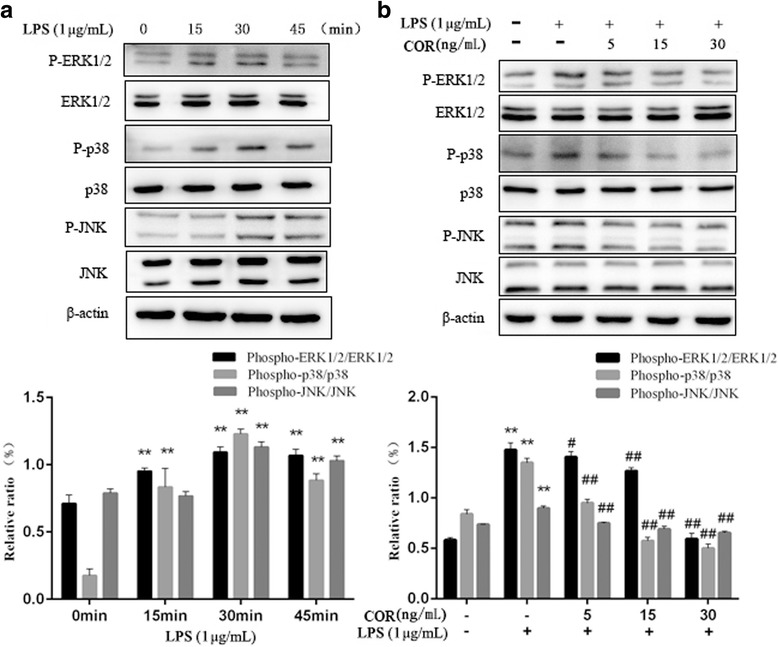
Inhibitory effects of cortisol on MAPK phosphorylation in RAW264.7 macrophages. a Cells were stimulated with LPS (1 μg/mL) alone for 0, 15, 30 and 45 min. b Cells were co-treated with cortisol (5, 15 and 30 ng/mL) and LPS (1 μg/mL) for 30 min. Total proteins were isolated and subjected to Western blotting. The data presented are the means±SEM. ** p < 0.01 vs. the control group; # p < 0.05, ## p < 0.01 vs. the LPS group
Discussion
In this study, we examined the anti-inflammatory activities of cortisol in LPS-induced RAW264.7 cells. Cortisol significantly inhibited the expression levels of inflammatory mediators and pro-inflammatory cytokines (Figs. 3 and 4). Moreover, the NF-κB and MAPK activities in LPS-induced RAW264.7 macrophages were obviously alleviated by cortisol (Figs. 5 and 6).
After parturition, dairy cows are more susceptible to endometritis, which is the primary cause of reproductive failure [2]. If not treated in a timely manner, the inflammatory response generates more serious consequences that lead to endometritis and even purulent uterine inflammation. Cortisol is a major regulator of inflammation and may play a role in preventing inflammation in the body [24]. Perinatal stress triggers the release of corticotropin-releasing hormone (CRH) from the hypothalamus, which acts on the pituitary to release adrenocorticotropin hormone (ACTH) and subsequently on the adrenal glands to release cortisol into blood circulation. In addition, cortisol production peaks due to the stress of parturition [16, 25].
A rapid inflammatory response is produced in LPS-stimulated RAW264.7 macrophages, which could release a large number of pro-inflammatory cytokines (TNFα, IL-1β, and IL-6) and inflammatory mediators (PGE2, COX-2 and iNOS) [6]. This is beneficial to attract circulating immune effector cells, such as neutrophils, to fight infection [26], but excessive inflammatory responses can injure tissues and organs. Therefore, the expression of inflammatory mediators and pro-inflammatory cytokines needs to be tightly regulated during an inflammatory response [27, 28]. In this study, we demonstrated the protective effect of cortisol against LPS-induced inflammation injury in the RAW264.7 macrophage cell line. The results showed that the gene expression and production of TNFα, IL-1β, and IL-6 were significantly increased in RAW264.7 macrophages stimulated with LPS, which induced a drastic inflammatory response. As expected, cortisol effectively inhibited the mRNA expression levels of IL-1β, IL-6, and TNFα in a dose-dependent manner, which protected macrophages from LPS-induced inflammation injury. Interestingly, there were inconsistencies between the mRNA expression and secretory protein levels of IL-6 and TNFα, which may be related to the translational regulation or the cytoplasm storage of these molecules. However, the specific mechanism behind this phenomenon requires further investigation.
NO and PGE2 are important inflammatory mediators that result in serious inflammatory diseases. iNOS catalyzes the oxidative deamination of L-arginine and ultimately leads to significant nitric oxide (NO) production. Similarly, COX-2 is a key enzyme involved in the biosynthesis of prostaglandin E2 (PGE2). Thus, reducing the levels of iNOS and COX-2 would be an effective strategy for suppressing inflammatory responses. Our study demonstrated that cortisol inhibited extracellular production of PGE2 in a dose-dependent manner at all time points. Moreover, cortisol inhibited LPS-induced iNOS and COX-2 mRNA and protein levels in a dose-dependent manner. These results indicated that cortisol could effectively inhibit the LPS-induced inflammatory response.
NF-κB is an important regulatory transcription factor that plays a critical role in regulating the expression of iNOS, COX-2 and pro-inflammatory cytokines such as TNFα, IL-1β and IL-6. Once activated by LPS, phosphorylation of IκBα is strongly enhanced (IκBα is the principal inhibitory protein of NF-κB), which leads to the rapid proteasomal degradation [29, 30] of IκBα. Nuclear factor κB dimers (p50:p65) are released and phosphorylated, which quickly enter the nucleus and bind specifically to defined DNA sequences to promote target gene expression [11, 12, 31]. Thus, we investigated the inhibitory effect of cortisol on LPS-induced NF-κB activation. The present study showed that the degradation of IκBα and the phosphorylation of IκBα and p65 were significantly increased after stimulation with LPS for 30 min, which suggested obviously increased NF-κB activation. However, the degradation of IκBα and the phosphorylation of IκBα and p65 were reduced after 45 min due to the synthesis and secretion of IκBα, which was consistent with the results of previous studies [32, 33]. Our results suggested that cortisol could reduce the degradation of IκBα and phosphorylation of IκB α and p65 at 30 min in a dose-dependent manner, demonstrating a significant inhibitory effect on NF-κB activity. Thus, cortisol could inhibit the inflammatory mediator and pro-inflammatory cytokine expression by downregulating the NF-κB pathway. Ultimately, inflammatory injury in the LPS-induced RAW264.7 macrophage cell line was significantly weakened.
In addition to NF-κB, much evidence has shown that the MAPK pathway also plays an important role in the inflammatory response. The MAPK family includes ERK1/2, JNK, and p38, which play a critical role in the transcriptional regulation of the LPS-induced expression of iNOS and COX-2 [34]. Moreover, MAPKs are known as upstream activators of NF-κB [35], as demonstrated by the inhibition of NF-κB transcriptional activation by specific MAPK inhibitors [36]. In the present study, the phosphorylation of ERK1/2, JNK, and p38 was significantly increased after LPS stimulation of RAW 264.7 macrophages, indicating that LPS activated the MAPK signaling pathway in the RAW264.7 macrophage cell line. Cortisol treatment obviously inhibited the phosphorylation of ERK1/2, JNK, and p38. These results suggested that the anti-inflammatory effects of cortisol are related to inhibition of MAPK phosphorylation in LPS-induced RAW 264.7 cells.
Conclusions
In conclusion, the current study clearly demonstrated the protective effect of cortisol on LPS-induced inflammation injury in the RAW264.7 macrophage cell line. Cortisol inhibited LPS-induced iNOS and COX-2 expression, as well as PGE2 production, in the macrophages. Equally, it also inhibited the expression of pro-inflammatory cytokines, including IL-1β, IL-6 and TNFα. The anti-inflammatory effect of cortisol on macrophages is mediated through inhibition of the NF-κB and MAPK signaling pathways.
Acknowledgements
None applicable.
Funding
The investigation was financially supported by the National Science Foundation of China (No.31672614, 31072176, 31302151), Natural Science Foundation of Jiangsu Province (No.BK20160062, BK2012265), Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), Top-notch Academic Programs Project of Jiangsu Higher Education Institutions (TAPP), Natural Science Foundation of the Higher Education Institutions of Jiangsu Province, China (17KJB230007), The National Natural Science Foundation of China (No.31502127) and Students’ Science and Technology Innovation Fund of Yangzhou University. The funders had no role in study design, data collection, analysis and interpretation, decision to publish, or preparation of the manuscript.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- COR
Cortisol
- ELISA
Enzyme-linked immunosorbent assay
- LPS
Lipopolysaccharide
- MAPK
Mitogen-activated protein kinase
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- qRT-PCR
Real-time quantitative reverse transcription PCR
Authors’ contributions
JD and JL conceived, designed and performed the majority of the experiments and drafted the manuscript. LC helped with experiments, provided valuable discussion and modified the final manuscript. YW, JL and YQ participated in experimental procedures and data analysis. HW conceived the study, participated in its coordination and helped to revise the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Junsheng Dong, Email: junshengsdau@163.com.
Jianji Li, Email: yzjjli@163.com.
Luying Cui, Email: dwyxcly@126.com.
Yefan Wang, Email: 425437445@qq.com.
Jiaqi Lin, Email: 240142830@qq.com.
Yang Qu, Email: 826114159@qq.com.
Heng Wang, Email: sdaulellow@163.com.
References
- 1.Sheldon IM, Noakes DE, Rycroft AN, Pfeiffer DU, Dobson H. Influence of uterine bacterial contamination after parturition on ovarian dominant follicle selection and follicle growth and function in cattle. Reproduction. 2002;123(6):837–845. doi: 10.1530/rep.0.1230837. [DOI] [PubMed] [Google Scholar]
- 2.Sheldon IM, Cronin J, Goetze L, Donofrio G, Schuberth HJ. Defining postpartum uterine disease and the mechanisms of infection and immunity in the female reproductive tract in cattle. Biol Reprod. 2009;81(6):1025–1032. doi: 10.1095/biolreprod.109.077370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim KS, Lee DS, Bae GS, Park SJ, Kang DG, Lee HS, Oh H, Kim YC. The inhibition of JNK MAPK and NF-κB signaling by tenuifoliside a isolated from Polygala Tenuifolia in lipopolysaccharide-induced macrophages is associated with its anti-inflammatory effect. Eur J Pharmacol. 2013;721(1–3):267–276. doi: 10.1016/j.ejphar.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 4.Zhong Y, Chiou YS, Pan MH, Shahidi F. Anti-inflammatory activity of lipophilic epigallocatechin gallate (EGCG) derivatives in LPS-stimulated murine macrophages. Food Chem. 2012;134(2):742–748. doi: 10.1016/j.foodchem.2012.02.172. [DOI] [PubMed] [Google Scholar]
- 5.Okkyoung K, Meeyoung L, Jieun Y, Seiryang O, Youngwon C, Hyeongkyu L, Kyungseop A. Anti-inflammatory effects of methanol extracts of the root of Lilium Lancifolium on LPS-stimulated Raw264.7 cells. J Ethnopharmacol. 2010;130(1):28–34. doi: 10.1016/j.jep.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Fairweather D, Rose NR. Inflammatory heart disease: a role for cytokines. Lupus. 2005;14(14):646–651. doi: 10.1191/0961203305lu2192oa. [DOI] [PubMed] [Google Scholar]
- 7.Chiu WT. Suppression of lipopolysaccharide-induced of inducible nitric oxide synthase and cyclooxygenase-2 by Sanguis Draconis, a dragon's blood resin, in RAW 264.7 cells. J Ethnopharmacol. 2008;115(3):455–462. doi: 10.1016/j.jep.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 8.Kim KS, Cui X, Lee DS, Sohn JH, Yim JH, Kim YC, Oh H. Anti-inflammatory effect of neoechinulin a from the marine fungus Eurotium sp. SF-5989 through the suppression of NF-кB and p38 MAPK pathways in lipopolysaccharide-stimulated RAW264.7 macrophages. Molecules. 2013;18(11):13245–13259. doi: 10.3390/molecules181113245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu Y, Yeh W, Ohashi P. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42(2):145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Shao J, Li Y, Wang Z, Xiao M, Yin P, Lu Y, Qian X, Xu Y, Liu J. 7b, a novel naphthalimide derivative, exhibited anti-inflammatory effects via targeted-inhibiting TAK1 following down-regulation of ERK1/2- and p38 MAPK-mediated activation of NF-κB in LPS-stimulated RAW264.7 macrophages. Int Immunopharmacol. 2013;17(2):216–228. doi: 10.1016/j.intimp.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Hoffmann A, Baltimore D. Circuitry of nuclear factor κB signaling. Immunol Rev. 2006;210(1):171–186. doi: 10.1111/j.0105-2896.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto M, Yamazaki S, Uematsu S, Sato S, Hemmi H, Hoshino K, Kaisho T, Kuwata H, Takeuchi O, Takeshige K. Regulation of toll/IL-1-receptor-mediated gene expression by the inducible nuclear protein IkappaBzeta. Nature. 2004;430(6996):218–222. doi: 10.1038/nature02738. [DOI] [PubMed] [Google Scholar]
- 13.Kamata H, Tsuchiya Y, Asano T. IκBβ is a positive and negative regulator of NF-κB activity during inflammation. Cell Res. 2010;20(11):1178–1180. doi: 10.1038/cr.2010.147. [DOI] [PubMed] [Google Scholar]
- 14.Li F, Fu Y, Bo L, Liu Z, Li D, Liang D, Wen Z, Cao Y, Zhang N, Zhang X. Stevioside suppressed inflammatory cytokine secretion by Downregulation of NF-κB and MAPK signaling pathways in LPS-stimulated RAW264.7 cells. Inflammation. 2012;35(5):1669–1675. doi: 10.1007/s10753-012-9483-0. [DOI] [PubMed] [Google Scholar]
- 15.Carter AB, Knudtson KL, Monick MM, Hunninghake GW. The p38 mitogen-activated protein kinase is required for NF-kappaB-dependent gene expression. The role of TATA-binding protein (TBP) J Biol Chem. 1999;274(43):30858–30863. doi: 10.1074/jbc.274.43.30858. [DOI] [PubMed] [Google Scholar]
- 16.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21(1):55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 17.Pontes GCS, Monteiro PLJ, Prata AB, Guardieiro MM, Pinto DAM, Fernandes GO, Wiltbank MC, Santos JEP, Sartori R. Effect of injectable vitamin E on incidence of retained fetal membranes and reproductive performance of dairy cows. J Dairy Sci. 2015;98(4):2437–2449. doi: 10.3168/jds.2014-8886. [DOI] [PubMed] [Google Scholar]
- 18.Pathak R, Prasad S, Kumaresan A, Kaur M, Manimaran A, Dang AK: Alterations in cortisol concentrations and expression of certain genes associated with neutrophil functions in cows developing retention of fetal membranes. Veterinary Immunology & Immunopathology 2015, 168(3–4):164-168. [DOI] [PubMed]
- 19.Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Molecular & Cellular Endocrinology. 2011;335(1):2–13. doi: 10.1016/j.mce.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hwang JH, Kim KJ, Ryu SJ, Lee BY. Caffeine prevents LPS-induced inflammatory responses in RAW264.7 cells and zebrafish. Chem Biol Interact. 2016;248:1. doi: 10.1016/j.cbi.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 21.Bi CL, Wang H, Wang YJ, Sun J, Dong JS, Meng X, Li JJ. Selenium inhibits Staphylococcus Aureus -induced inflammation by suppressing the activation of the NF-κB and MAPK signalling pathways in RAW264.7 macrophages. Eur J Pharmacol. 2016;780:159–165. doi: 10.1016/j.ejphar.2016.03.044. [DOI] [PubMed] [Google Scholar]
- 22.Shao CY, Wang H, Meng X, Zhu JQ, Wu YQ, Li JJ. Characterization of the innate immune response in goats after intrauterine infusion of E. Coli using histopathological, cytologic and molecular analyses. Theriogenology. 2012;78(3):593–604. doi: 10.1016/j.theriogenology.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy--from molecular mechanisms to therapeutic benefits. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics. 2005;1754(1–2):253–262. doi: 10.1016/j.bbapap.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 24.Mastorakos G, Ilias I. Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum. Ann N Y Acad Sci. 2003;997(997):136–149. doi: 10.1196/annals.1290.016. [DOI] [PubMed] [Google Scholar]
- 25.Patel OV, Takahashi T, Takenouchi N, Hirako M, Sasaki N, Domeki I. Peripheral cortisol levels throughout gestation in the cow: effect of stage of gestation and foetal number. Br Vet J. 1996;152(4):425–432. doi: 10.1016/S0007-1935(96)80036-4. [DOI] [PubMed] [Google Scholar]
- 26.Porcherie A, Cunha P, Trotereau A, Roussel P, Gilbert FB, Rainard P, Germon P. Repertoire of Escherichia Coli agonists sensed by innate immunity receptors of the bovine udder and mammary epithelial cells. Vet Res. 2012;43(1):1–8. doi: 10.1186/1297-9716-43-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shuster DE, Jr KM, Rainard P, Paape M. Complement fragment C5a and inflammatory cytokines in neutrophil recruitment during intramammary infection with Escherichia Coli. Infection & Immunity. 1997;65(8):3286–3292. doi: 10.1128/iai.65.8.3286-3292.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scheibel M, Klein B, Merkle H, Schulz M, Fritsch R, Greten FR, Arkan MC, Schneider G, Schmid RM. IκBβ is an essential co-activator for LPS-induced IL-1β transcription in vivo. J Exp Med. 2010;207(12):2621. doi: 10.1084/jem.20100864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karin M, Ben-Neriah Y. Phosphorylation meets Ubiquitination: the control of NF-κB activity. Immunology. 2000;18(18):621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 30.Ghosh S, Hayden MS. New regulators of NF-B in inflammation. Nat Rev Immunol. 2008;8(11):837–848. doi: 10.1038/nri2423. [DOI] [PubMed] [Google Scholar]
- 31.Kearns JD. Distinct functions of negative regulators of NF-kappaB. Dissertations & Theses - Gradworks. 2009;
- 32.Hoffmann A, Levchenko A, Scott ML, Baltimore D. The IκB-NF-κB signaling module: temporal control and selective gene activation. Science. 2002;298(5596):1241–1245. doi: 10.1126/science.1071914. [DOI] [PubMed] [Google Scholar]
- 33.Liu M, Song S, Li H, Jiang X, Yin P, Wan C, Liu X, Liu F, Xu J. The protective effect of caffeic acid against inflammation injury of primary bovine mammary epithelial cells induced by lipopolysaccharide. J Dairy Sci. 2014;97(5):2856–2865. doi: 10.3168/jds.2013-7600. [DOI] [PubMed] [Google Scholar]
- 34.Pratheeshkumar P, Kuttan G. Modulation of immune response by L. inhibits the proinflammatory cytokine profile, iNOS, and COX-2 expression in LPS-stimulated macrophages. Immunopharmacology & Immunotoxicology. 2011;33(1):73–83. doi: 10.3109/08923971003745977. [DOI] [PubMed] [Google Scholar]
- 35.Berghe WV, Plaisance S, Boone E, Bosscher KD, Schmitz ML, Fiers W, Haegeman G. p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways are required for nuclear factor-kappaB p65 transactivation mediated by tumor necrosis factor. J Biol Chem. 1998;273(6):3285–3290. doi: 10.1074/jbc.273.6.3285. [DOI] [PubMed] [Google Scholar]
- 36.Caivano M. Role of MAP kinase cascades in inducing arginine transporters and nitric oxide synthetase in RAW264 macrophages. FEBS Lett. 1998;429(3):249–253. doi: 10.1016/S0014-5793(98)00578-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.


