Abstract
Background
Hypertrophic cardiomyopathy (HCM) is a genetic disease was characterised by left ventricular hypertrophy (LVH), myocardial fibrosis, fiber disarray. The short-axis systolic function is important in left ventricle function.
Methods
Forty one healthy subjects and 37 HCM patients were enrolled for this research. Parasternal short-axis at the basal, middle, and apical levels were acquired by Echocardiography. The peak systolic circumferential strain of the endocardial, the middle and the epicardial layers, the peak systolic radial strain, and the peak systolic rotational degrees at different short-axis levels were measured by 2-dimensional speckle tracking imaging (2D–STI).
Results
The peak systolic circumferential strain of the septum and anterior walls in HCM patients was significantly lower than normal subjects. All of the peak systolic radial strain in HCM patients was significantly lower than normal subjects. The rotational degrees at the base and middle short-axis levels in HCM patients were larger than normal subjects. The interventricular septal thickness in end-diastolic period correlated to the peak systolic circumferential strain of the septum wall.
Conclusions
The short-axis systolic function was impaired in HCM patients. The peak circumferential systolic strain of the different layers, peak systolic radial strain and rotation degrees of the different short-axis levels detected by 2D–STI are very feasible for assessing the short-axis function in HCM patients.
Keywords: Two-dimensional speckle tracking imaging, Hypertrophic cardiomyopathy, Circumferential, Radial, Strain
Background
Hypertrophic cardiomyopathy (HCM) is a common cardiac disease [1, 2]. As the universal use of the echocardiography, computer tomography, and magnetic resonance imaging, the discovery of HCM have increased annually [3–6]. The disease is characterized by thickening the ventricular myocardium walls [7]. It is a genetic disorder of the myocardium caused by mutations in cardiac sarcomeric proteins. The pathology of HCM is the gross of the cardiac myocardial hypertrophy and fiber disarray. HCM patients often asymptomatic throughout life, but someone may have severe symptoms like sudden cardiac death at a young age [8, 9].
Two-dimensional speckle tracking imaging (2D–STI) can assess myocardial function accurately [10]. Currently, researches mainly focussed on myocardial function by detected the global myocardial strain, strain rate and torsion [11–16]. As we know, a normal myocardium is contained three layers: endocardial, middle myocardial and epicardial layers [17, 18]. Endocardial and epicardial layers are longitudinal oriented, and the middle myocardial is circumferential oriented. When the longitudinal and circumferential myocardium contract and relax, the cardiac myocardium deformation occurs in three directions: longitudinally, circumferentially, and radially. Our previous study showed that in HCM patients, the longitudinal function was damaged, even with normal LV ejection fraction [19], however, short-axis cardiac function as circumferentially and radially is also essential like longitudinally function. So, in this study, we mainly analysed the short-axis function in HCM patients.
Of data, detect the peak circumferential systolic strain of endocardial, middle myocardial, and epicardial layers in HCM patients is rare. The innovations of this study were ① Measure the peak systolic circumferential strain of endocardial, middle myocardial, and epicardial layers in patients with HCM. ② Measure the peak systolic radial strain in HCM patients. ③ Measure the peak systolic rotation degrees at the different short-axis levels in HCM patients, then to assess the changes in the left ventricular systolic function at the short-axis levels in HCM patients.
Methods
Ethical approvals
Recruitment to the study followed a full explanation of our methods including the fact that there was no risk of harm. Written informed consent was accepted. The Human Subjects Committee of Changzhou No. 2 People’s Hospital approved this study.
Study sample
Thirty seven HCM patients and 41 age- and gender- matched healthy subjects were enrolled for the research. The diagnosis of HCM was based on the transthoracic echocardiography findings, and the inclusion criteria were as follows [20]: M-mode and/or 2D echocardiographic evidence of wall thickness ≥ 15 mm in one or more LV myocardial segments and non-dilated left ventricle (LV). All enrolled HCM patients were had septal wall hypertrophy and with/without other LV walls hypertrophy, in the absence of another cardiac disease causing LVM hypertrophy, such as hypertensive heart diseases, aortic valve stenosis. The normal subjects had no evidence of family histories of HCM, hypertension, and any other diseases. All of the physical examination tests, the electrocardiogram and the echocardiography were normal.
Conventional 2D Doppler echocardiography
Thirty seven HCM patients and 41 normal subjects all had conventional 2D Doppler echocardiography (Vivid E9, GE Healthcare, Horten, Norway), Left atrial diameter (LAD), interventricular septal thickness in end-diastolic period (IVSD) and LV posterior wall thickness in end-diastolic period (LVPWD) were measured in the parasternal long axis view of the left ventricular by M-mode. Biplane Simpson’s method was used to measure the LV ejection fractions (LVEF). The peak velocities during early diastole (Ve) and late diastole (Va) of the anterior mitral valve were measured by pulsed-wave Doppler, and the ratio of Ve/Va was calculated. ECG leads were connected to each individual in all groups. Hold on the breath, standard high frame rate (60–90/s) of the parasternal short-axis views at the base, middle and apex of three consecutive were acquired for offline analysis.
Data analysis for LV systolic function
We analysis the short-axis views at the base, middle and apex using 2D–STI software (2D–Strain, EchoPac PC version 113, GE Healthcare, Horten, Norway). Used the button SAX-MV, SAX-PM and SAX-AP to sketched the endocardial, respectively, then the software would create a region of interest (ROI) automatically which contained endocardial, middle myocardial and epicardial layers, then adjusted the ROI to make the myocardium included well. Approved the ROI, the software would divide the LV into six segments, and then the peak systolic circumferential strain of endocardial, middle myocardial and epicardial layers, the peak radial systolic strain and the different short-axis rotation degrees could be calculated and recorded.
Statistical analysis
All of the analysis was performed using a commercially available package (SPSS 17.0. SPSS Inc., Chicago, IL, USA). Whether the distribution of the data in all subjects was normal were assessed by Kolmogorov-Smirnov’s test. If the data distribution was normal, differences between HCM patients and normal subjects were compared with an independent student t-test, for variables with a non-normal distribution, the nonparametric Mann-Whitney test was used. The correlation between the IVSD and the peak circumferential systolic strain of endocardial, middle myocardial, and epicardial layers, the radial systolic strain used the correlations test. Pearson correlation was used if the data distribution was normal, however, Spearman correlation was chosen if the data distribution was non-normal distribution. Data were presented as the mean ± s.d.. Difference was considered statistically significant in all tests when the P-value was less than 0.05.
Results
Basic information in HCM patients and the normal subjects
The values of LAD, IVSD and LVPWD in HCM patients were larger than normal subjects (p < 0.001). There were no significant difference in LVEDV, LVESV, LVEF, Ve, Va and Ve/Va (p > 0.05) (Table 1).
Table 1.
The basic Information in HCM patients and control subjects from conventional Two-Dimensional Doppler Echocardiography (mean ± s.d)
| HR (bpm) | LAD (mm) | IVSD (mm) | LVPWD (mm) | LVEDV (ml) | LVESV (ml) | LVEF (%) | Ve (m/s) | Va (m/s) | Ve/Va | |
|---|---|---|---|---|---|---|---|---|---|---|
| HCM (37) | 72 ± 13 | 42 ± 5 | 19 ± 4 | 10 ± 1 | 80 ± 17 | 27 ± 9 | 67 ± 6 | 0.75 ± 0.16 | 0.62 ± 0.23 | 1.39 ± 0.57 |
| Normal (41) | 72 ± 12 | 35 ± 3 | 9 ± 1 | 9 ± 1 | 80 ± 12 | 29 ± 7 | 65 ± 6 | 0.84 ± 0.15 | 0.69 ± 0.18 | 1.29 ± 0.39 |
| P-Value | 0.900 | < 0.001 | < 0.001 | < 0.001 | 0.845 | 0.354 | 0.137 | 0. 207 | 0.143 | 0.367 |
LAD left atrial diameter, HR heart rate, IVSD interventricular septal thickness in end-diastolic period, LVPWD left ventricular posterior wall thickness in end-diastolic period, LVEDV left ventricular end-diastolic volume, LVESV left ventricular end-systolic volume, LVEF left ventricular ejection fraction, Ve the peak velocity during early diastole of anterior mitral leftlet, Va the peak velocity during late diastole of anterior mitral leftlet
bold number is specify the significance of the comparision
The peak systolic circumferential strain in different myocardium layers
The trend of the peak systolic circumferential strain of endocardial, middle myocardial, and epicardial layers of all the subjects was: endocardial > middle myocardial > epicardial. The strain absolute values of the anter-septum and anterior walls (in all layers) in HCM patients had significant lower than normal subjects. The strain absolute values of the septum wall (middle and epicardial layers) in HCM patients had significant lower than normal subjects. The strain absolute values of the posterior wall (endocardial and middle layers) in HCM patients had significant larger than normal subjects. Although the other walls had no significant difference, the absolute values of HCM patients were larger than normal subjects. (Table 2, Fig. 1).
Table 2.
Comparision of the peak systolic circumferential strain of endocardial, the middle myocardial and epicardial layers and peak systolic radial strain in HCM patients and control subjects (mean ± s.d.)
| LV Walls | PSCS (%) | PSRS (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endocardial | Middle myocardial | Epicardial | ||||||||||
| HCM (37) | Normal (41) | P-value | HCM (37) | Normal (41) | P-value | HCM (37) | Normal (41) | P-value | HCM (37) | Normal (41) | P-value | |
| Ant-Septum | −29.16 ± 6.26 | −34.16 ± 5.42 | < 0.001 | −16.79 ± 4.58 | −23.62 ± 4.62 | < 0.001 | −9.36 ± 3.83 | −15.95 ± 4.29 | < 0.001 | 23.89 ± 9.82 | 40.22 ± 12.50 | < 0.001 |
| Anterior | −24.73 ± 6.43 | − 28.23 ± 7.01 | 0.025 | −13.92 ± 4.61 | −18.83 ± 5.53 | < 0.001 | −7.24 ± 3.73 | −11.91 ± 4.70 | < 0.001 | 27.12 ± 9.75 | 41.06 ± 11.92 | < 0.001 |
| Lateral | −22.33 ± 5.32 | −20.95 ± 6.45 | 0.310 | −12.45 ± 3.25 | −12.58 ± 4.56 | 0.884 | −6.18 ± 2.58 | −6.61 ± 3.82 | 0.568 | 33.65 ± 11.52 | 42.27 ± 11.73 | 0.002 |
| Posterior | −22.62 ± 8.15 | −17.66 ± 7.45 | 0.006 | −13.20 ± 4.95 | −10.58 ± 4.89 | 0.021 | −7.11 ± 3.22 | −5.60 ± 3.60 | 0.055 | 37.25 ± 13.33 | 43.26 ± 11.59 | 0.036 |
| Inferior | −26.47 ± 7.79 | −23.87 ± 7.64 | 0.141 | −15.89 ± 5.37 | −14.93 ± 5.46 | 0.437 | −8.94 ± 4.12 | −8.50 ± 4.16 | 0.639 | 35.66 ± 14.20 | 43.59 ± 11.83 | 0.009 |
| Septum | −31.73 ± 7.12 | −34.08 ± 6.58 | 0.134 | −19.68 ± 5.00 | −23.53 ± 5.28 | 0.002 | −12.08 ± 4.14 | −15.75 ± 4.89 | < 0.001 | 27.88 ± 11.55 | 42.39 ± 12.63 | < 0.001 |
PSCS peak systolic circumferential strain, PSRS peak systolic radial strain
bold number is specify the significance of the comparision
Fig. 1.
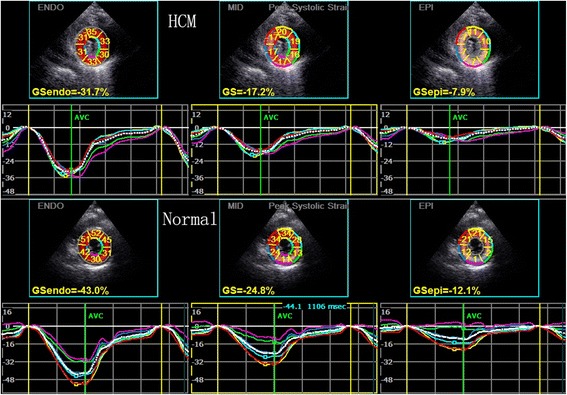
The peak systolic circumferential strain of the endocardial, the middle and the epicardial layers of left ventricular in HCM patients and normal subjects
The peak systolic radial strain
All of the peak systolic radial strain in HCM patients was significantly lower than normal subjects. (Table 2, Fig. 2).
Fig. 2.
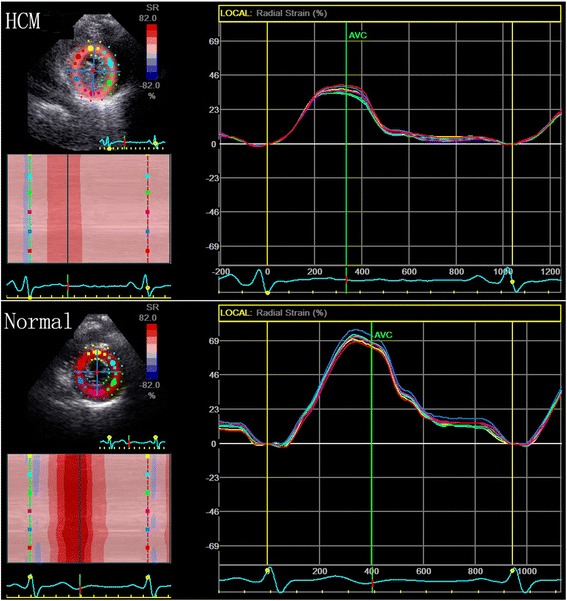
The peak systolic radial strain of left ventricular in HCM patients and normal subjects
Rotation degrees at different short-axis levels
In the systolic period, the LV apex wall rotated counter-clockwise, whereas the LV basal wall rotated clockwise in all subjects. Middle LV rotation was clockwise in HCM patients. The absolute values of peak systolic rotational degrees in the basal and middle short-axis levels in HCM patients had significant larger than normal subjects. (Table 3).
Table 3.
The rotational degrees at different short-axis levels in HCM patients and normal subjects (mean ± s.d.)
| Rotational degrees in different short-axis levels | |||
|---|---|---|---|
| Basal (°) | Middle (°) | Apex (°) | |
| HCM (37) | −11.27 ± 5.14 | −3.90 ± 4.86 | 4.84 ± 7.34 |
| Normal (41) | −4.65 ± 6.65 | −0.87 ± 5.98 | 6.42 ± 7.35 |
| P-Value | < 0.001 | 0.017 | 0.346 |
bold number is specify the significance of the comparision
The correlation between IVSD and the peak systolic circumferential strain in different myocardium layers, the peak systolic radial strain
The IVSD correlated well to the peak systolic circumferential strain of endocardial, middle myocardial, and epicardial layers of the septum wall (Endocardial: r = 0.445, p = 0.006, Middle myocardial: r = 0.458, p = 0.004, Epicardial: r = 0.373, p = 0.023). There was no correlation between IVSD and the peak systolic radial strain (r = − 0.230, p = 0.170) (Table 4, Fig. 3).
Table 4.
The correlation between IVSD and the peak systolic circumferential strain of endocardial, the middle myocardial and epicardial layers and peak radial strain in HCM patients
| PSCS | PSRS | |||
|---|---|---|---|---|
| Endocardial | Middle | Epicardial | ||
| r-value | 0.445 | 0.458 | 0.373 | −0.230 |
| p-value | 0.006 | 0.004 | 0.023 | 0.170 |
PSCS peak systolic circumferential strain, PSRS peak systolic radial strain
bold number is specify the significance of the comparision
Fig. 3.
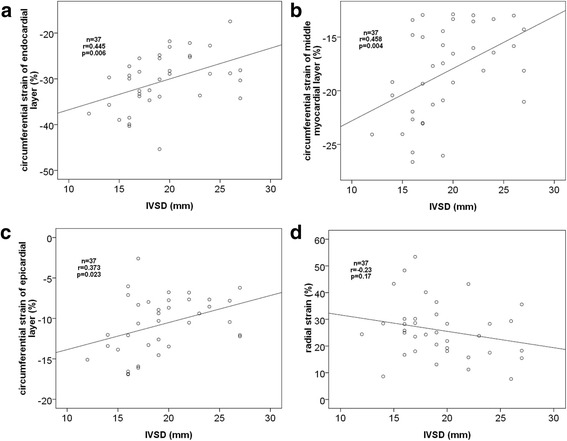
The correlation between IVSD and the peak systolic circumferential strain of the endocardial (a), the middle (b) and the epicardial layers (c), the peak systolic radial strain (d) in HCM patients. IVSD: Interventricular Septal Thickness in end-diastolic period
Discussion
HCM is a genetic disease, and mainly was characterized by left ventricular hypertrophy [21]. The systolic function detects by conventional echocardiography like LVEF is often normal, so the subclinical LV dysfunction cannot be identified by 2D conventional echocardiography. Peak short-axis systolic myocardial strain detect by 2D–STI can reflect the systolic function accurately. As we know, the peak circumferential strain of endocardial, middle myocardial, and epicardial layers in HCM patients is little reported.
Tigen K et al. [22] detected the LV systolic function by measuring the circumferential strain of the HCM patients, and found that the circumferential strain was significantly lower in patients with HCM compared with those of normal subjects. From the results, we found that, in the hypertrophied LVM, the LV peak systolic circumferential strain was decreased, and in non-hypertrophied myocardium, the strain value was increased. In HCM patients, LV hypertrophy, myocardial fibrosis, fiber disarray in the LV myocardium maybe a reason for the results. Once the LV was hypertrophy, the sequence of endocardial, middle myocardial, and epicardial layers had changed. The segmental systolic function was impaired. In order to maintain the normal LV systolic function, the non- hypertrophied myocardium enhanced their peak systolic circumferential strain. HCM patients with non- hypertrophied myocardium appeared to compensate for the early systolic changes via increased circumferential strain in order to keep the normal LV systolic function.
Yajima R et al. [8] found regional peak radial strain in basal, middle and apical levels were all significantly lower in HCM subjects than those in normal subjects. Our results were according to their results. Peak systolic radial strain in HCM patients was significantly lower than in the normal subjects. In HCM patients, decreased LV peak systolic radial strain appears not only in hypertrophied LVM, but also in non-hypertrophied myocardium. Peak systolic radial strain can reflect the systolic function very conveniently and accurately.
Zhang HJ et al. [23] investigated whether left ventricular twist analysis can detect the extent of myocardial fibrosis in patients with HCM, and found that, left ventricular twist mechanics are associated with the extent of myocardial fibrosis, and LV-twist assessment by STI may be clinically useful. Carasso S et al. [24] found middle LV rotation was clockwise (opposite to normal), they found that, in both HCM and normal subjects, LV rotation, viewed from the apex, was clockwise at the base, and count-clockwise at the apex, the difference was in the middle level. Ni XD et al. [25] found that in normal subject the transition from basal clockwise rotation to apical counterclockwise rotation is located at the papillary muscle level. Our research was according with the previous studies. The base-to-apex twist plane in HCM patients was changed. The peak rotational degrees at the base and middle short-axis levels in HCM patients were larger than normal subjects. Sengupta PP et al. [26] told us in the LV myocardial wall, the myofibers geometry changes smoothly from a right-handed helix in the endocardium to a left-handed helix in the epicardium such that the helix angle varies continuously from positive at the endocardium to negative at the epicardium. When the myofibres contract and relax, the cardiac have three motions: longitudinal, circumferential and radial, also produced the rotational motion. The pathogenesis of abnormal rotation at the middle level and the different rotation degrees are not clear. When the LV fibrosis, hypertrophied and stiffening, the LV myofibres were remodeling, the original balance of endocardial, middle myocardial, and epicardial myofibres was changed. Because the longitudinal and radial function were decreased in HCM patients, so for another possible reason was, in order to keep normal LV systolic function. HCM patients enhanced the peak rotational degrees at the base and middle short-axis levels.
The IVSD correlated well to the peak circumferential systolic strain of endocardial, middle myocardial, and epicardial layers of the septum wall, we concluded that, the more thickening of IVSD, the more circumferential systolic function was impaired. Through the correlation analysis, we found that the thickening of IVSD in HCM patients was consistent with its systolic function. There was not any correlation between IVSD and the peak systolic radial strain of the septum wall (r = − 0.230, p = 0.170), we concluded that, the thickening of IVSD had no internal relationship with peak systolic radial strain.
Conclusions
According to this research, we know the short-axis systolic function is impaired despite the presence of preserved LVEF in patients with HCM. The peak circumferential systolic strain of the different layers, peak systolic radial strain and rotation degrees of the different short-axis levels detected by 2D–STI are very sensitive for assessing the systolic function in HCM patients. In the clinical implication, the study can help us to know the early cardiac dysfunction of HCM patients, then to give them early treatment and assess the effect after the treatment.
Limitations
The greatest limitation of this study is that the relationship of decreasing short axis functions to a clinical outcome or event is not investigated. Another important limitation is the small number of patients. The third limitation is that we don’t evaluate the relationship with other image techniques, such as cardiac MR, SPECT.
Acknowledgements
The authors would like to thank the department of Echocardiography, the Affiliated Changzhou No.2 People’s Hospital of Nanjing Medical University.
Funding
none
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- 2D–STI
2-dimensional speckle tracking imaging
- HCM
Hypertrophic cardiomyopathy
- IVSD
Interventricular septal thickness in end-diastolic period
- LAD
Left atrial diameter
- LVEDV
Left ventricular end-diastolic volume
- LVEF
Left ventricular ejection fraction
- LVESV
Left ventricular end-systolic volume
- LVH
Left ventricular hypertrophy
- LVPWD
LV posterior wall thickness in end-diastolic period
- Va
The peak velocities during late diastole of the anterior mitral valve
- Ve
The peak velocities during early diastole of the anterior mitral valve
Authors’ contributions
HJ, YZN and RYF designed the study and carried out the study, data collection and analysis, HJ wrote and revised the manuscript, RYF and FL revised the manuscript, LC, LJ and FL designed part of the experiments, and collected the HCM patients and normal subjects. HJ and LJ performed the statistical analysis. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study complied with the Declaration of Helsinki and was reviewed and approved by the Human Subjects Committee of Changzhou No. 2 People’s Hospital approved this study. Written informed consent was obtained from the each people enrolled in the study.
Consent for publication
This manuscript does not include any individual person’s data.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jun Huang, Email: 305669112@qq.com.
Zi-Ning Yan, Email: docyanzining@163.com.
Yi-Fei Rui, Email: 2318472619@qq.com.
Li Fan, Email: 1900883927@qq.com.
Chang Liu, Email: 524341735@qq.com.
Jie Li, Email: 1165672447@qq.com.
References
- 1.Hensley N, Dietrich J, Nyhan D, Mitter N, Yee MS, Brady M. Hypertrophic cardiomyopathy: a review. Anesth Analg. 2015;120:554–569. doi: 10.1213/ANE.0000000000000538. [DOI] [PubMed] [Google Scholar]
- 2.He XW, Song ZZ. Evaluation Of left ventricular function, rotation, twist and untwist in patients with hypertrophic cardiomyopathy. Exp Clin Cardiol 2013;18: e47–e49. [PMC free article] [PubMed]
- 3.Shah PM, Gramiak R, Kramer DH. Ultrasound localization of left ventricular outflow obstruction in hypertrophic obstructive cardiomyopathy. Circulation. 1969;40:3–11. doi: 10.1161/01.CIR.40.1.3. [DOI] [PubMed] [Google Scholar]
- 4.Baccouche H, Maunz M, Beck T, Gaa E, Banzhaf M, Knayer U, et al. Differentiating cardiac amyloidosis and hypertrophic cardiomyopathy by use of three-dimensional speckle tracking echocardiography. Echocardiography. 2012;29:668–677. doi: 10.1111/j.1540-8175.2012.01680.x. [DOI] [PubMed] [Google Scholar]
- 5.Nagueh SF, Bierig SM, Budoff MJ, Desai M, Dilsizian V, Eidem B, Goldstein SA, et al. American Society of Echocardiography; American Society of Nuclear Cardiology; Society for Cardiovascular Magnetic Resonance; Society of Cardiovascular Computed Tomography. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with hypertrophic cardiomyopathy: endorsed by the American Society of Nuclear Cardiology, Society for Cardiovascular Magnetic Resonance, and Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr. 2011;24:473–498. doi: 10.1016/j.echo.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Olivotto I, Maron MS, Autore C, Lesser JR, Rega L, Casolo G, et al. Assessment and significance of left ventricular mass by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2008;52:559–566. doi: 10.1016/j.jacc.2008.04.047. [DOI] [PubMed] [Google Scholar]
- 7.Bing W, Knott A, Redwood C, Esposito G, Purcell I, Watkins H, et al. Effect of hypertrophic cardiomyopathy mutations in human cardiac muscle alpha -tropomyosin (Asp175Asn and Glu180Gly) on the regulatory properties of human cardiac troponin determined by in vitro motility assay. J Mol Cell Cardiol. 2000;32:1489–1498. doi: 10.1006/jmcc.2000.1182. [DOI] [PubMed] [Google Scholar]
- 8.Yajima R, Kataoka A, Takahashi A, Uehara M, Saito M, Yamaguchi C, et al. Distinguishing focal fibrotic lesions and non-fibrotic lesions in hypertrophic cardiomyopathy by assessment of regional myocardial strain using two-dimensional speckle tracking echocardiography: comparison with multislice CT. Int J Cardiol. 2012;158:423–432. doi: 10.1016/j.ijcard.2011.01.096. [DOI] [PubMed] [Google Scholar]
- 9.Kauer F, van Dalen BM, Michels M, Soliman OI, Vletter WB, van Slegtenhorst M, et al. Diastolic abnormalities in normal phenotype hypertrophic cardiomyopathy gene carriers: a study using speckle tracking echocardiography. Echocardiography. 2013;30:558–563. doi: 10.1111/echo.12076. [DOI] [PubMed] [Google Scholar]
- 10.Notomi Y, Lysyansky P, Setser RM, Shiota T, Popović ZB, Martin-Miklovic MG, et al. Measurement of ventricular torsion by two-dimensional ultrasound speckle tracking imaging. J Am Coll Cardiol. 2005;45:2034–2041. doi: 10.1016/j.jacc.2005.02.082. [DOI] [PubMed] [Google Scholar]
- 11.Hurlburt HM, Aurigemma GP, Hill JC, Narayanan A, Gaasch WH, Vinch CS, et al. Direct ultrasound measurement of longitudinal, circumferential, and radial strain using 2-dimensional strain imaging in normal adults. Echocardiography. 2007;24:723–731. doi: 10.1111/j.1540-8175.2007.00460.x. [DOI] [PubMed] [Google Scholar]
- 12.Sengupta PP, Tajik AJ, Chandrasekaran K, Khandheria BK. Twist mechanics of the left ventricle: principles and application. JACC Cardiovasc Imaging. 2008;1:366–376. doi: 10.1016/j.jcmg.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Hartlage GR, Kim JH, Strickland PT, Cheng AC, Ghasemzadeh N, Pernetz MA, et al. The prognostic value of standardized reference values for speckle-tracking global longitudinal strain in hypertrophic cardiomyopathy. Int J Cardiovasc Imaging. 2015;31:557–565. doi: 10.1007/s10554-015-0590-5. [DOI] [PubMed] [Google Scholar]
- 14.Takano H, Isogai T, Aoki T, Wakao Y, Fujii Y. Feasibility of radial and circumferential strain analysis using 2D speckle tracking echocardiography in cats. J Vet Med Sci. 2015;77:193–201. doi: 10.1292/jvms.13-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Urbano Moral JA, Arias Godinez JA, Maron MS, Malik R, Eagan JE, Patel AR, et al. Left ventricular twist mechanics in hypertrophic cardiomyopathy assessed by three-dimensional speckle tracking echocardiography. Am J Cardiol. 2011;108:1788–1795. doi: 10.1016/j.amjcard.2011.07.047. [DOI] [PubMed] [Google Scholar]
- 16.Orta Kilickesmez K, Baydar O, Bostan C, Coskun U, Kucukoglu S. Four-dimensional speckle tracking echocardiography in patients with hypertrophic cardiomyopathy. Echocardiography. 2015;32:1547–1453. doi: 10.1111/echo.12916. [DOI] [PubMed] [Google Scholar]
- 17.Greenbaum RA, Ho SY, Gibson DG, Becker AE, Anderson RH. Left ventricular fibre architecture in man. Br Heart. 1981;45:248–263. doi: 10.1136/hrt.45.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henein MY, Gibson DG. Editorial normal long axis function. Heart. 1999;81:111–113. doi: 10.1136/hrt.81.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang J, Yan ZN, Fan L, Rui YF, Song XT. Left ventricular systolic function changes in hypertrophic cardiomyopathy patients detected by the strain of different myocardium layers and longitudinal rotation. BMC Cardiovasc Disord. 2017;17:214. doi: 10.1186/s12872-017-0651-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, et al. American College of Cardiology Foundation/American Heart Association task force on practice guidelines; American Association for Thoracic Surgery; American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Failure Society of America; Heart Rhythm Society; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2011;124:e783–e831. doi: 10.1161/CIR.0b013e318223e2bd. [DOI] [PubMed] [Google Scholar]
- 21.Urbano-Moral JA, Rowin EJ, Maron MS, Crean A, Pandian NG. Investigation of global and regional myocardial mechanics with 3-dimensional speckle tracking echocardiography and relations to hypertrophy and fibrosis in hypertrophic cardiomyopathy. Circ Cardiovasc Imaging. 2014;7:11–19. doi: 10.1161/CIRCIMAGING.113.000842. [DOI] [PubMed] [Google Scholar]
- 22.Tigen K, Sunbul M, Karaahmet T, Dundar C, Ozben B, Guler A, et al. Left ventricular and atrial functions in hypertrophic cardiomyopathy patients with very high LVOT gradient: a speckle tracking echocardiographic study. Echocardiography. 2014;31:833–841. doi: 10.1111/echo.12580. [DOI] [PubMed] [Google Scholar]
- 23.Zhang HJ, Wang H, Sun T, Lu MJ, Xu N, Wu WC, et al. Assessment of left ventricular twist mechanics by speckle tracking echocardiography reveals association between LV twist and myocardial fibrosis in patients with hypertrophic cardiomyopathy. Int J Cardiovasc Imaging. 2014;30:1539–1548. doi: 10.1007/s10554-014-0509-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carasso S, Yang H, Woo A, Vannan MA, Jamorski M, Wigle ED, et al. Systolic myocardial mechanics in hypertrophic cardiomyopathy: novel concepts and implications for clinical status. J Am Soc Echocardiography: official Publ Am Soc Echocardiography. 2008;21:675–683. doi: 10.1016/j.echo.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 25.Ni XD, Huang J, Hu YP, Xu R, Yang WY, Zhou LM. Assessment of the rotation motion at the papillary muscle short-axis plane with normal subjects by two-dimensional speckle tracking imaging: a basic clinical study. PLoS One. 2013;8:e83071. doi: 10.1371/journal.pone.0083071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sengupta PP, Tajik AJ, Chandrasekaran K, Khandheria BK. Twist mechanics of the left ventricle. principles and application JACC Cardiovascular imaging. 2008;1:366–376. doi: 10.1016/j.jcmg.2008.02.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.


