Abstract
Staphylococcus aureus can cause numerous different diseases, which has been attributed to its large repertoire of virulence factors, many of which are under the control of the accessory gene regulator (agr) quorum sensing system. Under conditions of high cell density, agr increases the production of many virulence factors, decreases expression of several colonization factors, and is intimately associated with the pathogenesis and biofilm formation of S. aureus. This review summarizes our current understanding of the molecular mechanisms underlying agr quorum sensing and the regulation of agr expression. The discussion also examines subgroups of agr and their association with different diseases, and concludes with an analysis of strategies for designing drugs and vaccines that target agr to combat S. aureus infections.
Keywords: Staphylococcus aureus, agr, virulence factors, biofilms, autoinduction
Overview of Quorum Sensing and agr in Staphylococcus aureus
Quorum sensing is a bacterial cell to cell communication system that controls expression of many genes in response to population density (Fuqua et al., 1994). The phenomenon was first investigated in the marine bacterium Vibrio fischeri, in which it modulates the expression of bioluminescence (Engebrecht et al., 1983). Subsequently, quorum-sensing systems have been found in a wide variety of microbes, and the main similarities and differences in the mechanisms employed by Gram-positive and Gram-negative bacteria have been describe (Xavier and Bassler, 2003). Gram-negative bacteria primarily use the LuxI/LuxR system, in which homoserine lactone (HSL) autoinducers are synthesized by LuxI-type enzymes and detected by LuxR-type transcriptional regulators. Gram-positive bacteria typically use oligopeptide-mediated quorum sensing, and two-component sensor kinase phosphorylation cascades are employed for signal transmission (Bassler, 2002).
Staphylococcus aureus is a highly versatile and adaptable Gram-positive pathogen. It can inhabit the skin and mucous membranes as a harmless commensal (Novick, 2003). However, S. aureus can also proliferate in the bloodstream and in various tissues, causing serious disease (Krismer and Peschel, 2011), and is considered one of the leading causes of hospital- and community-acquired infections worldwide (Mandal et al., 2015). It can cause conditions ranging from minor skin infections to systemic, life-threatening illnesses, such as pneumonia, osteomyelitis, and endocarditis (Thammavongsa et al., 2015). A significant aspect of diseases caused by S. aureus is recurrence, which is seen in 8–33% of skin, soft-tissue, and bloodstream infections, resulting in severe human morbidity and mortality (Thammavongsa et al., 2015).
The ability of S. aureus to cause such a wide range of infections is attributed to its large arsenal of virulence factors (adhesins, toxins, and enzymes) (Tuchscherr and Loffler, 2016), many of which are under the control of the quorum-sensing accessory gene regulator (agr) system (Li et al., 2014). The agr locus was first described by Peng et al. (1988) and found to be widespread in staphylococci. The agr system serves a crucial role in pathogenesis by regulating virulence factors, biofilm formation, and the heterogeneous resistance of methicillin-resistant Staphylococcus aureus (MRSA) (Singh and Ray, 2014; Mohsenzadeh et al., 2015; Kavanaugh and Horswill, 2016).
The agr operon is organized around two divergent promoters, P2 and P3, and generates two primary transcripts, RNAII and RNAIII, respectively (Figure 1) (Ji et al., 1995). RNAII encodes AgrB, AgrD, AgrC, and AgrA. AgrD encodes the precursor of the autoinducing peptide (AIP) pheromone. AgrB is a multifunctional endopeptidase and chaperone protein that contributes to the maturation and export of AIP. AgrC and AgrA comprise a two-component signal transduction system in which AgrC is the membrane histidine kinase and AgrA is the response regulator (Novick et al., 1995). The agr system is activated when the extracellular AIP concentration reaches a threshold. Upon binding AIP, AgrC phosphorylates AgrA, which in turn activates the P2 and P3 promoters in addition to several other transcriptional targets (Ji et al., 1995; Queck et al., 2008). RNAIII is a posttranscriptional regulator of multiple virulence genes. Recognizable agr loci are subject to considerable sequence polymorphism. After cloning and initial characterization of the agr locus, Peng et al. (1988) identified four variants (agr types I through IV). These S. aureus strains are characterized by mutations in the sensor domain of the histidine kinase AgrC and polymorphisms in the sequences of secreted autoinducing peptides (Srivastava et al., 2014), affecting the three determinants of agr group specificity (AgrB, AgrD, and the sensor domain of AgrC) (Figure 1) (Wright et al., 2005b). Because agr is an integrated system, these variations must evolve in concert in order to maintain agr functionality which enable the bacteria to evade host defenses, spread within the host, and to degrade host cells and tissues (Kavanaugh and Horswill, 2016).
FIGURE 1.
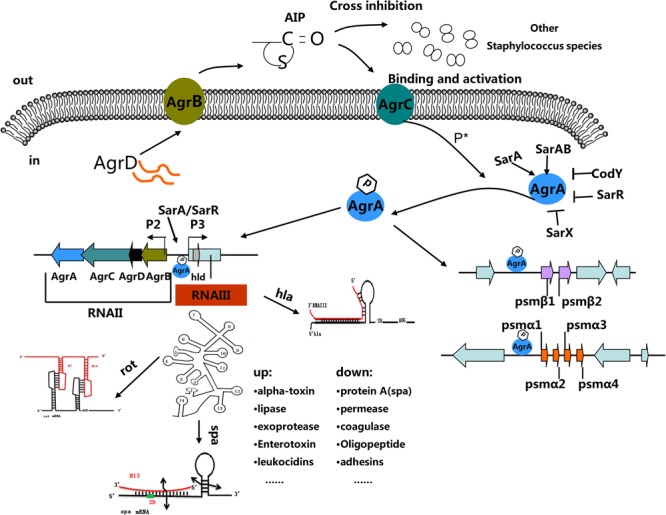
The Staphylococcal quorum-sensing system. The agr locus is composed of divergent transcripts designated RNAII and RNAIII, driven by promoters P2 and P3, respectively. The AIP signal is produced from the AgrD precursor, while the membrane-localized enzyme AgrB participates in the maturation and export of the AIP. At a critical threshold concentration, AIP activates the two-component signal transduction system, AgrC–AgrA, and causes the phosphorylation of AgrA. Once phosphorylated, AgrA binds to the P2 and P3 promoter regions, as well as promoters PSM-α and PSM-β, resulting in agr system transcription. RNAIII encodes the delta-toxin encoding gene hld, and 14 stem-loop motifs. These domains regulate the expression of numerous virulence factors. Other regulators (such as SarA, SrrAB, SarR and SarX) can enhance or inhibit agr activity.
Molecular Basis of the agr System in S. aureus
AgrB, the 22 kDa peptidase responsible for proteolysis of AgrD, is located in the cytoplasmic membrane. It has six transmembrane segments, consisting of four hydrophobic transmembrane α-helices and two hydrophilic loops with several positively charged amino acid residues (Zhang et al., 2002). AgrB is the most unusual feature of the staphylococcal agr system because its sequence has little in common with other quorum-sensing proteins. In staphylococcal species, the N-terminal domain of AgrB is highly conserved, the first 34 residues, located in the first transmembrane hydrophilic domain, are absolutely conserved among the four S. aureus agr types (Thoendel et al., 2011). Mutations in this conserved region will eliminate AgrB activity (Qiu et al., 2005). In particular, the histidine residue at position 77 (H77) and the cysteine residue at position 84 (C84) are required for the proteolytic processing of AgrD. Mutations in the second hydrophilic transmembrane domain have no effect on AgrB activity. All AgrB homologs are likely to utilize the same or similar mechanisms to process AgrD, but the mechanism of AgrD-dependent AIP maturation and the AIP secretion pathway are unknown. AgrB and AgrD are sufficient for AIP production, since heterologous expression of AgrB and D in Escherichia coli or Bacillus subtilis results in functional AIP (Thoendel et al., 2011). Chimeric AgrB proteins have been used to identify the group-specific segment(s) in AgrB that contribute to AgrD proteolysis. The results indicate that the interaction between AgrB and AgrD is group specific (Zhang and Ji, 2004). For example, in agr group I, the first transmembrane α-helix and the extracellular loop 1 of AgrB are critical for processing group I AgrD. In contrast, two hydrophilic parts of group II AgrB play a key role in the processing of group II AgrD (Zhang and Ji, 2004).
AgrD is the propeptide for AIP. The structure of AgrD has diverged across species, but is typically comprised of an N-terminal amphipathic leader, a middle region of seven to nine residues that is processed into the mature AIP thiolactone structure, and a negatively charged C-terminal recognition sequence (Ji et al., 1995). AgrB-catalyzed proteolysis cuts off the AgrD recognition sequence as a linear peptide and subsequently processes it into a thiolactone group at the C-terminus of the remaining fragment. The recognition sequence of staphylococcal AgrD has abundant acidic residues and is highly conserved (Thoendel and Horswill, 2009). The AgrD propeptide is integrated in the cytoplasmic membrane by a conserved amphipathic α-helical motif in its N-terminal region that is required for the stabilization of AgrD and the production of mature AIP. However, this region is not specifically involved in the interaction with AgrB (Thoendel et al., 2011). If the N-terminal amphipathic motif of AgrD is replaced by an artificial amphipathic peptide, production of AIP still occurs (Zhang et al., 2004). Evidence suggests that the conserved cysteine residue is required to generate the thiolactone ring structure of AIP. The C-terminal tail of AgrD plays an essential role in cleavage by AgrB and AIP production (Thoendel and Horswill, 2009). It is assumed that the highly conserved Glu-Asp pair of AIP is crucial for processing the C-terminal end of the AIP (Dufour et al., 2002). The first nine residues of AgrD are necessary for AIP production and AgrB endopeptidase activity. Mutations affecting glutamate 34 or leucine 41 inhibit AIP production and AgrB activity (Thoendel and Horswill, 2009). AgrD function has been extensively studied (Thoendel and Horswill, 2009; Schwartz et al., 2014). Schwartz et al. (2014) demonstrated that AgrD structure and function are similar to the PSM family of toxins. Similar to PSMs, N-AgrD is present in the amyloid fibrils of S. aureus biofilms, and can form and seed amyloid fibrils in vitro (Schwartz et al., 2014). An AgrD mutant displays significantly reduced biofilm formation in Listeria monocytogenes (Riedel et al., 2009).
AgrC, the critical receptor protein for signal recognition and transmission (George Cisar et al., 2009), is a 46 KDa membrane protein belonging to the class 10 receptor-histidine protein kinase (HPK) family. Its features include an N-terminal, membrane-integrated sensor module that detects and binds AIP, several transmembrane domains, and a C-terminal histidine kinase module (Grebe and Stock, 1999).
After AIP binds to the AgrC N-terminal sensor module, a conformational change occurs in the AgrC cytoplasmic helix that links the sensor and kinase domains, which then enables autophosphorylation and activation of the AgrC kinase. The AgrC–AIP and AgrC–AgrA interaction have been intensively studied. Geisinger et al. (2009) first reported constitutive mutants of AgrC and elucidated the mechanism of ligand-receptor interaction. Additionally the team found that substitution of amino acid isoleucine at position 171 led to altered activity of AgrC (Geisinger et al., 2009). Mairpady Shambat et al. (2016) found that substitution of tyrosine by cysteine at position 223 (Y233C) in AgrC destabilizes AgrC–AgrA interaction, affecting the regulation of virulence genes that switch the strain from a cytotoxin-mediated phenotype to a colonizing phenotype. The same laboratory used random mutagenesis to isolate AgrC mutants with constitutive activity, as well as those with altered specificity for divergent AIPs. Even changes at a single amino acid affect virulence properties and infection outcome (Mairpady Shambat et al., 2016).
AgrA, the 27 kDa response regulator for the agr system, belongs to a family of conserved response regulators with CheY-like receiver domains (Traber and Novick, 2006). Sequence comparison indicates that the amino acid sequences of AgrB, AgrC, and AgrD are strikingly variable among different S. aureus agr types, whereas AgrA is highly conserved. AgrA acts as a response regulator by binding to recognition sites in RNAIII and RNAII promoter domains (Koenig et al., 2004). Using electrophoretic mobility shift assays (EMSAs), Koenig et al. (2004) demonstrated that AgrA binds to the P2-P3 region of the agr locus with high affinity, and the affinity of phosphorylated AgrA is stronger for the P2 promoter than for P3. However, the frameshift mutation produced by inserting an extra adenine into the seven continuous adenines in the C-terminus of AgrA generates a partially defective AgrA that significantly delays the activation of the agr locus (Traber and Novick, 2006). In DNA microarray experiments, Queck et al. (2008) demonstrated that AgrA up-regulates three other chromosomal operons (psmα, psmβ, and MW00370/0372). The up-regulations of α and β PSM transcriptions are induced by direct binding of AgrA to their promoter domains, independently of RNAIII (Thoendel et al., 2011).
RNAIII
The 514 nucleotides of RNAIII contain 14 potential stem-loop structures. Regions in the folded molecule participate in two long-distance interactions (Figure 1) (Bronesky et al., 2016). The 3′-end of RNAIII which contains some C-rich sequence motifs and unpaired regions that contribute to the initiation of the binding of RNAIII to the ribosome binding sites of several target mRNAs (Bronesky et al., 2016). The 3′-end domain of RNAIII represses the synthesis of several surface and secreted proteins specific to S. aureus (Figure 1). RNAIII was the first example as an “antisense RNA” that stimulates translation of its target mRNA (Morfeldt et al., 1995). Following this discovery, many studies have investigated how RNAIII functions as an effector molecule in the agr system. In RNAIII mutants, low molecular weight toxins and the exoenzymes (Ecp protease and Geh lipase) are down-regulated (Xiong et al., 2002).
Aip Synthesis, Structure and Activity
The agr autoinducing peptide (AIP) varies from 7–9 amino acids in length and contains a 5-membered ring. For S. aureus, the AIP sequences of agr-I, II, III, and IV are YSTCDFTM, GVNACSSLF, YINCDFLL, and YSTCYFTM, respectively (Yarwood and Schlievert, 2003). S. aureus must produce sufficient amounts of thiolactone-containing AIP to enable quorum sensing. The proteolytic events and chemical steps that enable AIP production have been identified in vitro by reconstituting the AgrB-dependent proteolysis of the AgrD precursor (Wang et al., 2015). After removal of the C-terminal tail, the new C terminus forms a thiolactone bond by condensation of the sulfhydryl group of a conserved cys residue and the α-carboxyl group. Cleavage of the N-terminal domain then results in a molecule with a tail of 2–4 amino acid residues connected to a 16-membered macrocycle (Geisinger et al., 2009). Efficient thiolactone production is driven by association of the thiolactone-containing intermediate with the membrane, which stabilizes the macrocycle, and by rapid degradation of the C-terminal fragment of AgrD after proteolysis (Wang et al., 2015).
The four AIP molecules are sufficiently similar in structure that they can bind to the AgrC receptor from different group, although in such cases they do not activate the AgrA protein inside the cell (Jabbari et al., 2012). MDowell et al. (2001) found that synthetic group I AIP analogs can replace the authentic group I AIP to activate the agr system. This indicates that covalent modification of the AgrC receptor is not a necessary prerequisite for agr activation (MDowell et al., 2001). The C-terminal endocyclic amino acid residue (aspartate) and the central cysteine are critical for the function of S. aureus group I AIP. Replacement of them with alanine converts the AIP from an activator to a potent inhibitor (MDowell et al., 2001).
Autoinducing peptide interactions between different agr groups can result in cross-inhibition, leading to quorum sensing interference (Ji et al., 1997). Structure-activity analyses on AIP–AgrC interaction indicated that AIP macrocycle size and conformation are essential to its specific activity. Johnson et al. (2015) investigated that alterations of microcycle size and conformation of AIP drastically affected its ability to bind and activate the AgrC-I receptor (Johnson et al., 2015). Swapping the five divergent residues in the second extracellular loop of the AgrC-I and AgrC-IV receptors switches the activation specificity between AIP-I and AIP-IV (Geisinger et al., 2008). These results suggest that the inhibitory receptor conformation stabilized by non-cognate AIPs is critical for the ligand–receptor interaction (Johnson et al., 2015).
The Biological Activities of agr System-Mediated Regulation
Agr has various biological functions. The typical two are regulating the expression of staphylococcal virulence factors and facilitating the structuring and detachment of bacteria biofilms. These functions are crucial for the pathogenesis of staphylococci and are always associated with the pathogenicity of highly virulent S. aureus.
Agr Regulation of Staphylococcal Virulence
The agr system is a global regulator of staphylococci and exhibits a dual regulatory effect on staphylococcal virulence (Arvidson and Tegmark, 2001; Bronner et al., 2004; Singh and Ray, 2014). It can up-regulate the expressions of several exoproteins (e.g., α-, β-, γ-hemolysin, and leucotoxins), lipases, phenol-soluble modulins, and toxic shock syndrome toxins (TSST), and represses the transcription of some cell wall-associated proteins (e.g., protein A, coagulase, and fibronectin binding protein) (Bronner et al., 2004). Agr can regulate the expression of virulence factors directly and indirectly. For example, through direct binding of AgrA to PSM promoter regions, the agr system regulates the expression of PSM (Peschel and Otto, 2013). Also, the agr system controls the expression of genes encoding alpha-hemolysin (hla), beta-hemolysin (hlb), protein A (spa), exfoliative toxin A (etaA), toxic shock syndrome toxin-1 (tsst), and staphylococcal serine protease (sspA) by regulating RNAIII. Through direct base pairing with target gene cohorts, or indirect control of regulating transcriptional regulators such as Rot, SarT, and SarS, RNAIII up- or down-regulate virulence gene expression (Figure 1) (Arvidson and Tegmark, 2001; Le and Otto, 2015; Bronesky et al., 2016).
Agr-Mediated Biofilm Formation
Staphylococcus aureus is a leading cause of chronic relapsing infections such as implanted device related infections: intravenous catheters, urinary catheters, and orthopedic prosthesis (Singh and Ray, 2014). These types of infections all have a biofilm component which physically protects the bacteria from the immune system and cells within a biofilm are more tolerant to antibiotics (Waters et al., 2016). In Staphylococcus, the agr system appears to influence biofilm formation at structuring and dispersal stages. Many researches demonstrated that repression of agr is necessary for biofilm formation, while activation of the agr system is essential for the detachment of biofilm (Vuong et al., 2000; Boles and Horswill, 2008). Some dysfunctional agr mutants have been isolated from biofilm-associated infections and these form thicker, smoother biofilms (Yarwood and Schlievert, 2003).
The agr system can affect biofilms development in a variety of ways. In established biofilms, adding AIP could reactivate of agr and contribute to biofilm detachment by increasing secretion of extracellular proteases (Boles and Horswill, 2008). Solano et al. (2014) also showed that agr system influence biofilm development by interfering with protease expression. AgrB is also thought to regulate biofilm dispersal, because biofilm biomass (cells, extracellular polymeric substances, and extracellular DNA) is inversely correlated with agrB expression (Grande et al., 2014). The agrD mutant formed larger biofilms than did the parent strain in a static biofilm system (Yarwood et al., 2004). RNAIII controls both biofilm formation and accumulation (Coelho et al., 2008), and high RNAIII is thought to have anti-biofilm effects (Lauderdale et al., 2009). Moreover, AgrA-controlled PSM expression is also involved in biofilms detachement (Dastgheyb et al., 2015). As monomers, PSMs promote biofilm disassembly, but when polymerized in amyloid-like fibers, they favor biofilm development (Solano et al., 2014).
Regulation of the agr System
As a global regulator, the agr system controls the expression of numerous effectors. However, its activity is under the strict control of other regulators. In addition to the autoregulatory behavior of AgrA, which binds to the P2–P3 promoter region and regulates P2 and P3 transcriptions, other factors controlling agr expression have been described (Reyes et al., 2011). For example, the P2–P3 intergenic region contains SarA/SarR binding sites as well as the four AgrA boxes to which AgrA binds (Figure 1) (Reyes et al., 2011). It was reported that SarA activates whereas SarR represses P2 transcription (Reyes et al., 2011). Two-component system SrrAB can also affect the activity of agr system (Pragman et al., 2004). The global regulator CodY indirectly represses agr activity to prevent inappropriate agr expression at low cell densities (Painter et al., 2014). A lack of SigB activity leads to increased RNAIII expression, thus elevating extracellular protease levels and influencing the murein hydrolase activity (Lauderdale et al., 2009). Moreover, numerous environmental and metabolic factors such as pH, glucose concentration, reactive oxygen species (ROS), and nutrient availability, can also modulate agr quorum sensing system in S. aureus (James et al., 2013).
Association of agr Types With Specific Biological Characteristics
agr groups vary by clonal lineages distribution, antibiotic resistance profile, biofilm formation, expression of virulence factors, and AIP structures. Many studies have attempted to associate agr types with one or more of these characteristics (Gomes et al., 2005; Ikonomidis et al., 2009; Nichol et al., 2011).
Agr Types and Clonal Lineages
Specific S. aureus lineages may correlate with different Agr types. Agr group I is usually found in clonal lineages CC8, CC25, CC22, CC45, and CC395. CC5, CC12, and CC15 isolates usually harbor agr group II, CC30 is often characterized by agr group III, and CC121 harbors agr group IV (Holtfreter et al., 2007).
Biofilm Formation among agr Types
The association between agr groups and biofilm formation has been widely studied. Strains of agr groups II and III are the main biofilm producers among the four agr types. Ikonomidis et al. (2009) found that agr group II MRSAs exhibit higher biofilm formation capacity compared with the other agr groups. Cafiso et al. (2007) also reported that agr group II S. aureus strains are usually prolific biofilm formers, while strains in agr group III are less so. However, Khoramrooz et al. (2016) reported a significant association between agr group III and biofilm production in S. aureus isolates, and concluded that the type III isolates are potent biofilm producers. The relationship between S. aureus agr groups and antibiotic resistance is also of interest. For example, agr group I is more strongly associated with CA-MRSA genotypes, while agr group II is more correlated with HA-MRSA in human isolates (Nichol et al., 2011). In addition, another study reported that methicillin resistance of bovine isolates is more prevalent in agr group I than other groups (Mohsenzadeh et al., 2015).
Toxin Gene Distribution among agr Types
According to an analysis performed by Jarraud et al. (2002) on 198 S. aureus strains, toxin gene distribution is strongly related to agr phylogeny, as determined using AFLP clusters. The enterotoxin gene cluster (seg, sei, sem, sen, and seo) was relevant to group IV, and correlated negatively with agr groups I and II. lukD-lukE and hlg-2 correlated negatively with group III but were associated with other groups. Meanwhile, eta and etb were related to group IV (Jarraud et al., 2002). de Almeida et al. (2013) also evaluated the association of genes encoding cytotoxin, adhesins, and toxins with superantigen activity with S. aureus clones isolated from milk obtained from ewes exhibiting clinical and subclinical mastitis. The clfA gene was identified in all isolates, and hla and lukE-D genes were, respectively, detected from 77.3 and 82.8% clones. In contrast, bbp, ebpS, cna, fnbB, icaA, icaD, bap, hlg, lukM-lukF-PV, and se-a-b-d-e were not found (de Almeida et al., 2013).
Mobile genetic elements (MGEs) may also show agr group specificity. Staphylococcal chromosomal cassette mec (SCCmec) carries the mecA gene which encodes a penicillin-binding protein (PBP2a) and confers resistance to β-lactam antibiotics. Eleven distinct SCCmec elements have been identified in MRSA (Wright et al., 2005b). Interestingly, all are found in agr groups I, II, and III, but group IV strains have not acquired a SCCmec element (Wright et al., 2005b). Plasmids and phages, two common types of mobile genetic elements, are also agr type-specific. For example, phages and plasmids make frequent appearances in agr-IV strains that carry eta or etb. Agr-II strains harbor cna showed much lower frequencies than other types, while the TSST-1 prototype antigen is preferentially carried by agr-III strains (Wright et al., 2005b). Because MLST patterns occur within a single agr group, this suggests that agr groups evolved prior to MLST diversification (Wright et al., 2005b).
Agr Types and Disease
Several studies have demonstrated a strong relationship between agr types and particular diseases. Jarraud et al. (2002) found that phylogenetic group AF1 (agr group IV) strains are closely related to generalized exfoliative syndromes and bullous impetigo. Among suppurative infections, endocarditis is mainly caused by phylogenetic group AF2 (agr groups I and II) strains. Agr group III and IV strains are associated with TSST-1 (Gomes et al., 2005). Sakoulas et al. (2003) determined that more than half of clinical MRSA bloodstream isolates belong to agr group II. Although the precise relationship is unclear, the limited literature suggests a link between different agr types and certain staphylococcal syndromes. The inconsistencies in these reports may reflect ecological and geographical factors or different experimental designs, but the general lessons learned from them are comparable.
Future Perspective
Due to its importance in regulating virulence factor production and biofilm formation, the agr system is considered as an attractive therapeutic target. Interfering with the agr system or blocking it entirely may be an effective method for weakening the virulence of staphylococcal pathogens and controlling staphylococcal disease. Measures that target AgrB/D/C/A, AIP, or RNAIII are all of interest (Table 1).
Table 1.
Known targets in agr system (target AgrB/D/C/A, RNAIII, and AIP) with the potential to inhibit Staphylococcus aureus infections.
| Anti-agr compound | Mechanism of inhibition | Reference |
|---|---|---|
| RNAIII inhibiting protein | ||
| RIP | Inhibits synthesis of agr transcripts RNAII and RNAIII | Gov et al., 2001 |
| RIP derivatives (16P-AC) | Inhibits the expression of biofilm-related genes in S. aureus | Zhou et al., 2016 |
| RIP-V, RIP-L | Down-regulates RNAIII expression and α-hemolysin production | Ma et al., 2015 |
| AIP and AIP derivatives | ||
| Truncated AIP-I, II, III, | Inhibits autoinduction of all four S. aureus subgroups | Otto et al., 2001 |
| Vaccination with hapten-linked AIP IV | Provides passive immunity and reduces the pathology of agr IV strains | Tal-Gan et al., 2016 |
| Secondary metabolites | ||
| Solonamide/Solonamide B | From marine bacteria; functions via competitive inhibition of AgrC | Wang and Muir, 2016 |
| Cochinmicin | From actinomycetes, functions via competitive inhibition of AgrC | Wang and Muir, 2016 |
| Avellanin | From sponges; functions via competitive inhibition of AgrC | Wang and Muir, 2016 |
| 3-oxo-C12-HSL, (HQNO) | From Pseudomonas aeruginosa; quenches S. aureus autoinduction | Wang and Muir, 2016 |
| Naringenin | Reduces agrA and hla transcript levels | Zhang et al., 2013 |
| 2-(4-methylphenyl)-1,3- thiazole-4-carboxylic acid, 9H-xanthene-9-carboxylic acid, 4-phenoxyphenol | Binds C terminus of AgrA and disrupts AgrA-DNA binding activity | Leonard et al., 2012 |
| Savirin | Blocks S. aureus autoinduction | Sully et al., 2014 |
| ω-hydroxyemodin (OHM) | Prevents agr activity by all four S. aureus agr group strains | Daly et al., 2015 |
| Antisense oligonucleotides | ||
| PLNA34 | Specifically and significantly reduces agrA mRNA levels | Da et al., 2017 |
| Bacterial | ||
| Staphylococcus schleiferi | Functions by cross-inhibition of the pathogenic agr system | Canovas et al., 2016 |
AgrB/D/C/A As agr System Targets
As noted earlier, the agr operon is transcribed from divergent promoters, P2 and P3, to yield RNAII and RNAIII, respectively. Transcription depends on the specific binding of activated AgrA to the P2 and P3 promoter regions. Because activated AgrA itself is the final product of RNAII activation, RNAII transcription can be affected by targeting AgrB, D, C, or A, as well as AIP.
Since it has a key role in agr activation, AgrA can serve as a potential drug target for inhibition of agr quorum sensing. Blocking the binding of phosphorylated AgrA to the P2 and P3 promoters will repress the agr system, interfering with the expression of virulence factors such as α-hemolysin and PSMs, and attenuating the virulence of S. aureus (Khodaverdian et al., 2013). Pathogens affected in this way are less able to colonize host tissues and are ultimately eradicated by host immune system (Kong et al., 2016). Considerable effort has focused on identifying AgrA antagonists. Leonard et al. (2012) recently identified three compounds that target the LytTR DNA binding domain of AgrA and prevent its binding to the P3 promoter. Savirin, a small molecule that inhibits activation of P3 by AgrA in all four agr types, was also screened by Sully et al. (2014). This compound prevents the up-regulation of virulence genes in S. aureus, promotes clearance of agr+ S. aureus, and has a high efficacy in murine skin infection models (Sully et al., 2014). ω-hydroxyemodin (OHM), a polyhydroxyanthraquinone isolated from solid-phase cultures of penicillium restrictum, prevents interaction of AgrA with the P2 promoter, thus blocking agr activity of all S. aureus agr types (Daly et al., 2015). Naringenin significantly reduces AgrA and hla transcript levels in post-exponential cultures and protects mice from pneumonia caused by S. aureus (Zhang et al., 2013). Solonamide B cyclodepsipeptide isolated from the marine bacterium Photobacterium halotolerans, strongly down-regulates the expression of RNAIII and AgrA-controlled virulence genes in S aureus. Moreover, because the phosphorylation and activation of AgrA is catalyzed by AgrC, inhibitors targeting AgrC or AgrA are also potential ways to block disease development. For example, cochinmicin, avellanin, and Solonamide possess a 16-membered macrocycle and can function as competitive inhibitors of AgrC (Wang and Muir, 2016). Another approach for quenching the agr system is to use antisense locked nucleic acids that target the members of agr system. The antisense oligonucleotide PLNA34, designed to target agrA mRNA, specifically and significantly down-regulated agrA mRNA transcription with no bactericidal activity (Da et al., 2017).
AgrB and AgrD are responsible for producing autoinducing peptide (AIP), and inhibitors that target AgrB or prevent export of AgrD would be efficient antagonists of the agr system. Examples include peptide analogs that irreversibly antagonize the cleavage and cyclization of the AgrD active site, destabilize the enzyme structure of AgrB, or transform it to an inactive conformation (Gray et al., 2013).
Agr Targeting by Cross-Inhibitory AIP
Some secondary metabolites have the potential to interfere with bacterial signals (Packiavathy et al., 2014; Zhang et al., 2014), because they can serve as both autoinducer agonists and antagonists. Some AIPs from other staphylococcal species are able to repress the function of the S. aureus agr system. It may be possible to use the agr pheromone from a non-pathogenic strain of S. aureus or from other staphylococci for therapeutic purposes against S. aureus infection (Jabbari et al., 2012). For example, the culture supernatant of S. schleiferi exhibits potent inhibitory activity against the S. aureus agr system and is effective against all four agr classes. The expression of many virulence genes is also suppressed when S. aureus and S. schleiferi are co-inoculated in vivo (Canovas et al., 2016). Genes that contribute to colonization and virulence regulated by the agr system are also inhibited by coculture with other commensal strains (Ramsey et al., 2016). Furthermore, AIP can be inhibited by other AIP subtypes of S. aureus and analogs from staphylococci. AIP-II has been applied to prevent abscess formation caused by agr-I S. aureus in a murine model (Wright et al., 2005a). The agr system of S. aureus and S. epidermidis are also cross-inhibitory with each other. The AIPs of S. aureus groups I, II, and III are all sensitive to S. epidermidis pheromone, while subgroup 4 pheromones of S. aureus can also inhibit the S. epidermidis agr response (Otto et al., 2001). AIP-I, -II, -III, and their analogs have been successfully made into quorum sensing blocking agents capable of inhibiting the autoinduction of the four S. aureus subgroups (Otto et al., 2001; Tal-Gan et al., 2016).
RNAIII as a Target
As the main effector molecule of the agr system, RNAIII is responsible for the expression of a huge number of virulence genes. Inhibiting RNAIII may therefore be an effective method for reducing the production of toxins and other virulence factors. The RNA-III inhibiting peptide (RIP) effectively suppresses diseases caused by S. aureus (Gov et al., 2001). Native RIP (YSPWTNF-NH2) and some of its synthetic analogs were found to inhibit RNAII and RNAIII transcription (Gov et al., 2001). Furthermore, RIPs reduce bacterial adherence to mammalian cells and plastic substrates, and prevent biofilm formation in surgical transplantation by inhibiting RAP (Gov et al., 2001).
RNA-III inhibiting peptide derivatives have also been designed and evaluated for their potential as specific drug candidates for treating S. aureus infections. Ma et al. (2015) reported that RIP-V and RIP-L efficiently inhibit injury infection in a MRSA sepsis mouse model and increase the survival rate. However, due to rapid renal clearance and degradation, the plasma half-life of RIP and its derivatives in vivo is generally short, which greatly limits their clinical utility (Werle and Bernkop-Schnurch, 2006). RIP derivatives have been modified using amino acid substitution and oligomerization to improve their metabolic stability and activity in vivo and in vitro. 16P-AC (CH3CO-YKPVTNF-ST-YKPVTNF-CONH2), a hexadecapeptide RIP oligomer with an amidated C-terminal and an acetylated N-terminal, shows greatly enhanced stability and activity in vivo and is a promising drug candidate for treatment of MRSA-related infections (Zhou et al., 2016).
Other Methods
Strategies that directly antagonize toxins may help prevent and treat S. aureus infections by reducing toxin-binding affinities or by blocking the ability of toxins to elicit pro-inflammatory responses and cytolytic activities. Toxins encoded by the core genome, such as the PSMs, α-hemolysin, LukGH, and SElX, are essential virulence factors for pathogenic staphylococci and are attractive for vaccine development (Cheung and Otto, 2012). For example, PSMs can cause lysis of red and white blood cells, stimulate inflammatory responses, facilitate neutrophil lysis after phagocytosis, advance biofilm-related infection propagation, and are key virulence determinants (Li et al., 2014). Since the amino acid sequences of PSMs are highly conserved between S. aureus strains, they are good targets for therapeutic antibody development (Cheung and Otto, 2012). In addition, export of PSM peptides is controlled by the dedicated Pmt secretion system (Chatterjee et al., 2013). Approaches that target PSM secretion may efficiently disable these toxins and prevent host damage (Li et al., 2014). Antibodies against α-hemolysin confer protection against S. aureus infection in various animal models (Hua et al., 2014). Rouha et al. (2015) isolated a monoclonal antibody with high affinity and cross-reactivity toward α-hemolysin and 4 different bi-component leukocidins (HlgAB, HlgCB, LukED, and LukSF). The antibody provided high levels of protection in murine models of pneumonia and sepsis (Rouha et al., 2015). HuMAb-154, a human monoclonal antibody with high affinity for SEB, prolongs survival of SEB-challenged mice by neutralizing SEB-induced cytokines (Drozdowski et al., 2010).
Remaining Problems
Although agr systems have been targeted successfully in animal models, some problems remain. Although small molecule inhibitors that block the activity of the agr system and prevent gene expression are of great interest, most of them specifically block only one or two agr types. Inhibitors that can block all four S. aureus agr types need to be developed (Sully et al., 2014; Daly et al., 2015). Agr cross-inhibition may offer a basis for broader targeting, but a particular agr AIP or AIP derivative always exhibits varying activity in different staphylococcal strains. In addition, the therapeutic situation is complicated by the fact that different staphylococcal strains can coexist in a patient (Gomes et al., 2005). RNA-III inhibiting peptide has poor metabolic stability and activity. Although RIP derivatives can be stabilized by amino acid modification and oligomerization, more development is necessary before they can find use in treatment of human staphylococcus-associated infection. Another challenge is that numerous virulence factors are encoded by mobile genetic elements (MGES). This is a serious problem for target-oriented drug development due to their diversity and their parallel transfer between strains (Wright et al., 2005b). Toxins encoded by the core genome also vary. For example, the expression patterns for PSMs differ among Staphylococcus species, making it difficult to develop antibodies or vaccines against PSMs (Peschel and Otto, 2013).
A final puzzle is that agr-defective strains or agr- variants are detected in many infections (Traber et al., 2008). Most of these have lost the ability to disseminate in tissues and are often associated with biofilm formation (Painter et al., 2014). That a significant fraction of strains lacking agr activity have been isolated from cases of bacteremia has led to further consideration of the role of agr in invasive staphylococcal infection (Painter et al., 2014). One possibility is that the density of bacteria in the bloodstream is too low to activate the agr system. However, transcription analysis indicates that RNAIII expression is still low in blood even at high densities (James et al., 2013). Some studies suggest that AgrA and/or AIP activities may be inhibited by serum reactive oxygen species (ROS) (Kavanaugh and Horswill, 2016). Moreover, apolipoprotein B (apoB) in serum may sequester AIP from interaction with the sensor kinase AgrC and contribute to quorum-sensing inhibition (James et al., 2013).
Strategies to antagonize the components of the agr system will ultimately contribute to therapy against S. aureus-associated infections. However, the problems described above will need to be addressed before highly effective quorum sensing blockers can be developed to treat diseases caused by S. aureus.
Author Contributions
LT was mainly responsible for writing the manuscript. SiL was mainly responsible for literature collection and assisted in writing. BJ provided guidance in writing. XH and ShL provided guidance on the ideas and grammar for the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by awards from the Natural Science Foundation (grant nos. 31470241 and 31570127).
References
- Arvidson S., Tegmark K. (2001). Regulation of virulence determinants in Staphylococcus aureus. Int. J. Med. Microbiol. 291 159–170. 10.1078/1438-4221-00112 [DOI] [PubMed] [Google Scholar]
- Bassler B. L. (2002). Small talk. Cell-to-cell communication in bacteria. Cell 109 421–424. 10.1016/S0092-8674(02)00749-3 [DOI] [PubMed] [Google Scholar]
- Boles B. R., Horswill A. R. (2008). Agr-mediated dispersal of Staphylococcus aureus biofilms. PLOS Pathog. 4:e1000052. 10.1371/journal.ppat.1000052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronesky D., Wu Z., Marzi S., Walter P., Geissmann T., Moreau K., et al. (2016). Staphylococcus aureus RNAIII and its regulon link quorum sensing, stress responses, metabolic adaptation, and regulation of virulence gene expression. Annu. Rev. Microbiol. 70 299–316. 10.1146/annurev-micro-102215-095708 [DOI] [PubMed] [Google Scholar]
- Bronner S., Monteil H., Prevost G. (2004). Regulation of virulence determinants in Staphylococcus aureus: complexity and applications. FEMS Microbiol. Rev. 28 183–200. 10.1016/j.femsre.2003.09.003 [DOI] [PubMed] [Google Scholar]
- Cafiso V., Bertuccio T., Santagati M., Demelio V., Spina D., Nicoletti G., et al. (2007). agr-Genotyping and transcriptional analysis of biofilm-producing Staphylococcus aureus. FEMS Immunol. Med. Microbiol. 51 220–227. 10.1111/j.1574-695X.2007.00298.x [DOI] [PubMed] [Google Scholar]
- Canovas J., Baldry M., Bojer M. S., Andersen P. S., Grzeskowiak P. K., Stegger M., et al. (2016). Cross-Talk between Staphylococcus aureus and other Staphylococcal Species via the agr quorum sensing system. Front. Microbiol. 7:1733 10.3389/fmicb.2016.01733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S. S., Joo H. S., Duong A. C., Dieringer T. D., Tan V. Y., Song Y., et al. (2013). Essential Staphylococcus aureus toxin export system. Nat. Med. 19 364–367. 10.1038/nm.3047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung G. Y., Otto M. (2012). The potential use of toxin antibodies as a strategy for controlling acute Staphylococcus aureus infections. Expert Opin. Ther. Targets 16 601–612. 10.1517/14728222.2012.682573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho L. R., Souza R. R., Ferreira F. A., Guimaraes M. A., Ferreira-Carvalho B. T., Figueiredo A. M. (2008). agr RNAIII divergently regulates glucose-induced biofilm formation in clinical isolates of Staphylococcus aureus. Microbiology 154(Pt 11) 3480–3490. 10.1099/mic.0.2007/016014-0 [DOI] [PubMed] [Google Scholar]
- Da F., Yao L., Su Z., Hou Z., Li Z., Xue X., et al. (2017). Antisense locked nucleic acids targeting agrA inhibit quorum sensing and pathogenesis of community-associated methicillin-resistant Staphylococcus aureus. J. Appl. Microbiol. 122 257–267. 10.1111/jam.13321 [DOI] [PubMed] [Google Scholar]
- Daly S. M., Elmore B. O., Kavanaugh J. S., Triplett K. D., Figueroa M., Raja H. A., et al. (2015). omega-Hydroxyemodin limits Staphylococcus aureus quorum sensing-mediated pathogenesis and inflammation. Antimicrob. Agents Chemother. 59 2223–2235. 10.1128/AAC.04564-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dastgheyb S. S., Villaruz A. E., Le K. Y., Tan V. Y., Duong A. C., Chatterjee S. S., et al. (2015). Role of phenol-soluble modulins in formation of Staphylococcus aureus biofilms in synovial fluid. Infect. Immun. 83 2966–2975. 10.1128/IAI.00394-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida L. M., de Almeida M. Z., de Mendonça C. L., Mamizuka E. M. (2013). Comparative analysis of agr groups and virulence genes among subclinical and clinical mastitis Staphylococcus aureus isolates from sheep flocks of the Northeast of Brazil. Braz. J. Microbiol. 44 493–498. 10.1590/S1517-83822013000200026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drozdowski B., Zhou Y., Kline B., Spidel J., Chan Y. Y., Albone E., et al. (2010). Generation and characterization of high affinity human monoclonal antibodies that neutralize staphylococcal enterotoxin B. J. Immune Based Ther. Vaccines 8:9. 10.1186/1476-8518-8-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour P., Jarraud S., Vandenesch F., Greenland T., Novick R. P., Bes M., et al. (2002). High genetic variability of the agr locus in Staphylococcus species. J. Bacteriol. 184 1180–1186. 10.1128/jb.184.4.1180-1186.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engebrecht J., Nealson K., Silverman M. (1983). Bacterial bioluminescence: isolation and genetic analysis of functions from Vibrio fischeri. Cell 32 773–781. 10.1016/0092-8674(83)90063-6 [DOI] [PubMed] [Google Scholar]
- Fuqua W. C., Winans S. C., Greenberg E. P. (1994). Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J. Bacteriol. 176 269–275. 10.1128/jb.176.2.269-275.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisinger E., George E. A., Chen J., Muir T. W., Novick R. P. (2008). Identification of ligand specificity determinants in AgrC, the Staphylococcus aureus quorum-sensing receptor. J. Biol. Chem. 283 8930–8938. 10.1074/jbc.M710227200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisinger E., Muir T. W., Novick R. P. (2009). agr receptor mutants reveal distinct modes of inhibition by staphylococcal autoinducing peptides. Proc. Natl. Acad. Sci. U.S.A. 106 1216–1221. 10.1073/pnas.0807760106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George Cisar E. A., Geisinger E., Muir T. W., Novick R. P. (2009). Symmetric signalling within asymmetric dimers of the Staphylococcus aureus receptor histidine kinase AgrC. Mol. Microbiol. 74 44–57. 10.1111/j.1365-2958.2009.06849.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes A. R., Vinga S., Zavolan M., de Lencastre H. (2005). Analysis of the genetic variability of virulence-related loci in epidemic clones of methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 49 366–379. 10.1128/AAC.49.1.366-379.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gov Y., Bitler A., Dell’Acqua G., Torres J. V., Balaban N. (2001). RNAIII inhibiting peptide (RIP), a global inhibitor of Staphylococcus aureus pathogenesis: structure and function analysis. Peptides 22 1609–1620. 10.1016/S0196-9781(01)00496-X [DOI] [PubMed] [Google Scholar]
- Grande R., Nistico L., Sambanthamoorthy K., Longwell M., Iannitelli A., Cellini L., et al. (2014). Temporal expression of agrB, cidA, and alsS in the early development of Staphylococcus aureus UAMS-1 biofilm formation and the structural role of extracellular DNA and carbohydrates. Pathog Dis. 70 414–422. 10.1111/2049-632X.12158 [DOI] [PubMed] [Google Scholar]
- Gray B., Hall P., Gresham H. (2013). Targeting agr- and agr-Like quorum sensing systems for development of common therapeutics to treat multiple gram-positive bacterial infections. Sensors (Basel) 13 5130–5166. 10.3390/s130405130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebe T. W., Stock J. B. (1999). The histidine protein kinase superfamily. Adv. Microb. Physiol. 41 139–227. 10.1016/S0065-2911(08)60167-8 [DOI] [PubMed] [Google Scholar]
- Holtfreter S., Grumann D., Schmudde M., Nguyen H. T., Eichler P., Strommenger B., et al. (2007). Clonal distribution of superantigen genes in clinical Staphylococcus aureus isolates. J. Clin. Microbiol. 45 2669–2680. 10.1128/JCM.00204-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua L., Hilliard J. J., Shi Y., Tkaczyk C., Cheng L. I., Yu X., et al. (2014). Assessment of an anti-alpha-toxin monoclonal antibody for prevention and treatment of Staphylococcus aureus-induced pneumonia. Antimicrob. Agents Chemother. 58 1108–1117. 10.1128/AAC.02190-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomidis A., Vasdeki A., Kristo I., Maniatis A. N., Tsakris A., Malizos K. N., et al. (2009). Association of biofilm formation and methicillin-resistance with accessory gene regulator (agr) loci in Greek Staphylococcus aureus clones. Microb. Pathog. 47 341–344. 10.1016/j.micpath.2009.09.011 [DOI] [PubMed] [Google Scholar]
- Jabbari S., King J. R., Williams P. (2012). Cross-strain quorum sensing inhibition by Staphylococcus aureus. Part 2: a spatially inhomogeneous model. Bull. Math. Biol. 74 1326–1353. 10.1007/s11538-011-9702-0 [DOI] [PubMed] [Google Scholar]
- James E. H., Edwards A. M., Wigneshweraraj S. (2013). Transcriptional downregulation of agr expression in Staphylococcus aureus during growth in human serum can be overcome by constitutively active mutant forms of the sensor kinase AgrC. FEMS Microbiol. Lett. 349 153–162. 10.1111/1574-6968.12309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarraud S., Mougel C., Thioulouse J., Lina G., Meugnier H., Forey F., et al. (2002). Relationships between Staphylococcus aureus genetic background, virulence factors, agr groups (alleles), and human disease. Infect. Immun. 70 631–641. 10.1128/IAI.70.2.631-641.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G., Beavis R., Novick R. P. (1997). Bacterial interference caused by autoinducing peptide variants. Science 276 2027–2030. 10.1126/science.276.5321.2027 [DOI] [PubMed] [Google Scholar]
- Ji G., Beavis R. C., Novick R. P. (1995). Cell density control of staphylococcal virulence mediated by an octapeptide pheromone. Proc. Natl. Acad. Sci. U.S.A. 92 12055–12059. 10.1073/pnas.92.26.12055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. G., Wang B., Debelouchina G. T., Novick R. P., Muir T. W. (2015). Increasing AIP macrocycle size reveals key features of agr activation in Staphylococcus aureus. Chembiochem 16 1093–1100. 10.1002/cbic.201500006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanaugh J. S., Horswill A. R. (2016). Impact of environmental cues on Staphylococcal quorum sensing and biofilm development. J. Biol. Chem. 291 12556–12564. 10.1074/jbc.R116.722710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodaverdian V., Pesho M., Truitt B., Bollinger L., Patel P., Nithianantham S., et al. (2013). Discovery of antivirulence agents against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 57 3645–3652. 10.1128/AAC.00269-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoramrooz S. S., Mansouri F., Marashifard M., Malek Hosseini S. A., Akbarian Chenarestane-Olia F., Ganavehei B., et al. (2016). Detection of biofilm related genes, classical enterotoxin genes and agr typing among Staphylococcus aureus isolated from bovine with subclinical mastitis in southwest of Iran. Microb. Pathog. 97 45–51. 10.1016/j.micpath.2016.05.022 [DOI] [PubMed] [Google Scholar]
- Koenig R. L., Ray J. L., Maleki S. J., Smeltzer M. S., Hurlburt B. K. (2004). Staphylococcus aureus AgrA binding to the RNAIII-agr regulatory region. J. Bacteriol. 186 7549–7555. 10.1128/JB.186.22.7549-7555.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong C., Neoh H. M., Nathan S. (2016). Targeting Staphylococcus aureus toxins: a potential form of anti-virulence therapy. Toxins (Basel) 8:E72. 10.3390/toxins8030072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krismer B., Peschel A. (2011). Does Staphylococcus aureus nasal colonization involve biofilm formation? Future Microbiol. 6 489–493. 10.2217/fmb.11.37 [DOI] [PubMed] [Google Scholar]
- Lauderdale K. J., Boles B. R., Cheung A. L., Horswill A. R. (2009). Interconnections between Sigma B, agr, and proteolytic activity in Staphylococcus aureus biofilm maturation. Infect. Immun. 77 1623–1635. 10.1128/IAI.01036-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le K. Y., Otto M. (2015). Quorum-sensing regulation in staphylococci-an overview. Front. Microbiol. 6:1174. 10.3389/fmicb.2015.01174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard P. G., Bezar I. F., Sidote D. J., Stock A. M. (2012). Identification of a hydrophobic cleft in the LytTR domain of AgrA as a locus for small molecule interactions that inhibit DNA binding. Biochemistry 51 10035–10043. 10.1021/bi3011785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Huang H., Rao X., Chen W., Wang Z., Hu X. (2014). Phenol-soluble modulins: novel virulence-associated peptides of staphylococci. Future Microbiol. 9 203–216. 10.2217/fmb.13.153 [DOI] [PubMed] [Google Scholar]
- Ma B., Zhou Y., Li M., Yu Q., Xue X., Li Z., et al. (2015). RIP-V improves murine survival in a sepsis model by down-regulating RNAIII expression and alpha-hemolysin release of methicillin-resistant Staphylococcus aureus. Pharmazie 70 81–87. [PubMed] [Google Scholar]
- Mairpady Shambat S., Siemens N., Monk I. R., Mohan D. B., Mukundan S., Krishnan K. C., et al. (2016). A point mutation in AgrC determines cytotoxic or colonizing properties associated with phenotypic variants of ST22 MRSA strains. Sci. Rep. 6:31360. 10.1038/srep31360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal S. M., Ghosh A. K., Pati B. R. (2015). Dissemination of antibiotic resistance in methicillin-resistant Staphylococcus aureus and vancomycin-resistant S aureus strains isolated from hospital effluents. Am. J. Infect. Control 43 e87–e88. 10.1016/j.ajic.2015.08.015 [DOI] [PubMed] [Google Scholar]
- MDowell P., Affas Z., Reynolds C., Holden M. T., Wood S. J., Saint S., et al. (2001). Structure, activity and evolution of the group I thiolactone peptide quorum-sensing system of Staphylococcus aureus. Mol. Microbiol. 41 503–512. 10.1046/j.1365-2958.2001.02539.x [DOI] [PubMed] [Google Scholar]
- Mohsenzadeh M., Ghazvini K., Azimian A. (2015). Frequency of specific agr groups and antibiotic resistance in Staphylococcus aureus isolated from bovine mastitis in the northeast of Iran. Vet. Res. Forum 6 295–299. [PMC free article] [PubMed] [Google Scholar]
- Morfeldt E., Taylor D., von Gabain A., Arvidson S. (1995). Activation of alpha-toxin translation in Staphylococcus aureus by the trans-encoded antisense RNA, RNAIII. EMBO J. 14 4569–4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol K. A., Adam H. J., Hussain Z., Mulvey M. R., McCracken M., Mataseje L. F., et al. (2011). Comparison of community-associated and health care-associated methicillin-resistant Staphylococcus aureus in Canada: results of the CANWARD 2007-2009 study. Diagn. Microbiol. Infect. Dis. 69 320–325. 10.1016/j.diagmicrobio.2010.10.028 [DOI] [PubMed] [Google Scholar]
- Novick R. P. (2003). Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol. Microbiol. 48 1429–1449. 10.1046/j.1365-2958.2003.03526.x [DOI] [PubMed] [Google Scholar]
- Novick R. P., Projan S. J., Kornblum J., Ross H. F., Ji G., Kreiswirth B., et al. (1995). The agr P2 operon: an autocatalytic sensory transduction system in Staphylococcus aureus. Mol. Gen. Genet. 248 446–458. 10.1007/BF02191645 [DOI] [PubMed] [Google Scholar]
- Otto M., Echner H., Voelter W., Gotz F. (2001). Pheromone cross-inhibition between Staphylococcus aureus and Staphylococcus epidermidis. Infect. Immun. 69 1957–1960. 10.1128/IAI.69.3.1957-1960.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packiavathy I. A., Priya S., Pandian S. K., Ravi A. V. (2014). Inhibition of biofilm development of uropathogens by curcumin - an anti-quorum sensing agent from Curcuma longa. Food Chem. 148 453–460. 10.1016/j.foodchem.2012.08.002 [DOI] [PubMed] [Google Scholar]
- Painter K. L., Krishna A., Wigneshweraraj S., Edwards A. M. (2014). What role does the quorum-sensing accessory gene regulator system play during Staphylococcus aureus bacteremia? Trends Microbiol. 22 676–685. 10.1016/j.tim.2014.09.002 [DOI] [PubMed] [Google Scholar]
- Peng H. L., Novick R. P., Kreiswirth B., Kornblum J., Schlievert P. (1988). Cloning, characterization, and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. J. Bacteriol. 170 4365–4372. 10.1128/jb.170.9.4365-4372.1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschel A., Otto M. (2013). Phenol-soluble modulins and staphylococcal infection. Nat. Rev. Microbiol. 11 667–673. 10.1038/nrmicro3110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pragman A. A., Yarwood J. M., Tripp T. J., Schlievert P. M. (2004). Characterization of virulence factor regulation by SrrAB, a two-component system in Staphylococcus aureus. J. Bacteriol. 186 2430–2438. 10.1128/JB.186.8.2430-2438.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu R., Pei W., Zhang L., Lin J., Ji G. (2005). Identification of the putative staphylococcal AgrB catalytic residues involving the proteolytic cleavage of AgrD to generate autoinducing peptide. J. Biol. Chem. 280 16695–16704. 10.1074/jbc.M411372200 [DOI] [PubMed] [Google Scholar]
- Queck S. Y., Jameson-Lee M., Villaruz A. E., Bach T. H., Khan B. A., Sturdevant D. E., et al. (2008). RNAIII-independent target gene control by the agr quorum-sensing system: insight into the evolution of virulence regulation in Staphylococcus aureus. Mol. Cell 32 150–158. 10.1016/j.molcel.2008.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey M. M., Freire M. O., Gabrilska R. A., Rumbaugh K. P., Lemon K. P. (2016). Staphylococcus aureus shifts toward commensalism in response to Corynebacterium Species. Front. Microbiol. 7:1230. 10.3389/fmicb.2016.01230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes D., Andrey D. O., Monod A., Kelley W. L., Zhang G., Cheung A. L. (2011). Coordinated regulation by AgrA, SarA, and SarR to control agr expression in Staphylococcus aureus. J. Bacteriol. 193 6020–6031. 10.1128/JB.05436-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel C. U., Monk I. R., Casey P. G., Waidmann M. S., Gahan C. G., Hill C. (2009). AgrD-dependent quorum sensing affects biofilm formation, invasion, virulence and global gene expression profiles in Listeria monocytogenes. Mol. Microbiol. 71 1177–1189. 10.1111/j.1365-2958.2008.06589.x [DOI] [PubMed] [Google Scholar]
- Rouha H., Badarau A., Visram Z. C., Battles M. B., Prinz B., Magyarics Z., et al. (2015). Five birds, one stone: neutralization of alpha-hemolysin and 4 bi-component leukocidins of Staphylococcus aureus with a single human monoclonal antibody. MAbs 7 243–254. 10.4161/19420862.2014.985132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakoulas G., Eliopoulos G. M., Moellering R. C., Jr., Novick R. P., Venkataraman L., Wennersten C., et al. (2003). Staphylococcus aureus accessory gene regulator (agr) group II: is there a relationship to the development of intermediate-level glycopeptide resistance? J. Infect. Dis. 187 929–938. 10.1086/368128 [DOI] [PubMed] [Google Scholar]
- Schwartz K., Sekedat M. D., Syed A. K., O’Hara B., Payne D. E., Lamb A., et al. (2014). The AgrD N-terminal leader peptide of Staphylococcus aureus has cytolytic and amyloidogenic properties. Infect. Immun. 82 3837–3844. 10.1128/IAI.02111-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R., Ray P. (2014). Quorum sensing-mediated regulation of staphylococcal virulence and antibiotic resistance. Future Microbiol. 9 669–681. 10.2217/fmb.14.31 [DOI] [PubMed] [Google Scholar]
- Solano C., Echeverz M., Lasa I. (2014). Biofilm dispersion and quorum sensing. Curr. Opin. Microbiol. 18 96–104. 10.1016/j.mib.2014.02.008 [DOI] [PubMed] [Google Scholar]
- Srivastava S. K., Rajasree K., Fasim A., Arakere G., Gopal B. (2014). Influence of the AgrC-AgrA complex on the response time of Staphylococcus aureus quorum sensing. J. Bacteriol. 196 2876–2888. 10.1128/JB.01530-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sully E. K., Malachowa N., Elmore B. O., Alexander S. M., Femling J. K., Gray B. M., et al. (2014). Selective chemical inhibition of agr quorum sensing in Staphylococcus aureus promotes host defense with minimal impact on resistance. PLOS Pathog. 10:e1004174. 10.1371/journal.ppat.1004174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal-Gan Y., Ivancic M., Cornilescu G., Blackwell H. E. (2016). Characterization of structural elements in native autoinducing peptides and non-native analogues that permit the differential modulation of AgrC-type quorum sensing receptors in Staphylococcus aureus. Org. Biomol. Chem. 14 113–121. 10.1039/c5ob01735a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thammavongsa V., Kim H. K., Missiakas D., Schneewind O. (2015). Staphylococcal manipulation of host immune responses. Nat. Rev. Microbiol. 13 529–543. 10.1038/nrmicro3521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoendel M., Horswill A. R. (2009). Identification of Staphylococcus aureus AgrD residues required for autoinducing peptide biosynthesis. J. Biol. Chem. 284 21828–21838. 10.1074/jbc.M109.031757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoendel M., Kavanaugh J. S., Flack C. E., Horswill A. R. (2011). Peptide signaling in the staphylococci. Chem. Rev. 111 117–151. 10.1021/cr100370n [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traber K., Novick R. (2006). A slipped-mispairing mutation in AgrA of laboratory strains and clinical isolates results in delayed activation of agr and failure to translate delta- and alpha-haemolysins. Mol. Microbiol. 59 1519–1530. 10.1111/j.1365-2958.2006.04986.x [DOI] [PubMed] [Google Scholar]
- Traber K. E., Lee E., Benson S., Corrigan R., Cantera M., Shopsin B., et al. (2008). agr function in clinical Staphylococcus aureus isolates. Microbiology 154(Pt 8) 2265–2274. 10.1099/mic.0.2007/011874-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuchscherr L., Loffler B. (2016). Staphylococcus aureus dynamically adapts global regulators and virulence factor expression in the course from acute to chronic infection. Curr. Genet. 62 15–17. 10.1007/s00294-015-0503-0 [DOI] [PubMed] [Google Scholar]
- Vuong C., Saenz H. L., Gotz F., Otto M. (2000). Impact of the agr quorum-sensing system on adherence to polystyrene in Staphylococcus aureus. J. Infect. Dis. 182 1688–1693. 10.1086/317606 [DOI] [PubMed] [Google Scholar]
- Wang B., Muir T. W. (2016). Regulation of virulence in Staphylococcus aureus: molecular mechanisms and remaining puzzles. Cell Chem. Biol. 23 214–224. 10.1016/j.chembiol.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Zhao A., Novick R. P., Muir T. W. (2015). Key driving forces in the biosynthesis of autoinducing peptides required for staphylococcal virulence. Proc. Natl. Acad. Sci. U.S.A. 112 10679–10684. 10.1073/pnas.1506030112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters E. M., Rowe S. E., O’Gara J. P., Conlon B. P. (2016). Convergence of Staphylococcus aureus persister and biofilm research: can biofilms be defined as communities of adherent persister cells? PLOS Pathog. 12:e1006012. 10.1371/journal.ppat.1006012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werle M., Bernkop-Schnurch A. (2006). Strategies to improve plasma half life time of peptide and protein drugs. Amino Acids 30 351–367. 10.1007/s00726-005-0289-3 [DOI] [PubMed] [Google Scholar]
- Wright J. S., III, Jin R., Novick R. P. (2005a). Transient interference with staphylococcal quorum sensing blocks abscess formation. Proc. Natl. Acad. Sci. U.S.A. 102 1691–1696. 10.1073/pnas.0407661102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J. S., III, Traber K. E., Corrigan R., Benson S. A., Musser J. M., Novick R. P. (2005b). The agr radiation: an early event in the evolution of staphylococci. J. Bacteriol. 187 5585–5594. 10.1128/JB.187.16.5585-5594.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier K. B., Bassler B. L. (2003). LuxS quorum sensing: more than just a numbers game. Curr. Opin. Microbiol. 6 191–197. 10.1016/S1369-5274(03)00028-6 [DOI] [PubMed] [Google Scholar]
- Xiong Y. Q., Van Wamel W., Nast C. C., Yeaman M. R., Cheung A. L., Bayer A. S. (2002). Activation and transcriptional interaction between agr RNAII and RNAIII in Staphylococcus aureus in vitro and in an experimental endocarditis model. J. Infect. Dis. 186 668–677. 10.1086/342046 [DOI] [PubMed] [Google Scholar]
- Yarwood J. M., Bartels D. J., Volper E. M., Greenberg E. P. (2004). Quorum sensing in Staphylococcus aureus biofilms. J. Bacteriol. 186 1838–1850. 10.1128/JB.186.6.1838-1850.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarwood J. M., Schlievert P. M. (2003). Quorum sensing in Staphylococcus infections. J. Clin. Invest. 112 1620–1625. 10.1172/JCI20442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Rui X., Wang L., Guan Y., Sun X., Dong M. (2014). Polyphenolic extract from Rosa rugosa tea inhibits bacterial quorum sensing and biofilm formation. Food Control 42 125–131. 10.1016/j.foodcont.2014.02.001 [DOI] [Google Scholar]
- Zhang L., Gray L., Novick R. P., Ji G. (2002). Transmembrane topology of AgrB, the protein involved in the post-translational modification of AgrD in Staphylococcus aureus. J. Biol. Chem. 277 34736–34742. 10.1074/jbc.M205367200 [DOI] [PubMed] [Google Scholar]
- Zhang L., Ji G. (2004). Identification of a staphylococcal AgrB segment(s) responsible for group-specific processing of AgrD by gene swapping. J. Bacteriol. 186 6706–6713. 10.1128/JB.186.20.6706-6713.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Lin J., Ji G. (2004). Membrane anchoring of the AgrD N-terminal amphipathic region is required for its processing to produce a quorum-sensing pheromone in Staphylococcus aureus. J. Biol. Chem. 279 19448–19456. 10.1074/jbc.M311349200 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wang J. F., Dong J., Wei J. Y., Wang Y. N., Dai X. H., et al. (2013). Inhibition of alpha-toxin production by subinhibitory concentrations of naringenin controls Staphylococcus aureus pneumonia. Fitoterapia 86 92–99. 10.1016/j.fitote.2013.02.001 [DOI] [PubMed] [Google Scholar]
- Zhou Y., Zhao R., Ma B., Gao H., Xue X., Qu D., et al. (2016). Oligomerization of RNAIII-inhibiting peptide inhibits adherence and biofilm formation of methicillin-resistant Staphylococcus aureus In Vitro and In Vivo. Microb. Drug Resist. 22 193–201. 10.1089/mdr.2015.0170 [DOI] [PubMed] [Google Scholar]


