Introduction
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome is a life-threatening adverse drug reaction with an up to 10% mortality rate. It is characterized by fever, skin eruption, lymphadenopathy, and systemic organ dysfunction. Aromatic anticonvulsants were the first identified culprit medications.1, 2, 3 This condition has a prolonged latency period with clinical disease developing up to 2 months after exposure.1, 4, 5, 6 We report a case of severe DRESS syndrome secondary to a combination of the antihypertensive medications, perindopril and amlodipine.
Case report
A 77-year-old retired Hungarian accountant presented to our hospital with a generalized morbilliform eruption. The pruritic eruption initially started on his chest and then extended onto the limbs over 2 days. The patient had completed a course of cephalexin 3 weeks prior as prophylaxis after an elective nasal polypectomy. He also had pruritus without rash 1 week after finishing cephalexin, which was diagnosed as mold allergy by his immunologist. His other medical history included hypertension, past hepatitis B and C infection from blood transfusions, and right upper lobe lobectomy for pulmonary tuberculosis. The only abnormal laboratory result on admission was a mild eosinophilia (0.06 x109/L), which normalized to 0.0 x 109 the next day. His renal and liver functions were within normal limits. The histology findings from the skin biopsy were suggestive of a drug reaction, as they showed spongiosis, exocytosis of lymphocytes, and necrotic keratinocytes. By then, the patient's only medication was perindopril, 5 mg, in combination with amlodipine, 5 mg. These medications were stopped, and prednisone, 25 mg daily was started and produced cutaneous improvement. The discharge diagnosis was drug reaction secondary to either cephalexin or perindopril/amlodipine.
Three months after discharge, the patient restarted the combination antihypertensive drugs and had an erythematous skin eruption 3 days later. This time, there was associated fever, lethargy, neck swelling, and peripheral edema. He also reported a 5-kg unintentional weight loss over the 4 weeks. On physical examination, he was erythrodermic, tachycardic, and febrile. There was an exfoliative facial dermatitis and a diffuse maculopapular eruption over the arms, trunk, and lower limbs (Fig 1). We also noted facial and acral swelling and prominent nontender left cervical lymphadenopathy (Fig 2).
Fig 1.
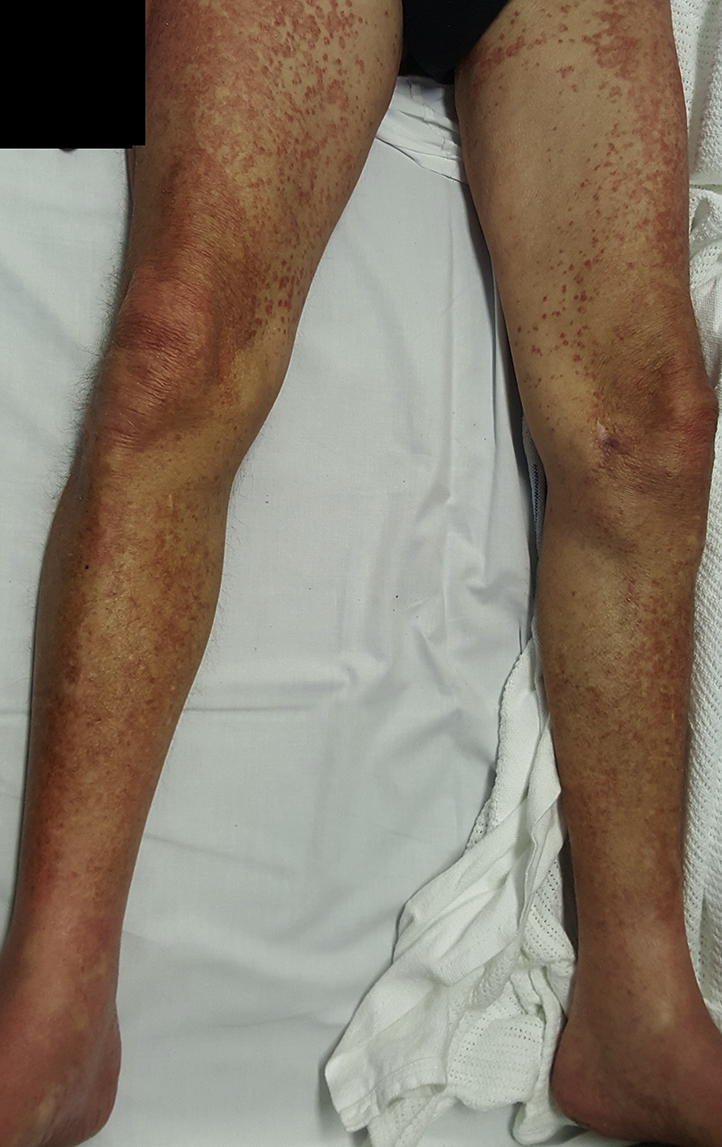
DRESS syndrome secondary to perindopril/amlodipine. Characteristic maculopapular rash on both lower extremities.
Fig 2.
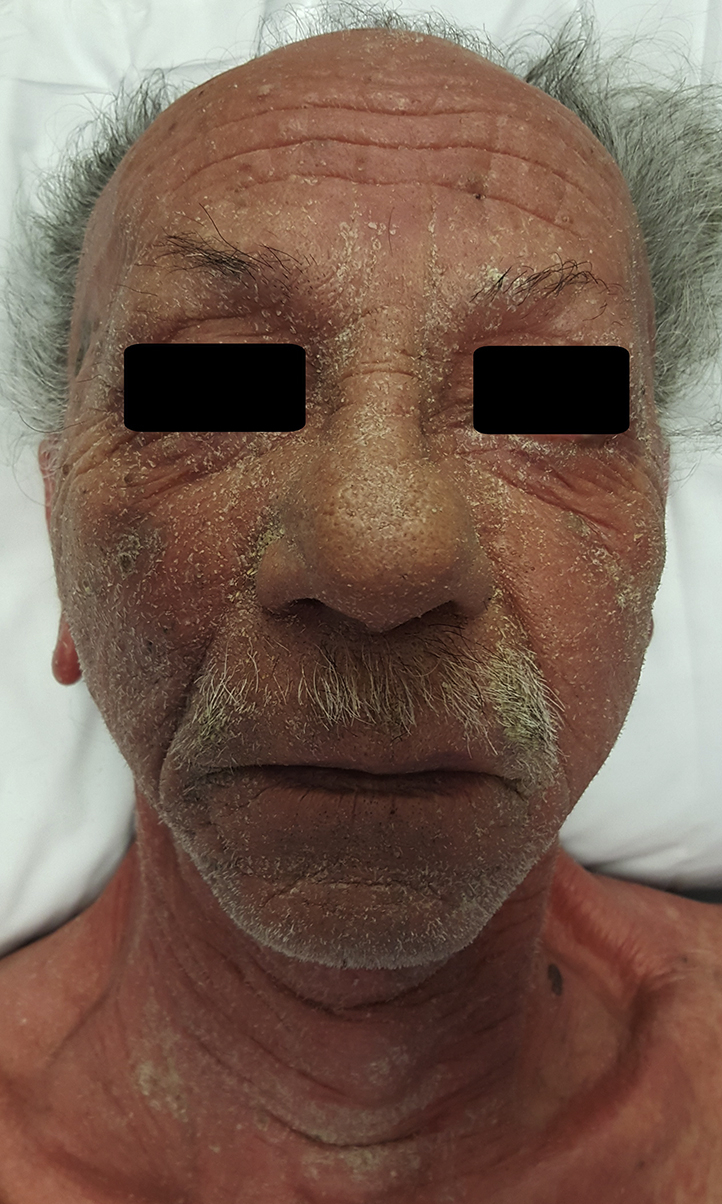
DRESS syndrome with marked facial exfoliative dermatitis and swelling.
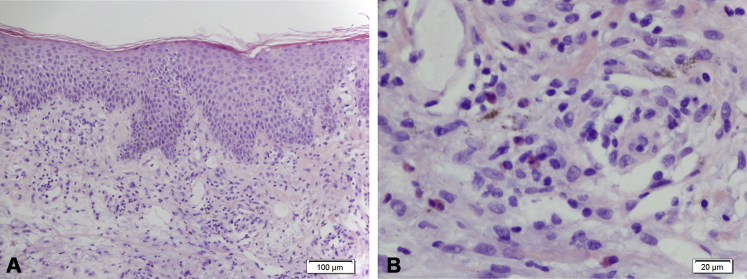
Laboratory results showed anemia (111 g/L), leukocytosis (white cell count, 13.6 x 109/L), isolated eosinophilia (4.6 x 109/L), high C-reactive protein (87 mg/L), and hepatic dysfunction (alkaline phosphatase, 267 U/L; γ-glutamyl transferase, 202 U/L; alanine aminotransferase, 63 U/L; aspartate aminotransferase, 62 U/L). There was also acute renal impairment with creatinine level of 148 μm/L and estimated glomerular filtration rate of 39 mL/min/1.73 m2. An autoimmune panel showed antinuclear antibody titer of 160 with a centromere pattern, normal double-stranded DNA antibody levels (0.1 U/mL), and normal complement levels (C3, 0.98 g/L; C4, 0.21 g/L). To exclude paraneoplastic causes, a computerized tomography scan of the neck, chest, abdomen, and pelvis was performed, which found a suspicious right lung nodule and multisite lymphadenopathy (left supraclavicular, 13 × 9 mm; bilateral axillary largest, 29 × 6 mm; inguinal largest, 25 × 9 mm). Bronchoscopic cell washings were negative for malignancy and infection. Viral serology found previous hepatitis B and C viruses and Epstein-Barr virus infections. Multiple sputum, blood, and urine cultures were negative. The 2 skin biopsies found moderate spongiosis and superficial dermal infiltrate of lymphocytes with eosinophils (Fig 3). A lymph node and bone marrow biopsy were performed by the consulting hematologist to exclude malignancy, and both showed eosinophilic infiltrates without malignant cells. Similarly, the patient's renal biopsy found numerous eosinophils with florid tubulointerstitial nephritis, which is consistent with renal involvement in DRESS syndrome.
Fig 3.
A, Low power. The skin biopsy from the left thigh shows mild spongiosis and parakeratosis. There is a mild superficial perivascular lymphocytic infiltrate with scattered interstitial neutrophils, eosinophils, and mast cells. B, High power. Eosinophils. (Original magnifications: A, ×200; B, ×400.)
DRESS syndrome secondary to amlodipine/perindopril was diagnosed by applying the European Registry of Severe Cutaneous Adverse Reactions (RegiSCAR) scoring system and obtaining a Naranjo Score.7, 8 A RegiSCAR score of 7 places the patient in the “definite case” category for DRESS syndrome, whereas a Naranjo score of 6 leaves perindopril/amlodipine in the “probable” category as the culprit medications. Although treatment with prednisone, 1 mg/kg daily, wet dressings, and antihistamine produced rapid cutaneous improvement, the patient soon had right lower lobe pneumonia with patchy consolidation on radiograph and negative sputum and blood cultures. Then on day 13, the patient suffered a non-ST segment elevation myocardial infarction. The serial troponin T enzymes were elevated at 401 ng/L to 776 ng/L. On day 15, he received emergency hemodialysis for critical hyperkalemia (6.4 mmol/L), and his care was transferred to the renal team who started pulse intravenous methyl-prednisone. On day 30, the patient was discharged with 50 mg oral prednisolone. On his 1-month follow up examination, there was residual exfoliative dermatitis and truncal hyperpigmentation. However, the serum eosinophilia had resolved, and the renal function was stabilizing at a new baseline of creatinine of 150 μm/L and estimated glomerular filtration rate of 40 mL/min/1.73.
Discussion
The most common cutaneous pattern in this disease is urticated papular exanthem on the neck, torso, and upper extremities.3 Erythroderma is seen in 18% of cases.3, 4, 5 Common systemic involvements are fever (90%), lymphadenopathy (75%), and hematologic and liver abnormalities. Renal dysfunction is reported in 11% of cases and usually occurs in allopurinol-induced DRESS syndrome.1, 2, 5 The DRESS syndrome–related bronchopneumonia and myocardial infarction in this case are uncommon systemic associations. A combination of excess drug metabolites, pharmacogenetics, and herpes virus reactivation are thought to operate synergistically to produce the clinical syndrome.9, 10
Approximately 44 medications have been identified as potential causal agents for DRESS syndrome.5, 9 To our knowledge, there are no reports of amlodipine or perindopril individually or in combination being implicated in this condition.
Diagnosis is established by applying RegiSCAR scoring system shown in Table I.7 In the recent French Society of Dermatology management recommendations, withdrawal of suspect medications and supportive care with emollients and H1-antihistamines are recommended for all cases. Severe DRESS syndrome was defined as transaminase levels greater than 5 times the normal and any renal involvement, pneumonia, or hemophagocytosis.11 For these patients, prednisone, 1 mg/kg/d, or an equivalent is recommended. In those with life-threatening systemic disease such as bone marrow failure or fulminant hepatitis, Intravenous immunoglobulin at 2 g/kg over 5 days should be added.3 Tapering of prednisone can occur over 6 to 8 weeks with at least 6 months of follow up with basic blood tests to capture relapse.2, 5, 11
Table I.
RegiSCAR Diagnostic Criteria for diagnosis of DRESS syndrome7
| Criteria | Minimum | Maximum | |||
|---|---|---|---|---|---|
| Fever ≥ 38.5 | No (−1) | Yes (0) | (−1) | (0) | |
| Enlarged lymph nodes (minimum of 2 sites, at least 1 cm) | No (0) | Yes (1) | (0) | (1) | |
| Eosinophils greater than the normal range | 0.7-1.499 × 109 L−1 (1) |
>1.5 × 109 L−1 (2) |
(0) | (2) | |
| If leucocytes <4 × 109 L−1 | 10-19.9% Eosinophils (1) |
≥20% Eosinophils (2) |
|||
| Atypical lymphocytes | No (0) | Yes (1) | (0) | (1) | |
| Skin rash | (−2) | (2) | |||
| Body surface area affected (%) | Unknown (0) | >50% affected (1) | |||
| Rash suggestive of DRESS | No (−1) | Unknown (0) | Yes (1) | ||
| Biopsy suggestive of DRESS | No (−1) | Unknown (0) | Yes (0) | ||
| Organ involvement (after excluding other causes): liver, kidney, lung, muscle/heart, pancreas, other organs | No (0) | Unknown (0) | Yes (1) for each organ, up to two organs | (0) | (2) |
| Resolution of symptoms ≥15 days | No (−1) | Unknown (−1) | Yes (0) | (−1) | (0) |
| Other potential causes | |||||
| Antinuclear antibody | If none positive and ≥3 negatives (1) | (0) | (1) | ||
| Blood culture | |||||
| Serology for HAV/HBV/HCV | |||||
| Chlamydia and mycoplasma | |||||
| Final Score | Minimum [−4] | Maximum [9] | |||
|
Final score: <2 = no case 2-3 = possible case 4-5 = probable case >5 = definite case for DRESS syndrome |
|||||
Poor prognostic indicators are pancytopenia, leukocytosis, coagulopathy, gastrointestinal bleeding, pre-existing chronic renal insufficiency, and multiple comorbidities.1, 11 Persistent tachycardia and tachypnea were also associated with poorer outcomes.1, 11 Autoantibody production and autoimmune diseases such as autoimmune thyroiditis, type 1 diabetes mellitus, and systemic lupus erythematosus can occur years after the initial DRESS syndrome. This was more common in younger patients and in those who did not receive oral corticosteroids.3
We report an unusual case of DRESS syndrome likely secondary to combination antihypertensive amlodipine and perindopril. The patient suffered acute tubular nephritis necessitating hemodialysis, pneumonia, acute coronary syndrome and chronic renal failure. This case highlights the life-threatening potential of this condition and alerts clinicians to the potential adverse effect of this commonly used antihypertensive combination.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Chen Y.C., Chiu H.C., Chu C.Y. Drug reaction with eosinophilia and systemic symptoms – a retrospective study of 60 cases. Arch Dermatol. 2010;146(12):1373–1379. doi: 10.1001/archdermatol.2010.198. [DOI] [PubMed] [Google Scholar]
- 2.Husain Z., Reddy B.Y., Schwartz R.A. DRESS syndrome: Part I. Clinical perspectives. J Am Acad Dermatol. 2013;68(5):693.e1–693.e14. doi: 10.1016/j.jaad.2013.01.033. [DOI] [PubMed] [Google Scholar]
- 3.Walsh S., Diaz-Cano S., Higgins E. Drug reaction with eosinophilia and systemic symptoms: is cutaneous phenotype a prognostic marker for outcome? A review of clinicopathological features of 27 cases. Br J Dermatol. 2013;168(2):391–401. doi: 10.1111/bjd.12081. [DOI] [PubMed] [Google Scholar]
- 4.Sasidharanpillai S., Riyaz N., Rajan U. Drug reaction with eosinophilia and systemic symptoms: observations from a tertiary care institution. Indian J Dermatol Venereol Leprol. 2014;80(3):221–228. doi: 10.4103/0378-6323.132249. [DOI] [PubMed] [Google Scholar]
- 5.Sheikh J., Sameem F., Ashraf M. Drug reaction with eosinophilia and systemic symptoms: manifestations, treatment and outcome in 17 patients. Int J Dermatol. 2015;54(5):537–542. doi: 10.1111/ijd.12331. [DOI] [PubMed] [Google Scholar]
- 6.Skowron F., Bensaid B., Balme B. Drug reaction with eosinophilia and systemic symptoms (DRESS): clinicopathological study of 45 cases. J Eur Acad Dermatol Venereol. 2015;29(11):2199–2205. doi: 10.1111/jdv.13212. [DOI] [PubMed] [Google Scholar]
- 7.Peyriere H., Dereure O., Breton H. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2006;155(2):422–428. doi: 10.1111/j.1365-2133.2006.07284.x. [DOI] [PubMed] [Google Scholar]
- 8.Naranjo C.A., Busto U., Sellers E.M. A method for estimating the probability of adverse drug reactions. Clin Pharmcol Ther. 1981;30(2):239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 9.Fernando S. Drug-reaction eosinophilia and systemic symptoms and drug-induced hypersensitivity syndrome. Australas J Dermatol. 2014;55(1):15–23. doi: 10.1111/ajd.12085. [DOI] [PubMed] [Google Scholar]
- 10.Choudhary S., McLeod M., Torchia D., Romanelli P. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome. J Clin Aesthet Dermatol. 2013;6(6):31–37. [PMC free article] [PubMed] [Google Scholar]
- 11.Husain Z., Reddy B.Y., Schwartz R.A. DRESS syndrome: Part II. Management and therapeutics. J Am Acad Dermatol. 2013;68(5):709.e1–709.e9. doi: 10.1016/j.jaad.2013.01.032. [DOI] [PubMed] [Google Scholar]