Abstract
No previous event-related potentials (ERPs) study has explored the error-related negativity (ERN) - an ERP component indexing performance monitoring - associated to cancer and chemotherapy-induced cognitive impairment in a lung cancer population. The aim of this study was to examine differences in performance monitoring in a small-cell lung cancer group (SCLC, C +) 1-month following chemotherapy and two control groups: a non-small cell lung cancer patient group (NSCLC, C −) prior to chemotherapy and a healthy control group (HC).
Seventeen SCLC (C +) underwent a neuropsychological assessment and an ERP study using a flanker and a stop-signal paradigm. This group was compared to fifteen age-, gender- and education-matched NSCLC (C −) and eighteen HC.
Between 20 and 30% of patients in both lung cancer groups (C + and C −) met criteria for cognitive impairment. Concerning ERPs, lung cancer patients showed lower overall hit rate and a severe ERN amplitude reduction compared to HC.
Lung cancer patients exhibited an abnormal pattern of performance monitoring thus suggesting that chemotherapy and especially cancer itself, may contribute to cognitive deterioration. ERN appeared as an objective laboratory tool sensitive to cognitive dysfunction in cancer population.
Keywords: Event-related potentials-ERP, Error-related negativity (ERN), Performance monitoring, Lung cancer, Cognitive impairment, Chemobrain
Highlights
-
•
This is the first study to explore error-related negativity in lung cancer patients.
-
•
Lung cancer patients showed a severe ERN amplitude reduction.
-
•
ERN resulted a potential biomarker of cognitive impairment in lung cancer population.
1. Introduction
Chemotherapy-induced cognitive impairment or ‘chemobrain’ is a well-recognized clinical syndrome, consisting of subtle to moderate cognitive changes across various domains (Jim et al., 2012). Although acute cognitive changes during chemotherapy are common (Ahles et al., 2002), long-term cognitive changes post-treatment seem to persist only in a subgroup (17–34%) of cancer survivors (Jim et al., 2012, Ahles and Saykin, 2007). In addition, cognitive deficits have also been described in cancer patients prior to chemotherapy (Ahles and Saykin, 2002). In recent years, several studies using neuroimaging techniques, concretely magnetic resonance imaging (MRI) and positron emission tomography, as well as event-related brain potentials (ERPs), have reported structural and functional changes associated with cancer and chemotherapy (Simo et al., 2015, Kaiser and Dietrich, 2014).
The fined-grained analysis of electrophysiological measures (such as ERPs), widely used in cognitive neuroscience, allows us to functionally characterize certain cognitive processes with higher temporal precision when compared to other metabolic based neuroimaging techniques (Münte et al., 2001, Marco-Pallares et al., 2009). However, ERPs have been scarcely used to investigate the neural correlates underlying cancer and chemotherapy-related cognitive impairments (see for a review, Kaiser and Dietrich (2014)). Most of the ERPs studies focused on cancer population yielded converging evidence of changes in the well-known P300 attention-related component associated with chemotherapy (Kreukels et al., 2005, Kreukels et al., 2006, Kreukels et al., 2008a, Kreukels et al., 2008b, Heukrodt et al., 1988, Kam et al., 2016). More specifically, these studies showed a reduced amplitude and a prolonged latency for the P300 component in chemotherapy-treated patients, suggesting impairments of cognitive function including attention and slow information processing and none of them included a cancer control group prior to receive chemotherapy to study more general cancer-related changes (Kreukels et al., 2005, Kreukels et al., 2006, Kreukels et al., 2008a, Kreukels et al., 2008b, Heukrodt et al., 1988, Kam et al., 2016).
Cognitive impairments in performance monitoring can also be assessed focusing on other stimulus-triggered ERP components, such as the frontocentral N200 and the P300 components observed after the presentation of the stimulus array. The amplitude of the N200 component is larger in conflict-related or incongruent trials and it has been associated to conflict monitoring (Gehring et al., 1992, Yeung et al., 2004, van Veen and Carter, 2002b, Kopp et al., 1996). Some authors have proposed that the conflict N2 component and the ERN share similar neural generators (Burgess et al., 2000, Stuss et al., 1995, Damasio, 1995, Holroyd and Coles, 2002). Besides, in incongruent trials, this N2 component is followed by a delayed P300 peak latency, which has been associated to a slowing of stimulus evaluation processes in incongruent trials (Münte et al., 2001, Gehring et al., 1992, Kok, 1997). Finally, it is important to mention that the amplitude of the P300 component has been associated to cognitive and executive functions including attention, amount of resources involved in a particular task and context updating in tasks that require stimulus evaluation and memory updating (Münte et al., 2001, Kok, 1997, Ferdinand et al., 2015).
The error monitoring is the ability to control our performance as well as to correct our errors in a highly flexible manner and is part of the executive function system (or cognitive control network), which includes planning, problem solving, working memory, and performance monitoring, among other regulatory functions (Burgess et al., 2000, Stuss et al., 1995, Damasio, 1995). The ERN, with a very specific frontal central scalp distribution, is elicited immediately after the detection of a performance error (with a latency in between 60 and 100 ms after error commission) and it is originated most probably in the anterior cingulate cortex (ACC) (Holroyd and Coles, 2002, Marco-Pallares et al., 2008, Gehring and Knight, 2000, Ullsperger and von Cramon, 2001, Ridderinkhof et al., 2004). The functional aspects of the ERN has been linked to reinforcement learning processes regulated by the mesencephalic dopaminergic system (Holroyd and Coles, 2002). To the best of our knowledge, no previous ERP study in cancer population has explored this relevant neurophysiological marker associated to error monitoring process.
Interestingly, one of the most important cognitive domains in which changes due to chemotherapy have been described (see for a review Jim et al. (2012)) is executive functioning. Most of the investigations on chemotherapy and cancer-related cognitive changes have focused on breast cancer patients whilst research focused on lung cancer population has been scarce. Early studies found that lung cancer patients exhibit cognitive impairments soon after chemotherapy treatment (Kaasa et al., 1988a, Kaasa et al., 1988b, Komaki et al., 1995, Grosshans et al., 2008). However, more recent studies have shown that lung cancer population exhibit cognitive impairments not only following chemotherapy but also prior to chemotherapy (cancer effect), accompanied by structural and functional neural changes (Simo et al., 2015, Simo et al., 2016, Horky et al., 2014, Simo et al., 2017). Thus, the study of the toxic effects of cancer and chemotherapy on cognition in lung cancer population remains challenging and under-represented in the literature.
Based on previous studies pointing out the inherent problems in cognitive control of lung cancer patients, we hypothesized that both lung cancer groups (C + and C– groups) will show a disruption in the performance monitoring system as measured using the ERN component. We further investigated the frontocentral N2 and P300 components as additional measures of conflict monitoring (concurrent activation of multiple-competing responses in incongruent trials). For this purpose, we used a well-known paradigm that combines a flanker and a stop-signal paradigm (measuring motor inhibition), which has been widely used to investigate performance monitoring (Rodriguez-Fornells et al., 2002, Kramer et al., 2007). This paradigm is well-suited in this particular case, since it allows the observation of error monitoring during stop-inhibited trials (i.e., patients cannot avoid responding), a condition that induces a large amount of errors.
2. Methods
2.1. Patients
Patients were prospectively recruited from December 2010 to December 2013 from the Lung Cancer Unit of the ICO L'Hospitalet-Hospital Universitari de Bellvitge (n = 32). Patients were eligible if they presented histologically proven diagnosis of either NSCLC or SCLC, were between the ages of 40 and 70 years, had no severe concomitant systemic illness or psychiatric disorder with a negative impact on cognitive function, or had no other contraindication to undergo an MRI scan. Patients were excluded if they had evidence of brain metastases on MRI. This cross-sectional analysis is the baseline analysis of a longitudinal study designed to examine the effects of further prophylactic cranial irradiation (PCI) in SCLC patients (Simo et al., 2015). C + (n = 17) who were eligible to receive PCI and were anti-HU negative were enrolled one month following completion of chemotherapy but prior to receive PCI. C– (n = 15) who were eligible to receive a platinum-based chemotherapy were enrolled in the study just after cancer diagnosis and before the initiation of chemotherapy. NSCLC was selected as the cancer control group because they underwent the same platinum-based chemotherapy as SCLC patients but they would not receive PCI, therefore facilitating the study of the long-term effects of chemotherapy in the longitudinal study. Age- and education-matched HC (n = 18) who met the same inclusion (except for cancer diagnosis) and exclusion criteria were recruited through community advertisements. Vascular risk factors were collected and classified in low-risk (if the patient had none or one risk factor) and high-risk (if the patient had two or more risk factors) groups (Welzel et al., 2008). The study protocol was approved by the Ethical Committee of Hospital Universitari de Bellvitge-ICO L'Hospitalet and written informed consent was obtained from all participants. All methods were performed in accordance with relevant guidelines and regulations.
2.2. Neuropsychological assessment
Patients were evaluated using: Mattis Dementia Rating Scale–2 (MDRS-2); selected subtests of the Spanish version of the Wechsler Adult Intelligence Scale-III (Vocabulary, Information, Similarities, Digit Span, Letter Number Sequencing, Block Design, Matrix Reasoning, and Picture Completion); Rey Auditory Verbal Learning Test (RAVLT); Wechsler Memory Scale–III Logical Memory I–II; Rey-Osterreith Complex Figure Test Copy, Immediate and Delayed; Spanish Version of the Boston Naming Test; Verbal Fluency test (Phonemic and Semantic); Trail Making Test (A–B); and Beck Depression Inventory (BDI). Intelligence quotient was estimated using Vocabulary performance. Raw cognitive test scores were compared with the validated Spanish normative values, corrected for age and education, and converted into z-scores. Cognitive impairment was defined as a MDRS-2 raw score < 123 (Mattis, 1988), one test ≥ 2 or two tests ≥ 1.5 standard deviations below the sample mean (Correa et al., 2013). All statistical analyses were conducted in SPSS 18.0 (SPSS, Chicago, IL). One-way analysis of variance and Chi-square tests were used to test group differences with a critical p-threshold of 0.05. Results are reported both uncorrected and Bonferroni corrected for multiple comparisons.
2.3. Paradigm
Our paradigm was a modified variant of the Eriksen flanker task (Eriksen and Eriksen, 1974) which has been previously used and described in U.M. Kramer et al. (2007). Briefly, in go trials, the task required the participants to respond to the central arrow in an array of five horizontal arrows, with the right hand after a right-directed arrow and vice versa. The four surrounding arrows could either be compatible (33.3% of trials) or incompatible (50% of trials) to the central arrow. Following a variant of the stop-signal paradigm (Band et al., 2003), the remaining 16.6% of the trials were ‘no-go’ trials, where the inhibition of the response was required after the central green arrow changed to red after a variable delay. The stop-signal delay was initially set to 140 ms and after a successful (or failed) inhibition the stop-signal delay increased (or decreased) by 10 ms, making the inhibition harder (or easier) (Band and van Boxtel, 1999).
The stop-signal reaction time (SSRT) was computed by subtracting the participant's mean stop-signal delay from the median reaction time of correct ‘go’ responses (Band et al., 2003). They were encouraged to correct their errors in the ‘go’ trials as fast as possible. The experiment was divided in eight blocks, each comprising 240 trials, resulting in a total of 1920 trials.
2.4. Neurophysiological study
2.4.1. Behavioral analysis
The percentage of correct responses was analyzed by a one-way ANOVA on ‘group’ (HC, C +, C −). During all the analyses, in case a main effect of ‘group’ was found, post-hoc tests were performed to evaluate which group yielded different results. Levene's tests were performed to test for equality of variances and if the assumption of homogeneity of variances was met, Tukey's HSD (honest significant difference) tests were conducted. On the contrary, if the variance was not equally distributed, Games Howell post hoc tests were conducted. The percentage of errors were analyzed by a two-way mixed-model ANOVA with repeated measurement on the between-subjects variable ‘group’ and the within-subjects variable ‘compatibility’ (compatible, incompatible). In addition, the percentage of corrected errors was analyzed by a one-way ANOVA on group. Reaction time (RT) was defined as the time between stimulus onset and the button press. The RT was analyzed by a two-way ANOVA on ‘group’ and ‘response’ (correct response, error) and the RT of correct responses was investigated by an ANOVA on ‘group’ and ‘compatibility’. The RT of corrected errors was analyzed by a two-way ANOVA on ‘group’ and ‘condition’ (corrected, non-corrected). Lastly, post error slowing, post non-inhibited slowing and SSRT were analyzed with a one-way ANOVA on ‘group’, provided that the participant reached a minimum of 5 trials per condition in order to compute a reliable average.
2.4.2. EEG recording and analysis
The electroencephalogram (EEG) was recorded from 29 tin electrodes mounted in an elastic cap (electrode positions: Fp1/2, F3/4, C3/4, P3/4, O1/2, F7/8, T3/4, T5/6, FC1/2, FC5/6, CP1/2, CP5/6, PO1/2, Fz, Cz, Pz) with reference electrodes placed on the right and left mastoids. During the recording, all scalp electrodes were referenced against an average reference, and electrode impedances were kept below 5 kΩ. Vertical eye movements and blinks were monitored by an electrode placed below the right eye. EEG and electrooculogram (EOG) were recorded continuously and digitized with a sampling rate of 250 Hz (bandpass from 0.01–70 Hz). EEG data was analyzed using EEGLAB v13.4.4b (Delorme and Makeig, 2004) and custom routines written in MatLab R2008b (The Mathworks, Natick, MA, USA). ERP data was re-referenced off-line to the average of both mastoids (Luck, 2005) and filtered with a 30 Hz low-pass. Trials with base-to-peak EOG amplitude of > 75 μV, amplifier saturation, or a baseline shift exceeding 200 μV/s were automatically rejected (Cunillera et al., 2008). After individualized rejection of artifacts, stimulus- and response-locked averages were obtained for the different conditions. To obtain reliable averages, we required each condition to have a minimum of 15 trials per participant (Rodriguez-Fornells et al., 2002, Gurtubay-Antolin and Rodriguez-Fornells, 2017, Gurtubay-Antolin et al., 2015).
2.4.3. ERPs: response-locked data
For the response-locked averages and after rejection of eye and muscle artifacts, epochs of 200 ms before and 600 ms after the response were extracted from the continuous EEG and baseline-corrected using a − 50–0 ms pre-response window following previously methodology (Rodriguez-Fornells et al., 2002, Kramer et al., 2007, Gurtubay-Antolin and Rodriguez-Fornells, 2017). In a similar manner as done by U.M. Kramer et al. (2007), after visually inspecting the 30 Hz low-pass filtered data, we decided to bandpass-filter (2–8 Hz) the EEG in order to obtain the response-locked ERPs in the band in which the ERN is best detectable. In the following, errors in ‘go’ trials will be referred to as ‘choice errors’ or Choice ERN and errors in ‘no-go’ trials will be referred to as ‘stop errors’ or Stop ERN. To note, corrected and uncorrected choice errors were included in the ‘choice error’ condition, since the number of uncorrected and corrected choice errors separately did not meet the criterion of a minimum of 15 trials in all the participants.
In ‘go’ trials, the amplitude of the error-related negativity in choice errors (Choice ERN) was defined as the mean voltage in the 50–150 ms interval following erroneous responses at the frontal and central midline electrodes (Fz, Cz). The same time interval and location was used to quantify the correct response amplitude (Choice Correct-related Negativity or CRN), following the emission of correct responses.
To analyze ‘no-go’ trials, the amplitude of the ERN in stop errors (Stop ERN) was measured between 100 and 200 ms after the non-inhibited response. In order to compare the amplitude of stop errors with the amplitude of correct responses, stop CRN, was defined as the mean voltage in the 100–200 ms interval following the emission of the correct response. These temporal windows corresponded to the maximum amplitude intervals (see panel A, Fig. 1, Fig. 2). In ‘go’ trials, we submitted choice CRN and choice ERN amplitude values to a three-way repeated-measures ANOVA that included the between-subject variable ‘group’ and two within-subject factors: ‘response’ (Correct: choice CRN, error: choice ERN) and ‘electrode’ (Fz, Cz). In case a main effect of ‘group’ was found, post-hoc Tukey tests were conducted. In ‘no-go’ trials, we submitted the stop CRN and the stop ERN amplitudes to the same three-way repeated-measures ANOVA.
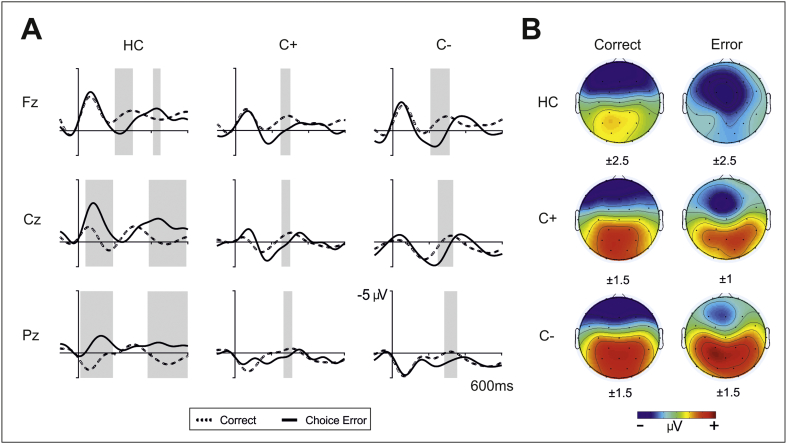
Fig. 1.
A. Grand averages of response-locked event-related potentials in ‘go’ trials at midline electrodes (Fz, Cz, Pz) for the three groups (controls, C + group and C– group). Depicted are ERPs for correct trials (dashed lines) and choice errors (thick solid lines). Data were bandpass filtered (bandpass 2–8 Hz) (U.M. Kramer et al., 2007). The grey shadows indicate the interval where the difference between conditions is statistically significant. B. Three-dimensional isovoltage topographical maps for correct responses (left column) and choice errors (right column) in the 50–150 ms interval for each group. Numbers below each map indicate the scale used.
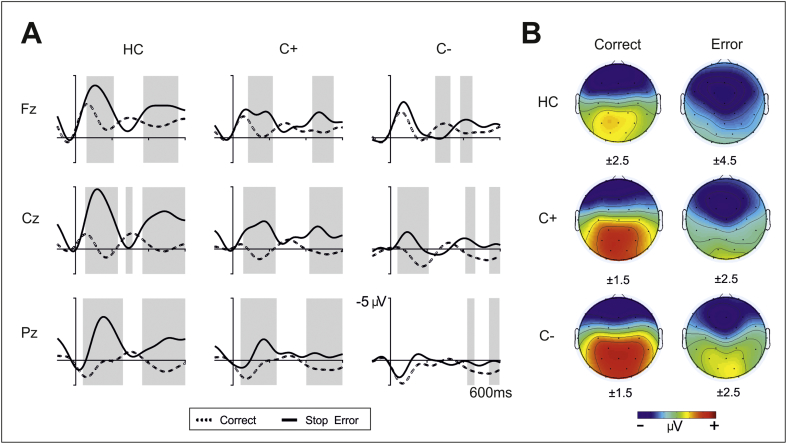
Fig. 2.
A. Grand averages of response-locked event-related potentials in ‘no-go’ trials at midline electrodes (Fz, Cz, Pz) for the three groups (controls, C + group and C– group). Depicted are ERPs for correct trials (dashed lines) and stop errors (thick solid lines). Data were bandpass filtered (bandpass 2–8 Hz). The grey shadows indicate the interval where the difference between conditions is statistically significant. B. Three-dimensional isovoltage topographical maps for correct responses (left column) and stop errors (right column) in the 100–200 ms interval for each group. Numbers below each map indicate the scale used.
2.4.4. ERPs: stimulus-locked data
In order to show that any reported group differences were not due to the general effects of medication, we analyzed the exogenous N1 and P2 components (where we did not expect to find between-group differences). To test this, the amplitude values of compatible trials in a 50 ms time-window centered around the most negative (and positive) peaks between 100 and 150 ms (and 150–200 ms) were entered to a repeated measures ANOVA with the factors ‘group’ and ‘electrode’ (Fz, Cz, Pz).
Finally, to investigate detrimental effects of chemotherapy on ERPs, we investigated the N2 and the P300 in the stimulus-locked averages. After rejection of eye and muscle artifacts, epochs of 100 ms before and 924 ms after the response were extracted from the continuous EEG and baseline-corrected using a − 100–0 ms pre-response window following previously validated methodology (U.M. Kramer et al., 2007). To probe the N2 incompatibility effect for group differences, we entered the mean amplitude value between 180 and 280 ms (100 ms time-window centered around the peak of the grand average N2) into a three-way repeated-measures ANOVA that included the between-subject variable ‘group’ and two within-subject factors: ‘compatibility’ (compatible, incompatible) and ‘electrode’ (Fz, Cz). Moreover, the P300 was defined as the most positive peak between 350 and 450 ms and its amplitude was considered the mean voltage between 350 and 450 ms post stimulus-onset. Latencies of the P300 were entered to a two-way repeated-measures ANOVA on ‘group’ and ‘electrode’ (Cz, Pz), while the amplitude values were included in a three-way repeated-measures ANOVA on ‘group’, ‘compatibility’ (compatible, incompatible) and ‘electrode’ (Cz, Pz).
3. Results
3.1. Patient characteristics
Characteristics of the entire cohort are described in Table 1. There were no significant differences between groups in age, gender, education or grouped vascular risk factors. When analyzed independently, only smoking history showed a significant difference between lung cancer patients and HC (X2 (1, Yates continuity correction) = 11.4, p < 0.001), but no differences were observed between both cancer groups (C + and C −).
Table 1.
Baseline demographics and vascular risk factors of the entire cohort.
| C + (n = 17) | C– (n = 15) | HC (n = 18) | p-Value | |
|---|---|---|---|---|
| Age (years)a | 61.69 ± 7.67 | 57.44 ± 3.43 | 62.12 ± 8.10 | 0.30 |
| Gender (F/M) | 2/15 | 0/15 | 0/18 | 0.13 |
| Education (years)b | 6 (4,17) | 7 (4,14) | 8 (6,19) | 0.30 |
| KPSb | 80 (70,90) | 80 (70,100) | 100 (90,100) | 0.0001 |
| Smokingc | 17 (100) | 15 (100) | 11 (61) | 0.001 |
| Alcoholc | 6 (35) | 5 (33) | 11 (61) | 0.19 |
| Hypertensionc | 5 (29) | 6 (40) | 8 (44) | 0.65 |
| DM type IIc | 5 (29) | 4 (27) | 1 (5) | 0.16 |
| Dyslipidemiac | 5 (29) | 6 (40) | 10 (55.5) | 0.29 |
| Vascular risk factorsc | ||||
| Low-risk (0 or 1) | 8 (47) | 2 (13) | 6 (33) | 0.12 |
| High-risk (≥ 2) | 9 (53) | 13 (87) | 12 (66) |
C +: chemotherapy-treated small-cell lung cancer group; C −: non-chemotherapy treated non-small cell lung cancer group; HC: healthy control group; F: female; M: male; KPS: Karnofsky performance status; IQ: intelligence quotient; DM: diabetes mellitus.
Statistically significant results are marked in bold.
Mean ± SD.
Median (range).
n (%).
3.2. Neuropsychological assessment
Both cancer groups exhibited a higher rate of cognitive impairment (29% of C + and 20% of C −) compared to healthy controls (X2 (1, Yates continuity correction) = 1.78, p = 0.18) (see Table 2). Concerning specific neuropsychological assessment, there were no differences between groups after applying Bonferroni correction. However, uncorrected results showed that lung cancer patients performed significantly worse than healthy controls in several subtests. Specifically C + patients performed worse than healthy controls in WAIS-III Vocabulary, in speed-processing and executive functions (Trail Making Test part B and part B minus part A) as well as in visuospatial abilities (ROCF first copy). C– patients performed worse than HC in working verbal memory (AVLT immediate recall (A1)).
Table 2.
Summary of neuropsychological evaluation.
| C + (n = 17) | C– (n = 15) | HC (n = 18) | p-Value | |
|---|---|---|---|---|
| Cognitive impairmentc | 5 (29%) | 3 (20%) | 1 (6%) | 0.18 |
| Mattis dementia rating scale-2a | 144 (133–144) | 144 (140–144) | 144 (139–144) | 0.91 |
| BDI (≥ 13)c | 5 (29) | 4 (27) | 2 (11) | 0.30 |
| Estimated verbal IQ | ||||
| WAIS-III vocabulary sub-test | 0 ± 1.03⁎ | 0.21 ± 0.73 | 1.05 ± 1.13 | 0.01 |
| Language | ||||
| Brief Spanish adaptation of the Boston naming test | 0.33 ± 0.86 | 0.25 ± 0.59 | 0.56 ± 0.68 | 0.59 |
| Verbal fluency | ||||
| Semantic fluency | 0.43 ± 0.70 | 0.28 ± 0.65 | 0.30 ± 0.76 | 0.67 |
| Phonemic fluency | − 0.33 ± 1.23 | − 0.23 ± 1.12 | 0.5 ± 0.72 | 0.05 |
| Processing speed/executive functions | ||||
| Trail Making test A (TMT-A) | − 0.45 ± 0.87 | − 0.08 ± 0.72 | 0.31 ± 0.97 | 0.06 |
| Trail Making test B (TMT-B) | − 0.83 ± 0.82⁎ | − 0.41 ± 0.99 | − 0.02 ± 0.75 | 0.04 |
| TMT-B minus TMT-A (in seconds)a | 144 ± 96⁎ | 112 ± 118 | 66 ± 45 | 0.02 |
| Attention/working memory | ||||
| WAIS-III digits sub-test | 0.15 ± 1.12 | 0.28 ± 0.57 | 0.65 ± 0.80 | 0.20 |
| Visuospatial abilities | ||||
| ROCF first copy | 0.60 ± 1.05⁎ | 1.23 ± 1.24 | 1.68 ± 1.03 | 0.03 |
| Visual memory | ||||
| ROCF delayed | 0.43 ± 0.80 | 1 ± 0.97 | 0.78 ± 0.73 | 0.26 |
| Verbal memorya | ||||
| AVLT immediate recall (A1) | 4.06 ± 2.01 | 3.43 ± 1.28⁎⁎ | 5.06 ± 1.62 | 0.03 |
| AVLT immediate recall (B) | 4.50 ± 0.85 | 4.86 ± 1.77 | 5 ± 1.37 | 0.60 |
| AVLT short-delay recall(A6) | 5.86 ± 1.61 | 6.71 ± 3.45 | 7.72 ± 3.23 | 0.23 |
| AVLT long-delay recall(A7) | 5.38 ± 2.55 | 6.21 ± 3.11 | 7.39 ± 3.52 | 0.29 |
All results are z-scores mean ± SD except for araw score, bmedian (range), cn (%).
C +: chemotherapy-treated small-cell lung cancer group; C −: non-chemotherapy treated non-small cell lung cancer group; HC: healthy control group; BDI: Beck Depression Inventory test, IQ: intelligence quotient, WAIS-III: Wechsler Adult Intelligence Scale-III, ROCF: Rey-Osterrieth Complex Figure, AVLT: Auditory Verbal Learning Test.
Statistically significant results are marked in bold.
Significant differences were between C + and HC.
Significant differences were between C– and HC.
3.3. Neurophysiological correlates
3.3.1. Behavioral results
Behavioral data for each group is depicted in Table 3. The percentage of correct responses, was significantly different between the groups (F(2,47) = 9.9, p < 0.001). Games-Howell post hoc tests revealed that control subjects (mean ± SD: 84.2 ± 19.3%) responded more accurately than the C + group (61.1 ± 19.3%) and the C– group (56.8 ± 19.3%) (p = 0.03 and p = 0.01 respectively). In line with previously reported results applying the usual Flanker task, a main effect of ‘compatibility’ (F(1,47) = 18.6, p < 0.001) showed that the percentage of errors 13.6 ± 11.0%, was higher for incompatible trials (15.4 ± 12.7%) than for compatible trials (10.9 ± 9.7%). A significant difference across groups was found in the percentage of errors (F(2,47) = 6.1, p = 0.005). Games-Howell post hoc tests revealed a trend towards significance with controls (7.1 ± 9.7%) committing less errors than the C + group (18.3 ± 9.7%) (p = 0.007) and the C– group (14.6 ± 9.7%) (p = 0.04). Furthermore, the percentage of corrected errors did not differ across groups (F(2,47) = 2.6, p = 0.09).
Table 3.
Summary of behavioral and ERP results.
| C + (n = 17) | C– (n = 15) | HC (n = 18) | p-Value | |
|---|---|---|---|---|
| RT (compatible) | 465.7 ± 63.9 | 476.1 ± 84.6 | 467.6 ± 52.5 | 0.89 |
| RT (incompatible) | 490.2 ± 84.8 | 499.8 ± 95.9 | 486.5 ± 51.1 | 0.89 |
| RT difference | 24.5 | 23.7 | 18.9 | |
| Percentage of choice errors | 18 ± 13⁎ | 15 ± 11⁎⁎ | 7 ± 4 | 0.005 |
| RT (choice errors) | 286.0 ± 84.3 | 302.2 ± 75.4 | 323.4 ± 61.5 | 0.33 |
| Percentage of corrected errors | 31 ± 25 | 33 ± 25 | 48 ± 23 | 0.09 |
| Post-error slowing | − 19.3 ± 112.6 | 8.8 ± 86.4 | 66.4 ± 84.0 | 0.11 |
| Percentage of inhibited no-go | 41.5 ± 15.3 | 47.5 ± 13.5 | 48.9 ± 9.3 | 0.20 |
| Post-non-inhibition slowing | 18.3 ± 44.5 | 31.6 ± 62.4 | 38.8 ± 84.6 | 0.69 |
| Stop-signal delay | 121.9 ± 82.4 | 135.2 ± 68.7 | 161.0 ± 51.7 | 0.24 |
| SSRT | 334.1 ± 104.8 | 300.6 ± 51.7 | 292.5 ± 51.9 | 0.24 |
| Percentage artifact rejections | 26.5 ± 23.6 | 22.0 ± 19.4 | 22.4 ± 21.0 | |
| Included choice errors | 123.3 ± 94.7 | 106.2 ± 57.9 | 56.6 ± 31.5 | |
| Included stop errors | 72.9 ± 37.3 | 74.2 ± 32.5 | 77.7 ± 17.7 |
RT: reaction time of correct responses in milliseconds; percentage of choice errors: percentage of error trials of all ‘go’ trials; percentage of corrected errors: number of corrected ‘go’ errors relative to all choice errors; percentage of artifact rejection: average percentage of rejected EEG epochs; included ‘go’ and stop errors: the average number of included error trials after artifact rejection; SSRT: stop-signal reaction time; C +: chemotherapy-treated small-cell lung cancer group; C −: non-chemotherapy treated non-small cell lung cancer group; HC: healthy control group.
Statistically significant results are marked in bold.
Significant differences were between C + and HC.
Significant differences were between C– and HC.
Regarding reaction time measures, errors (392 ± 112 ms) were faster than correct responses (479 ± 60 ms) (F(1,47) = 30.5, p < 0.001) and correct responses were faster in compatible trials (470 ± 66 ms) than in incompatible trials (492 ± 77 ms) (F(1,47) = 7.8; p = 0.007), as expected, with no differences between groups. Lastly, RTs in corrected errors (304 ± 74 ms) were faster than in non-corrected errors (431 ± 193 ms) (F(1,47) = 17.2, p < 0.001) and no differences were found between groups.
Despite remarkably different means in each group, the post error slowing (global mean ± SD: 16 ± 102 ms), post non-inhibited slowing (30 ± 67 ms) and the SSRT (309 ± 76 ms) did not differ between groups, mainly due to their large variability (see Table 3).
3.3.2. Event-related potentials (ERP)
Both choice and stop errors led to a negative component peaking after the error, resembling the well-known ERN component with its characteristic frontal central distribution (Falkenstein et al., 1990, Gehring et al., 1993) while the correct responses yielded a more anterior negative deflection (CRN) localized at Fz (see panel A, Fig. 1, Fig. 2). The choice ERN peaked at ≈ 100 ms after the response, whereas the stop ERN peaked slightly later at ≈ 150 ms after the response (see U.M. Kramer et al. (2007)). Results for both conditions will be reported separately.
3.3.2.1. ERPs: response-locked data
3.3.2.1.1. ‘Go’ trials: choice ERN
For illustrative purposes, ERPs of correct responses, choice errors and the difference waveforms have been plotted for each group at midline locations (Fz, Cz and Pz electrodes) (see Fig. 1A). Likewise, isovoltage topographical maps for choice errors in the 50–150 ms interval are depicted separately for each group (see Fig. 1B).
Differences in the choice CRN and choice ERN between the groups were reflected by a significant main effect of group (F(2,47) = 3.9, p = 0.03) and post hoc Tukey's tests showed that the healthy control and the C– groups differed significantly (p = 0.05), with healthy controls showing a more negative amplitude overall (− 1.8 ± 1.7 μV) than the C– group (0.3 ± 1.2 μV). Likewise, the interaction of ‘electrode’ by ‘response’ (correct vs. error trials) (F(2,47) = 32.8, p < 0.001) indicated that the ERN effect was larger at central regions, where the difference between the ERN and CRN is larger than at frontal sites [mean CRN and ERN values across groups at Fz (CRN: − 1.4 ± 2.1 μV; ERN: − 1.3 ± 2.8 μV) and at Cz (CRN: 0.1 ± 1.4 μV; ERN: − 0.8 ± 2.8 μV)].
The significant interaction of electrode by response by group (F(2,47) = 4.1, p = 0.02) showed that the amplitude of the ERN and CRN components differed depending on the electrode and the group (see difference waveforms in Fig. 4A). This interaction reflects the differential amplitude observed across groups in the negativity associated with erroneous responses (see Fig. 3B). While the ERN component (see difference waveform, Error – Correct) is clearly observed at Cz location in the control group and as expected considering previous studies, the amplitude is drastically diminished in the other two groups (C + and C −).
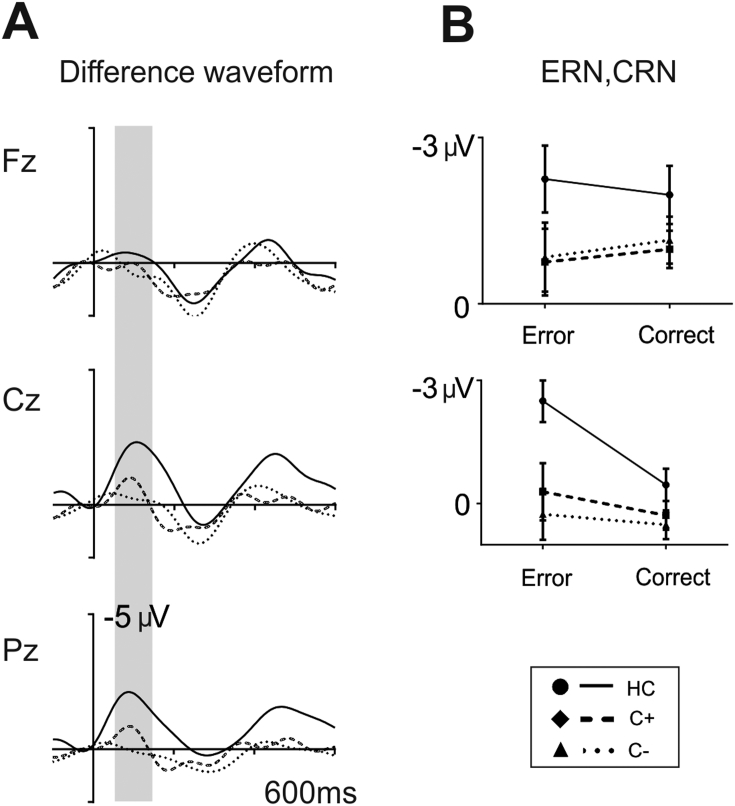
Fig. 4.
A. Grand averages of response-locked difference waveforms in ‘go’ trials (stop errors – correct trials) at midline electrodes (Fz, Cz, Pz) for each group (controls: solid line, C + group: dashed line, and C– group: dotted line). The grey shadows indicate the time interval used to define the ERN and CRN amplitudes (100–200 ms). B. Box plot illustrating the amplitude in correct trials and stop errors in the 100–200 ms interval for each group.
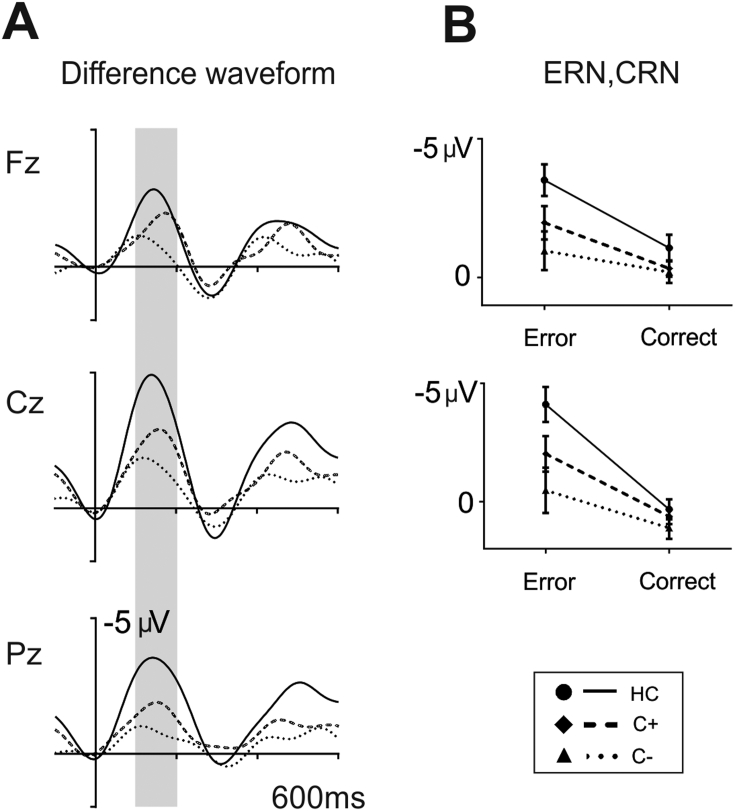
Fig. 3.
A. Grand averages of response-locked difference waveforms in ‘go’ trials (choice errors – correct trials) at midline electrodes (Fz, Cz, Pz) for each group (Controls: solid line, C + group: dashed line, and C– group: dotted line). The grey shadows indicate the time interval used to define the ERN and CRN amplitudes (50–150 ms). B. Box plot illustrating the amplitude in correct trials and choice errors in the 50–150 ms interval for each group.
To further analyze this interaction we conducted independent sample t-tests comparing the difference ERN (ERN – CRN) at Cz between each pair of groups. Healthy controls were found to show a greater difference between errors and correct trials compared to the C– group (t(31) = − 2.9, p = 0.007) (HC: − 2.0 ± 1.7 μV; C −: − 0.3 ± 1.7 μV). The remaining main effects and interaction did not reach significance.
3.3.2.1.2. ‘No-go’ trials: stop ERN
For the sake of illustration, ERPs of correct responses, stop errors and the difference waveforms have been plotted for each group and Fz, Cz and Pz electrodes separately (see Fig. 2A). Furthermore, isovoltage topographical maps for stop errors in the 100–200 ms interval have been plotted for each group (see Fig. 2B).
In line with the results obtained for the choice errors, differences in the CRN and stop ERN between the groups were reflected by a significant main effect of group (F(2,47) = 4.6, p = 0.02) and post hoc Tukey's tests showed that controls and the C– group differed significantly at p = 0.01 with controls showing a more negative amplitude overall (− 2.1 ± 3.1 μV) than the C– group (− 0.1 ± 3.4 μV). Moreover, the main effect of ‘response’ (F(1,47) = 40.0, p < 0.001) showed a clear negativity in erroneous stop trials when compared to correct trials (ERN: − 2.2 ± 2.8 μV; CRN: 0.08 ± 1.4 μV). The interaction of ‘electrode’ by ‘response’ (F(2,47) = 42.5, p < 0.001) showed the different scalp distribution of the ERN and CRN (larger negativity for the CRN in frontal regions; similar values for the ERN in frontal and central locations) [mean values of CRN (Fz: − 0.5 ± 1.4 μV; Cz: 0.7 ± 1.4 μV) and ERN (Fz: − 2.1 ± 2.9 μV; Cz: − 2.2 ± 3.2 μV)]. As in the case of choice errors, the interaction of ‘response’ by group (F(2,47) = 3.4, p = 0.04) and ‘electrode’ by ‘response’ by group (F(2,47) = 3.4, p = 0.04) showed that the amplitude of the difference ERN (error vs. correct trials) was larger for the control group mainly at Cz when compared to both patient groups (see difference waveforms in Fig. 4A/B). Analogous to the analysis performed in choice errors, to further analyze the interaction we conducted independent sample t-tests comparing the difference ERN (ERN – CRN) at Cz between each pair of groups. As in choice errors, healthy controls were found to show a greater difference between errors and correct trials compared to the C– group (t(31) = − 3.1, p = 0.005) (HC: − 4.5 ± 2.7 μV; C −: − 1.6 ± 2.6 μV). Neither the interaction of ‘electrode’ by group nor the remaining effects did reach significance.
3.3.2.2. ERPs: stimulus-locked data
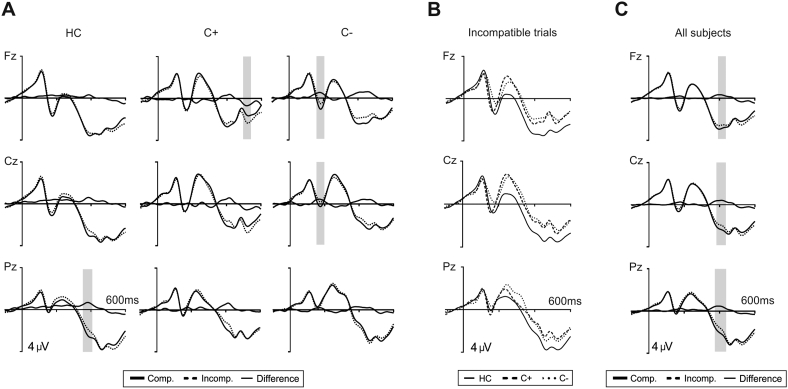
Stimulus-locked averages are depicted in Fig. 5. Visual inspection did not show large amplitude differences between groups except for enlarged amplitude in the N2 component for the C + and C– patient groups (Fig. 5A/B).
Fig. 5.
A. Grand averages of stimulus-locked event-related potentials at midline electrodes (Fz, Cz, Pz) for the three groups (controls, C + group and C– group). Depicted are ERPs for compatible trials (thick solid lines), incompatible trials (dashed lines) and the difference waveforms (thin solid lines). Data were lowpass filtered (30 Hz). The grey shadows indicate the interval where the difference between conditions is statistically significant. B. Grand averages of stimulus-locked event-related potentials in incompatible trials at midline electrodes (Fz, Cz, Pz) for each group (controls: solid line, C + group: dashed line, and C– group: dotted line). C. Grand averages of stimulus-locked event-related potentials at midline electrodes (Fz, Cz, Pz) pooling together the three groups. Depicted are ERPs for compatible trials (thick solid lines), incompatible trials (dashed lines) and the difference waveforms (thin solid lines). The grey shadows indicate the interval where the difference between conditions is statistically significant.
N1/P2: As expected, no group differences were found in the amplitude of the N1 (F(2,47) = 0.2, p = 0.9) (HC: − 1.7 ± 2.6 μV; C +: − 1.7 ± 2.7 μV; C −: − 1.8 ± 2.9 μV) or in the P2 component (F(2,47) = 0.3, p = 0.7) (HC: 0.7 ± 4.0 μV; C +: 0.5 ± 4.2 μV; C −: 0.0 ± 4.4 μV). The interaction terms did not reached significance either (see Fig. 1).
N2: In contrast to the apparent enhancement of the N2 for the C + and C– patient groups (Fig. 5B), and probably due to the large variability observed, no differences between groups were found in the amplitude of this component (F(2,47) = 1.3, p = 0.3) (HC: − 0.1 ± 5.0 μV; C +: − 1.7 ± 5.2 μV; C −: − 1.2 ± 5.5 μV) and no other effects emerged.
P300: Regarding the peak latency of the P300 component, no differences between groups were found (F(2,47) = 0.4, p = 0.6) nor the remaining effects reached significance. The ANOVA on the P300 amplitude revealed a main effect of compatibility (F(2,47) = 6.5, p = 0.01) denoting a larger P300 for compatible (2.1 ± 2.7 μV) than for incompatible stimuli (1.7 ± 2.5 μV). No group differences were found (F(2,47) = 1.1, p = 0.4) and no other effects were significant.
Overall, stimulus-locked ERP components did not differ across groups, ruling out the possibility that the effects above observed in response-locked averages could have been affected by overall amplitude differences across groups.
4. Discussion
Cognitive control can be tested by using paradigms exploring error monitoring and error compensation mechanisms. The ERN has been previously described as a neurophysiological marker of error detection, showing alteration in aging and several neurodegenerative diseases. The current investigation is the first exploring the ERN component in a lung-cancer population. Our main goal was to study differences in performance monitoring in a small-cell lung cancer group (SCLC, C +) after chemotherapy, a non-small cell lung cancer patient group (NSCLC, C −) prior to chemotherapy and a healthy control group (HC). We found that both lung cancer patients, and specially the C– group, exhibited lower overall accuracy as well as a reduced difference between both choice and stop CRN and ERN, indicating potential error monitoring deficits in lung cancer patients both prior and following chemotherapy.
ERN is considered to be an electrophysiological marker of error detection originated in the medial prefrontal cortex, most probably the anterior cingulate cortex (ACC) and supplementary motor regions (Holroyd and Coles, 2002, Falkenstein et al., 1991). Further contributions from fronto-striatal circuits in the elicitation of error-related signals have been confirmed using functional MRI (Marco-Pallares et al., 2009, Ullsperger and von Cramon, 2001) and lesion data (Gehring and Knight, 2000, Turken and Swick, 2008, Ullsperger et al., 2002, Ullsperger and von Cramon, 2006, Stemmer et al., 2004). Recently, error-related activity has been associated not only to the widely recognized ACC but also to frontal and parietal cortex as well as subcortical and cerebellar regions (see for review, Neta et al. (2015)). A second but smaller ERN-like medial-frontal negativity related to response monitoring can also be elicited after correct responses (CRN) (Ford et al., 1999, Falkenstein et al., 2000). In contrast to the ERN, and being less studied, the CRN presumably plays a more general role in performance monitoring, maybe associated to the amount of uncertainty in response monitoring and the initiation of future performance adaptation or implementation of remedial actions (Falkenstein et al., 2000, Bartholow et al., 2005, Sebastian-Galles et al., 2006).
Both performance monitoring and adaptation effects have been shown to be more difficult with aging (Band and Kok, 2000, Eppinger et al., 2007). Several studies reported reduced ERN amplitudes in healthy older individuals compared to younger adults (Band and Kok, 2000, Falkenstein et al., 2001, Hoffmann and Falkenstein, 2011, Schreiber et al., 2011) and also in patients with neurodegenerative diseases (Mathalon et al., 2003, Ito and Kitagawa, 2005, Pietto et al., 2016). In fact, it has been proposed that the larger the difference between the ERN and CRN components, the better the performance in cognitive testing (Thurm et al., 2013). In this sense, increases in the amplitude of the CRN in older adults could be observed in several studies (Schreiber et al., 2011, Thurm et al., 2013, Endrass et al., 2012, Eppinger et al., 2008), suggesting an impairment in the flexible adaptation to conflict processing related to aging (Eppinger et al., 2008). Furthermore, Gehring and Knight (2000) observed equal amplitude for the ERN and CRN in patients with focal lateral prefrontal cortex lesions, resulting also in no difference between error and correct trials components. Lateral prefrontal damage has also been associated to reduced ERNs (Ullsperger et al., 2002, Ullsperger and von Cramon, 2006, Kopp et al., 2014) as well as lesions in the thalamus (Seifert et al., 2011), frontal white matter (Hogan et al., 2006), and basal ganglia (Ullsperger and von Cramon, 2006). More recently, some studies focused in aging and mild cognitive impairment described a persevered difference between ERN and CRN in older adults while it was diminished in cognitive impaired patients (Thurm et al., 2013).
According to the previous literature, in the present study we observed that both lung cancer patients, and specially the C– group, exhibited a reduced difference between both choice and stop CRN and ERN. While CRN remained stable through all groups, the ERN was drastically diminished in both lung cancer groups. Since no differences were observed across groups in stimulus-locked ERP components, this result could not be due to overall reduction in ERP amplitude in patients due to their illness or medication (see Fig. 5). Additionally, the CRN was larger in frontal regions while the ERN showed similar values for frontal and central locations. Previous studies in lung cancer population, also pre- and post-chemotherapy, described a functionally impairment of the default mode network as well as functional and structural changes in temporal and cerebellar networks (Simo et al., 2015, Simo et al., 2017). This findings thus evidence that lung cancer patients prior to chemotherapy exhibit cognitive deficits associated with structural and functional changes in subcortical diffuse brain regions while, following chemotherapy, patients show grey and white matter structural damage more focused in temporal regions bilaterally (Simo et al., 2015, Simo et al., 2017).
Our study highlights potential deficits in performance monitoring (executive functioning) of lung cancer patients prior and following chemotherapy. In terms of behavioral performance, lung cancer patients showed lower overall accuracy when compared to the control group. Notice however, that reaction times were very similar across groups (see Table 3) facilitating the comparison of the ERP responses across groups, but suggesting that the patient groups may be performing in a different speed/accuracy trade off. Thus, it could be the case that reducing response speed in the patient groups might have diminished the amount of errors performed and the differences observed when compared to the control group. Taken together, these findings suggest potential error monitoring deficits in lung cancer patients both prior and following chemotherapy, compared to an age-matched HC group.
One possible explanation for the diminished amplitude of the ERN in lung cancer patients, as described in the aging literature (Nieuwenhuis et al., 2002), is the potential degradation of error monitoring processes associated to impoverished executive control (J.H. Kramer et al., 2007). Lung cancer patients showed clear differences in accuracy at the behavioral level (but being equally fast as HC), suggesting that the diminished amplitude in the ERN component could be related to a reduced accuracy in error monitoring processes. An alternative explanation might be that, in contrast to the elderly, lung cancer patients are not able to compensate their reduced cognitive control capacity by recruiting redundant cognitive control regions, and thus their accuracy is reduced. However, it remains to be observed if patients, performing in a slower pace and reducing the amount of errors, could induced an ERN component of equal amplitude as the one observed in the control group. The strongest emphasis of the patient group in speed vs. accuracy might have induced larger uncertainty and noise in decision-related processes, thus impacting on patients' overall performance monitoring abilities. This could explain also the lack of differences (equal amplitude due to reduced ERN amplitude) observed between the ERN and CRN in the patient groups as they might be performing in a more stringent speed/accuracy trade-off regime. Important to this idea is the fact that no behavioral differences were observed in other reliable measures of cognitive control included in the present study. For example, no group differences were observed for the reaction time between incompatible and compatible trials, post-error slowing related processes or motor inhibition (by means of the percentage of inhibited trials and the speed of motor inhibition - SSRT) (Marco-Pallares et al., 2008, Kramer et al., 2007, Barch et al., 2009). Thus, the present findings speak in favor of certain selectivity in the deficits observed in performance monitoring that do not extent to all domains of cognitive control processes. Further research is needed in this direction to understand the sources of the differences observed here in performance monitoring in cancer patients.
Although we specially focused in the ERN because its relevance in error detection and conflict monitoring, the frontocentral N2 component also reflects the activation of the ACC during correct conflict trials and prior to the response (Van Veen and Carter, 2002a). In fact, both component have similar scalp distributions, strongly suggesting that these two components could share similar neural sources (Van Veen and Carter, 2002a, Gehring et al., 2012). There is also considerable evidence showing that amplitude of the ERN is sensitive to the degree of response conflict and consistent with its role in conflict monitoring (Larson et al., 2014). Additionally, the P300 component has been associated to cognitive and executive functions including attention and context updating in tasks that require stimulus evaluation and memory updating (Ferdinand et al., 2015).
In our study no differences emerged between groups concerning the frontocentral N2 and peak latency of the P300 components. Although both lung cancer groups exhibited an apparent enhancement of the N2 amplitude and a diminished P300 in comparison to the healthy control group, these differences did not reach a statistically significant threshold. The lack of significant differences between groups in the N2 and the P300, might be explained by the fact that the present task (the Eriksen flanker task) is highly demanding, very fast and a manual response is always required. The inherent difficulty of the task might explain the differences to other studies in which a diminished P300 amplitude has been observed in much less demanding tasks (e.g., oddball task) (Kreukels et al., 2005, Kreukels et al., 2006, Kreukels et al., 2008a, Kreukels et al., 2008b, Heukrodt et al., 1988, Kam et al., 2016). Increased N2 amplitudes in the patient groups could have been associated to an increased conflict monitoring which might converge with a diminished amplitude in the P300 (amplitude reductions are observed in more resource-demanding tasks). An alternative hypothesis is the large variability observed in all groups which prevented to observe these effects at the statistical level.
Regarding cognitive deficits, around 20–30% of patients in both lung cancer groups met criteria for cognitive impairment; however, the neuropsychological profile was quite different. The C– group performed worse than HC and C + in long-term verbal memory, as has been previously described in cancer patients before the initiation of therapy (Shilling et al., 2005, Wefel et al., 2004). Following treatment, and in line with previous literature (Jim et al., 2012), the C + group performed worse than the HC group in visuospatial measures and verbal phonemic fluency. Although cognitive changes associated with either cancer or cancer treatment have been extensively recognized, the pathogenesis of these neurocognitive changes remains unclear. In this setting, several hypotheses have been proposed including the biology of cancer, as well as common risk factors for the development of both cancer and mild cognitive changes in normal aging (Ahles and Saykin, 2007).
Our study presents some limitations that should be taken into account. The cross-sectional design of the study may have limited the possibility to clearly isolate the effect of chemotherapy from more general cancer-related changes. However, our results suggest that the cancer by itself may be associated with cognitive and functional deficits. Supporting these findings, several studies have hypothesized that the biology of cancer, the inflammatory response triggering neurotoxic cytokines or common risk factors for the development of both cancer and mild cognitive changes may be responsible for the cognitive decline found in these patients (Ahles and Saykin, 2007).
To conclude, lung cancer patients prior to chemotherapy (C −, cancer-related effect) and 1-month following chemotherapy (chemo-induced) exhibited an abnormal pattern of performance monitoring, suggesting that both cancer and chemotherapy have an impact on cognitive functioning of lung cancer patients, being ERN an objective laboratory tool sensitive to cognitive dysfunction in this population.
Acknowledgments
Acknowledgment
This work was supported by la Fundació Marató-TV3 [Acquired Spinal Cord and Brain Injuries Program (2012–2015), 111710, awarded to ARF], Spanish Government Grant (Ministerio de Economía y Competitividad), PSI2015-69178-P, MINECO/FEDER, awarded to ARF, Catalan Government (Awarded Advanced Catalan Research Group, 2014SGR1413, to ARF) and Hospital of Bellvitge Research Award 2016, awarded to MS. MS is a recipient of a Juan Rodés contract from the Carlos III National Health Institute (Spanish Government)-European Social Fund (ESF). AGA is a recipient of a predoctoral grant (MINECO-FPI program) from the Spanish government. The authors would like to kindly thank all the patients and their families, as well as the healthy volunteers for their engagement in the study.
Conflicts of interest
All the signing authors have seen and approved the manuscript content and there is no conflict of interest.
References
- Ahles T.A., Saykin A.J. Breast cancer chemotherapy-related cognitive dysfunction. Clin. Breast Cancer. 2002;3(Suppl. 3):S84–90. doi: 10.3816/cbc.2002.s.018. [DOI] [PubMed] [Google Scholar]
- Ahles T.A., Saykin A.J. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat. Rev. Cancer. 2007;7:192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles T.A. Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2002;20:485–493. doi: 10.1200/JCO.2002.20.2.485. [DOI] [PubMed] [Google Scholar]
- Band G.P., Kok A. Age effects on response monitoring in a mental-rotation task. Biol. Psychol. 2000;51:201–221. doi: 10.1016/s0301-0511(99)00038-1. [DOI] [PubMed] [Google Scholar]
- Band G.P., van Boxtel G.J. Inhibitory motor control in stop paradigms: review and reinterpretation of neural mechanisms. Acta Psychol. 1999;101:179–211. doi: 10.1016/s0001-6918(99)00005-0. [DOI] [PubMed] [Google Scholar]
- Band G.P., Ridderinkhof K.R., van der Molen M.W. Speed-accuracy modulation in case of conflict: the roles of activation and inhibition. Psychol. Res. 2003;67:266–279. doi: 10.1007/s00426-002-0127-0. [DOI] [PubMed] [Google Scholar]
- Barch D.M., Braver T.S., Carter C.S., Poldrack R.A., Robbins T.W. CNTRICS final task selection: executive control. Schizophr. Bull. 2009;35:115–135. doi: 10.1093/schbul/sbn154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholow B.D. Strategic control and medial frontal negativity: beyond errors and response conflict. Psychophysiology. 2005;42:33–42. doi: 10.1111/j.1469-8986.2005.00258.x. [DOI] [PubMed] [Google Scholar]
- Burgess P.W., Veitch E., de Lacy Costello A., Shallice T. The cognitive and neuroanatomical correlates of multitasking. Neuropsychologia. 2000;38:848–863. doi: 10.1016/s0028-3932(99)00134-7. [DOI] [PubMed] [Google Scholar]
- Correa D.D. A prospective evaluation of changes in brain structure and cognitive functions in adult stem cell transplant recipients. Brain Imaging Behav. 2013 doi: 10.1007/s11682-013-9221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunillera T., Gomila A., Rodriguez-Fornells A. Beneficial effects of word final stress in segmenting a new language: evidence from ERPs. BMC Neurosci. 2008;9:23. doi: 10.1186/1471-2202-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio A.R. On some functions of the human prefrontal cortex. Ann. N. Y. Acad. Sci. 1995;769:241–251. doi: 10.1111/j.1749-6632.1995.tb38142.x. [DOI] [PubMed] [Google Scholar]
- Delorme A., Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Endrass T., Schreiber M., Kathmann N. Speeding up older adults: age-effects on error processing in speed and accuracy conditions. Biol. Psychol. 2012;89:426–432. doi: 10.1016/j.biopsycho.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Eppinger B., Kray J., Mecklinger A., John O. Age differences in task switching and response monitoring: evidence from ERPs. Biol. Psychol. 2007;75:52–67. doi: 10.1016/j.biopsycho.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Eppinger B., Kray J., Mock B., Mecklinger A. Better or worse than expected? Aging, learning, and the ERN. Neuropsychologia. 2008;46:521–539. doi: 10.1016/j.neuropsychologia.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Eriksen B., Eriksen C. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept. Psychophys. 1974;16:143–149. [Google Scholar]
- Falkenstein M., Hohnsbein, Hoormann J. In: Psychophysiological Brain Mearch. Brunia A.W.K. Gaillard C.H.M., Kok A., editors. vol. 1. Tilburg University Press; 1990. pp. 192–195. [Google Scholar]
- Falkenstein M., Hohnsbein J., Hoormann J., Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalogr. Clin. Neurophysiol. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Falkenstein M., Hoormann J., Christ S., Hohnsbein J. ERP components on reaction errors and their functional significance: a tutorial. Biol. Psychol. 2000;51:87–107. doi: 10.1016/s0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- Falkenstein M., Hoormann J., Hohnsbein J. Changes of error-related ERPs with age. Exp. Brain Res. 2001;138:258–262. doi: 10.1007/s002210100712. [DOI] [PubMed] [Google Scholar]
- Ferdinand N.K., Mecklinger A., Opitz B. Learning context modulates the processing of expectancy violations. Brain Res. 2015;1629:72–84. doi: 10.1016/j.brainres.2015.10.017. [DOI] [PubMed] [Google Scholar]
- Ford J.M. P300 amplitude is related to clinical state in severely and moderately ill patients with schizophrenia. Biol. Psychiatry. 1999;46:94–101. doi: 10.1016/s0006-3223(98)00290-x. [DOI] [PubMed] [Google Scholar]
- Gehring W.J., Knight R.T. Prefrontal-cingulate interactions in action monitoring. Nat. Neurosci. 2000;3:516–520. doi: 10.1038/74899. [DOI] [PubMed] [Google Scholar]
- Gehring W.J., Gratton G., Coles M.G.H., Donchin E. Probability effects on stimulus evaluation and response processes. J. Exp. Psychol. Hum. Percept. Perform. 1992;18:198–216. doi: 10.1037/0096-1523.18.1.198. [DOI] [PubMed] [Google Scholar]
- Gehring W., Goss B., Coles M.G., Meyer D.E., Donchin E. A neural system for error detection and compensation. Psychol. Sci. 1993;4:385–390. [Google Scholar]
- Gehring W.J., L. Y., Orr J.M., Carp J. In: Oxford Handbook of Event-related Potential Components. Kappenman E., Luck S.J., editors. Oxford University Press; 2012. pp. 231–291. [Google Scholar]
- Grosshans D.R., Meyers C.A., Allen P.K., Davenport S.D., Komaki R. Neurocognitive function in patients with small cell lung cancer: effect of prophylactic cranial irradiation. Cancer. 2008;112:589–595. doi: 10.1002/cncr.23222. [DOI] [PubMed] [Google Scholar]
- Gurtubay-Antolin A., Rodriguez-Fornells A. Neurophysiological evidence for enhanced tactile acuity in early blindness in some but not all haptic tasks. NeuroImage. 2017;162:23–31. doi: 10.1016/j.neuroimage.2017.08.054. [DOI] [PubMed] [Google Scholar]
- Gurtubay-Antolin A., Rodriguez-Herreros B., Rodriguez-Fornells A. The speed of object recognition from a haptic glance: event-related potential evidence. J. Neurophysiol. 2015;113:3069–3075. doi: 10.1152/jn.00836.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heukrodt C. Electrophysiological signs of neurocognitive deficits in long-term leukemia survivors. J. Pediatr. Psychol. 1988;13:223–236. doi: 10.1093/jpepsy/13.2.223. [DOI] [PubMed] [Google Scholar]
- Hoffmann S., Falkenstein M. Aging and error processing: age related increase in the variability of the error-negativity is not accompanied by increase in response variability. PLoS One. 2011;6 doi: 10.1371/journal.pone.0017482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan A.M., Vargha-Khadem F., Saunders D.E., Kirkham F.J., Baldeweg T. Impact of frontal white matter lesions on performance monitoring: ERP evidence for cortical disconnection. Brain J. Neurol. 2006;129:2177–2188. doi: 10.1093/brain/awl160. [DOI] [PubMed] [Google Scholar]
- Holroyd C.B., Coles M.G. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol. Rev. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Horky L.L., G. V., Zaitsev A., Plesniak W., Hainer J., Govindarajulu U., Kikinis R., Dietrich J. Systemic chemotherapy decreases brain glucose metabolism. Ann. Clin. Transl. Neurol. 2014 doi: 10.1002/acn3.121. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J., Kitagawa J. Error processing in patients with Alzheimer's disease. Pathophysiology. 2005;12:97–101. doi: 10.1016/j.pathophys.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Jim H.S. Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012;30:3578–3587. doi: 10.1200/JCO.2011.39.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaasa S., Olsnes B.T., Mastekaasa A. Neuropsychological evaluation of patients with inoperable non-small cell lung cancer treated with combination chemotherapy or radiotherapy. Acta Oncol. 1988;27:241–246. doi: 10.3109/02841868809093532. [DOI] [PubMed] [Google Scholar]
- Kaasa S., Olsnes B.T., Thorud E., Host H. Reduced short-term neuropsychological performance in patients with nonsmall-cell lung cancer treated with cisplatin and etoposide. Antibiot. Chemother. 1988;41:226–231. doi: 10.1159/000416209. [DOI] [PubMed] [Google Scholar]
- Kaiser J., Dietrich J. Challenges in research on the neural basis of “chemobrain”. J. Translat. Neurosci. 2014;5:222. [Google Scholar]
- Kam J.W. Sustained attention abnormalities in breast cancer survivors with cognitive deficits post chemotherapy: an electrophysiological study. Clin. Neurophysiol. 2016;127:369–378. doi: 10.1016/j.clinph.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Kok A. Event-related-potential (ERP) reflections of mental resources: a review and synthesis. Biol. Psychol. 1997;45:19–56. doi: 10.1016/s0301-0511(96)05221-0. [DOI] [PubMed] [Google Scholar]
- Komaki R. Evaluation of cognitive function in patients with limited small cell lung cancer prior to and shortly following prophylactic cranial irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1995;33:179–182. doi: 10.1016/0360-3016(95)00026-U. [DOI] [PubMed] [Google Scholar]
- Kopp B., Rist F., Mattler U. N200 in the flanker task as a neurobehavioral tool for investigating executive control. Psychophysiology. 1996;33:282–294. doi: 10.1111/j.1469-8986.1996.tb00425.x. [DOI] [PubMed] [Google Scholar]
- Kopp B., Lange F., Howe J., Wessel K. Age-related changes in neural recruitment for cognitive control. Brain Cogn. 2014;85:209–219. doi: 10.1016/j.bandc.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Kramer U.M. The impact of catechol-O-methyltransferase and dopamine D4 receptor genotypes on neurophysiological markers of performance monitoring. J. Neurosci. Off. J. Soc. Neurosci. 2007;27:14190–14198. doi: 10.1523/JNEUROSCI.4229-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer J.H. Longitudinal MRI and cognitive change in healthy elderly. Neuropsychology. 2007;21:412–418. doi: 10.1037/0894-4105.21.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreukels B.P. Electrophysiological correlates of information processing in breast-cancer patients treated with adjuvant chemotherapy. Breast Cancer Res. Treat. 2005;94:53–61. doi: 10.1007/s10549-005-7093-3. [DOI] [PubMed] [Google Scholar]
- Kreukels B.P. Effects of high-dose and conventional-dose adjuvant chemotherapy on long-term cognitive sequelae in patients with breast cancer: an electrophysiologic study. Clin. Breast Cancer. 2006;7:67–78. doi: 10.3816/CBC.2006.n.015. [DOI] [PubMed] [Google Scholar]
- Kreukels B.P. ERP amplitude and latency in breast cancer survivors treated with adjuvant chemotherapy. Clin. Neurophysiol. 2008;119:533–541. doi: 10.1016/j.clinph.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Kreukels B.P., van Dam F.S., Ridderinkhof K.R., Boogerd W., Schagen S.B. Persistent neurocognitive problems after adjuvant chemotherapy for breast cancer. Clin. Breast Cancer. 2008;8:80–87. doi: 10.3816/CBC.2008.n.006. [DOI] [PubMed] [Google Scholar]
- Larson M.J., Clayson P.E., Clawson A. Making sense of all the conflict: a theoretical review and critique of conflict-related ERPs. Int. J. Psychophysiol. 2014;93:283–297. doi: 10.1016/j.ijpsycho.2014.06.007. [DOI] [PubMed] [Google Scholar]
- Luck S., editor. An Introduction to the Event-related Potential Technique. The MIT Press; 2005. pp. 1–50. [Google Scholar]
- Marco-Pallares J., Camara E., Munte T.F., Rodriguez-Fornells A. Neural mechanisms underlying adaptive actions after slips. J. Cogn. Neurosci. 2008;20:1595–1610. doi: 10.1162/jocn.2008.20117. [DOI] [PubMed] [Google Scholar]
- Marco-Pallares J., Camara E., Münte T.F., Rodriguez-Fornells A. In: Handbook of Quantitative Methods in Psychology. Millsap R.E., Maydeu-Olivares A., editors. SAGE; 2009. [Google Scholar]
- Mathalon D.H. Response-monitoring dysfunction in aging and Alzheimer's disease: an event-related potential study. Neurobiol. Aging. 2003;24:675–685. doi: 10.1016/s0197-4580(02)00154-9. [DOI] [PubMed] [Google Scholar]
- Mattis S. Psychological Assessment Resources. 1988. Dementia rating scale: professional manual. [Google Scholar]
- Münte T.F., Urbach T.P., Düzel E., Kutas M. In: Handbook of Neuropsychology. Boller F., Grafmann J., Rizolatti G., editors. Elsevier Science; 2001. pp. 139–235. [Google Scholar]
- Neta M. Spatial and temporal characteristics of error-related activity in the human brain. J. Neurosci. Off. J. Soc. Neurosci. 2015;35:253–266. doi: 10.1523/JNEUROSCI.1313-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S. A computational account of altered error processing in older age: dopamine and the error-related negativity. Cogn. Affect. Behav. Neurosci. 2002;2:19–36. doi: 10.3758/cabn.2.1.19. [DOI] [PubMed] [Google Scholar]
- Pietto M. Behavioral and electrophysiological correlates of memory binding deficits in patients at different risk levels for Alzheimer's disease. J. Alzheimers Dis. 2016;53:1325–1340. doi: 10.3233/JAD-160056. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof K.R., Ullsperger M., Crone E.A., Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Fornells A., Kurzbuch A.R., Munte T.F. Time course of error detection and correction in humans: neurophysiological evidence. J. Neurosci. Off. J. Soc. Neurosci. 2002;22:9990–9996. doi: 10.1523/JNEUROSCI.22-22-09990.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber M., Pietschmann M., Kathmann N., Endrass T. ERP correlates of performance monitoring in elderly. Brain Cogn. 2011;76:131–139. doi: 10.1016/j.bandc.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Sebastian-Galles N., Rodriguez-Fornells A., de Diego-Balaguer R., Diaz B. First- and second-language phonological representations in the mental lexicon. J. Cogn. Neurosci. 2006;18:1277–1291. doi: 10.1162/jocn.2006.18.8.1277. [DOI] [PubMed] [Google Scholar]
- Seifert S., von Cramon D.Y., Imperati D., Tittgemeyer M., Ullsperger M. Thalamocingulate interactions in performance monitoring. J. Neurosci. Off. J. Soc. Neurosci. 2011;31:3375–3383. doi: 10.1523/JNEUROSCI.6242-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilling V., Jenkins V., Morris R., Deutsch G., Bloomfield D. The effects of adjuvant chemotherapy on cognition in women with breast cancer—preliminary results of an observational longitudinal study. Breast. 2005;14:142–150. doi: 10.1016/j.breast.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Simo M. Cognitive and brain structural changes in a lung cancer population. J. Thorac. Oncol. 2015;10:38–45. doi: 10.1097/JTO.0000000000000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simo M. Longitudinal brain changes associated with prophylactic cranial irradiation in lung cancer. J. Thorac. Oncol. 2016 doi: 10.1016/j.jtho.2015.12.110. [DOI] [PubMed] [Google Scholar]
- Simo M. Brain functional connectivity in lung cancer population: an exploratory study. Brain Imaging Behav. 2017 doi: 10.1007/s11682-017-9697-8. [DOI] [PubMed] [Google Scholar]
- Stemmer B., Segalowitz S.J., Witzke W., Schonle P.W. Error detection in patients with lesions to the medial prefrontal cortex: an ERP study. Neuropsychologia. 2004;42:118–130. doi: 10.1016/s0028-3932(03)00121-0. [DOI] [PubMed] [Google Scholar]
- Stuss D.T., Shallice T., Alexander M.P., Picton T.W. A multidisciplinary approach to anterior attentional functions. Ann. N. Y. Acad. Sci. 1995;769:191–211. doi: 10.1111/j.1749-6632.1995.tb38140.x. [DOI] [PubMed] [Google Scholar]
- Thurm F. Effects of aging and mild cognitive impairment on electrophysiological correlates of performance monitoring. J. Alzheimers Dis. 2013;35:575–587. doi: 10.3233/JAD-121348. [DOI] [PubMed] [Google Scholar]
- Turken A.U., Swick D. The effect of orbitofrontal lesions on the error-related negativity. Neurosci. Lett. 2008;441:7–10. doi: 10.1016/j.neulet.2008.05.115. [DOI] [PubMed] [Google Scholar]
- Ullsperger M., von Cramon D.Y. Subprocesses of performance monitoring: a dissociation of error processing and response competition revealed by event-related fMRI and ERPs. NeuroImage. 2001;14:1387–1401. doi: 10.1006/nimg.2001.0935. [DOI] [PubMed] [Google Scholar]
- Ullsperger M., von Cramon D.Y. The role of intact frontostriatal circuits in error processing. J. Cogn. Neurosci. 2006;18:651–664. doi: 10.1162/jocn.2006.18.4.651. [DOI] [PubMed] [Google Scholar]
- Ullsperger M., von Cramon D.Y., Muller N.G. Interactions of focal cortical lesions with error processing: evidence from event-related brain potentials. Neuropsychology. 2002;16:548–561. doi: 10.1037//0894-4105.16.4.548. [DOI] [PubMed] [Google Scholar]
- Van Veen V., Carter C.S. The timing of action-monitoring processes in the anterior cingulate cortex. J. Cogn. Neurosci. 2002;14:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- van Veen V., Carter C.S. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol. Behav. 2002;77:477–482. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- Wefel J.S., Lenzi R., Theriault R.L., Davis R.N., Meyers C.A. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: results of a prospective, randomized, longitudinal trial. Cancer. 2004;100:2292–2299. doi: 10.1002/cncr.20272. [DOI] [PubMed] [Google Scholar]
- Welzel T. Diffusion tensor imaging screening of radiation-induced changes in the white matter after prophylactic cranial irradiation of patients with small cell lung cancer: first results of a prospective study. AJNR Am. J. Neuroradiol. 2008;29:379–383. doi: 10.3174/ajnr.A0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung N., Botvinick M.M., Cohen J.D. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychol. Rev. 2004;111:931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]