Abstract
Exosomes are extracellular membrane vesicles of 50- to 130-nm diameter secreted by most tumor cells. Exosomes can mediate the intercellular transfer of proteins and RNAs, including microRNAs (miRNAs), and promote both tumorigenesis and premetastatic niche formation. In this study, we performed exosomal RNA sequencing to identify candidate exosomal miRNAs that could be associated with colorectal cancer (CRC) and its distant metastasis. The expression profiles of exosomal miRNA, as secreted by isogenic human primary CRC cell line SW480 and highly metastatic cell line SW620, were analyzed and the potential targets related to tumorigenesis and metastatic progression were investigated. We found that 25 miRNAs had been up-regulated and 5 miRNAs had been down-regulated in exosomes purified from SW620 culture supernatant. Candidate miRNAs were further evaluated for CRC diagnosis using quantitative real-time polymerase chain reaction in CRC patients. Higher expression levels of circulating exosomal miR-17-5p and miR-92a-3p were significantly associated with pathologic stages and grades of the CRC patients. CONCLUSIONS: Circulating exosomal miR-17-5p and miR-92a-3p may provide a promising noninvasive prognostic biomarker for primary and metastatic CRC.
Introduction
Exosomes are small extracellular vesicles secreted by most cultured cells. They could mediate intercellular communication, providing opportunity for the exchange of DNA, mRNAs, microRNAs (miRNAs), proteins, and other molecules between donor cells and recipient cells and acting to regulate their function [1], [2], [3]. Recent studies suggest that tumor cell–derived exosomes can act to promote both tumorigenesis and metastasis by influencing other types of cells in tissue microenvironment [4], [5], [6]. Exosomes have been noted as the major carriers for miRNAs in serum [7]. They can be extracted from various body fluids including serum, urine, milk, lymph, bile, and saliva and remain stable for long-term storage and expression profiling. The exosomal miRNAs in these biofluids can reflect many aspects of physiological change and disease progression. Recent studies have begun to apply exosomal miRNAs as diagnostic markers for a number of human diseases [8], [9], [10]. In addition, exosomal miRNAs show more significant differences between healthy people and patients than do other serum parameters [11], [12]. Therefore, specific miRNA or miRNA combinations in exosomes can potentially be effective diagnostic markers correlating to several human diseases such as cancers. Currently, detailed information about the types and abundance of exosomal miRNAs can be obtained through next-generation sequencing. Therefore, we can effectively screen disease-related exosomal miRNAs as diagnostic markers.
Colorectal cancer (CRC) is the third most common cancer and the second leading cause of cancer-related death worldwide with an estimated 1.2 million new cases and a half million deaths each year [13]. To investigate the molecular mechanism of primary and metastatic CRC, we compared the miRNA expression profiles of exosomes secreted by two isogenic human CRC cell lines (primary SW480 cell line and its lymph node metastatic variant SW620) using the Illumina HiSeq 2500 system. Exosomal miRNA expression profiling analysis identified several differentially expressed miRNAs related to inflammation and tumor progression (e.g., oncogenic miR-17-92 cluster, miR-7, miR-181a, miR-192, miR-194, miR-375). The predicted genes of these miRNAs included factors related to inflammation, transcription, angiogenesis, DNA replication, transcriptional control and cell-matrix adhesion, cancer-related genes, signal transduction molecules, fibrosis associated components, transforming growth factors and receptors, and others. We then performed Gene Ontology (GO) annotation and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis based on predicted target genes. This helped us to understand the possible biological process and pathways involved in cells with higher tumorigenesis and metastasis capacity. Dysregulated expressed miRNAs were then confirmed by individual quantitative real-time polymerase chain reaction (qRT-PCR) in 29 CRC patients and 10 normal controls. We verified the correlation of candidate exosomal miRNAs expression levels with CRC progression and identified circulating exosomal miR-17-5p and miR-92a-3p as promising prognostic biomarkers for CRC patients.
Materials and Methods
Cell Culture
Normal human colon mucosal epithelial cell NCM460 was obtained from Jennio Biotechnology (Guangzhou, China). SW480 primary CRC cell lines and SW620 lymph node metastatic variants were obtained from the State Key Lab of Diagnostic and Treatment of Infectious Diseases, the First Affiliated Hospital, Zhejiang University School of Medicine. All media and supplements were from Hyclone (Logan, UT) and Sigma. Other CRC cell lines were obtained from ATCC.
Cells were cultured in DMEM supplemented with 10% FBS, and 100 IU/ml penicillin-streptomycin at 37°C with 5% CO2. For exosome collection, 2×106 cells were seeded in 15-cm dishes with 30 ml of the complete culture. Cells were washed once with DMEM upon reaching 70% confluence and then cultured in 25 ml medium supplemented with 10% exosome-depleted FBS and penicillin-streptomycin for 48 to 72 hours. Bovine exosomes were removed from FBS by ultracentrifugation at 100,000×g for 15 hours.
Serum Sample Collection
Clinical samples were processed as previously described [14]. Five milliliters of blood was collected from each patient or healthy control individuals with overnight fasting. All samples were centrifuged within 12 hours after collection at 1500 rpm for 10 minutes, followed by centrifugation at 12,000 rpm for 2 minutes to remove cell debris. The obtained serum samples were stored at −80°C until use.
Exosome Isolation, Characterization, and Analysis
Exosomes of cell culture supernatants were prepared by differential ultracentrifugation as previously described [1]. Briefly, cell culture supernatants were collected and subjected to centrifugation at 300×g for 10 minutes then 2000×g for 10 minutes for removal of floating cells and cell debris. They were then further centrifuged at 10,000×g at 4°C (Beckman Ti70) for 30 minutes to eliminate large membranous particles. Exosomes were gathered through centrifugation at 100,000×g for 70 minutes. Pellets were then washed by being resuspended in 20 ml PBS to eliminate any protein contamination and finally collected by centrifugation at the same high speed.
Exosomes in the serum were purified by qEV Size Exclusion Columns (Izon Science Ltd., Christchurch, New Zealand). Briefly, 1 ml of processed serum was overlaid on columns followed by rinsing with PBS. Five-hundred-microliter fractions were collected. Particle size and concentration of exosomes were determined using tunable resistive pulse sensing (TRPS). Residual exosomes were concentrated using the RiboTM Exosome Isolation Reagent (ribobio, China) to extract total RNA. Anti-CD63 (25682-1-AP), Tsg101 (14497-1-AP, 46KD), and Alix (12422-1-AP, 96KD) were from Proteintech.
TRPS
Exosome size and particle concentration were analyzed with TRPS (qNano, Izon Science Ltd.) according to instructions. Particle concentration was standardized by calibration beads of 1.0×1011 particles/ml. Data were analyzed using the Izon Control Suite software version 3.2.
Total RNA Isolation
For exosomal RNA extraction, total RNA was extracted using a HiPure Liquid RNA/miRNA Kit (Magen, China). The concentration and purification of RNA were determined using nanodrop. The RNA Integrity Number was analyzed using an Agilent 2200 TapeStation.
Library Construction and Sequencing
The exosomal miRNA-Seq experiment was performed by RiboBio company (GuangZhou, China). Briefly, approximately 100 ng total RNA was used to construct a library using the NEBNext Multiplex Small RNA Library Prep Set for Illumina (Illumina, San Diego, CA) according to the manual. Libraries were then amplified and sequenced using HiSeq Rapid SBS Kit V2 (50 cycles) and HiSeq Rapid SR Cluster Kit V2 at the HiSeqTM 2500 system (Illumina).
Small RNA Sequence Analysis
Obtained sequences were aligned with sequences in database (miRBase) to verify known miRNAs. The relative expression level of miRNAs was normalized using the following formula: RPM = (number of reads mapping to miRNA /number of reads in clean data) × 1,000,000. Correlation coefficients were determined by the Pearson method (R2 > 0.8). The criteria of |log2(fold change)| ≥ 1 and FDR < 0.05 were used for determining significantly differentially expressed miRNAs.
The Functional Analysis of Differentially Expressed miRNAs and Predicted Target Genes
Functional analysis of significantly dysregulated expressed miRNAs was performed as previous study [15]. We used four databases to predict the mutual potential target genes of candidate miRNAs. These databases included TargetScan Release 6.0 (http://www.targetscan.org/), miRanda (http://www.microrna.org/microrna/home.do), miRDB (http://mirdb.org/), and CLIP (http://starbase.sysu.edu.cn/). These predicted target genes were further analyzed by GO annotation (DAVID, Version 6.7, http://david.abcc.ncifcrf.gov) and KEGG pathway analysis (http://www.genome.jp/).
Validation of miRNA Expression Using Stem-Loop qRT-PCR
Differentially expressed were further validated by stem-loop qRT-PCR as previously described [16], [17]. Fifty to 500 ng total RNA was reverse transcribed to cDNAs using specific miRNA stem-loop RT primer with Bestar QPCR RT Kit (DBI Bioscience, Germany) and was then amplified using specific forward primer and universal reverse primer using Bestar SybrGreen qPCR Mastermix (DBI Bioscience, Germany). U6 was used as an internal reference in cells, while cel-miR-39 was used as external controls in exosomes from cell culture supernatants and clinical samples. Expression levels were considered statistically different at P < .05 using Student’s t test. Primer sequences used for RT-qPCR are shown in Table 1.
Table 1.
Primers Used for RT-qPCR
| miRNA | Stem Loop RT Primer | Forward Primer |
|---|---|---|
| hsa-miR-17-5p | CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGACTACCTG | ACACTCCAGCTGGGCAAAGTGCTTACAGTGCA |
| hsa-miR-19a-3p | CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTCAGTTTT | ACACTCCAGCTGGGTGTGCAAATCTATGCAA |
| hsa-miR-20a-5p | CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCTACCTGC | ACACTCCAGCTGGGTAAAGTGCTTATAGTGC |
| hsa-miR-92a-3p | CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGACAGGCCGG | ACACTCCAGCTGGGTATTGCACTTGTCCC |
| hsa-miR-7-5p | CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCAACAAAA | ACACTCCAGCTGGGTGGAAGACTAGTGATT |
| hsa-miR-181a-5p | CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGACTCACCG | ACACTCCAGCTGGGAACATTCAACGCTGTCG |
| hsa-miR-375 | CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTCACGCGA | ACACTCCAGCTGGGTTTGTTCGTTCGGCTC |
| hsa-miR-194-5p | CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTCCACATG | ACACTCCAGCTGGGTGTAACAGCAACTCCA |
| hsa-miR-30d-5p | CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCTTCCAGT | ACACTCCAGCTGGGTGTAAACATCCCCGAC |
| hsa-miR-192-5p | CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTGTCAATT | ACACTCCAGCTGGGCTGACCTATGAA |
| hsa-miR-146a-5p | CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGAACCCATG | ACACTCCAGCTGGGTGAGAACTGAATTCCA |
| Universal Reverse primer | TGGTGTCGTGGAGTCG | |
| U6 | AACGCTTCACGAATTTGCGT | CTCGCTTCGGCAGCACA |
Human Studies
Human studies were approved by the First Affiliated Hospital, Zhejiang University School of Medicine. Human serum samples were collected from control healthy individuals and CRC patients with or without metastasis at the First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, China. All aspects of the disease process of the patients were pathologically confirmed. All patients provided informed consent for blood donation and approved protocols.
Mouse Strains and Treatments
Six-week-old female Balb/C nu-nu mice were obtained from SHANGHAI SLAC LABORATORY ANIMAL CO. LTD. (ShangHai, China) and maintained under specific pathogen-free conditions. Mice were injected as previously described [18]. For intrasplenic injection (IS), 5E+06 cells were obtained and resuspended in 100 μl PBS. Mice were anesthetized, and a midline abdominal incision was made. The spleen was exteriorized via an abdominal midline incision, and tumor cell suspension was injected slowly into the spleen. For assays of tumorigenicity and liver metastasis, mice were euthanized 4 weeks after injection. Organs were weighed before fixation.
Statistical Analysis
Significance was calculated using two-tailed Student's t test or by Mann-Whitney U test. Differences were considered to be significant when P < .05. Error bars represent means ± SEM. ROC curves and AUC values were used to evaluate the diagnostic potential of the candidate miRNAs for CRC and metastasis. A Spearman Pearson correlation was used to evaluate correlation performance. GraphPad Prism software and SPSS (version 19.0, SPSS Inc.) were used for statistics. The experiments were not randomized.
Results
In Vivo Tumorigenicity and Metastasis Potential of CRC Cell Lines
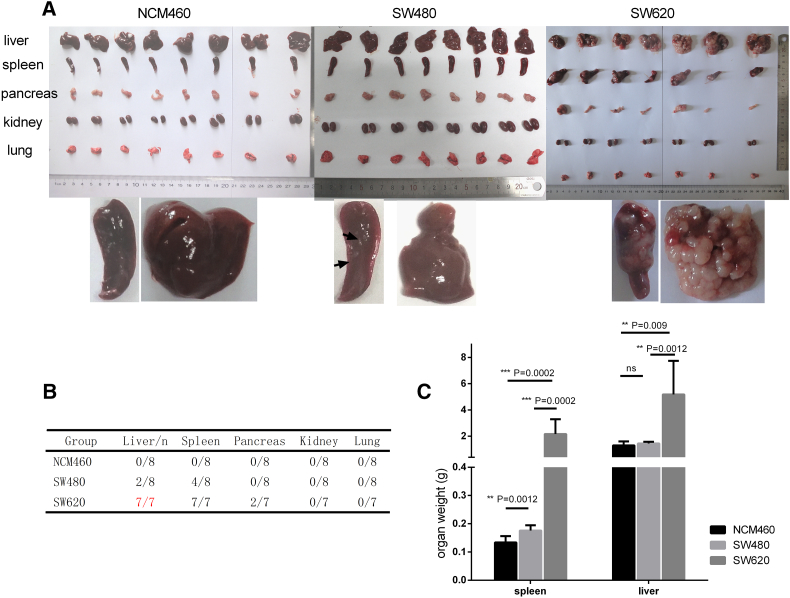
Three cell lines, including normal human colon mucosal epithelium NCM460 and CRC cell line SW480 and its lymph node metastatic variant SW620, were utilized to verify the in vivo tumorigenesis and metastasis ability in nude mice by IS injections. Previous studies had suggested the higher metastasis capacity of SW620 [18], [19], and our data confirmed that SW620 cells displayed a higher tumorigenesis and metastasis capacity (especially liver metastasis through IS) as compared to NCM460 and SW480 cells (Figure 1).
Figure 1.
Spleen injection assay to compare tumorigenicity and metastatic potential. (A) Tumorigenicity and metastatic potential of NCM460, SW480, and SW620 cells. Each group (n = 8) of mice was injected IS with 5E+06 cells. After euthanasia, autopsies were performed 4 weeks after injection. (B) Metastases analyzed. (C) Mean weights of liver following IS injection are shown.
As shown in Figure 1A, in mice injected with SW620 cells, splenic tumors and numerous liver metastatic nodules were observed after 4 weeks. In contrast, no tumors were observed in any of the spleens or livers from the NCM460 group, and only few liver nodules were observed in mice injected with SW480 cells. The tumorigenicity and metastasis rates in organs are listed in Figure 1B. Metastatic tumors were visible in all seven of seven livers, and these were especially prominent in the SW620 group. The mean weight of the spleens and livers in the SW620 group was significantly higher than in other groups (Figure 1C).
Exosome Isolation and Characterization
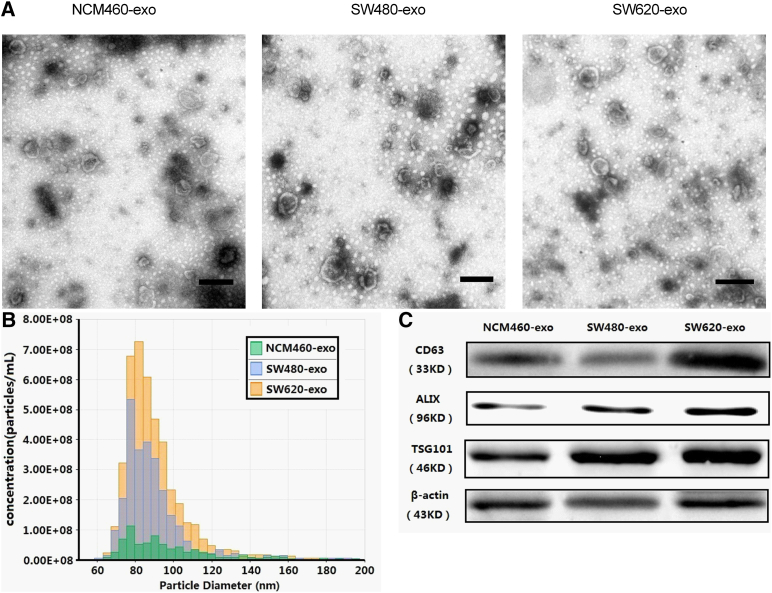
BCA assays showed that the yield of the three purified exosomes (NCM460-exo, SW480-exo, and SW620-exo) by differential ultracentrifugation was approximately 10 to 50 μg/108 cells. This was consistent with the results of previous studies [1], [20], [21]. The EM of purified exosomes derived from three cell lines revealed that three exosomes were essentially homogeneous and of 40 to 100 nm in diameter (Figure 2A). Exosome size and particle number were analyzed with TRPS. Results showed that the three exosomes had similar particle size distribution (Figure 2B). Western blot showed that CD63, ALIX, and TSG101 were expressed in all three exosomes (Figure 2C).
Figure 2.
Characteristics of exosomes from cell lines. (A) Representative TEM photograph of exosomes (scale bar, 200nm). (B) Exosome size and particle number were analyzed with TRPS. (C) Western blot of exosomes and cells for CD63 and β-actin.
MirRNA Profiling Comparison of the SW480 and SW620 Exosome
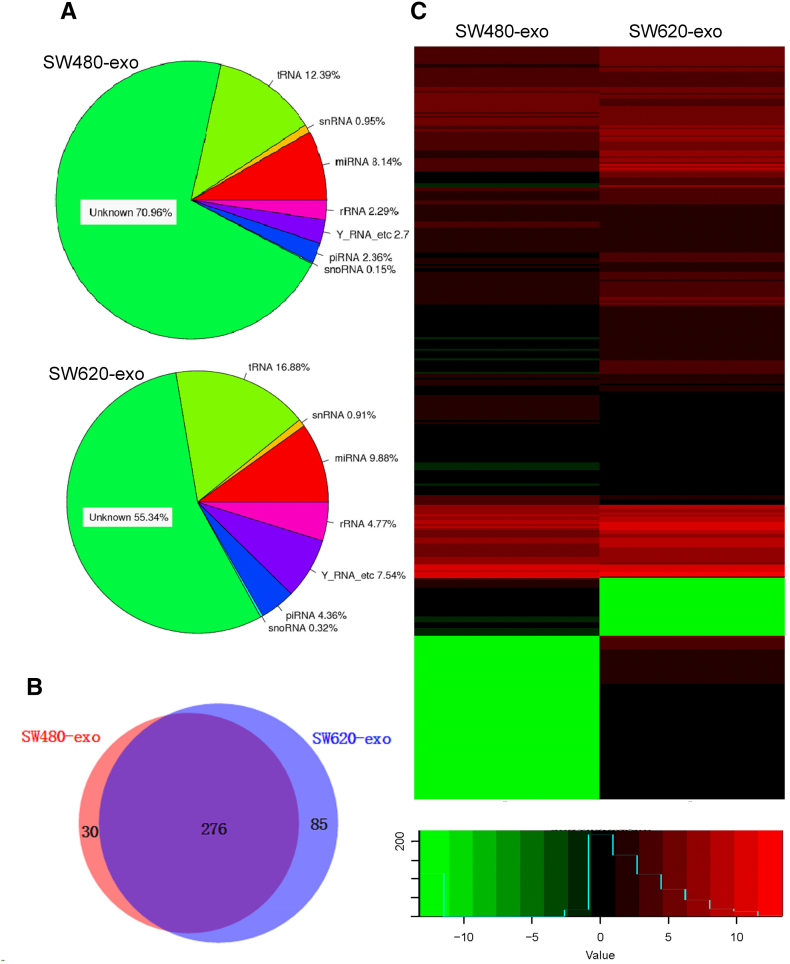
The miRNA profilings of the exosomes from SW480 and SW620 cells were analyzed using miRNA-Seq (Illumina HiSeq 2500). For purified exosomes, RNA sequencing resulted in 17,873,867 reads in SW620-exo and 22,089,590 reads in SW480-exo. For further analysis after a quality and length filter, the proportion of annotated miRNAs was separated as 8.14% and 9.88% in these exosomes, respectively (Figure 3A). Results identified 361 known miRNAs in the SW620-exo and 306 known miRNAs in the SW480-exo.
Figure 3.
MiRNA expressions profiles of exosomes. (A) Profiling of small RNAs in exosome samples. (B) A Venn diagram showing the co-expressed and specifically expressed miRNAs in exosomes. (C) Heat map of sequencing results. Gene expression data obtained using next-generation sequencing on the Illumina HiSeq 2500 platform. P < .05 and fold change > 2 were considered significant
The miRNAs profiles of the two exosomes were different. The results showed that 238 miRNAs were expressed in both exosomes and 23 and 6 miRNAs were uniquely expressed in SW620-exo and SW480-Exo, respectively (Figure 3B). The expression of the miR-17-92 cluster, miR-192, miR-375, miR-7, miR-200b, miR-549 and other 25 miRNAs was up-regulated, while miR-146, miR-5787, miR-29a, miR-372, miR-3910, and miR-7704 were downregulated in the SW620-exo (Figure 3C). Candidates were chosen from those showing significantly dysregulated expression of miRNAs (|log2(fold change)| ≥ 1 and FDR < 0.05).
Functions and Pathways Analysis of Significantly Dysregulated Expressed miRNAs in SW480 and SW620 Exosomes
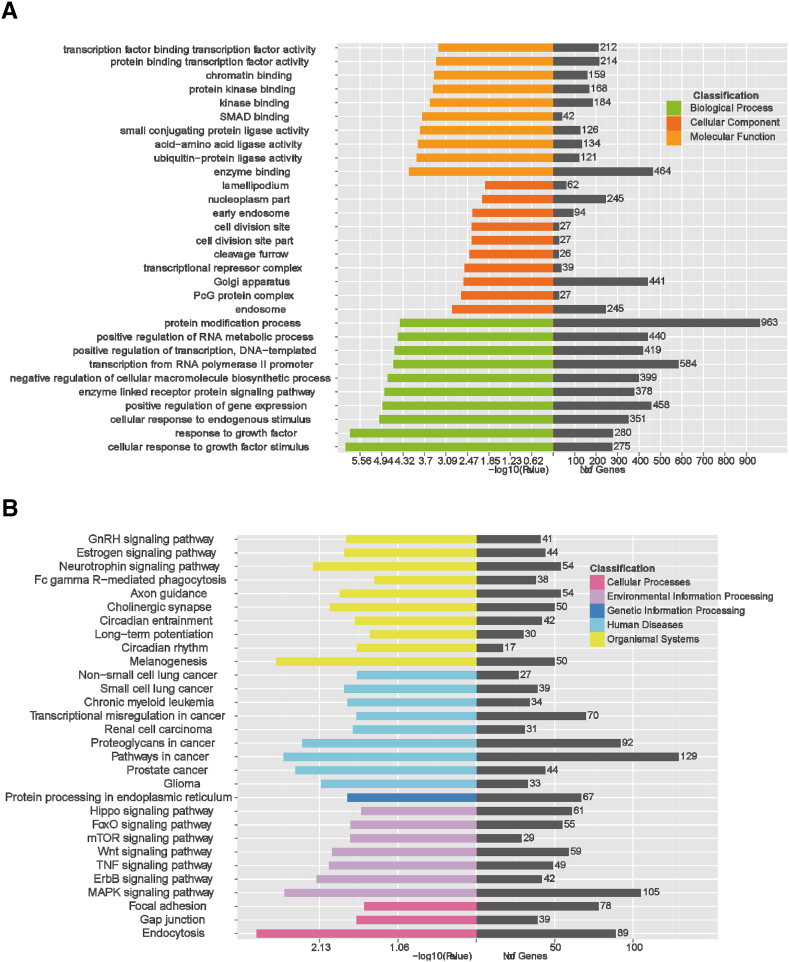
Exosomal miRNA expression profiling analysis identified significantly dysregulated expression in several key miRNAs related to inflammation and tumor progression (oncogenic miR-17-92 cluster, miR-7, miR-181a-5p, miR-192, miR-194, and miR-375). The target gene prediction and enrichment analysis of 25 up-regulated miRNAs and the 6 down-regulated miRNAs were performed. Based on commonly predicted targets of miRNAs in four databases (miRanda/miRDB/TargetScan/CLIP), we obtained a total of 7326 candidate target genes of these miRNAs. These mainly included chemokine/cytokine/receptors and other inflammation factors (such as TNF,CXCL2, IL1A, STAT3, IL13RA1, CCL2, HMGB1, S100PBP, HIF1A), oncogenes/tumor suppressor genes/and other cancer-related genes (RAF1, NRAS, JUN, FOS, PTEN, PIK3CA), transcription factors (SP1, SP3, RUNX2, IRF4, NFAT5), signal transduction molecules (NOTCH2, MTOR, JAK1), fibrosis-associated components/transforming growth factors and receptors (FN1,TGFBR1, TGFBR2), angiogenesis factors (EGFR, VAMP3), autophage/apoptosis-related factors (FAS), DNA replication and transcriptional control factors (E2F1, EIF5), and cell-matrix adhesion moleculars (CD44, ITGB1, ITGA11).
GO annotation of predicted genes significantly targeted those related to biological processes such as cellular responses to growth factors or other endogenous stimulus; the protein modification process, relating to cellular components such as endosomes; and those that function in enzyme binding and the positive regulation of gene expression and others (Figure 4A). Putative targets were also assigned to the KEGG analysis to characterize predominant pathways. The top signaling pathways were related to carcinogenesis or inflammation including those related to human diseases such as melanogenesis/prostate cancer/CRC, those related to environmental information processing functions including the MAPK signaling pathway/TNF signaling pathway/mTOR signaling pathway, and those related to cellular processes such as endocytosis (Figure 4B). Our analysis indicated that these dysregulated expressed miRNAs may be involved in the tumorigenicity and metastasis of CRC cells.
Figure 4.
GO annotation and KEGG pathway analysis. (A) The top GO terms of predicted targets belong to the dysregulated expressed miRNAs. (B) Pathway analysis of differential expression genes by KEGG.
Experimental Validation of miRNA Expression in Cells and Exosomes
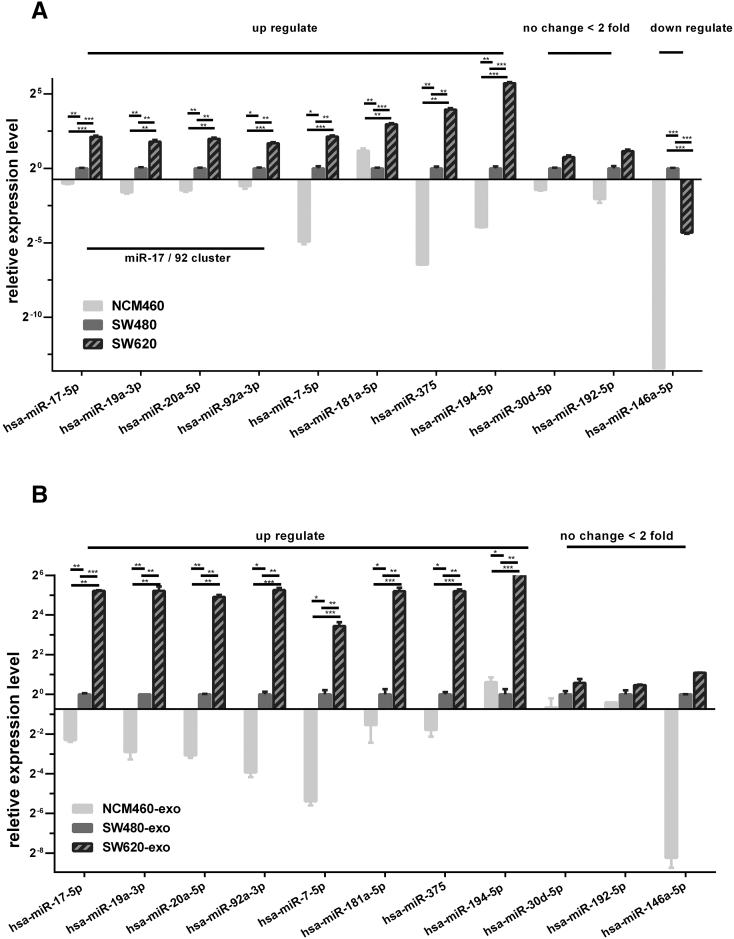
Eleven dysregulated expressed miRNAs identified from miRNA-seq (miR-17, miR-19a, miR-20, miR-92a, miR-7, miR-181a, miR-375, miR-194, miR-30d, miR-192, and miR-146) were chosen for further confirmation by stem-loop qRT-PCR in both cells and exosomes. Four miRNAs of the oncogenic miR-17-92 cluster (miR-17, miR-19a, miR-20, miR-92a) showed similar variation tendency as detected by the miRNA-seq. However, the change of miR-17-92 cluster in three different exosomes was much higher (>30-fold; Figure 5B) than in cells (<10-fold; Figure 5A). This was possibly due to the selective enrichment of specific miRNAs in tumor cell–derived exosomes.
Figure 5.
Validation of miRNA expression in cells and exosomes. The expression levels of 11 candidate differentially expressed miRNAs in cells (A) and exosomes (B) confirmed by qRT-PCR (two-tailed Student's t test) (error bars represent means ± SEM). Experiments were performed in triplicate. P< .01.
Circulating Exosomal miR-17-5p and miR-92a-3p Levels Were Significantly Associated With Pathologic Stage and Grade in CRC Patients
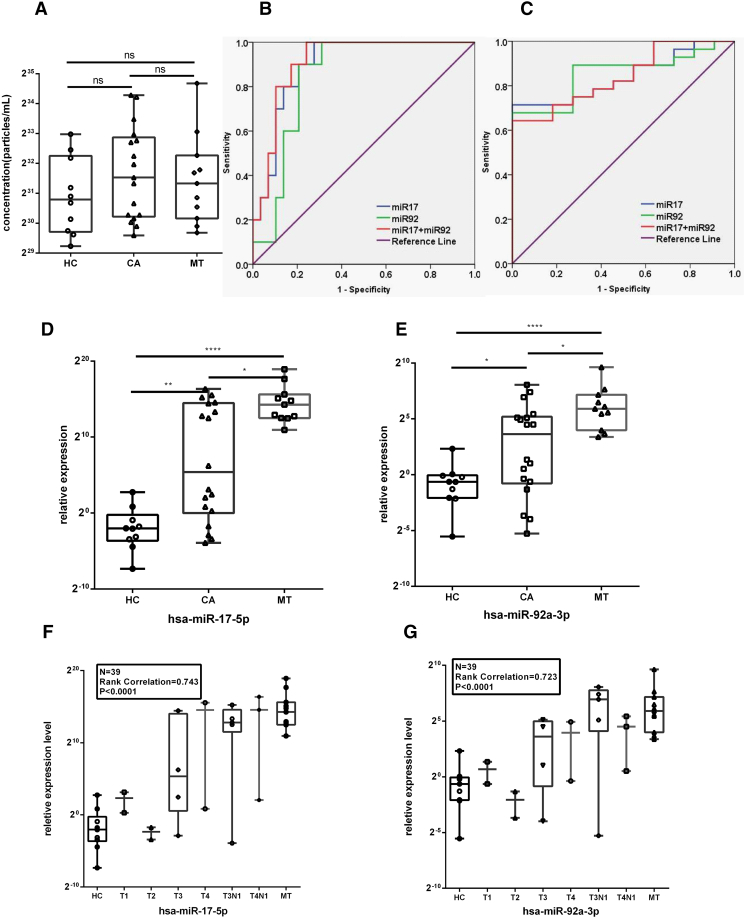
Stem-loop qRT-PCR assays were then performed to validate the expression levels of the 11 candidate miRNAs in CRC patients using healthy volunteers as a control. We isolated and characterized exosomes from the serum of 10 NCs and 18 CRC patients (classified from T1 to T4 according to the TNM staging system) and 11 metastasis patients (as shown in Table 2). The size distribution and particle concentration of the purified exosomes showed no significant differences between healthy control and patients as measured by TRPS (Figure 6A). Total RNA was subsequently extracted from circulating exosomes. Consistent with expression levels of candidate miRNAs in cell-derived exosomes, 2 out of the 11 miRNAs (miR-17, miR-92a) showed significantly higher levels in the serum of CRC and metastasis groups than in control groups (Mann-Whitney U test) (Figure 6, D and E). Using ROC curves to obtain the best decisive threshold for distinguishing two groups (CRC vs. NCs, metastasis vs. nonmetastasis; Figure 6, B and C). The AUC of serum exosomal miR-17-5p showed value of 0.897 (95% CI, 0.800-0.994) for CRC and 0.841 (95% CI, 0.720-0.962) for metastasis. The AUC of serum exosomal miR-92a-3p showed value of 0.845 (95% CI, 0.724-0.966) for CRC and 0.854 (95% CI, 0.735-0.973) for metastasis. The combination of the two miRNAs showed no further improvement with the AUC of 0.910 (95% CI, 0.820-1) for CRC and 0.841 (95% CI, 0.718-0.964) for metastasis. There was significant correlation of miR-17/miR-92a expression level with CRC pathologic stage (Figure 6, C and D, P value < .0001, R = 0.736/0.698). Spearman Pearson correlation was used to assess performance.
Table 2.
Characteristics of Clinical Samples
| Variables | Controls (%) | Patient (%) |
|---|---|---|
| Number (n) | 10 (26%) | 29 (74%) |
| Gender | ||
| Male | 5 (50%) | 16 (55%) |
| Female | 5 (50%) | 13 (45%) |
| Age | ||
| <60 | 8 (80%) | 8 (28%) |
| ≥60 | 2 (20%) | 21 (72%) |
| Location | ||
| Colon | 14 (48%) | |
| Rectum | 15 (52%) | |
| TNM stage | ||
| T1 | 2 (7%) | |
| T2 | 2 (7%) | |
| T3 | 9 (31%) | |
| T4 | 5 (17%) | |
| Distant metastasis | 11 (37%) |
Figure 6.
Circulating exosomal miR-17-5p and miR-92a-3p levels correlated with pathologic stage and grade in CRC patients. (A) The size distribution and particle concentration of purified exosomes in different groups (two-tailed Student's t test) (error bars in graphical data represent means ± SEM). (B) ROC curves to differentiate CRC patients from NCs. (C) ROC curves to differentiate metastasis from nonmetastasis. (D and E) Circulating exosomal miR-17-5p and miR-92a-3p levels in clinical CRC serum specimens tested by qPCR (Mann-Whitney U test; error bars in graphical data represent means ± SEM; P < .05). (F and G) Significant correlation of exo-miR level with CRC pathologic stage (P value < .0001). A Spearman Pearson correlation was used to evaluate performance.
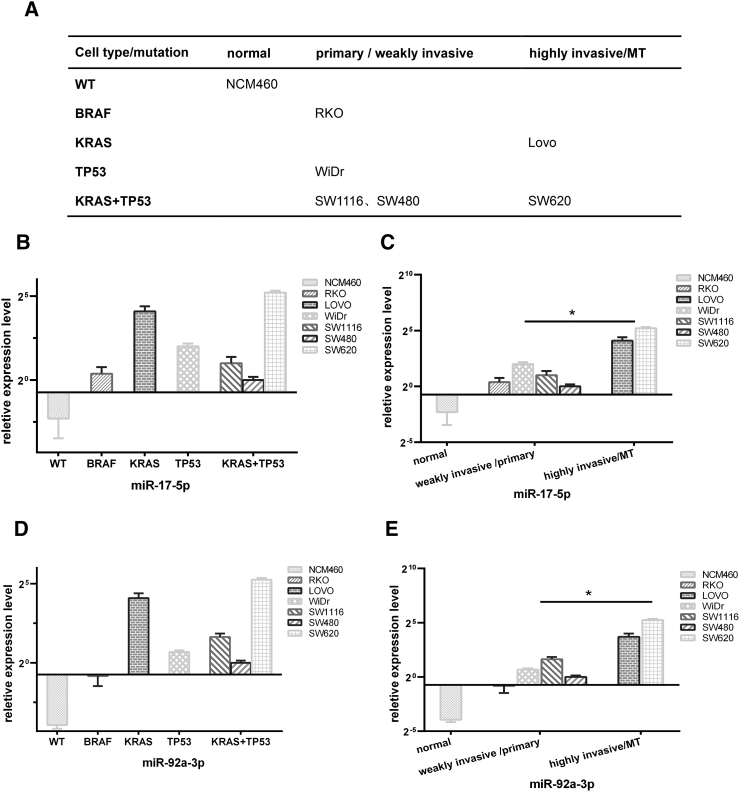
We have also tested several other colon cancer cell lines with different gene mutations and different ability of metastasis; the results and conclusions are shown in Figure 7. Results suggested that miR-17-5p and miR-92a-3p levels were more associated with invasive ability and metastasis potentials but had no significant correlation with the mutation type.
Figure 7.
Exosomal miR-17-5p and miR-92a-3p levels in several other colon cancer cell lines with different gene mutations and different ability of metastasis. (A) Gene mutations and metastasis ability of CRC cell lines. (B) Exosomal miR-17-5p levels in CRC cell lines classified by different gene mutations (error bars in graphical data represent means ± SEM). (C) Exosomal miR-17-5p levels in CRC cell lines classified by invasive ability and metastasis potentials (one-way ANOVA) (error bars in graphical data represent means ± SEM). (D) Exosomal miR-92a-3p levels in CRC cell lines classified by different gene mutations (error bars in graphical data represent means ± SEM). (E) Exosomal miR-92a-3p levels in CRC cell lines classified by invasive ability and metastasis potentials (one-way ANOVA) (error bars in graphical data represent means ± SEM).
Discussion
A unique feature of SW480 and SW620 colon carcinoma cell lines is that they are derived from primary and secondary tumors resected from one single patient with different tumorigenicity and metastasis potential [18]. Since then, they have represented a reliable resource which can be used to explore genetic changes during CRC progression.
Exosomes are extracellular membrane vesicles of 50 to 130 nm diameter secreted by most tumor cells. Recent studies suggested that exosomal miRNAs may be promising biomarkers for tumor diagnosis [14], [22], [23]. To precisely identify sera specific miRNAs correlated to CRC metastasis, we compared the exosomal miRNA profiling of these two cell lines to screen candidate exosomal miRNAs. Then, we chose 11 top dysregulated expressed miRNAs obtained in the screening phase for further validation in cells and exosomes. In the RT-qPCR validation phase, we added normal human colon mucosal epithelium NCM460 as a normal control. We found that the variation tendency between normal and cancer cell lines was broadly consistent within and between the primary and metastasis groups (Figure 5). We further explored serum exosomal miRNAs levels of normal controls and CRC patients, and finally, two miRNAs (miR-17-5p, miR-92a-3p), belonging to the miR-17-92 cluster, were confirmed to be up-regulated in the circulating exosomes of CRC patients. Most notably, the expression levels of these were significantly correlated with the pathological stage and grade of the patients. The expression levels of miR-17-5p and miR-92a-3p were consistent with CRC progression, and this suggested that these miRNAs may play important roles in both the tumorigenesis and metastasis of CRC. In conclusion, the two miRNAs in circulating exosomes may serve as noninvasive biomarkers. However, large-scale sample analysis and validation will be required for further confirmation.
The oncogene Mir-17-92 cluster encodes six individual miRNAs including miR-17, miR-18a, miR-19a, miR-20a, miR-19b, and miR-92a. Mir-17-92 is highly expressed in cancers including lung cancers, liver cancers, and colon cancers. The highly oncogenic features of MiR-17-92 cluster have been implicated in several cancers [24], [25], [26], [27]. Mir-17-92 promotes tumorigenicity and metastasis through regulating multiple cellular functions, such as cell proliferation, apoptosis, and angiogenesis [28].
MiR-17a-5p could also enhance cell proliferation and metastasis in colon cancer as a member of the miR-17-92 cluster [21] and has been considered as a promising biomarker for CRC in several previous researches. MiR-17-5p was found to be significantly deregulated in CRC [29], and higher expression levels of miR-17-5p were found in CRC patients with distant metastases and higher clinical stages using in situ hybridization and immunohistochemistry analysis [30]. It was found that miR-92a was also involved in the metastasis of CRC through a PTEN-mediated PI3K/AKT pathway [31]. It also suggested that miRNA-92a might be a novel promising biomarker in the detection of CRC [32]. However, these previous studies mainly focused on the tissue samples or circulating cell-free miRNAs which may provide a less stable and less sensitive measure than that of circulating exosomal miRNAs.
As our data showed, the change in candidate miRNAs within three different exosomes was much higher (>30-fold) than within three cells (<10-fold) (Figure 5). This may be due to the selective enrichment of specific miRNAs in tumor cell–derived exosomes. Where in sera analysis of exosomal miRNAs may exhibit a more significant difference between healthy individuals and patients than in situ tissue analysis. Furthermore, a recent study discovered that exosomes in serums were the main carriers of the circulating miRNAs [7]. This may also indicate that noninvasive exosomal miRNAs assays could be much more specific and sensitive than conditional methods. Our study suggests that exosomal miR-17-5p and miR-92a-3p could represent a step forward as noninvasive biomarkers for CRC and metastasis progression.
The tissue microenvironment is essential for the initiation and progression of carcinogenesis. Besides primary tumorigenesis, metastasis of malignant tumor requires specific microenvironments in distant metastatic organs which facilitate the adhesion, colonization, and proliferation of circulating tumor cells. Tumor cell–derived exosomes with prometastatic factors, including miRNAs, have been demonstrated to initiate and facilitate premetastatic niche formation that permits the survival and outgrowth of disseminated cancer cells [4], [5]. Our candidate miRNAs including miR-7, miR-181a-5p, miR-192, miR-194, and miR-375, which related to multiple biofunctions such as inflammation, fibrosis, and angiogenesis, may also play important roles in distant metastasis of CRC. The effects and mechanisms of these candidate miRNAs in tumorigenesis and premetastatic niche formation require further investigation.
Acknowledgments
Acknowledgements
We thank Hangping Yao (First Affiliated Hospital, Zhejiang University, School of Medicine, Hangzhou, China) for his technical support and Chris Wood (Zhejiang University, School of Medicine, Hangzhou, China) for manuscript revising. This work was supported by the grants from Chinese National Natural and Science Foundation (81272679) in China and the Key R & D Projects of Zhejiang province (2015C03045).
Conflicts of Interest
The authors confirm that this article content has no conflict of interest.
References
- 1.Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006:3.22.1–3.22.29. doi: 10.1002/0471143030.cb0322s30. https://doi.org/10.1002/0471143030.cb0322s30 [Chapter 3: Unit 3 22] [DOI] [PubMed] [Google Scholar]
- 2.Park JE, Tan HS, Datta A, Lai RC, Zhang H, Meng W, Lim SK, Sze SK. Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes. Mol Cell Proteomics. 2010;9:1085–1099. doi: 10.1074/mcp.M900381-MCP200. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paggetti J, Haderk F, Seiffert M, Janji B, Distler U, Ammerlaan W, Kim YJ, Adam J, Lichter P, Solary E. Exosomes released by chronic lymphocytic leukemia cells induce the transition of stromal cells into cancer-associated fibroblasts. Blood. 2015;126:1106–1117. doi: 10.1182/blood-2014-12-618025. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, Huang WC, Li P, Li M, Wang X, Zhang C. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527:100–104. doi: 10.1038/nature15376. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17:816–826. doi: 10.1038/ncb3169. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, Yu Y, Chow A, O'Connor ST, Chin AR. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014;25:501–515. doi: 10.1016/j.ccr.2014.03.007. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao K, Liang G, Sun X, Guan le L. Comparative miRNAome analysis revealed different miRNA expression profiles in bovine sera and exosomes. BMC Genomics. 2016;17:630. doi: 10.1186/s12864-016-2962-1. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ye SB, Zhang H, Cai TT, Liu YN, Ni JJ, He J, Peng JY, Chen QY, Mo HY, Jun C. Exosomal miR-24-3p impedes T-cell function by targeting FGF11 and serves as a potential prognostic biomarker for nasopharyngeal carcinoma. J Pathol. 2016 doi: 10.1002/path.4781. [DOI] [PubMed] [Google Scholar]
- 9.Huang X, Yuan T, Liang M, Du M, Xia S, Dittmar R, Wang D, See W, Costello BA, Quevedo F. Exosomal miR-1290 and miR-375 as prognostic markers in castration-resistant prostate cancer. Eur Urol. 2015;67:33–41. doi: 10.1016/j.eururo.2014.07.035. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halkein J, Tabruyn SP, Ricke-Hoch M, Haghikia A, Nguyen NQ, Scherr M, Castermans K, Malvaux L, Lambert V, Thiry M. MicroRNA-146a is a therapeutic target and biomarker for peripartum cardiomyopathy. J Clin Invest. 2013;123:2143–2154. doi: 10.1172/JCI64365. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashby J, Flack K, Jimenez LA, Duan Y, Khatib AK, Somlo G, Wang SE, Cui X, Zhong W. Distribution profiling of circulating microRNAs in serum. Anal Chem. 2014;86:9343–9349. doi: 10.1021/ac5028929. [DOI] [PubMed] [Google Scholar]
- 12.Xie JX, Fan X, Drummond CA, Majumder R, Xie Y, Chen T, Liu L, Haller ST, Brewster PS, Dworkin LD. MicroRNA profiling in kidney disease: plasma versus plasma-derived exosomes. Gene. 2017;627:1–8. doi: 10.1016/j.gene.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 14.Zhu M, Huang Z, Zhu D, Zhou X, Shan X, Qi LW, Wu L, Cheng W, Zhu J, Zhang L. A panel of microRNA signature in serum for colorectal cancer diagnosis. Oncotarget. 2017;8:17081–17091. doi: 10.18632/oncotarget.15059. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang G, Malmuthuge N, McFadden TB, Bao H, Griebel PJ, Stothard P, Guan le L. Potential regulatory role of microRNAs in the development of bovine gastrointestinal tract during early life. PLoS One. 2014;9:e92592. doi: 10.1371/journal.pone.0092592. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang F, Hajkova P, Barton SC, Lao K, Surani MA. MicroRNA expression profiling of single whole embryonic stem cells. Nucleic Acids Res. 2006;34:e9. doi: 10.1093/nar/gnj009. https://doi.org/10.1093/nar/gnj009 [doi: 34/2/e9 pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. doi: 10.1038/cr.2008.282. [cr2008282 [pii]] [DOI] [PubMed] [Google Scholar]
- 18.Hewitt RE, McMarlin A, Kleiner D, Wersto R, Martin P, Tsokos M, Stamp GW, Stetler-Stevenson WG. Validation of a model of colon cancer progression. J Pathol. 2000;192:446–454. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH775>3.0.CO;2-K. [pii 10.1002/1096-9896(2000)9999:9999<::AID-PATH775>3.0.CO;2-K]. [DOI] [PubMed] [Google Scholar]
- 19.Park SY, Lee SJ, Cho HJ, Kim TW, Kim JT, Kim JW, Lee CH, Kim BY, Yeom YI, Lim JS. Dehydropeptidase 1 promotes metastasis through regulation of E-cadherin expression in colon cancer. Oncotarget. 2016;7:9501–9512. doi: 10.18632/oncotarget.7033. [7033 [pii]] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji H, Greening DW, Barnes TW, Lim JW, Tauro BJ, Rai A, Xu R, Adda C, Mathivanan S, Zhao W. Proteome profiling of exosomes derived from human primary and metastatic colorectal cancer cells reveal differential expression of key metastatic factors and signal transduction components. Proteomics. 2013;13:1672–1686. doi: 10.1002/pmic.201200562. [DOI] [PubMed] [Google Scholar]
- 21.van Niel G, Raposo G, Candalh C, Boussac M, Hershberg R, Cerf-Bensussan N, Heyman M. Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology. 2001;121:337–349. doi: 10.1053/gast.2001.26263. doi: S001650850128055X [pii] [DOI] [PubMed] [Google Scholar]
- 22.Zhou X, Wen W, Shan X, Zhu W, Xu J, Guo RH, Cheng WF, Wang F, Qi LW, Chen Y. A six-microRNA panel in plasma was identified as a potential biomarker for lung adenocarcinoma diagnosis. Oncotarget. 2017;8:6513–6525. doi: 10.18632/oncotarget.14311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Q, Yu Z, Yuan S, Xie W, Li C, Hu Z, Xiang Y, Wu N, Wu L, Bai L. Circulating exosomal microRNAs as prognostic biomarkers for non-small-cell lung cancer. Oncotarget. 2017;8:13048–13058. doi: 10.18632/oncotarget.14369. 14369 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu H, Han C, Wu T. MiR-17-92 cluster promotes hepatocarcinogenesis. Carcinogenesis. 2015;36:1213–1222. doi: 10.1093/carcin/bgv112. [bgv112 [pii]] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang JX, Song W, Chen ZH, Wei JH, Liao YJ, Lei J, Hu M, Chen GZ, Liao B, Lu J. Prognostic and predictive value of a microRNA signature in stage II colon cancer: a microRNA expression analysis. Lancet Oncol. 2013;14:1295–1306. doi: 10.1016/S1470-2045(13)70491-1. [S1470-2045(13)70491-1 [pii]] [DOI] [PubMed] [Google Scholar]
- 26.Yu Z, Wang C, Wang M, Li Z, Casimiro MC, Liu M, Wu K, Whittle J, Ju X, Hyslop T. A cyclin D1/microRNA 17/20 regulatory feedback loop in control of breast cancer cell proliferation. J Cell Biol. 2008;182:509–517. doi: 10.1083/jcb.200801079. [jcb.200801079 [pii]] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ren P, Gong F, Zhang Y, Jiang J, Zhang H. MicroRNA-92a promotes growth, metastasis, and chemoresistance in non-small cell lung cancer cells by targeting PTEN. Tumour Biol. 2015 doi: 10.1007/s13277-015-4150-3. [10.1007/s13277-015-4150-3 [pii]] [DOI] [PubMed] [Google Scholar]
- 28.Olive V, Li Q, He L. mir-17-92: a polycistronic oncomir with pleiotropic functions. Immunol Rev. 2013;253:158–166. doi: 10.1111/imr.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kara M, Yumrutas O, Ozcan O, Celik OI, Bozgeyik E, Bozgeyik I, Tasdemir S. Differential expressions of cancer-associated genes and their regulatory miRNAs in colorectal carcinoma. Gene. 2015;567:81–86. doi: 10.1016/j.gene.2015.04.065. [DOI] [PubMed] [Google Scholar]
- 30.Fang LK, Li HR, Wang L, Hu J, Jin TR, Wang JP, Yang BB. MicroRNA-17-5p promotes chemotherapeutic drug resistance and tumour metastasis of colorectal cancer by repressing PTEN expression. Oncotarget. 2014;5:2974–2987. doi: 10.18632/oncotarget.1614. [doi:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ke TW, Wei PL, Yeh KT, Chen WT, Cheng YW. MiR-92a promotes cell metastasis of colorectal cancer through PTEN-mediated PI3K/AKT pathway. Ann Surg Oncol. 2015;22:2649–2655. doi: 10.1245/s10434-014-4305-2. [DOI] [PubMed] [Google Scholar]
- 32.Yang X, Zeng Z, Hou Y, Yuan T, Gao C, Jia W, Yi X, Liu M. MicroRNA-92a as a potential biomarker in diagnosis of colorectal cancer: a systematic review and meta-analysis. PLoS One. 2014;9:e88745. doi: 10.1371/journal.pone.0088745. [PONE-D-13-50342 [pii]] [DOI] [PMC free article] [PubMed] [Google Scholar]