SUMMARY
Although it is now well established that the hippocampus supports memory encoding [1, 2], little is known about hippocampal activity during spontaneous memory retrieval. Recent intracranial electroencephalographic (iEEG) work has shown that hippocampal activity during encoding predicts subsequent temporal organization of memories [3], supporting a role in contextual binding. It is an open question, however, whether the hippocampus similarly supports contextually mediated processes during retrieval. Here, we analyzed iEEG recordings obtained from 215 epilepsy patients as they performed a free recall task. To identify neural activity specifically associated with contextual retrieval, we compared correct recalls, intrusions (incorrect recall of either items from prior lists or items not previously studied), and deliberations (matched periods during recall when no items came to mind). Neural signals that differentiate correct recalls from both other retrieval classes reflect contextual retrieval, as correct recalls alone arise from the correct context. We found that in the hippocampus, high-frequency activity (HFA, 44–100 Hz), a proxy for neural activation [4], was greater prior to correct recalls relative to the other retrieval classes, with no differentiation between intrusions and deliberations. This pattern was not observed in other memory-related cortical regions, including DLPFC, thus supporting a specific hippocampal contribution to contextually mediated memory retrieval.
RESULTS
The goal of our study was to assess whether the hippocampus specifically supports spontaneous retrieval of contextually mediated memories, whereby temporal contextual information is used to guide retrieval processes [5, 6]. We collected intracranial electroencephalographic (iEEG) data from 215 epilepsy patients who participated in a free recall experiment. We measured high-frequency activity (HFA, 44–100 Hz) in the field potential, a signal that correlates with both multi-unit activity and the fMRI BOLD signal [7, 8], during the 500 ms preceding correct recalls (C) and recall errors (intrusions, I). Additionally, we assessed HFA during “deliberation” periods (D), 500-ms intervals of silence during which participants attempted to recall items without making overt vocalizations. We measured retrieval effects in four regions of interest (ROIs) that broadly encompass the “core memory network” [9, 10], the hippocampus, frontal, temporal, and parietal lobes (Figures 1A–1D, left panel). Whereas general retrieval should be characterized by greater HFA for correct recalls and intrusions relative to deliberations (e.g., [11]), contextually mediated retrieval processes should be reflected by both (1) reliable differences between correct recalls and intrusions and (2) non-reliable differences between intrusions and deliberation periods, as only correct recalls reflect retrieval of correct contextual information.
Figure 1. High-Frequency Activity during Free Recall in the Core Memory Network.
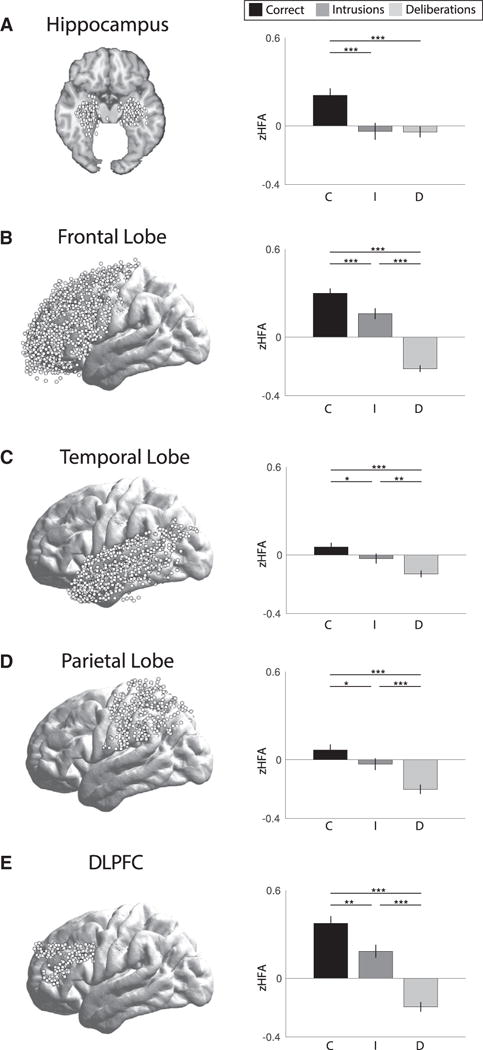
(A–E) The left panel shows electrode locations in hippocampus (A), frontal lobe (B), temporal lobe (C), parietal lobe (D), and DLPFC (E). The right panel shows Z scored high-frequency activity (zHFA, 44–100 Hz) for each of three retrieval classes: correct recalls (black), intrusions (dark gray), and deliberation periods (light gray). Error bars are standard error of the mean. *p < 0.05, **p < 0.01, ***p < 0.001.
We predicted that the pattern of activity in hippocampus would be consistent with contextually mediated retrieval, with increased activity for correct recalls only, given the role of hippocampus in contextual encoding [3], source memory [12, 13] and recollection [14, 15]. In comparison, we predicted that our other ROIs would differentiate intrusions and deliberations. Specifically, retrieval search and monitoring processes in the frontal lobe [16, 17] and sensory reactivation processes in temporal lobe [18–20] should differentiate any retrieval event, whether correct or incorrect, from a failed memory search that does not result in a retrieval event (e.g., deliberation periods). Likewise, activity in parietal lobe should be greater for intrusions than deliberations, given that intrusions are reported because they are subjectively believed to be correct and the parietal lobe supports subjective memory [21]. We found a pattern consistent with contextually mediated retrieval in the hippocampus (Figure 1A, right panel), with increased HFA for correct recalls relative to intrusions and deliberations, and no reliable difference between intrusions and deliberations (Table 1). We found reliable differences between all three retrieval classes in each of three large cortical regions: frontal, temporal, and parietal lobe (see Table 1).
Table 1.
Comparison of Retrieval Classes within Each ROI
| Region | C> I | I > D | C> D |
|---|---|---|---|
| Hippocampus (76) | 3.5*** | .06 | 4.1*** |
| Frontal lobe (123) | 4.4*** | 8.6*** | 12.7*** |
| Temporal lobe (131) | 2.1* | 2.9** | 6.2*** |
| Parietal lobe (110) | 2.3* | 4.2*** | 6.1*** |
| DLPFC (98) | 3.4** | 6.8*** | 8.8*** |
T statistics for the paired comparison of Z scored high-frequency activity (zHFA, 44–100 Hz) across retrieval classes (correct recalls, C; intrusions, I; deliberations, D) within each ROI. Numbers in parentheses denote number of participants with electrodes in each region.
p < 0.05
p < 0.01
p < 0.001.
A potentially interesting question concerns the nature of intrusions, which can be divided into prior-list intrusions, recall of items from previous study lists, and extra-list intrusions, recall of items not previously studied. Among the 25 participants who contributed a minimum of five prior- and extra-list intrusions, we found no reliable difference in hippocampal HFA preceding each intrusion type (t(24) = 0.18, p = 0.86). We therefore collapsed across both intrusion types for all remaining analyses.
We next sought to directly assess whether the pattern of HFA increases exclusively preceding correct recalls in hippocampus was reliably different from the patterns observed in frontal, temporal, and parietal lobes. We ran repeated-measures ANOVAs with retrieval class (C, I, D) and region (either hippocampus and frontal lobe, hippocampus and temporal lobe, or hippocampus and parietal lobe) as factors, followed by post hoc comparisons of contrasts between retrieval classes (Figure 2). That is, we were not interested in differences in retrieval class across regions, e.g., whether correct items had more or less HFA in the hippocampus versus frontal lobe. Instead, we wanted to test whether the components of contextually mediated retrieval, a large positive effect for the CI contrast and a non-significant effect for the ID contrast, were unique to the hippocampus.
Figure 2. Retrieval Contrasts.
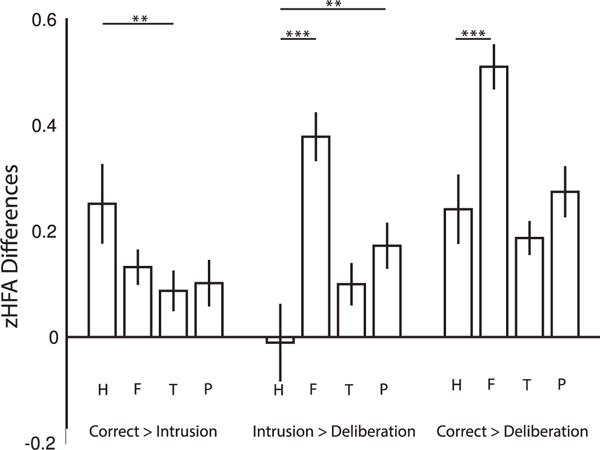
The figure shows zHFA differences between each retrieval class (correct recalls, intrusions, deliberations) for each of four ROIs (hippocampus, H; frontal lobe, F; temporal lobe, T; parietal lobe, P). Error bars are standard error of the mean. **p < 0.01, ***p < 0.001.
A 2 × 3 repeated-measures ANOVA between hippocampus and frontal lobe revealed a marginal effect of region, a main effect of retrieval class, and a reliable interaction between retrieval class and region (Table 2). Post hoc comparisons showed reliable regional differences in the ID and CD contrasts, but not the CI contrast (Table 2). A 2 × 3 repeated-measures ANOVA between hippocampus and temporal lobe revealed a main effect of region and of retrieval class and a reliable interaction between retrieval class and region (Table 2). Post hoc comparisons showed reliable region differences in the CI contrast, but not the ID or CD contrast (Table 2). A 2 × 3 repeated-measures ANOVA between hippocampus and parietal lobe likewise revealed a main effect of region and of retrieval class and a reliable interaction between retrieval class and region (Table 2). Post hoc comparisons showed reliable regional differences in the ID contrast, but not the CI or CD contrast (Table 2). Thus, all region by retrieval class comparisons showed that the pattern of contextually mediated retrieval was specific to the hippocampus.
Table 2.
Comparison of Retrieval Classes and Retrieval Class Contrasts across Regions
| F Statistics | T Statistics | |||||
|---|---|---|---|---|---|---|
| Region | Retrieval Class | Interaction | CI | ID | CD | |
| Frontal lobe (63) | 3.7~ | 29.9*** | 11.6*** | 1.1 | 4.2*** | 3.9*** |
| Temporal lobe (76) | 4.3* | 14.0*** | 4.1* | 2.7** | 1.8 | .09 |
| Parietal lobe (57) | 4.4* | 17.7*** | 3.6* | 1.7 | 2.7** | .91 |
| DLPFC (48) | 2.4 | 18.7*** | 6.4** | 1.4 | 1.9~ | 4.2*** |
F statistics for each 2 × 3 repeated-measures ANOVA comparing region (hippocampus and the region shown) and retrieval class (correct recalls, C; intrusions, I; deliberations, D). T statistics for each post hoc paired comparison between class contrasts (CI, ID, CD) across regions (hippocampus and the region shown). Numbers in parentheses denote number of participants with electrodes in both hippocampus and the region shown.
p = 0.06
p < 0.05
p < 0.01
p < 0.001.
As the ROIs we investigated outside of the hippocampus were quite broad, we performed an additional, more focused analysis on the dorsolateral prefrontal cortex (DLPFC), which has been shown to support both organizational processes during encoding [17] and monitoring processes during retrieval [16]. The pattern of HFA in DLPFC mirrored that in the broader frontal lobe (Figure 1E, right panel), with reliable differences between all three retrieval classes (Table 1). Likewise, a 2 × 3 repeated-measures ANOVA between hippocampus and DLPFC revealed no main effect of region, a main effect of retrieval class, and a reliable interaction between retrieval class and region (Table 2). Post hoc comparisons showed reliable regional differences in the CD contrast and a marginal difference in the ID contrast, with no differences in the CI contrast (Table 2), again showing that the pattern of contextually mediated retrieval is specific to the hippocampus.
DISCUSSION
The goal of the current study was to elucidate the neural mechanisms that give rise to contextually mediated retrieval. Based on prior work showing that hippocampal activity correlates with contextual encoding [3], we hypothesized that the hippocampus may also play a unique role in the retrieval of temporal contextual information. It was an open question whether other brain regions within the core memory network would similarly support contextually mediated retrieval. We measured HFA (44–100 Hz) during the 500-ms interval preceding retrieval of correct items, intrusions, and during periods of searching without a response (deliberations) in regions that broadly cover the core memory network [9, 10]: the hippocampus, the DLPFC, and the frontal, temporal, and parietal lobes. We found a pattern of activity specific to the hippocampus whereby HFA increased for correct recalls relative to both intrusions and deliberation periods. Together, these results suggest that the hippocampus specifically reflects contextually mediated retrieval.
Increased hippocampal activity is a correlate of successful memory encoding [1, 9, 12] and retrieval [22–24]. Although evidence from recognition memory studies suggests that the hippocampus supports source memory retrieval [20, 25, 26], the contribution of hippocampus to spontaneous memory retrieval is less well understood. By assessing HFA, a signal thought to reflect general cortical activation [4], during free recall, we were able to measure neural signals associated with the retrieval of items from correct and incorrect contexts (correct recalls and intrusions, respectively). General retrieval processes would be characterized by similar activation for correct recalls and intrusions relative to deliberations, as the first two cases both result in a retrieval event. Contextually mediated retrieval, however, would be characterized by both activation differences between correct recalls and intrusions and no activation differences between intrusions and deliberations, as the latter two cases both reflect failure to retrieve correct contextual information. We find the latter pattern in the hippocampus, suggesting that it supports contextually mediated retrieval.
Previous research has shown that item reinstatement occurs in the hippocampus [27] and that context is reinstated during retrieval [28]. The current work presents an intersection between these findings, suggesting that hippocampus specifically reflects the retrieval of context information. A speculative interpretation of these findings is that hippocampal pattern completion [29–31] may only occur for items retrieved from the correct temporal context, which would be consistent with findings that univariate hippocampal activity scales with parahippocampal representations of context [32]. Such pattern completion may be dependent on differential contributions of hippocampal sub-fields supporting temporal and spatial contextual information [33], a possible avenue for future research.
The pattern of contextually mediated retrieval was specific to the hippocampus as the frontal, temporal, and parietal lobes did not show both increased activation for correct recalls relative to intrusions and equivalent activation for intrusions and deliberations. Across all regions outside of the hippocampus, including DLPFC, we found greatest activation for correct recalls, followed by intrusions, with the least activation for deliberations. The frontal lobe has been shown to support a variety of retrieval processes including controlled retrieval [17, 34], selection [34, 35], and monitoring [36, 37]. The present results appear most consistent with a monitoring account whereby the frontal lobe acts as an evidence accumulator [38]. Likewise, the pattern of results in temporal cortex may reflect the degree of sensory reactivation [18], with relatively greater sensory reactivation for intrusions reflecting retrieval of item information without hippocampally mediated contextual reinstatement. Finally, our findings in the parietal lobe are consistent with evidence suggesting that this region supports vivid remembering [39] and that parietal lobe retrieval activity may reflect a retrieval buffer [40] and/or subjective memory experiences ([21] cf. [41]).
A major question not addressed by the present work includes the potential impact of epileptiform activity on memory retrieval. Given that our data are from neurological patients, epileptic activity may potentially contaminate the effects of interest. Indeed, epileptiform activity during retrieval can negatively impact memory performance [42], and thus, further research may help to clarify the degree to which electrophysiological differences between successful and unsuccessful retrieval periods reflect pathological neural activity.
During spontaneous retrieval, we must perform a search in order to successfully retrieve a memory. Several computational models of human memory (e.g., [5, 6]) propose that temporal context acts as a cue to guide this search process. Our study provides evidence that the hippocampus supports such contextually mediated retrieval and, in doing so, is distinct from other regions within the core memory network.
EXPERIMENTAL PROCEDURES
Participants
83 patients (37 female; age range: 15–57, mean = 36) participated in experiment 1, and 132 patients (68 female; age range: 16–64, mean = 36) participated in experiment 2. As the two experiments differed only in inter-stimulus and retrieval interval duration (see below), we merged data from both experiments and performed identical analyses on both datasets. All patients had medication-resistant epilepsy and underwent a surgical procedure in which electrodes were implanted subdurally on the cortical surface and deep within the brain parenchyma. In each case, the clinical team determined electrode placement so as to best localize epileptogenic regions. Data were collected as part of a long-term multicenter study; experiment 1 data were collected at the following centers: Boston Children’s Hospital (Boston), Hospital of the University of Pennsylvania (Philadelphia), Freiburg University Hospital (Freiburg), and Thomas Jefferson University Hospital (Philadelphia). Experiment 2 data were collected at the following centers: Thomas Jefferson University Hospital (Philadelphia), Mayo Clinic (Rochester), Hospital of the University of Pennsylvania (Philadelphia), Emory University Hospital (Atlanta), University of Texas Southwestern Medical Center (Dallas), Dartmouth-Hitchcock Medical Center (Lebanon), Columbia University Medical Center (New York), and the National Institutes of Health (Bethesda). The institutional review board at each hospital approved the research protocol. We obtained informed consent from the participants or their guardians. Participants were left-hemispheric language dominant as assessed by either the participants’ handedness or a clinically administered intracarotid injection of sodium amobarbital (Wada test). Clinical need determined the electrode placements. The raw, de-identified data as well as the associated codes used in this study can be accessed at the Cognitive Electrophysiology Data Portal (http://memory.psych.upenn.edu/Electrophysiological_Data).
Intracranial Recordings
iEEG data were recorded using a Bio-Logic, DeltaMed, Nicolet, GrassTelefactor, Nihon Kohden EEG system, Natus XLTek EMU 128, or Grass Aura-LTM64. Depending on the amplifier and the discretion of the clinical team, the signals were sampled at 500 to 2,000 Hz. Signals were referenced to a common contact placed either intracranially or on the scalp or mastoid process. Contact localization was accomplished by co-registering the post-operative CTs with the MRIs using FSL Brain Extraction Tool (BET) and FLIRT software packages. Contact locations were then mapped to both MNI and Talairach space using an indirect stereotactic technique. Depth electrodes were manually localized by a neuroradiologist experienced in neuroanatomical localization utilizing post-operative MRIs and CT images. For each participant and electrode, the raw EEG signal was downsampled to 500 Hz, and a fourth order 2-Hz stop-band butterworth notch filter was applied at 50 or 60 Hz to eliminate electrical line noise.
Free Recall Task
Participants studied lists of 12, 15, or 20 high-frequency, non-semantically-related nouns (http://memory.psych.upenn.edu/WordPools) for a delayed free recall task. The computer displayed each word for 1,600 ms, followed by an 800 to 1,200 ms (experiment 1) or 750 to 1,000 ms (experiment 2) blank interstimulus interval. No encoding task was used. Immediately following the final word in each list, participants performed a distractor task (20 s) consisting of a series of arithmetic problems of the form A + B + C = ?, where A, B, and C were randomly chosen integers ranging from 1 to 9. After the distractor, participants had 45 s (experiment 1) or 30 s (experiment 2) to freely recall as many words as possible from the list in any order. Vocalizations were digitally recorded and later manually scored for analysis. On average, participants participated in two sessions and studied 19 lists.
Data Analyses and Spectral Power
Two concerns when analyzing bivariate interactions between closely spaced intracranial contacts are volume conduction and confounding interactions with the reference line. We used bipolar referencing to eliminate such confounds when analyzing the neural signal [43]. We found the difference in voltage between pairs of immediately adjacent electrodes [2]. The resulting bipolar signals were treated as new virtual electrodes and are referred to as such throughout the text. Analog pulses synchronized the electrophysiological recordings with behavioral events.
We applied the Morlet wavelet transform (wave number 6) to all bipolar electrode EEG signals from 4,500 ms preceding to 1,000 ms following vocalization, across 46 logarithmically spaced frequencies (2–100 Hz). We included a 1,000-ms buffer on both sides of the data to minimize edge effects. After log transforming the power, we downsampled the data by taking a moving average across 100-ms time windows and sliding the window every 50 ms, resulting in 109 time intervals (54 non-overlapping) from 4,500 ms preceding to 1,000 ms following vocalization. Power values were then Z transformed within session by subtracting the mean and dividing by the standard deviation power. Mean and standard deviation power were calculated across all retrieval events and time points in a session for each frequency. We then reduced the Z-transformed power to a single band of HFA (44–100 Hz).
We analyzed three classes of retrieval events: correctly recalled items, intrusions (items recalled that were not from the preceding list, excluding repetitions of previously recalled items), and deliberation periods. Any vocalization that was preceded by 5,000 ms of “silence,” i.e., no other vocalizations, was included in our analysis. We then divided this 5,000-ms interval into a deliberation period and a correct recall or intrusion event. For correct recalls and intrusions, we collapsed HFA across the 500-ms interval preceding vocalization, so as not to include signals associated with speech production [44]. Deliberation periods were 500-ms intervals of silence from 3,000 to 2,500 ms preceding vocalization during which participants were attempting to recall items but made no overt vocalizations [11]. By yoking deliberation periods to both correct recalls and intrusions in this way, we ensured that output position did not reliably differ across retrieval classes (mean output position by retrieval class: C, 3.0; I, 3.0, D, 2.9; repeated-measures one-way ANOVA of output position across class, F(2,284) = 0.13, p = 0.88). Across participants there were on average 38 correctly recalled items, 12 intrusions, and 51 deliberation periods. A participant had to have a minimum of five items per retrieval class to be included in the analysis [3]; 143 participants met this criterion.
ROI Selection and Analysis
Both to maximize the number of participants per ROI and to broadly encompass regions in the core memory network [9, 10], we analyzed data in the hippocampus and frontal, temporal, and parietal lobes. For increased spatial specificity, we also assessed retrieval effects in DLPFC, defined based on Brodmann areas (9, 46). When accounting for the minimum event requirement (see above), the number of patients with electrodes in a given region was as follows: 76 hippocampal, 123 frontal, 131 temporal, 110 parietal, and 98 DLPFC. For each participant, we averaged HFA values across electrodes within an ROI as we were interested in effects consistent across an ROI and not regional differences within an ROI. Therefore, each participant contributed a single HFA value for each retrieval class for each ROI.
Highlights.
We used iEEG recordings to assess the neural correlates of spontaneous retrieval
HFA patterns specific to the hippocampus reflect contextually mediated retrieval
Acknowledgments
We thank Blackrock Microsystems for providing neural recording equipment. This work was supported by the DARPA Restoring Active Memory program (Cooperative Agreement N66001-14-2-4032) and National Institutes of Health grant MH55687. We are indebted to all patients who have selflessly volunteered their time to participate in our study. The views, opinions, and/or findings contained in this material are those of the authors and should not be interpreted as representing the official views or policies of the Department of Defense or the U.S. Government.
Footnotes
AUTHOR CONTRIBUTIONS
N.M.L. and M.J.K. designed experiments. N.M.L. analyzed the data. J.M.S. localized the electrodes. M.R.S., G.A.W., K.A.D., R.E.G., B.C.L., B.C.J., S.A.S., and K.Z. recruited participants and provided general assistance. N.M.L. and M.J.K. wrote the manuscript. All authors provided feedback on the manuscript. M.J.K. supervised the research.
References
- 1.Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- 2.Long NM, Burke JF, Kahana MJ. Subsequent memory effect in intracranial and scalp EEG. Neuroimage. 2014;84:488–494. doi: 10.1016/j.neuroimage.2013.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Long NM, Kahana MJ. Modulation of task demands suggests that semantic processing interferes with the formation of episodic associations. J Exp Psychol Learn Mem Cogn. 2017;43:167–176. doi: 10.1037/xlm0000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burke JF, Ramayya AG, Kahana MJ. Human intracranial high-frequency activity during memory processing: neural oscillations or stochastic volatility? Curr Opin Neurobiol. 2015;31:104–110. doi: 10.1016/j.conb.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polyn SM, Norman KA, Kahana MJ. A context maintenance and retrieval model of organizational processes in free recall. Psychol Rev. 2009;116:129–156. doi: 10.1037/a0014420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lohnas LJ, Polyn SM, Kahana MJ. Expanding the scope of memory search: modeling intralist and interlist effects in free recall. Psychol Rev. 2015;122:337–363. doi: 10.1037/a0039036. [DOI] [PubMed] [Google Scholar]
- 7.Manning JR, Jacobs J, Fried I, Kahana MJ. Broadband shifts in local field potential power spectra are correlated with single-neuron spiking in humans. J Neurosci. 2009;29:13613–13620. doi: 10.1523/JNEUROSCI.2041-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mukamel R, Gelbard H, Arieli A, Hasson U, Fried I, Malach R. Coupling between neuronal firing, field potentials, and FMRI in human auditory cortex. Science. 2005;309:951–954. doi: 10.1126/science.1110913. [DOI] [PubMed] [Google Scholar]
- 9.Kim H. Neural activity that predicts subsequent memory and forgetting: a meta-analysis of 74 fMRI studies. Neuroimage. 2011;54:2446–2461. doi: 10.1016/j.neuroimage.2010.09.045. [DOI] [PubMed] [Google Scholar]
- 10.Burke JF, Long NM, Zaghloul KA, Sharan AD, Sperling MR, Kahana MJ. Human intracranial high-frequency activity maps episodic memory formation in space and time. Neuroimage. 2014;85:834–843. doi: 10.1016/j.neuroimage.2013.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lega BC, Jacobs J, Kahana MJ. Human hippocampal theta oscillations and the formation of episodic memories. Hippocampus. 2012;22:748–761. doi: 10.1002/hipo.20937. [DOI] [PubMed] [Google Scholar]
- 12.Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci USA. 2003;100:2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayama HR, Vilberg KL, Rugg MD. Overlap between the neural correlates of cued recall and source memory: evidence for a generic recollection network? J Cogn Neurosci. 2012;24:1127–1137. doi: 10.1162/jocn_a_00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ranganath C, Yonelinas AP, Cohen MX, Dy CJ, Tom SM, D’Esposito M. Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia. 2004;42:2–13. doi: 10.1016/j.neuropsychologia.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Yonelinas AP. The hippocampus supports high-resolution binding in the service of perception, working memory and long-term memory. Behav Brain Res. 2013;254:34–44. doi: 10.1016/j.bbr.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayama HR, Rugg MD. Right dorsolateral prefrontal cortex is engaged during post-retrieval processing of both episodic and semantic information. Neuropsychologia. 2009;47:2409–2416. doi: 10.1016/j.neuropsychologia.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Long NM, Öztekin I, Badre D. Separable prefrontal cortex contributions to free recall. J Neurosci. 2010;30:10967–10976. doi: 10.1523/JNEUROSCI.2611-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slotnick SD, Schacter DL. A sensory signature that distinguishes true from false memories. Nat Neurosci. 2004;7:664–672. doi: 10.1038/nn1252. [DOI] [PubMed] [Google Scholar]
- 19.Kuhl BA, Rissman J, Chun MM, Wagner AD. Fidelity of neural reactivation reveals competition between memories. Proc Natl Acad Sci USA. 2011;108:5903–5908. doi: 10.1073/pnas.1016939108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gordon AM, Rissman J, Kiani R, Wagner AD. Cortical reinstatement mediates the relationship between content-specific encoding activity and subsequent recollection decisions. Cereb Cortex. 2014;24:3350–3364. doi: 10.1093/cercor/bht194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rissman J, Greely HT, Wagner AD. Detecting individual memories through the neural decoding of memory states and past experience. Proc Natl Acad Sci USA. 2010;107:9849–9854. doi: 10.1073/pnas.1001028107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eldridge LL, Knowlton BJ, Furmanski CS, Bookheimer SY, Engel SA. Remembering episodes: a selective role for the hippocampus during retrieval. Nat Neurosci. 2000;3:1149–1152. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- 23.Fernandes MA, Moscovitch M, Ziegler M, Grady C. Brain regions associated with successful and unsuccessful retrieval of verbal episodic memory as revealed by divided attention. Neuropsychologia. 2005;43:1115–1127. doi: 10.1016/j.neuropsychologia.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 24.Cohn M, Moscovitch M, Lahat A, McAndrews MP. Recollection versus strength as the primary determinant of hippocampal engagement at retrieval. Proc Natl Acad Sci USA. 2009;106:22451–22455. doi: 10.1073/pnas.0908651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cansino S, Maquet P, Dolan RJ, Rugg MD. Brain activity underlying encoding and retrieval of source memory. Cereb Cortex. 2002;12:1048–1056. doi: 10.1093/cercor/12.10.1048. [DOI] [PubMed] [Google Scholar]
- 26.Yu SS, Johnson JD, Rugg MD. Hippocampal activity during recognition memory co-varies with the accuracy and confidence of source memory judgments. Hippocampus. 2012;22:1429–1437. doi: 10.1002/hipo.20982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gelbard-Sagiv H, Mukamel R, Harel M, Malach R, Fried I. Internally generated reactivation of single neurons in human hippocampus during free recall. Science. 2008;322:96–101. doi: 10.1126/science.1164685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manning JR, Polyn SM, Baltuch GH, Litt B, Kahana MJ. Oscillatory patterns in temporal lobe reveal context reinstatement during memory search. Proc Natl Acad Sci USA. 2011;108:12893–12897. doi: 10.1073/pnas.1015174108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marr D. Simple memory: a theory for archicortex. Philos Trans R Soc Lond B Biol Sci. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- 30.Guzowski JF, Knierim JJ, Moser EI. Ensemble dynamics of hippocampal regions CA3 and CA1. Neuron. 2004;44:581–584. doi: 10.1016/j.neuron.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Horner AJ, Bisby JA, Bush D, Lin WJ, Burgess N. Evidence for holistic episodic recollection via hippocampal pattern completion. Nat Commun. 2015;6:7462. doi: 10.1038/ncomms8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Staresina BP, Henson RN, Kriegeskorte N, Alink A. Episodic reinstatement in the medial temporal lobe. J Neurosci. 2012;32:18150–18156. doi: 10.1523/JNEUROSCI.4156-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mankin EA, Diehl GW, Sparks FT, Leutgeb S, Leutgeb JK. Hippocampal CA2 activity patterns change over time to a larger extent than between spatial contexts. Neuron. 2015;85:190–201. doi: 10.1016/j.neuron.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Badre D, Poldrack RA, Paré-Blagoev EJ, Insler RZ, Wagner AD. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47:907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 35.Thompson-Schill SL, Jonides J, Marshuetz C, Smith EE, D’Esposito M, Kan IP, Knight RT, Swick D. Effects of frontal lobe damage on interference effects in working memory. Cogn Affect Behav Neurosci. 2002;2:109–120. doi: 10.3758/cabn.2.2.109. [DOI] [PubMed] [Google Scholar]
- 36.Achim AM, Lepage M. Dorsolateral prefrontal cortex involvement in memory post-retrieval monitoring revealed in both item and associative recognition tests. Neuroimage. 2005;24:1113–1121. doi: 10.1016/j.neuroimage.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 37.Westphal AJ, Reggente N, Ito KL, Rissman J. Shared and distinct contributions of rostrolateral prefrontal cortex to analogical reasoning and episodic memory retrieval. Hum Brain Mapp. 2016;37:896–912. doi: 10.1002/hbm.23074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Philiastides MG, Auksztulewicz R, Heekeren HR, Blankenburg F. Causal role of dorsolateral prefrontal cortex in human perceptual decision making. Curr Biol. 2011;21:980–983. doi: 10.1016/j.cub.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 39.Kuhl BA, Chun MM. Successful remembering elicits event-specific activity patterns in lateral parietal cortex. J Neurosci. 2014;34:8051–8060. doi: 10.1523/JNEUROSCI.4328-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vilberg KL, Rugg MD. The neural correlates of recollection: transient versus sustained FMRI effects. J Neurosci. 2012;32:15679–15687. doi: 10.1523/JNEUROSCI.3065-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Urgolites ZJ, Smith CN, Squire LR. True and false memories, parietal cortex, and confidence judgments. Learn Mem. 2015;22:557–562. doi: 10.1101/lm.038349.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horak PC, Meisenhelter S, Song Y, Testorf ME, Kahana MJ, Viles WD, Bujarski KA, Connolly AC, Robbins AA, Sperling MR, et al. Interictal epileptiform discharges impair word recall in multiple brain areas. Epilepsia. 2016 doi: 10.1111/epi.13633. http://dx.doi.org/10.1111/epi.13633. [DOI] [PMC free article] [PubMed]
- 43.Nunez PL, Srinivasan R. Electric Fields of the Brain. Oxford University Press; 2006. [Google Scholar]
- 44.Sederberg PB, Schulze-Bonhage A, Madsen JR, Bromfield EB, Litt B, Brandt A, Kahana MJ. Gamma oscillations distinguish true from false memories. Psychol Sci. 2007;18:927–932. doi: 10.1111/j.1467-9280.2007.02003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


