Abstract
5′-AMP-activated protein kinase (AMPK) plays a pivotal role in maintaining energy and redox homeostasis under various metabolic stress conditions. Metabolic adaptation, which can be triggered by the activation of AMPK during metabolic stress, is the critical process for cell survival through the maintenance of ATP and NADPH levels. The importance of such regulation of fundamental process poses the AMPK signaling pathway in one of the most attractive therapeutic targets in many pathologies such as diabetes and cancer. In cancer, however, accumulating data suggest that the role of AMPK would not be simply defined as anti- or pro-tumorigenic, but it seems to have two faces like a double-edged sword. Importantly, recent studies showed that the anti-tumorigenic effects of many ‘indirect’ AMPK activators such as anti-diabetic biguanides are not dependent on AMPK; rather the activation of AMPK induces the resistance to their cytotoxic effects, emphasizing the pro-tumorigenic effect of AMPK. In this review, we summarize and discuss recent findings suggesting the two faces of AMPK in cancer, and discuss how we can exploit this unique feature of AMPK for novel therapeutic intervention.
Keywords: AMPK, LKB1, CAMKK2, mTORC1, ACC, NADPH, ROS, Fatty acids, Metabolic stress, Mitochondria, Biguanides, Cancer
Introduction
In 1987, AMPK has been discovered as a key regulator of fatty acid and cholesterol biosynthesis after the identification of AMPK as an upstream kinase of acetyl-CoA carboxylase (ACC) and 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMGR) (Carling et al. 1987). After the discovery of AMPK as a key regulator of lipid metabolism, it has now become one of the most versatile key players involved in the diverse and fundamental metabolic processes that counter metabolic stresses (Hardie and Alessi 2013). Upon activation of AMPK under these conditions, anabolism is shut down to prevent ATP and NADPH consumption while catabolism is turned on to increase ATP and NADPH production (Hardie et al. 2012; Jeon and Hay 2012). As such, AMPK plays a pivotal role in maintaining energy and redox homeostasis at the cellular and organismal level, and deregulation of this balance can lead to major metabolic diseases such as diabetes and cancer (Steinberg and Kemp 2009; Hardie et al. 2012).
The first link between AMPK and cancer came from the finding that AMPK can mediate the tumor suppressive signaling of liver kinase B1 (LKB1) to the inhibition of mechanistic target of rapamycin complex 1 (mTORC1), which is activated in most cancers (Shackelford and Shaw 2009). Since this discovery, the LKB1-AMPK axis has been considered as a tumor suppressor pathway through the inhibition of mTORC1. Consistent with this hypothesis, it has been shown that metabolic inhibitors such as anti-diabetic biguanides metformin and phenformin, which indirectly activate AMPK inhibits tumor growth through the inhibition of mTORC1 (Shackelford and Shaw 2009). However, despite much pharmacological evidence, there has been little genetic evidence supporting this tumor suppressive role of AMPK.
Recently and in contrast to prior expectation, multiple research groups have shown that AMPK actually plays a pivotal role in tumor survival and growth, both in vitro and in vivo (Jeon and Hay 2012; Faubert et al. 2014b; Liang and Mills 2013; Hardie and Alessi 2013). The accumulated data suggest that the reprogramming of metabolism, which is triggered by the activation of AMPK, is the critical process for cell survival during metabolic stress that can often occur under pathophysiological conditions such as those found in the tumor microenvironment. Thus, it would not be surprising if AMPK could promote survival of not only normal cells but also tumor cells in such cases, and consequently play an essential role as a tumor promoter. In this review, we summarize and discuss the various causes and two opposing consequences of AMPK activation in tumors in the context of the tumor microenvironment, and propose a novel therapeutic opportunity conferred by this unique feature of AMPK and its role in cancer.
AMPK as a converging point of various stress signals arising from the tumor microenvironment
As a key player in metabolic adaptation, AMPK mediates diverse metabolic stress signals arising from nutrient starvation, matrix detachment, oxidative stress and hypoxia (Hardie et al. 2012; Jeon and Hay 2012; Cardaci et al. 2012). Importantly, all of these stresses are well-known factors that are actually present in the tumor microenvironment (Fig. 1). In addition, recent studies have shown that AMPK can be also activated by a variety of oncogenic signaling triggered by the activation of proto-oncogenes or the inactivation of tumor suppressor genes (Rios et al. 2013, Tennakoon et al. 2013; Possik et al. 2014; Yan et al. 2014). The activation of AMPK is mainly mediated by an increase in the phosphorylation of T172 in the α-catalytic subunit of AMPK; a heterotrimer consists of one catalytic α-subunit and two regulatory β- and γ-subunits. In recent decades, major upstream kinases and phosphatases have been identified that are responsible for the phosphorylation and dephosphorylation of this residue: LKB1, calcium/calmodulin-dependent kinase kinase 2 (CAMKK2), and protein phosphatase 2C and 2A (PP2C and PP2A) (Woods et al. 2003; Hawley et al. 2003, 2005; Woods et al. 2005; Davies et al. 1995).
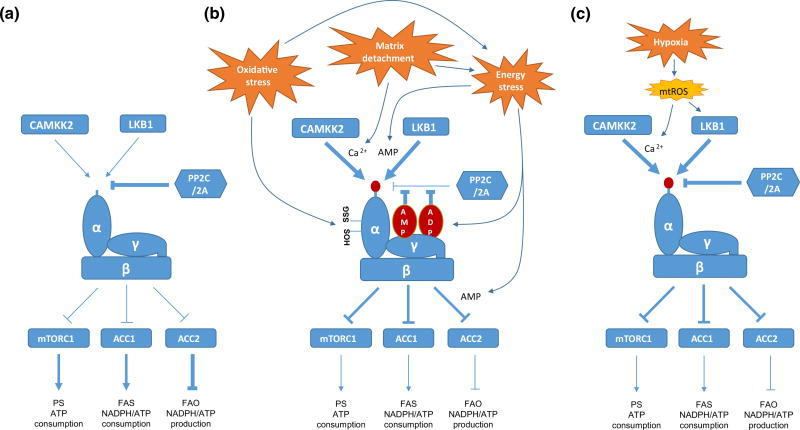
Fig. 1.
The mechanisms of AMPK activation by various factors present in the tumor microenvironment. a The regulation of AMPK activity under normal conditions. b The mechanisms of AMPK activation by energy stress, matrix detachment and oxidative stress. c The mechanisms of AMPK activation by hypoxia. See text for details. Arrows denote activation, whereas horizontal bars indicate inhibition. PS protein synthesis, FAS fatty acid synthesis, FAO fatty acid oxidation, mtROS mitochondrial reactive oxygen species
Energetic stress
Under normal circumstances, PP2C and PP2A can freely access the T172 residue and keep it unphosphorylated (Fig. 1a) (Hardie et al. 2012). However, the energetic stress driven increase in the levels of AMP and ADP leads to an increase in their binding with the γ-subunit of AMPK, and this induces conformational changes that prevent the access of the phosphatases to this residue, resulting in an increase in T172 phosphorylation (Fig. 1b) (Hardie et al. 2012). Moreover, conformational changes induced by the binding of AMP but not ADP can induce allosteric activation of AMPK without T172 phosphorylation (Fig. 1b) (Hardie et al. 2012). Since LKB1 is considered to be constitutively active while CAMKK2 can be only activated by Ca2+ flux, it has been accepted that LKB1 does play a major role in AMPK phosphorylation and activation during energetic stress. Recently, further mechanisms of AMPK activation by LKB1 under energetic stress have been reported. Hardie and colleagues proposed that AMP, in spite of being at a lower cellular concentration, is a greater physiological regulator of AMPK than ADP; they showed that AMP is ten times more potent than ADP in inhibiting dephosphorylation of T172 and, more importantly, AMP but not ADP can enhance LKB1-induced AMPK phosphorylation (Fig. 1b) (Gowans et al. 2013). In addition, Lin and colleagues proposed the mechanism by which LKB1-induced T172 phosphorylation of AMPK under energetic stress. Interestingly, they showed that conformational change of AMPK induced by the binding of AMP recruits AMPK to the scaffold protein Axin-LKB1 complex, which enables LKB1 to phosphorylate AMPK in close proximity (Zhang et al. 2013). These two independent observations are consistent, in that only AMP and not ADP can enhance LKB1-induced AMPK phosphorylation, while both AMP and ADP can inhibit dephosphorylation of T172 by blocking access by phosphatases.
Matrix detachment
Recently, several groups have shown that matrix detachment that can occur during solid tumor growth and metastasis, can also activate AMPK (Jeon et al. 2012; Fung et al. 2008; Ng et al. 2012; Hindupur et al. 2014). Matrix detachment has been reported to inhibit glucose uptake and glycolysis, thereby inducing energetic stress (Schafer et al. 2009), which suggests that LKB1 may play a major role in AMPK activation in such cases. Recently, we provided the first evidence showing that the activation of AMPK upon matrix detachment is mediated through both LKB1 and CAMKK2 (Fig. 1b) (Jeon et al. 2012). We found that LKB1-null cells, such as A549 cells, could still substantially activate AMPK upon matrix detachment, although this activity is less than in LKB1-reconstituted A549 cells, and so this suggests that LKB1-independent mechanisms are also present. Furthermore, we showed that this phosphorylation is dependent on CAMKK2, since the treatment of A549 cells with the CAMKK2 inhibitor STO609 almost completely inhibited the AMPK activation induced by matrix detachment. Thus, these data suggest that matrix detachment may also stimulate Ca2+ flux in these cells, although there is no direct evidence for this and the mechanisms are currently unknown.
Oxidative stress
The first report demonstrating that exogenous H2O2 can activate AMPK in cultured cells was published more than 10 years ago (Choi et al. 2001). Although they showed that H2O2 can increase the AMP/ATP ratio, further mechanisms have not been extensively studied until recently. Interestingly, a recent study showed that reactive oxygen species (ROS) that are constantly generated in the medium in cell culture by the addition of glucose oxidase can induce the oxidative modification including S-glutathionylation of two conserved cysteine residues within the α-catalytic subunit of AMPK, which is responsible for the activation of AMPK by ROS (Zmijewski et al. 2010). They showed that this activation and oxidation of AMPK occurs before any changes in intracellular ADP/ATP ratio and non-oxidative mutation of the cysteine residues within the α-catalytic subunit was sufficient to prevent the activation of AMPK by ROS. Conversely, Hardie and colleagues used AMP/ADP insensitive γ-subunit mutant (RG) expressing cells in order to thoroughly investigate the involvement of AMP/ADP levels in ROS-induced AMPK activation (Auciello et al. 2014; Hawley et al. 2010). They showed that ROS fail to activate AMPK in RG expressing cells suggesting that ROS-induced activation of AMPK requires AMP/ADP binding of the γ-subunit. They also showed that ROS can inhibit mitochondrial oxygen consumption, resulting in the increase of AMP/ADP levels. Furthermore, using the cell lines expressing glucose oxidase, which induces constant and physiological levels of H2O2 in the cells, they showed that both exogenous and endogenous ROS can activate AMPK in an AMP/ADP dependent manner (Auciello et al. 2014). Thus, it seems that ROS may activate AMPK by two distinct mechanisms involving direct oxidation of cysteine residues in AMPK as well as energetic stress through the inhibition of mitochondrial metabolism (Fig. 1b).
Hypoxia
Although hypoxia is a well-known feature of tumor microenvironments, the underlying mechanisms of AMPK activation remain elusive. At least two groups have reported mechanisms for the hypoxic activation of AMPK. Using mouse embryonic fibroblasts (MEFs), Chandel and colleagues showed that hypoxia activates AMPK by increasing the levels of mitochondrial ROS (mtROS) but not the AMP/ATP ratio (Emerling et al. 2009). Despite no change in the AMP/ATP ratio, they demonstrated that this activation of AMPK is dependent on LKB1. Interestingly, in constrast to hypoxic ROS, exogenous H2O2 (100 µM) under normoxia could significantly activate AMPK even in the absence of LKB1, suggesting that hypoxic ROS and normoxic ROS may have different mechanisms for AMPK activation. Conversely, Schumacker and colleagues proposed different mechanisms for the hypoxic activation of AMPK using MEFs and 143B osteosarcoma cells (Mungai et al. 2011). Although they also found that mtROS but not AMP/ATP ratio are responsible for the hypoxic activation, they showed that the hypoxia-induced ROS activate AMPK through an increase in Ca2+ levels and the activation of CAMKK2 but not LKB1. Since these two groups have reported two conflicting mechanisms (Fig. 1c), further studies will be necessary to elucidate the mechanisms underlying the hypoxic activation of AMPK.
Oncogenic signaling
Although AMPK signaling was originally suggested as a tumor suppressive pathway, as mentioned above, recent studies have shown that several oncogenic signaling molecules can activate AMPK. Oncogene SRC, which is overexpressed and activated in many cancers, has been shown to activate AMPK through the activation of the PKCα-LKB1 pathway in certain cancer cell lines (Mizrachy-Schwartz et al. 2011). Similarly, the expression of MYC and H-RasV12 in osteosarcoma cell lines and astrocytic tumors, respectively, also activate AMPK, although the mechanisms are not clear (Liu et al. 2012; Rios et al. 2013). Moreover, androgen receptor (AR) signaling also activates AMPK through the transactivation of CAMKK2 in prostate cancer (Massie et al. 2011, Tennakoon et al. 2013; Frigo et al. 2011). In addition to the activation of oncogenes, the loss of the tumor suppressor gene folliculin (FLCN), which is associated with Birt-Hogg-Dubé syndrome (BHD), has been shown to activate AMPK (Possik et al. 2014; Yan et al. 2014); this is discussed later.
AMPK as a pro-tumorigenic regulator: changing the old paradigm
As described above, many factors arising in the tumor microenvironment, as well as some oncogenic signaling, are bona fide AMPK activators in cancer. Indeed, AMPK has been shown to be strongly activated in vivo in both rodent and human glioblastomas (Rios et al. 2013; Jang et al. 2011). Thus, the main question arising from this is what are the consequences of AMPK activation in tumors; are they pro-tumorigenic or anti-tumorigenic? This has been debated in recent years, but it is now becoming clear that the activation of AMPK under these conditions can be a pro-tumorigenic signal in cancer.
The initial observations that LKB1 and AMPK have pro-tumorigenic roles came from the setting of oncogene-induced transformation experiments using MEFs. Two groups independently showed that both LKB1-null MEFs and AMPKα1/α2-null (AMPKα-KO) MEFs are resistant to oncogenes such as H-RasV12/SV40T-induced anchorage-independent growth as well as solid tumor growth in vivo (Bardeesy et al. 2002; Laderoute et al. 2006). Remarkably, three recent studies have shown that AMPK is also essential for other oncogene-induced tumorigenesis, such as the MYC-induced development of hepatocellular carcinoma (HCC), H-RasV12 ± Pten−/− induced astrocytic tumor cell proliferation, and kinase suppressor of ras 2 (KSR2) induced anoikis resistance of MEFs and some cancer cell lines (Liu et al. 2012; Rios et al. 2013; Fernandez et al. 2012). In addition, multiple groups have recently reported that AMPK has an essential role in a variety of cancer types. The silencing of AMPK in human breast and pancreatic cancer cells impairs their ability to grow in an anchorage-independent manner and to form tumors in vivo (Laderoute et al. 2014; Hindupur et al. 2014; Kato et al. 2002). In addition, the LKB1-AMPK signaling pathway is also required for glioma cell survival and spheroid migration in low-glucose conditions (Godlewski et al. 2010; Rios et al. 2013). Interestingly, two recent unbiased approaches have identified AMPKα1 and AMPKβ1 subunits as essential pro-tumorigenic genes in melanomas and prostate cancers, respectively. By chemical proteomic screening of melanoma cells, AMPKα1 was identified as playing a key role in maintaining cell viability through the regulation of micro-phthalmia-associated transcription factor (MITF), a melanoma oncoprotein (Borgdorff et al. 2014). Conversely, the expression of the AMPK β1 subunit in prostate cancer was found to be essential for cancer cell survival and was highly elevated in metastatic prostate cancers when compared with primary prostate cancers (Ros et al. 2012). Importantly, the AR-CAMKK2-AMPK axis was recently reported to have critical roles in prostate cancer. An interdisciplinary approach integrating genomic studies and metabolomic profiling found that CAMKK2, which is overexpressed in prostate cancers, is the critical transcriptional target of AR, and that the CAMKK2-AMPK axis is critical for AR-induced prostate cancer cell growth and survival (Massie et al. 2011). Consistently, other groups have also shown that the CAMKK2-AMPK pathway is a critical downstream effector of AR-dependent prostate cancer cell growth, migration and invasion, suggesting that the CAMKK2-AMPK axis is a promising therapeutic target in AR-dependent prostate cancer (Tennakoon et al. 2013; Frigo et al. 2011).
In general, the mechanisms underlying the pro-tumorigenic role of AMPK have been attributed to the maintenance of ATP levels by reprogramming energy metabolism, since this is the best-known function of AMPK. Supporting this view, it was shown that the activation of AMPK by MYC maintains the expression of mitochondrial respiratory chain complexes and respiratory capacity, which would be required for efficient glutamine metabolism for energy production (Liu et al. 2012). Similarly, the activation of AMPK by loss of tumor suppressor FLCN increases mitochondrial biogenesis by inducing PGC1α expression and consequently resulting in ROS production (Yan et al. 2014). They also showed that high levels of ROS activate HIF1α transcriptional activity resulting in an increase in the expression of glycolytic enzymes, which drive ATP production. Other studies of prostate cancer also proposed that the AR-CAMKK2-AMPK axis increases aerobic glycolysis and anabolic metabolism in part through PGC1α-mediated mitochondrial biogenesis, which can increase the supply of both the energy and the building blocks required for rapid cell growth (Massie et al. 2011; Tennakoon et al. 2013).
Recently, different mechanisms have been proposed, other than those involving the provision of energy or building blocks; these mechanisms are associated with the metabolic functions of AMPK. We showed that, in the absence of AMPK activity, oxidative stress is the major cause of cancer cell death during metabolic stress, such as glucose deprivation and matrix detachment (Jeon et al. 2012). Under these conditions, it is the maintenance of NADPH levels, rather than of ATP levels through phosphorylation and inhibition of acetyl-CoA carboxylases (ACCs), that are critical mechanisms of AMPK-induced tumor cell survival, anchorage-independent growth and solid tumor formation in vivo. The inhibition of ACC1 and ACC2, which reduce NADPH consumption by inhibition of fatty acid synthesis and increase NADPH production by activation of fatty acid oxidation, respectively, is required for maintaining the GSH/GSSG ratio and for reducing hydrogen peroxide levels, thereby protecting against oxidative stress induced cell death. Concordantly, recent studies have shown that AMPK protects against H2O2 induced cell death through NADPH maintenance in osteosarcoma and loss of folliculin was shown to confer an AMPK-dependent resistance to oxidative stress induced cell death (She et al. 2014; Possik et al. 2014).
Conversely, recent studies have also proposed different mechanisms that are not associated with the metabolic functions of AMPK. As mentioned above, AMPK has been found to inhibit the degradation of the oncoprotein MITF, which is required to maintain cell viability in melanoma (Borgdorff et al. 2014). MITF has been shown to be a melanoma-specific oncogene as it is amplified or mutated in a significant proportion of melanomas. However, the mechanisms by which the AMPK-MITF axis regulates cell viability in melanoma are not well understood. In breast cancer cells, AMPK has been shown to phosphorylate phosphoprotein enriched in astrocytes 15 (PEA15), a protein that is critical for mammosphere formation and solid tumor growth in vivo (Hindupur et al. 2014). Although they suggested that the AMPK-PEA15 axis can inhibit apoptosis, at least in part, through the inhibition of FADD-mediated apoptosis signaling, further studies would be needed to elucidate the significance of this mechanism.
AMPK as an anti-tumorigenic regulator: re-evaluation is needed
Although many recent studies have suggested the pro-tumorigenic role of AMPK, it was first suggested as having anti-tumorigenic activity after the discovery of LKB1 and mTORC1 as an upstream kinase and a downstream effector of AMPK, respectively (Hawley et al. 2003; Woods et al. 2003; Corradetti et al. 2004; Inoki et al. 2003). LKB1 was originally identified as a tumor suppressor gene since its germline mutations were found in Peutz-Jeghers Syndrome (PJS), which is characterized by intestinal hamartomas and increased cancer predisposition in certain tissues (Hemminki et al. 1998). In addition, somatic mutations in LKB1 were also found in certain cancers including lung and cervical cancers underscoring the tumor suppressive function of LKB1 (Shackelford and Shaw 2009). mTORC1, which is inactivated by the LKB1-AMPK axis, is a central integrator of growth factor and nutrient signals that regulate protein synthesis or cell growth, and are hyperactivated in most human cancers (Shackelford and Shaw 2009). Thus, the findings that LKB1 activates AMPK, and that AMPK inhibits mTORC1 have made it reasonable to classify AMPK under the tumor suppressive signaling pathway.
Indeed, recent studies using genetically modified mouse models supported the tumor suppressive role of LKB1. Although LKB1 deletion alone is not sufficient to develop lung tumors, it enhances K-Ras activation- or Pten deletion-induced lung tumorigenesis with elevated mTORC1 and SRC activity (Ji et al. 2007; Carretero et al. 2010; Xu et al. 2014). Similarly, LKB1 deletion has been shown to accelerate ERBB2-induced breast tumorigenesis with elevated mTORC1 activity (Dupuy et al. 2013). However, LKB1 deficiency in breast tumors reduces the lung metastatic burden, possibly due to the impaired metabolic flexibility during metastasis. Notably and in contrast to lung and breast, LKB1 deletion alone in the endometrium is sufficient to develop invasive endometrial carcinoma, which is highly responsive to rapamycin, an mTORC1 inhibitor, suggesting that mTORC1 activation could be the major downstream effector of LKB1 inhibition (Cheng et al. 2014; Contreras et al. 2010, 2008). Interestingly, recent studies have also suggested that LKB1 has an AMPK-mTORC1-independent tumor suppressive role, which would be dependent on MARK-mediated regulation of Snail or Hippo signaling (Mohseni et al. 2014; Goodwin et al. 2014).
In contrast with the several lines of evidence supporting the anti-tumorigenic role of LKB1, only one study to date has provided the genetic evidence supporting the anti-tumorigenic role of AMPK in vivo. A recent study by Jones and colleagues showed that deletion of AMPKα1, which is the sole catalytic subunit expressed in B lymphocytes, accelerates MYC-induced lymphomagenesis (Faubert et al. 2013), which seems to oppose the findings of the previous study that showed that AMPK has an essential role in MYC-induced hepatocellular carcinoma (HCC) development (Liu et al. 2012). Faubert et al. also provided evidence that the deletion of either LKB1 or AMPKα1 induces mTORC1-HIF1α-dependent metabolic reprograming that supports the energetic and anabolic demands of cancer cells (Faubert et al. 2013, 2014a). This also conflicts with another study mentioned above (Yan et al. 2014), which suggested that AMPK activates aerobic glycolysis through the PGC1α-ROS-HIF1α axis in the absence of FLCN. Although this discrepancy could be explained by the tissue-specific and context-dependent functions of AMPK, further work would be needed to better understand this complexity.
In addition to the genetic evidence, much conflicting pharmacological evidence regarding the role of AMPK in cancer has been reported. Retrospective epidemiological analysis proposed that long-term administration of the anti-diabetic drug metformin, which is known to indirectly activate AMPK through the inhibition of mitochondrial metabolism, has reduced the incidence and/or mortality of breast cancer among diabetic patients (Evans et al. 2005; Decensi et al. 2010). Moreover, numerous studies have shown that AMPK activators, such as AICAR and the anti-diabetic drugs metformin and phenformin, can inhibit cancer cell growth and proliferation (Rosilio et al. 2013; El-Masry et al. 2012; Buzzai et al. 2007; Appleyard et al. 2012). However, three recent studies have challenged these conclusions, which were drawn by the use of indirect or non-specific AMPK activators. Dasgupta and colleagues showed that AICAR and metformin, while non-specific or indirect activators of AMPK, can reduce glioblastoma proliferation and viability (Liu et al. 2014). Furthermore, a direct AMPK activator A769662 has no effect on proliferation, suggesting that the anti-tumorigenic effects of AICAR and metformin are AMPK-independent (Liu et al. 2014). Shaw and colleagues also showed that phenformin preferentially kills LKB1-deficient lung tumor cells in vivo, suggesting that the anti-tumor effect of phenformin is not dependent on the activation of AMPK, rather activation of AMPK in the presence of LKB1 actually compromises the cytotoxic effect of phenformin (Shackelford et al. 2013). Importantly, Jones and colleagues recently showed that all these nonspecific and indirect AMPK activators strongly inhibit cell proliferation and viability in the absence of AMPK, while these cytotoxic effects are reduced in the presence of AMPK; this suggests that the activation of AMPK actually antagonizes the cytotoxic effect of these indirect AMPK activators (Vincent et al. 2014). Furthermore, Jones and colleagues also showed that the direct AMPK agonist A769662 actually enhances cell proliferation under metabolic stress conditions, which supports the suggestion that AMPK has a pro-tumorigenic role in the tumor microenvironment. Thus, in contrast to the relatively solid experimental and genetic evidence supporting the anti-tumorigenic role of LKB1 in cancer, it seems that the role of AMPK in cancer need to be re-evaluated as an essential tumor promoter.
Resolving the LKB1/CAMKK2-AMPK paradox in cancer
While the original view suggested a tumor suppressive role for the LKB1-AMPK pathway, due to the finding of a loss of function of LKB1 in certain cancers and the inhibition of mTORC1 by the LKB1-AMPK pathway, the accumulated data also suggest that LKB1 might be a pro-tumorigenic regulator in other contexts. Thus, the key question arising is how can LKB1 be lost in certain cancers despite the disadvantages to metabolic adaptation and survival in the tumor microenvironment since the LKB1-AMPK pathway is essential for metabolic adaptation through the maintenance of energy and redox balance?
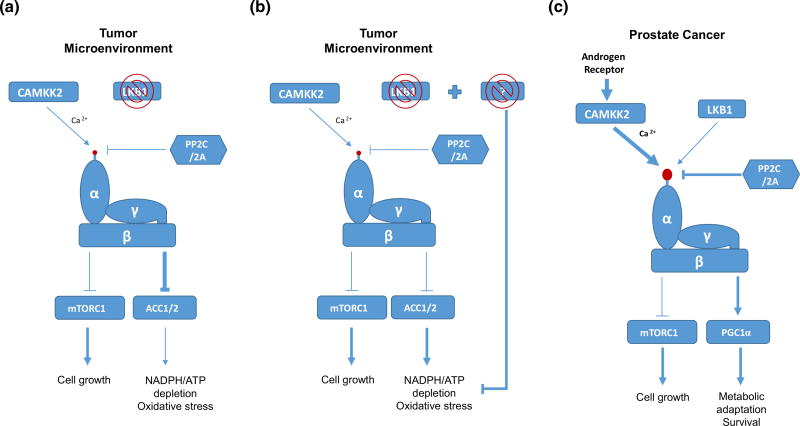
One possibility is that the levels of AMPK activity determine the different consequences of the AMPK activation. A recent study suggested that although AMPKα1-KO MEFs (α1 is the major isoform of AMPK in MEFs) and AMPKα1α2-DKO MEFs are resistant to oncogene-induced transformation, AMPKα2-KO MEFs (KO of a minor isoform) are sensitive to oncogene-induced transformation (Phoenix et al. 2012). These data raise the interesting possibility that in contrast to severe depletion of AMPK, slight or partial reduction of AMPK activity may be sufficient to trigger metabolic adaptation, while insufficient to inhibit mTORC1 activity (Fig. 2a). However, we cannot exclude the possibility that AMPKα1 and AMPKα2 may have different substrates and functions. Based on this observation, it may be plausible to speculate that in the absence of LKB1, moderate activation of AMPK by another upstream kinase such as CAMKK2 would be sufficient to trigger metabolic adaptation in tumor microenvironment, while insufficient to inhibit mTORC1 signaling (Fig. 2a). Indeed, it has been shown that matrix detachment of LKB1-deficient cancer cells can moderately activate AMPK in a CAMKK2-dependent manner, which is critical for anchorage-independent growth (Jeon et al. 2012). However, this hypothesis cannot fully explain the paradox since, unlike matrix detachment, nutrient deprivation-induced AMPK activation is mostly dependent on LKB1 (Hardie et al. 2012). This suggests that additional mechanisms that could compensate for the loss of LKB1-induced metabolic adaptation may be operating in LKB1-null cancer cells (Fig. 2b). Since previous studies have shown that the combination of K-Ras activation and LKB1 deficiency developed lung cancer (Ji et al. 2007), it would be interesting to investigate how K-Ras activation can compensate for the loss of LKB1-induced metabolic adaptation in K-Ras/LKB1 mutated lung cancers. Moreover, analysis of human cancer genome sequencing data should be very informative for finding the co-existing mutations in LKB1 mutated cancers, which synergize tumorigenesis by complementing the loss of the LKB1-AMPK pro-tumorigenic signaling (Fig. 2b).
Fig. 2.
The hypotheses to explain the paradox of the LKB1-AMPK pathway and the CAMKK2-AMPK pathway in cancer. a The LKB1-AMPK pathway. Partial activity of AMPK in the absence of LKB1 may be sufficient to trigger metabolic adaptation, while insufficient to inhibit mTORC1 in the tumor microenvironment. b The LKB1-AMPK pathway. Additional mutations that could compensate for the loss of LKB1-induced metabolic adaptation may exist in LKB1-deficient cancer. c The CAMKK2-AMPK pathway. Unlike to LKB1 induced activation of AMPK, AR-CAMKK2 induced activation of AMPK can only activate metabolic adaptation signaling but not growth inhibitory signaling. See text for details. Arrows denote activation, whereas horizontal bars indicate inhibition
In addition, although the LKB1-AMPK pathway in cancer has conflicting roles in cancer, the CAMKK2-AMPK pathway has been mostly reported as a pro-tumorigenic regulator, at least in prostate cancer (Frigo et al. 2011; Massie et al. 2011; Tennakoon et al. 2013). Thus, another interesting issue is to determine whether the activation of AMPK by LKB1 versus CAMKK2 results in different outcome or not. For example, it would be interesting to speculate that, unlike the LKB1-AMPK pathway, the CAMKK2-AMPK pathway may only activate metabolic adaptation pathways without inhibiting mTORC1 signaling, and thus can promote tumor growth (Fig. 2c). Supporting this possibility, a recent study showed that the inhibition of CAMKK2 cannot activate mTORC1 in prostate cancer (Massie et al. 2011). Therefore, further investigation of the differential consequences of AMPK activation by LKB1 and CAMKK2 may reveal novel mechanisms that determine the roles of AMPK in cancer.
Therapeutic implications in cancer: re-connection to biguanides
Since the discovery of the LKB1-AMPK-mTORC1 signaling axis, the activation of AMPK has been considered a reasonable anti-cancer strategy through its inhibition of mTORC1 (Kim and He 2013; Hadad et al. 2008). However, as discussed above, the roles of AMPK in cancer have turned out to be more complicated, suggesting that there are ‘two faces’ to the activity of AMPK in cancer (Fig. 3a). Importantly, the finding that the cytotoxic effects of metabolic inhibitors, which can indirectly activate AMPK, are enhanced in the absence of LKB1 or AMPK. This strongly suggests that AMPK activation could have more pro-survival effects in a therapeutic setting (Vincent et al. 2014). Thus, the combination of AMPK inhibitors with various metabolic inhibitors may be a reasonable therapeutic strategy for cancer.
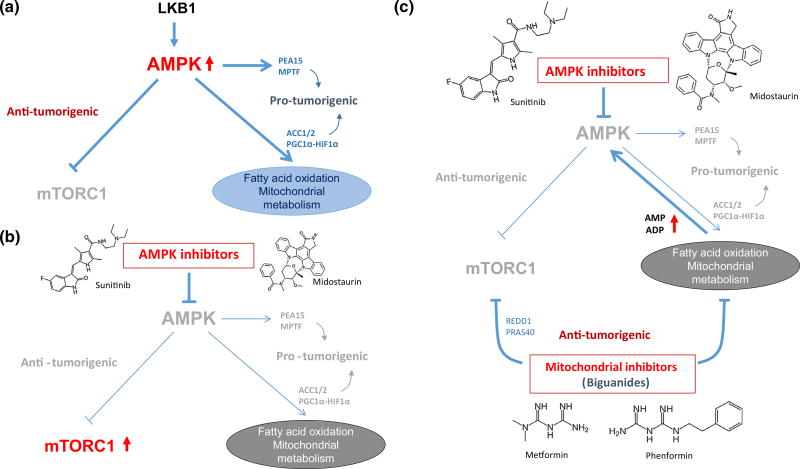
Fig. 3.
Novel therapeutic strategies targeting AMPK and mitochondrial metabolism. a The dual effects of AMPK activation on tumorigenesis. b The effects of AMPK inhibitors such as sunitinib or midostaurine on anti- and pro-tumorigenic signaling of AMPK. c The effects of biguanides in combination with AMPK inhibitors on anti- and pro- tumorigenic signaling of AMPK. See text for details. Arrows denote activation, whereas horizontal bars indicate inhibition. Gray text indicates inactivation. REDD1 regulated in development and DNA damage responses 1, PRAS40 proline-rich Akt substrate of 40 kDa
Currently, only one molecule, compound C, has been identified as a selective AMPK inhibitor (Zhou et al. 2001). Compound C was identified in a high-throughput kinase assay and is known to bind to the AMPKα subunit. However, many studies have shown that compound C can actually inhibit many other kinases as well as bone morphogenetic protein (BMP) receptor, indicating its promiscuity (Sinnett and Brenman 2014). Interestingly, a recent study showed that sunitinib, a multiple tyrosine kinase inhibitor that is used clinically against advanced renal cell carcinoma (RCC) and gastrointestinal stromal tumor (GIST), can directly inhibit AMPK activity by binding to the AMPKα subunit (Laderoute et al. 2010). Laderoute et al. also demonstrated that sunitinib is a more potent inhibitor of AMPK than compound C, both in vitro and in vivo. Congruently, another study showed that AMPKα1 was pulled-down with sunitinib and midostaurin when they were treated in melanoma cell lines (Borgdorff et al. 2014). They demonstrated that these two inhibitors can inhibit AMPK resulting in the degradation of MITF, and consequently induce cell death in melanoma cell lines; this was discussed earlier. Therefore, it is possible that the cytotoxic effects of sunitinib and midostaurin can be attributed, at least in part, to their inhibition of AMPK (Fig. 3a, b).
However, one concern and drawback of the inhibition of AMPK would be the hyperactivation of mTORC1, which can compromise the anti-cancer effect of the AMPK inhibition (Fig. 3b). Among the various metabolic inhibitors, the anti-diabetic biguanides phenformin and metformin, which inhibit mitochondrial metabolism, have been shown to inhibit mTORC1 in both AMPK-dependent and independent mechanisms (Fig. 3c) (Nair et al. 2014; Liu et al. 2014; Ben Sahra et al. 2011; Vincent et al. 2014). Moreover, given that the pro-survival effects of AMPK activation are mediated, at least in part, through the activation of mitochondrial metabolism, the use of biguanides should be an attractive strategy for bypassing part of their pro-survival effects since they are mitochondrial inhibitors (Fig. 3c). Furthermore, recent studies have shown that cancer stem cells are dependent on mitochondrial metabolism, and metformin and phenformin preferentially kill various cancer stem cells (Janzer et al. 2014; Honjo et al. 2014; Lonardo et al. 2013; Shank et al. 2012; Song et al. 2012; Vazquez-Martin et al. 2011; Hirsch et al. 2009; Viale et al. 2014). Indeed, a number of clinical trials of metformin are ongoing as therapy for a variety of cancers (Pollak 2013). Thus, we propose that the combination of biguanides with AMPK inhibitors would be the ‘best bet’ as this strategy would strongly enhance metabolic stress with preventing pro-tumorigenic signals such as the activation of mTORC1 and mitochondrial metabolism (Fig. 3c). For proof of concept, although no specific AMPK inhibitors are currently available, it would be important to examine if sunitinib or midostaurin in combination with phenformin or metformin do indeed have synergistic anti-cancer effects.
Concluding remarks
Although AMPK was originally suggested as suppressing tumors by mediating LKB1 signaling to mTORC1 inhibition, now the accumulated data suggest that AMPK could be a tumor promoter by protecting cancer cells from death in harsh tumor microenvironments. Importantly, it turns out that the secondary activation of AMPK by many metabolic inhibitors actually induces resistance to cell death. This suggests that, rather than activation, inhibition of AMPK with concurrent induction of metabolic stress should be a more reasonable strategy for treating cancers and this in combination with biguanides should be the best combination strategy. Thus, this would be reasonable approach for investigating the synergistic anti-cancer effects of the combination of clinically relevant AMPK inhibitors with anti-diabetic biguanides, which will change the paradigm of AMPK inhibitors as a novel and promising anti-cancer strategy.
Acknowledgments
This work was supported by grants from the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (S2014-A0251-00001) and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (S2014-A0403-00067).
Contributor Information
Sang-Min Jeon, College of Pharmacy, Ajou University, Suwon, Gyeonggi-do 443-749, Republic of Korea.
Nissim Hay, Department of Biochemistry and Molecular Genetics, College of Medicine, University of Illinois at Chicago, Chicago, IL 60607, USA.
References
- Appleyard MV, Murray KE, Coates PJ, Wullschleger S, Bray SE, Kernohan NM, Fleming S, Alessi DR, Thompson AM. Phenformin as prophylaxis and therapy in breast cancer xenografts. British Journal of Cancer. 2012;106:1117–1122. doi: 10.1038/bjc.2012.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auciello FR, Ross FA, Ikematsu N, Hardie DG. Oxidative stress activates AMPK in cultured cells primarily by increasing cellular AMP and/or ADP. FEBS Letters. 2014;588:3361–3366. doi: 10.1016/j.febslet.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardeesy N, Sinha M, Hezel AF, Signoretti S, Hathaway NA, Sharpless NE, Loda M, Carrasco DR, Depinho RA. Loss of the Lkb1 tumour suppressor provokes intestinal polyposis but resistance to transformation. Nature. 2002;419:162–167. doi: 10.1038/nature01045. [DOI] [PubMed] [Google Scholar]
- Borgdorff V, Rix U, Winter GE, Gridling M, Muller AC, Breitwieser FP, Wagner C, Colinge J, Bennett KL, Superti-Furga G, Wagner SN. A chemical biology approach identifies AMPK as a modulator of melanoma oncogene MITF. Oncogene. 2014;33:2531–2539. doi: 10.1038/onc.2013.185. [DOI] [PubMed] [Google Scholar]
- Buzzai M, Jones RG, Amaravadi RK, Lum JJ, Deberardinis RJ, Zhao F, Viollet B, Thompson CB. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Research. 2007;67:6745–6752. doi: 10.1158/0008-5472.CAN-06-4447. [DOI] [PubMed] [Google Scholar]
- Cardaci S, Filomeni G, Ciriolo MR. Redox implications of AMPK-mediated signal transduction beyond energetic clues. Journal of Cell Science. 2012;125:2115–2125. doi: 10.1242/jcs.095216. [DOI] [PubMed] [Google Scholar]
- Carling D, Zammit VA, Hardie DG. A common bicyclic protein kinase cascade inactivates the regulatory enzymes of fatty acid and cholesterol biosynthesis. FEBS Letters. 1987;223:217–222. doi: 10.1016/0014-5793(87)80292-2. [DOI] [PubMed] [Google Scholar]
- Carretero J, Shimamura T, Rikova K, Jackson AL, Wilkerson MD, Borgman CL, Buttarazzi MS, Sanofsky BA, Mcnamara KL, Brandstetter KA, Walton ZE, Gu TL, Silva JC, Crosby K, Shapiro GI, Maira SM, Ji H, Castrillon DH, Kim CF, Garcia-Echeverria C, Bardeesy N, Sharpless NE, Hayes ND, Kim WY, Engelman JA, Wong KK. Integrative genomic and proteomic analyses identify targets for Lkb1-deficient metastatic lung tumors. Cancer Cell. 2010;17:547–559. doi: 10.1016/j.ccr.2010.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Liu P, Zhang F, Xu E, Symonds L, Ohlson CE, Bronson RT, Maira SM, Di Tomaso E, Li J, Myers AP, Cantley LC, Mills GB, Zhao JJ. A genetic mouse model of invasive endometrial cancer driven by concurrent loss of Pten and Lkb1 Is highly responsive to mTOR inhibition. Cancer Research. 2014;74:15–23. doi: 10.1158/0008-5472.CAN-13-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SL, Kim SJ, Lee KT, Kim J, Mu J, Birnbaum MJ, Soo Kim S, Ha J. The regulation of AMP-activated protein kinase by H(2)O(2) Biochemical and biophysical research communications. 2001;287:92–97. doi: 10.1006/bbrc.2001.5544. [DOI] [PubMed] [Google Scholar]
- Contreras CM, Akbay EA, Gallardo TD, Haynie JM, Sharma S, Tagao O, Bardeesy N, Takahashi M, Settleman J, Wong KK, Castrillon DH. Lkb1 inactivation is sufficient to drive endometrial cancers that are aggressive yet highly responsive to mTOR inhibitor monotherapy. Disease models & mechanisms. 2010;3:181–193. doi: 10.1242/dmm.004440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras CM, Gurumurthy S, Haynie JM, Shirley LJ, Akbay EA, Wingo SN, Schorge JO, Broaddus RR, Wong KK, Bardeesy N, Castrillon DH. Loss of Lkb1 provokes highly invasive endometrial adenocarcinomas. Cancer Research. 2008;68:759–766. doi: 10.1158/0008-5472.CAN-07-5014. [DOI] [PubMed] [Google Scholar]
- Corradetti MN, Inoki K, Bardeesy N, Depinho RA, Guan KL. Regulation of the TSC pathway by LKB1: evidence of a molecular link between tuberous sclerosis complex and Peutz-Jeghers syndrome. Genes & Development. 2004;18:1533–1538. doi: 10.1101/gad.1199104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SP, Helps NR, Cohen PT, Hardie DG. 5′-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2C alpha and native bovine protein phosphatase-2AC. FEBS Letters. 1995;377:421–425. doi: 10.1016/0014-5793(95)01368-7. [DOI] [PubMed] [Google Scholar]
- Decensi A, Puntoni M, Goodwin P, Cazzaniga M, Gennari A, Bonanni B, Gandini S. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer prevention research. 2010;3:1451–1461. doi: 10.1158/1940-6207.CAPR-10-0157. [DOI] [PubMed] [Google Scholar]
- Dupuy F, Griss T, Blagih J, Bridon G, Avizonis D, Ling C, Dong Z, Siwak DR, Annis MG, Mills GB, Muller WJ, Siegel PM, Jones RG. LKB1 is a central regulator of tumor initiation and pro-growth metabolism in ErbB2-mediated breast cancer. Cancer & metabolism. 2013;1:18. doi: 10.1186/2049-3002-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Masry OS, Brown BL, Dobson PR. Effects of activation of AMPK on human breast cancer cell lines with different genetic backgrounds. Oncology letters. 2012;3:224–228. doi: 10.3892/ol.2011.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerling BM, Weinberg F, Snyder C, Burgess Z, Mutlu GM, Viollet B, Budinger GR, Chandel NS. Hypoxic activation of AMPK is dependent on mitochondrial ROS but independent of an increase in AMP/ATP ratio. Free Radical Biology and Medicine. 2009;46:1386–1391. doi: 10.1016/j.freeradbiomed.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faubert B, Boily G, Izreig S, Griss T, Samborska B, Dong Z, Dupuy F, Chambers C, Fuerth BJ, Viollet B, Mamer OA, Avizonis D, Deberardinis RJ, Siegel PM, Jones RG. AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metabolism. 2013;17:113–124. doi: 10.1016/j.cmet.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faubert B, Vincent EE, Griss T, Samborska B, Izreig S, Svensson RU, Mamer OA, Avizonis D, Shackelford DB, Shaw RJ, Jones RG. Loss of the tumor suppressor LKB1 promotes metabolic reprogramming of cancer cells via HIF-1alpha. Proceedings of the National Academy of Sciences of the United States of America. 2014a;111:2554–2559. doi: 10.1073/pnas.1312570111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faubert B, Vincent EE, Poffenberger MC, Jones RG. The AMP-activated protein kinase (AMPK) and cancer: Many faces of a metabolic regulator. Cancer letters. 2014b;356(2):165–70. doi: 10.1016/j.canlet.2014.01.018. [DOI] [PubMed] [Google Scholar]
- Fernandez MR, Henry MD, Lewis RE. Kinase suppressor of Ras 2 (KSR2) regulates tumor cell transformation via AMPK. Molecular and Cellular Biology. 2012;32:3718–3731. doi: 10.1128/MCB.06754-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigo DE, Howe MK, Wittmann BM, Brunner AM, Cushman I, Wang Q, Brown M, Means AR, Mcdonnell DP. CaM kinase kinase beta-mediated activation of the growth regulatory kinase AMPK is required for androgen-dependent migration of prostate cancer cells. Cancer Research. 2011;71:528–537. doi: 10.1158/0008-5472.CAN-10-2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung C, Lock R, Gao S, Salas E, Debnath J. Induction of autophagy during extracellular matrix detachment promotes cell survival. Molecular Biology of the Cell. 2008;19:797–806. doi: 10.1091/mbc.E07-10-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godlewski J, Nowicki MO, Bronisz A, Nuovo G, Palatini J, De Lay M, Van Brocklyn J, Ostrowski MC, Chiocca EA, Lawler SE. MicroRNA-451 regulates LKB1/AMPK signaling and allows adaptation to metabolic stress in glioma cells. Molecular Cell. 2010;37:620–632. doi: 10.1016/j.molcel.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin JM, Svensson RU, Lou HJ, Winslow MM, Turk BE, Shaw RJ. An AMPK-independent signaling pathway downstream of the LKB1 tumor suppressor controls Snail1 and metastatic potential. Molecular Cell. 2014;55:436–450. doi: 10.1016/j.molcel.2014.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowans GJ, Hawley SA, Ross FA, Hardie DG. AMP is a true physiological regulator of AMP-activated protein kinase by both allosteric activation and enhancing net phosphorylation. Cell Metabolism. 2013;18:556–566. doi: 10.1016/j.cmet.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadad SM, Fleming S, Thompson AM. Targeting AMPK: a new therapeutic opportunity in breast cancer. Critical reviews in oncology/hematology. 2008;67:1–7. doi: 10.1016/j.critrevonc.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Alessi DR. LKB1 and AMPK and the cancer-metabolism link - ten years after. BMC Biology. 2013;11:36. doi: 10.1186/1741-7007-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nature Reviews Molecular Cell Biology. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. Journal of biology. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metabolism. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Hawley SA, Ross FA, Chevtzoff C, Green KA, Evans A, Fogarty S, Towler MC, Brown LJ, Ogunbayo OA, Evans AM, Hardie DG. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metabolism. 2010;11:554–565. doi: 10.1016/j.cmet.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemminki A, Markie D, Tomlinson I, Avizienyte E, Roth S, Loukola A, Bignell G, Warren W, Aminoff M, Hoglund P, Jarvinen H, Kristo P, Pelin K, Ridanpaa M, Salovaara R, Toro T, Bodmer W, Olschwang S, Olsen AS, Stratton MR, De La Chapelle A, Aaltonen LA. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998;391:184–187. doi: 10.1038/34432. [DOI] [PubMed] [Google Scholar]
- Hindupur SK, Balaji SA, Saxena M, Pandey S, Sravan G, Heda N, Kumar M, Mukherjee G, Dey D, Rangarajan A. Identification of a novel AMPK-PEA15 axis in the anoikis-resistant growth of mammary cells. Breast cancer research : BCR. 2014;16:420. doi: 10.1186/s13058-014-0420-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Research. 2009;69:7507–7511. doi: 10.1158/0008-5472.CAN-09-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo S, Ajani JA, Scott AW, Chen Q, Skinner HD, Stroehlein J, Johnson RL, Song S. Metformin sensitizes chemotherapy by targeting cancer stem cells and the mTOR pathway in esophageal cancer. International Journal of Oncology. 2014;45:567–574. doi: 10.3892/ijo.2014.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- Jang T, Calaoagan JM, Kwon E, Samuelsson S, Recht L, Laderoute KR. 5′-AMP-activated protein kinase activity is elevated early during primary brain tumor development in the rat. International journal of cancer. Journal international du cancer. 2011;128:2230–2239. doi: 10.1002/ijc.25558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzer A, German NJ, Gonzalez-Herrera KN, Asara JM, Haigis MC, Struhl K. Metformin and phenformin deplete tricarboxylic acid cycle and glycolytic intermediates during cell transformation and NTPs in cancer stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:10574–10579. doi: 10.1073/pnas.1409844111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon SM, Chandel NS, Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 2012;485:661–665. doi: 10.1038/nature11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon SM, Hay N. The dark face of AMPK as an essential tumor promoter. Cellular logistics. 2012;2:197–202. doi: 10.4161/cl.22651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Ramsey MR, Hayes DN, Fan C, Mcnamara K, Kozlowski P, Torrice C, Wu MC, Shimamura T, Perera SA, Liang MC, Cai D, Naumov GN, Bao L, Contreras CM, Li D, Chen L, Krishnamurthy J, Koivunen J, Chirieac LR, Padera RF, Bronson RT, Lindeman NI, Christiani DC, Lin X, Shapiro GI, Janne PA, Johnson BE, Meyerson M, Kwiatkowski DJ, Castrillon DH, Bardeesy N, Sharpless NE, Wong KK. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448:807–810. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- Kato K, Ogura T, Kishimoto A, Minegishi Y, Nakajima N, Miyazaki M, Esumi H. Critical roles of AMP-activated protein kinase in constitutive tolerance of cancer cells to nutrient deprivation and tumor formation. Oncogene. 2002;21:6082–6090. doi: 10.1038/sj.onc.1205737. [DOI] [PubMed] [Google Scholar]
- Kim I, He YY. Targeting the AMP-activated protein kinase for cancer prevention and therapy. Frontiers in oncology. 2013;3:175. doi: 10.3389/fonc.2013.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laderoute KR, Amin K, Calaoagan JM, Knapp M, Le T, Orduna J, Foretz M, Viollet B. 5′-AMP-activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments. Molecular and Cellular Biology. 2006;26:5336–5347. doi: 10.1128/MCB.00166-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laderoute KR, Calaoagan JM, Chao WR, Dinh D, Denko N, Duellman S, Kalra J, Liu X, Papandreou I, Sambucetti L, Boros LG. 5′-AMP-activated protein kinase (AMPK) supports the growth of aggressive experimental human breast cancer tumors. The Journal of biological chemistry. 2014;289:22850–22864. doi: 10.1074/jbc.M114.576371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laderoute KR, Calaoagan JM, Madrid PB, Klon AE, Ehrlich PJ. SU11248 (sunitinib) directly inhibits the activity of mammalian 5′-AMP-activated protein kinase (AMPK) Cancer Biology & Therapy. 2010;10:68–76. doi: 10.4161/cbt.10.1.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Mills GB. AMPK: a contextual oncogene or tumor suppressor? Cancer Research. 2013;73:2929–2935. doi: 10.1158/0008-5472.CAN-12-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Ulbrich J, Muller J, Wustefeld T, Aeberhard L, Kress TR, Muthalagu N, Rycak L, Rudalska R, Moll R, Kempa S, Zender L, Eilers M, Murphy DJ. Deregulated MYC expression induces dependence upon AMPK-related kinase 5. Nature. 2012;483:608–612. doi: 10.1038/nature10927. [DOI] [PubMed] [Google Scholar]
- Liu X, Chhipa RR, Pooya S, Wortman M, Yachyshin S, Chow LM, Kumar A, Zhou X, Sun Y, Quinn B, Mcpherson C, Warnick RE, Kendler A, Giri S, Poels J, Norga K, Viollet B, Grabowski GA, Dasgupta B. Discrete mechanisms of mTOR and cell cycle regulation by AMPK agonists independent of AMPK. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E435–E444. doi: 10.1073/pnas.1311121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonardo E, Cioffi M, Sancho P, Sanchez-Ripoll Y, Trabulo SM, Dorado J, Balic A, Hidalgo M, Heeschen C. Metformin targets the metabolic achilles heel of human pancreatic cancer stem cells. PLoS ONE. 2013;8:e76518. doi: 10.1371/journal.pone.0076518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massie CE, Lynch A, Ramos-Montoya A, Boren J, Stark R, Fazli L, Warren A, Scott H, Madhu B, Sharma N, Bon H, Zecchini V, Smith DM, Denicola GM, Mathews N, Osborne M, Hadfield J, Macarthur S, Adryan B, Lyons SK, Brindle KM, Griffiths J, Gleave ME, Rennie PS, Neal DE, Mills IG. The androgen receptor fuels prostate cancer by regulating central metabolism and biosynthesis. The EMBO journal. 2011;30:2719–2733. doi: 10.1038/emboj.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrachy-Schwartz S, Cohen N, Klein S, Kravchenko-Balasha N, Levitzki A. Up-regulation of AMP-activated protein kinase in cancer cell lines is mediated through c-Src activation. The Journal of biological chemistry. 2011;286:15268–15277. doi: 10.1074/jbc.M110.211813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohseni M, Sun J, Lau A, Curtis S, Goldsmith J, Fox VL, Wei C, Frazier M, Samson O, Wong KK, Kim C, Camargo FD. A genetic screen identifies an LKB1-MARK signalling axis controlling the Hippo-YAP pathway. Nature Cell Biology. 2014;16:108–117. doi: 10.1038/ncb2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungai PT, Waypa GB, Jairaman A, Prakriya M, Dokic D, Ball MK, Schumacker PT. Hypoxia triggers AMPK activation through reactive oxygen species-mediated activation of calcium release-activated calcium channels. Molecular and Cellular Biology. 2011;31:3531–3545. doi: 10.1128/MCB.05124-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair V, Sreevalsan S, Basha R, Abdelrahim M, Abudayyeh A, Rodrigues Hoffman A, Safe S. Mechanism of metformin-dependent inhibition of mammalian target of rapamycin (mTOR) and Ras activity in pancreatic cancer: role of specificity protein (Sp) transcription factors. The Journal of biological chemistry. 2014;289:27692–27701. doi: 10.1074/jbc.M114.592576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng TL, Leprivier G, Robertson MD, Chow C, Martin MJ, Laderoute KR, Davicioni E, Triche TJ, Sorensen PH. The AMPK stress response pathway mediates anoikis resistance through inhibition of mTOR and suppression of protein synthesis. Cell Death and Differentiation. 2012;19:501–510. doi: 10.1038/cdd.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phoenix KN, Devarakonda CV, Fox MM, Stevens LE, Claffey KP. AMPKalpha2 suppresses murine embryonic fibroblast transformation and tumorigenesis. Genes and cancer. 2012;3:51–62. doi: 10.1177/1947601912452883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak M. Potential applications for biguanides in oncology. The Journal of Clinical Investigation. 2013;123:3693–3700. doi: 10.1172/JCI67232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possik E, Jalali Z, Nouet Y, Yan M, Gingras MC, Schmeisser K, Panaite L, Dupuy F, Kharitidi D, Chotard L, Jones RG, Hall DH, Pause A. Folliculin regulates ampk-dependent autophagy and metabolic stress survival. PLoS Genetics. 2014;10:e1004273. doi: 10.1371/journal.pgen.1004273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios M, Foretz M, Viollet B, Prieto A, Fraga M, Costoya JA, Senaris R. AMPK activation by oncogenesis is required to maintain cancer cell proliferation in astrocytic tumors. Cancer Research. 2013;73:2628–2638. doi: 10.1158/0008-5472.CAN-12-0861. [DOI] [PubMed] [Google Scholar]
- Ros S, Santos CR, Moco S, Baenke F, Kelly G, Howell M, Zamboni N, Schulze A. Functional metabolic screen identifies 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 as an important regulator of prostate cancer cell survival. Cancer discovery. 2012;2:328–343. doi: 10.1158/2159-8290.CD-11-0234. [DOI] [PubMed] [Google Scholar]
- Rosilio C, Lounnas N, Nebout M, Imbert V, Hagenbeek T, Spits H, Asnafi V, Pontier-Bres R, Reverso J, Michiels JF, Sahra IB, Bost F, Peyron JF. The metabolic perturbators metformin, phenformin and AICAR interfere with the growth and survival of murine PTEN-deficient T cell lymphomas and human T-ALL/T-LL cancer cells. Cancer Letters. 2013;336:114–126. doi: 10.1016/j.canlet.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Sahra IB, Regazzetti C, Robert G, Laurent K, Le Marchand-Brustel Y, Auberger P, Tanti JF, Giorgetti-Peraldi S, Bost F. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Research. 2011;71(13):4366–4372. doi: 10.1158/0008-5472.CAN-10-1769. [DOI] [PubMed] [Google Scholar]
- Schafer ZT, Grassian AR, Song L, Jiang Z, Gerhart-Hines Z, Irie HY, Gao S, Puigserver P, Brugge JS. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature. 2009;461:109–113. doi: 10.1038/nature08268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackelford DB, Abt E, Gerken L, Vasquez DS, Seki A, Leblanc M, Wei L, Fishbein MC, Czernin J, Mischel PS, Shaw RJ. LKB1 inactivation dictates therapeutic response of non-small cell lung cancer to the metabolism drug phenformin. Cancer Cell. 2013;23:143–158. doi: 10.1016/j.ccr.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nature Reviews Cancer. 2009;9:563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank JJ, Yang K, Ghannam J, Cabrera L, Johnston CJ, Reynolds RK, Buckanovich RJ. Metformin targets ovarian cancer stem cells in vitro and in vivo. Gynecologic Oncology. 2012;127:390–397. doi: 10.1016/j.ygyno.2012.07.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She C, Zhu LQ, Zhen YF, Wang XD, Dong QR. Activation of AMPK protects against hydrogen peroxide-induced osteoblast apoptosis through autophagy induction and NADPH maintenance: new implications for osteonecrosis treatment? Cellular Signalling. 2014;26:1–8. doi: 10.1016/j.cellsig.2013.08.046. [DOI] [PubMed] [Google Scholar]
- Sinnett SE, Brenman JE. Past strategies and future directions for identifying AMP-activated protein kinase (AMPK) modulators. Pharmacology & Therapeutics. 2014;143:111–118. doi: 10.1016/j.pharmthera.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song CW, Lee H, Dings RP, Williams B, Powers J, Santos TD, Choi BH, Park HJ. Metformin kills and radiosensitizes cancer cells and preferentially kills cancer stem cells. Scientific reports. 2012;2:362. doi: 10.1038/srep00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg GR, Kemp BE. AMPK in health and disease. Physiological Reviews. 2009;89:1025–1078. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- Tennakoon JB, Shi Y, Han JJ, Tsouko E, White MA, Burns AR, Zhang A, Xia X, Ilkayeva OR, Xin L, Ittmann MM, Rick FG, Schally AV, Frigo DE. Androgens regulate prostate cancer cell growth via an AMPK-PGC-1alpha-mediated metabolic switch. Oncogene. 2013;33(45):5251–5261. doi: 10.1038/onc.2013.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Martin A, Oliveras-Ferraros C, Del Barco S, Martin-Castillo B, Menendez JA. The anti-diabetic drug metformin suppresses self-renewal and proliferation of trastuzumab-resistant tumor-initiating breast cancer stem cells. Breast Cancer Research and Treatment. 2011;126:355–364. doi: 10.1007/s10549-010-0924-x. [DOI] [PubMed] [Google Scholar]
- Viale A, Pettazzoni P, Lyssiotis CA, Ying H, Sanchez N, Marchesini M, Carugo A, Green T, Seth S, Giuliani V, Kost-Alimova M, Muller F, Colla S, Nezi L, Genovese G, Deem AK, Kapoor A, Yao W, Brunetto E, Kang Y, Yuan M, Asara JM, Wang YA, Heffernan TP, Kimmelman AC, Wang H, Fleming JB, Cantley LC, Depinho RA, Draetta GF. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature. 2014;514(7524):628–632. doi: 10.1038/nature13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent EE, Coelho PP, Blagih J, Griss T, Viollet B, Jones RG. Differential effects of AMPK agonists on cell growth and metabolism. Oncogene. 2014 doi: 10.1038/onc.2014.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. Ca2 +/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metabolism. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Current biology: CB. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Xu C, Fillmore CM, Koyama S, Wu H, Zhao Y, Chen Z, Herter-Sprie GS, Akbay EA, Tchaicha JH, Altabef A, Reibel JB, Walton Z, Ji H, Watanabe H, Janne PA, Castrillon DH, Rustgi AK, Bass AJ, Freeman GJ, Padera RF, Dranoff G, Hammerman PS, Kim CF, Wong KK. Loss of Lkb1 and Pten leads to lung squamous cell carcinoma with elevated PD-L1 expression. Cancer Cell. 2014;25:590–604. doi: 10.1016/j.ccr.2014.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan M, Gingras MC, Dunlop EA, Nouet Y, Dupuy F, Jalali Z, Possik E, Coull BJ, Kharitidi D, Dydensborg AB, Faubert B, Kamps M, Sabourin S, Preston RS, Davies DM, Roughead T, Chotard L, Van Steensel MA, Jones R, Tee AR, Pause A. The tumor suppressor folliculin regulates AMPK-dependent metabolic transformation. The Journal of Clinical Investigation. 2014;124:2640–2650. doi: 10.1172/JCI71749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YL, Guo H, Zhang CS, Lin SY, Yin Z, Peng Y, Luo H, Shi Y, Lian G, Zhang C, Li M, Ye Z, Ye J, Han J, Li P, Wu JW, Lin SC. AMP as a low-energy charge signal autonomously initiates assembly of AXIN-AMPK-LKB1 complex for AMPK activation. Cell Metabolism. 2013;18:546–555. doi: 10.1016/j.cmet.2013.09.005. [DOI] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. The Journal of Clinical Investigation. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmijewski JW, Banerjee S, Bae H, Friggeri A, Lazarowski ER, Abraham E. Exposure to hydrogen peroxide induces oxidation and activation of AMP-activated protein kinase. The Journal of biological chemistry. 2010;285:33154–33164. doi: 10.1074/jbc.M110.143685. [DOI] [PMC free article] [PubMed] [Google Scholar]