Abstract
Human papillomaviruses (HPVs) are the causative agents of cervical and other cancers. The oncoprotein E7 activates the cell cycle and makes possible replication of the viral genome in differentiating epithelia. The HPV16 late promoter is activated upon cellular differentiation and regulates late gene expression. We investigated the effect of E7 on the late promoter and found that E7 was able to activate the promoter. In contrast, the other known viral transcriptional regulator, E2, had no effect on the late promoter. Promoter activation by E7 occurred despite inhibition of promoter activity by factors involved in the cell cycle, such as cyclin dependent kinases and E2F transcription factors, and by the ability of E7 to disrupt several aspects of cellular differentiation. These results suggest a new role for E7 in the context of the viral life cycle and shed light on the complex regulation of viral gene expression in infected, differentiating epithelia.
Keywords: papillomavirus, E7, E2, differentiation, late promoter, CDK, E2F
Introduction
Human papillomaviruses (HPVs) are small, non-enveloped DNA viruses that persistently infect the keratinocytes of stratified squamous epithelia (zur Hausen, 2009). Genital HPVs are the most common sexually transmitted disease (STD) agents, and certain “high risk” HPV types are necessary for the development of cervical cancer, which is a leading cause of cancer mortality in women worldwide (Parkin and Bray, 2006; Weinstock et al., 2004). In normal stratified epithelia, the only actively dividing cells are the basal cells located adjacent to the basement membrane. As basal cells divide, one daughter detaches from the basement membrane and begins to differentiate. Differentiating cells withdraw from the cell cycle and undergo a complex, highly regulated series of changes in gene expression (Simpson et al., 2011; Watt, 1998). Ultimately the cells die and are shed into the environment. HPV establishes infection in the long-lived cells of the basal layer, where viral genomes are maintained at approximately 50-100 copies per cell and viral gene expression is low. As host cells move from the basal layer and undergo differentiation, viral replication and gene expression are activated in a tightly regulated and stepwise manner resulting in the formation of virus particles in the most superficial layers and shedding into the environment (Bodily and Laimins, 2011).
High risk HPV infections typically last 1-2 years (Moscicki et al., 2006; Richardson et al., 2003; Woodman et al., 2001), but persistence by HPV over longer periods facilitates the accumulation of secondary cellular mutations and is thus the single most important risk factor for the development of HPV-induced cancers (Markowitz et al., 2007; Stanley, 2008; Woodman et al., 2001). Each step of the HPV life cycle is designed to maximize the ability of the virus to remain undetected in tissue for long periods (Bodily and Laimins, 2011). Since the capsid proteins L1 and L2 are major antigens, high level viral protein synthesis is restricted by a variety of mechanisms to the highly differentiated layers where immune surveillance is thought to be less stringent (Frazer, 2009). One of these mechanisms is that the late promoter, which drives expression of these genes, depends on cellular differentiation for its activity (Middleton et al., 2003; Peh et al., 2002; Ruesch and Laimins, 1998). In addition to driving expression of the capsid genes, the late promoter controls expression of viral regulatory proteins (E1, E2, E1ˆE4, and E5) involved in amplification of the viral genome (Fehrmann et al., 2003; Hummel et al., 1992; Ozbun and Meyers, 1997, 1998; Peh et al., 2004; Wilson et al., 2005). As a result, viral copy number increases to several thousand copies per cell in a differentiation-dependent manner (Bedell et al., 1991). Thus the late promoter contributes to HPV persistence by targeting high level genome replication and capsid protein expression to the proper place in the tissue. Some work has been done to map which differentiation-specific cellular factors contribute to promoter activity (Bodily et al., 2006; Bodily and Meyers, 2005; Gunasekharan et al., 2012; Kukimoto and Kanda, 2001; Kukimoto et al., 2006; Spink and Laimins, 2005), but details of its regulation remain elusive.
Differentiation-dependent compartmentalization of HPV gene expression is an important persistence strategy, but it creates a difficulty for the virus. Because the virus does not encode its own DNA replication factors, cellular DNA synthesis proteins are needed for productive viral replication. However, keratinocytes withdraw from the cell cycle upon differentiation and these cellular factors become unavailable to the virus (Watt, 1998). To circumvent this problem, the HPV forces differentiating cells to reenter the cell cycle, thus enabling viral DNA replication in cells that would otherwise be non-permissive (Munger et al., 2004). The viral protein primarily responsible for driving the cell cycle and maintaining replication competence in differentiating cells is E7. The major known activity of E7 is to bind to and inactivate members of the pRb family, resulting in the release of E2F transcription factors to promote expression of genes that produce an S phase-like environment. Degradation of the pocket protein p130 by E7 is thought to delay differentiation, allowing the virus a chance to replicate its genome (Klingelhutz and Roman, 2012). Additional activities of E7 are also reported, including regulation of transcription through binding to a wide variety of transcriptional regulatory factors, primarily through the C terminus. The roles of these interactions in the viral life cycle or in cancer development are unclear.
One consequence of viral oncogene expression is the creation of an unusual cellular environment in which cell cycling and differentiation, which are normally mutually exclusive processes, can occur simultaneously, albeit in modified form (Davy et al., 2005; McCance et al., 1988). How these two important processes interrelate in HPV-infected cells is not entirely clear, but late events in the viral life cycle, including transcription from the late promoter, have undoubtedly evolved to occur under these specific conditions. Because forced cell cycling is also central to the tendency of high risk HPV-induced lesions to progress to malignancy, understanding the interaction between differentiation and the cell cycle during HPV infections will illuminate the conditions under which HPV-induced cancers arise.
This study grew out of previous work in which we performed extensive mutagenesis of the E7 open reading frame (ORF) in the context of the complete viral genome in order to better understand the functions of E7 in the viral life cycle (Bodily et al., 2011a). Because the late promoter is imbedded within the E7 ORF, there was a chance that our mutations would impact cis promoter elements, which turned out to be the case for some mutants. However, it was also possible that mutation of E7 could somehow affect promoter activity through an effect on E7 protein in trans. Because of the antagonistic relationship between cell cycling, which is induced by E7, and differentiation, which is required for promoter activity, we expected that any effect of E7 protein expression on promoter activity was likely to be negative. Instead, we uncovered a novel and unexpected ability of E7 to activate the HPV16 late promoter. Activation by E7 was independent of transcriptional regulation by E2, which we found does not regulate the HPV16 late promoter. Late promoter activation was mapped to motifs in E7 that regulate the cell cycle. Despite the ability of E7 to promote cell cycle progression, we found that factors that promote cell cycle progression markedly antagonized promoter activation. These data describe a new function for E7 and highlight the tension between differentiation-dependence and promotion of cell cycling in the HPV life cycle.
Materials and Methods
Cell culture
Human foreskin keratinocytes (HFKs) were isolated from neonatal foreskins by enzymatic disaggregation with trypsin (Wilson and Laimins, 2005). Cell lines immortalized by episomal HPV16 genomes or by expressing HPV oncogenes from retroviral vectors were derived by transfection or infection of HFKs, followed by selection and maintenance in E medium with 5% fetal bovine serum and fibroblast feeders as described previously (Bodily et al., 2011a). Differentiation was induced by trypsinizing the cells and suspending them in E medium containing 1.6% methylcellulose (MC; Wilson and Laimins, 2005). U2OS cells were cultivated in DMEM containing 10% bovine growth serum.
Plasmids and drugs
The late promoter luciferase reporter, expression vectors for E6 and E7, and mutants of E7 were described previously (Bodily et al., 2011a; Bodily et al., 2011b). Expression vectors were gifts from Laimonis Laimins (pCMV-E2F1, -E2F2, -E2F3) and Bayar Thimmapaya (pcDNA flag-pRb) of Northwestern University School of Medicine; Christine Suetterlin (pcEGFP CDK1-DN, CDK2-DN) of the University of California, Irvine; and Andrew Wells (pMINR1-E2F6) of the University of Pennsylvania. CDK4/6 inhibitor was from EMD Millipore (cat. # 219492). The Y411C mutation of E1F1 was created using the primers in Table 1 to mutagenize pCMV-E2F1 using the QuickChange II Site Directed Mutagenesis kit (Agilent).
Table 1. PCR primers used in this study.
| Primer name | Sequence |
|---|---|
| E2F1 Y411C 5′ | GGCCCTCGACTGCCACTTCGGCCTCGAGGA |
| E2F1 Y411C 3′ | TCCTCGAGGCCGAAGTGGCAGTCGAGGGCC |
| qCyc 5′ | CTTGGGCCGCGTCTCC |
| qCyc 3′ | GCAGGAACCCTTATAACCAAATCC |
| INV 5′ | CTCTGCCTCAGCCTTACTGTGA |
| INV 3′ | GCTCCTGATGGGTATTGACTGG |
| TGM1 5′ | GCTGGAGATGGCACCATCC |
| TGM1 3′ | AGCTCGTCGTACTCATACTCGTCTG |
| K1 5′ | CAAGTCACTCAACAACCAATTTGC |
| K1 3′ | GGTATCTACCTGCTGCAGCAGC |
| K10 5′ | AGCCTCGTGACTACAGCAAATACTAC |
| K10 3′ | CTACCTCATTCTCATACTTCAGCCTG |
Transfection and reporter assays
Transient transfections were performed using polyethyleneimine (PEI; Polysciences) as described (Bodily et al., 2011b). Keratinocytes were transfected with reporter plasmids overnight using PEI. The next day, cultures were divided and half of the cells were plated in monolayer in 6 well plates, and half suspended in 5-10 ml 1.6% MC. Drugs were added to MC or monolayer media prior to adding the cells. Following 24 hours of incubation, lysates were prepared and assayed for luciferase activity using the Dual-Luciferase® Reporter Assay System (Promega) according to manufacturer's instructions, with Renilla luciferase as an internal control. The total level of DNA in each transfection was kept constant in each sample by addition of empty pcDNA vector DNA. SDS-PAGE and Western blotting were performed as described previously (Nakamura et al., 2009).
RNA extraction, Northern blotting, and qPCR
RNA-STAT 60 (TelTest, Inc) was used to isolate total RNAs. Northern analysis was performed by subjecting total RNA to agarose gel electrophoresis, transferring to a nylon membrane, and using the complete HPV16 genome as a probe. Alternatively, RNAs were DNAse treated and subjected to reverse transcription using qScript™ cDNA SuperMix (Quanta). cDNA was subjected to qPCR using the PerfeCTa® SYBR® Green SuperMix ROX (Quanta) on an Applied Biosystems StepOne Plus™ real time PCR machine. Data are presented as a fold change in gene expression normalized to Cyclophilin A as an internal control and normalized to the reference sample. PCR primers used in this study are shown in Table 1.
Results
HPV contributes to activation of its own late promoter
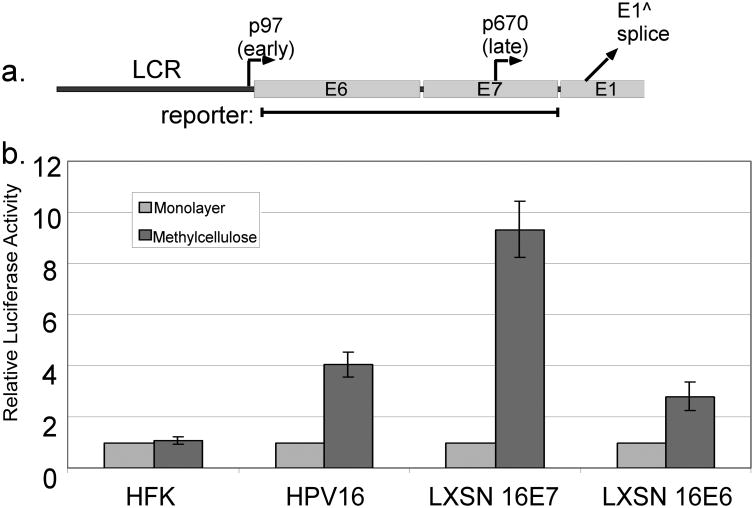
To test whether viral oncogenes including E7 would have an effect on late promoter activation, we cloned a portion of the HPV genome extending from just downstream of the early promoter to the beginning of the E1 ORF, into a luciferase reporter plasmid (Figure 1a). The reporter was transfected into human foreskin keratinocytes (HFKs), which do not contain any HPV gene products, or into HFKs expressing the viral oncogenes from the complete HPV16 genome. Following transfection, the cells were divided and cultured as monolayers (which models the undifferentiated condition) or suspended in semisolid medium containing 1.6% methylcellulose (MC, which induces differentiation of the keratinocytes). After 24 hours of culture, lysates were harvested and assayed for luciferase reporter activity. The activity of this reporter was increased upon differentiation in cells containing HPV16 genomes, as expected (Figure 1b). However, no increased activity was seen upon differentiation of uninfected HFKs. Transfection of HFKs and HPV16 cells had no effect on their ability to differentiate in MC, as measured increased levels of the differentiation marker involucrin (not shown). This result indicated that some HPV gene product is required for late promoter activation. It also indicated that the late promoter responds to signals in addition to differentiation alone.
Figure 1.
HPV genes needed for late promoter activation. a. The early region of the HPV16 genome, with the locations of the early and late promoters and the E1 splice donor. The region contained in the late promoter luciferase reporter is indicated. b. HFK cells, HFKs containing the episomal HPV16 genome, or HFKs expressing E6 or E7 from retroviral vectors were transfected with the late promoter reporter and incubated for 24 hours in monolayer or MC culture. Luciferase values were normalized to Renilla luciferase internal controls and to the monolayer value for each cell type. Values are the mean of 5-12 experiments. Bars represent ± one standard error of the mean.
E7 increases late promoter activation in differentiated keratinocytes
We next sought to identify the viral gene product responsible for activation of the late promoter. We transfected the late promoter reporter into HFKs that stably express either HPV16 E6 or E7 from retroviral vectors. Transfected cells were divided and grown in monolayer or MC culture as before, and luciferase activity was measured. As shown in Figure 1b, E6 and E7 were each sufficient in the absence of other viral factors to support late promoter activation to some degree upon differentiation, but E7 was much more efficient than E6. Thus we focused primarily on E7 in subsequent studies.
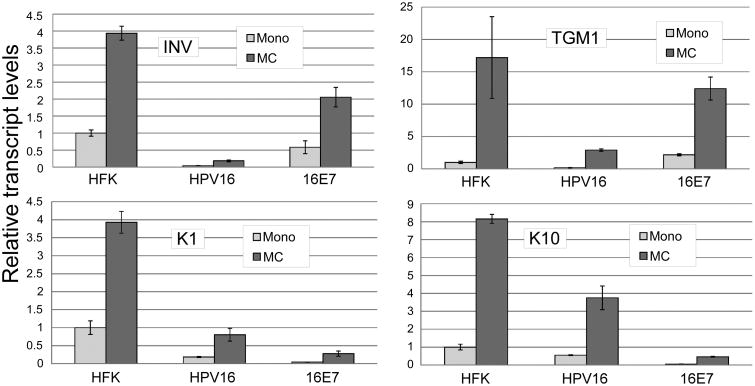
We were surprised to find that E7 could support differentiation-dependent late transcription because E7 is reported to interfere with certain aspects of differentiation (McCance et al., 1988). To clarify this issue, we examined the effect of the complete HPV16 genome and of E7 alone on induction of transcripts for several well-characterized cellular differentiation markers upon culture in MC using RT-qPCR. We found that the complete HPV16 genome dramatically reduced transcript levels for each of the cellular transcripts tested (Figure 2). Inhibition was seen both in basal conditions in monolayer, and upon induction of differentiation in MC. These data demonstrate that the late promoter is activated in a cellular differentiation environment that differs significantly from that seen in an uninfected keratinocyte undergoing differentiation, suggesting that the promoter has evolved to respond not to the normal keratinocyte differentiation signals but to the modified differentiation program present in a cell containing viral oncogenes. In keratinocytes immortalized by E7 alone, activation of differentiation markers was more variable and seemed to fall into two groups: keratin 1 (K1) and K10 were both efficiently inhibited by E7, while involucrin and transglutaminase 1 transcripts were not. The effect of E7 on the late differentiation markers loricrin and filaggrin was inconsistent, although both markers were consistently inhibited by the complete HPV16 genome (not shown).
Figure 2.
HPV16 reduces expression of cellular differentiation marker transcripts. The indicated cell types were cultured for 24 hours in monolayer or suspended in MC. Total RNAs were isolated and subjected to RT-qPCR using primers specific for involucrin (INV), transglutaminase 1 (TGM1), Keratin 1 (K1) or Keratin 10 (K10). Values represent a fold change in gene expression normalized to Cyclophilin A as an internal control and relative to HFK monolayer samples. Values represent the means of 2-3 experiments using cells from at least two genetic backgrounds each with 6-9 individual PCR reactions. Bars represent ± 1 standard error of the mean.
Transfected E7 can activate the late promoter
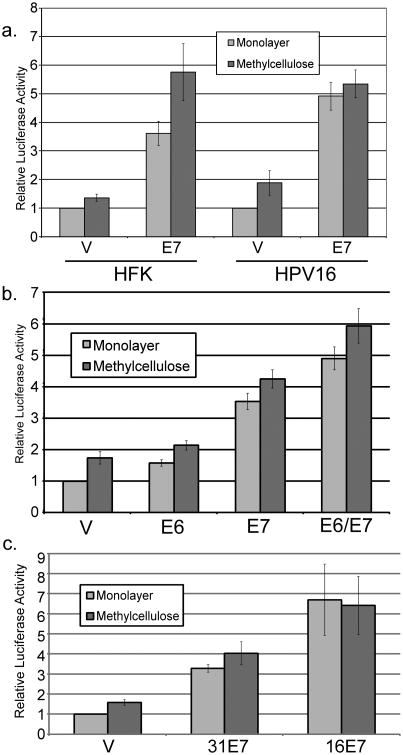
Because E7 is required for immortalization of primary keratinocytes, mutating E7 or manipulating expression levels under stable expression conditions, such as from the complete viral genome, is problematic. Although overexpression may generate non-physiological E7 levels, transient transfection provides flexibility for further molecular analyses. Thus we wished to know whether E7 expressed from a plasmid vector in a transient transfection would be able to activate the late promoter. For these experiments we transfected the late promoter reporter, with or without an expression vector for E7, into HFK cells or HFKs maintaining episomal HPV16. We then assayed luciferase activity after 24 hours of culture in monolayer or MC. E7 was able to activate the late promoter in HFKs under transient conditions (p<0.01, Figure 3a). Activation by E7 was seen in both monolayer and MC. We found that E7 was also able to activate the promoter in HPV-containing cells, both in monolayer and in MC (p<0.01). This effect was found to be responsive to the amount of E7 transfected (not shown). E7 was also able to increase late promoter activity when transfected into U2OS cells, which are derived from an osteosarcoma (not shown), indicating that the mechanism of E7-mediated promoter activation is not keratinocyte specific. By contrast, when E6 was transiently cotransfected, significant activation of the promoter in MC was not seen (p>0.05); modest augmentation of E7's activity was seen when it was cotransfected with E6 (p<0.02; Figure 3b). To determine whether E7-mediated activation was specific to the HPV16 late promoter or to 16E7, we transfected the HPV31 late promoter (Spink and Laimins, 2005) with expression vectors for 31E7 or 16E7 into HPV16-containing HFKs. Enhanced levels of promoter activity were seen with both 16E7 and 31E7 p<0.05; Figure 3c). Because the activating effect of E7 was seen in HPV-containing cells, and these cells grow more easily and consistently than HFK cells, we performed most of our subsequent experiments in cells containing episomal HPV16.
Figure 3.
E7 alone can activate the late promoter. Uninfected HFK cells (a) or HPV16-containing keratinocytes (a-c) were cotransfected with luciferase reporters for the HPV16 (a, b) or HPV31 (c) late promoters along with the indicated E7 expression vectors. Following culture in monolayer or MC for 24 hours, luciferase activities were measured and normalized as described in Figure 1. V, pcDNA empty vector. Values represent the means of 3-17 independent experiments and bars represent ± one standard error of the mean.
E7 and E2 have independent transcriptional functions
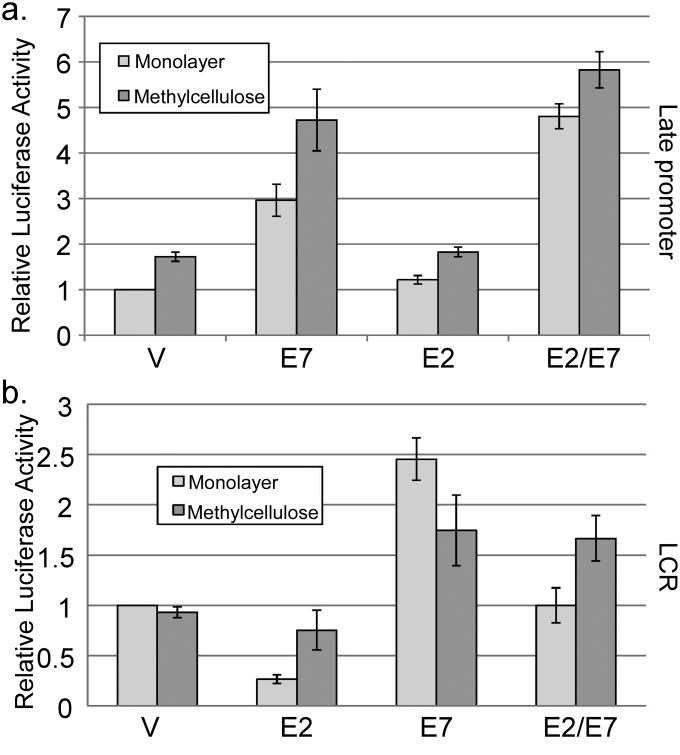
Our results suggested that E7 could act as a regulator of viral transcription. To understand the mechanism, we first considered a role for the HPV E2 protein, which is the best characterized regulator of HPV transcription. E2 is thought to perform its transcriptional regulatory activities by binding to specific sites in the viral regulatory region and, among other proposed mechanisms, interacting with the cellular chromatin binding protein Brd4, which serves to recruit the elongation factor P-TEFb (Schweiger et al., 2007; Wu and Chiang, 2007; Yan et al., 2010). Recent data have shown that E7 and E2 are able to interact physically, and that E2 can interfere with E7's transforming activities (Gammoh et al., 2006; Gammoh et al., 2009). The ability of E2 to regulate the late promoter has not been tested, and its transcriptional activity in differentiated conditions is also not well understood. Having found that E7 could activate the late promoter, we were curious whether E7 might act by reversing an inhibitory effect of E2. To test this possibility, we measured late promoter reporter activity with or without cotransfection of E7 and E2 expression vectors. There was no effect of E2 alone on late promoter reporter activity or the activity of E7 in MC (p>0.1, Figure 4a). A modest increase in reporter activity by E2/E7 as compared to E7 alone was seen in monolayer (p<0.05). Cotransfection of the viral E8ˆE2C repressor protein had no effect (not shown). Because E2 had no real effect on late promoter activity, we conclude that E7's ability to activate the late promoter is unlikely to be due to alterations in the activity of E2.
Figure 4.
Role of E2 and E7 in late and early promoter activity. HPV16-containing keratinocytes were cotransfected with luciferase reporters for the late promoter (a) or the LCR-driven early promoter (b) and expression vectors for E7 and/or E2. Following culture in monolayer or MC for 24 hours, luciferase activities were measured and normalized as described in Figure 1. V, pcDNA empty vector. Values represent the means of 5 independent experiments and bars represent ± one standard error of the mean.
Next, we wondered whether E7-mediated activation was specific to the late promoter. E2 is well known to inhibit the activity of the viral early promoter, which is contained in the long control region (LCR) in the viral genome (Thierry, 2009). Because E2 expression is driven from the early promoter, repression by E2 represents an autoregulatory loop controlling viral early promoter activity. E7 expression is also driven from the early promoter, so we tested whether E7 could likewise regulate the early promoter or modify early promoter inhibition by E2. Using a luciferase reporter containing the HPV16 LCR, we found that E2 was able to inhibit transcription from the LCR in monolayer culture as expected (p<0.01; Figure 4b). However, inhibition by E2 largely disappeared in cells differentiated in MC (p>0.2). E7 was able to increase activity of the LCR reporter, but only by about 2 fold (p<0.05), which is much less than the effect of E7 on the late promoter. When both viral proteins were expressed together, a combination effect was seen, in which E2 was able to reduce activity relative to E7 alone and E7 was able to increase the activity relative to E2 alone (p<0.01). Again, E2 had no effect on LCR-driven reporter activity in MC (p>0.4). Together, these data suggest that the activity of E2 is primarily directed toward the early promoter, whereas E7 most clearly affects late promoter activity. They also suggest that modifying the activity of E2 cannot account for the ability of E7 to activate late transcription and that E2 does not act as an inhibitor of the early promoter under differentiating conditions.
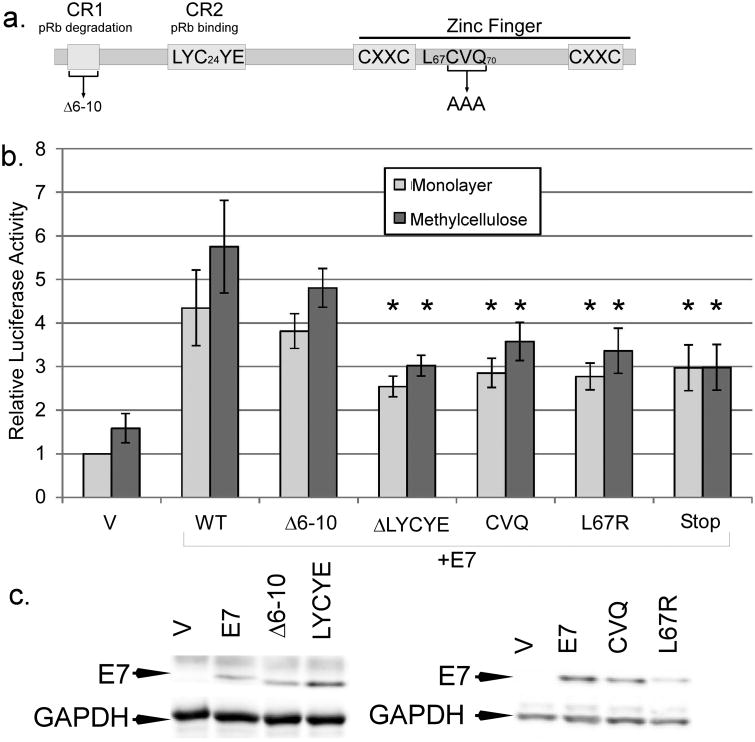
pRb binding and C terminal domains of E7 contribute to late promoter activation
Our previous studies found that HPV16 genomes harboring certain E7 mutants were less efficient at activating the late promoter and producing infectious particles than wild type (Bodily et al., 2011a). These included L67R, which disrupts the C terminal zinc-finger like domain and cannot bind histone deacetylases (Brehm et al., 1999), and CVQ-AAA, which has been shown to be defective in preventing p21CIP1-mediated growth arrest by E7 (Helt et al., 2002) and to have reduced binding to E2F-6 (McLaughlin-Drubin et al., 2008; Figure 5a). To confirm that these E7 mutants have a defect specifically in supporting late promoter activation, we cotransfected expression vectors for these mutants along with the late promoter reporter into HPV-containing keratinocytes and measured luciferase activity after culture in monolayer and MC. Neither L67R nor CVQ was able to activate the promoter as well as wild-type, consistent with our data from the complete genome (p<0.05). The LYCYE deletion mutant of E7 is unable to bind pRb, and because genomes containing this mutant are not maintained episomally, we were not previously able to discover an effect of this mutant on late viral activities (Bodily et al., 2011a). In transient transfections, however, we found that LYCYE was reduced in its ability to increase promoter activation (p<0.05; Figure 5b). In contrast, the Δ6-10 mutant, which can bind pRb but not degrade it, could activate the late promoter as well as wild type (p>0.2), suggesting that pRb binding may be important for promoter activation but that pRb degradation is not. This raises the possibility that the E7s of low-risk HPV types could also stimulate their respective late promoters, although further experiments are needed to test it. Mutants in the casein kinase II phosphorylation site were indistinguishable from wild type (not shown), consistent with the observation that these mutations in the context of the complete genome have no defect in promoter activation. We confirmed by western blotting that our mutants were all expressed at comparable levels (Figure 5c). Thus, the effects of the different mutations were consistent with our previous results in the context of the complete HPV16 genome (Bodily et al., 2011a). We conclude that the C terminus, which is implicated in p21CIP1, HDAC, and E2F-6 interactions, and also the pRb binding site, have roles in E7-mediated late promoter activation.
Figure 5.
Mutations of E7 in late promoter activation. a. A diagram of important domains in the E7 protein, with the locations of mutations used in this study. b. HPV16-containing keratinocytes were cotransfected with luciferase reporters for the late promoter and expression vectors for E7 mutants. Following culture in monolayer or MC for 24 hours, luciferase activities were measured and normalized as described in Figure 1. V, pcDNA empty vector; CVQ, CVQ68-70AAA. Values represent the means of 9-14 independent experiments and bars represent ± one standard error of the mean. Samples marked with (*) were significantly different from wild-type E7 in the corresponding culture condition (monolayer or MC, p<0.05). c. To determine expression levels of mutant E7s, U2OS cells were transfected with the indicated expression vectors for E7 and subjected to Western blotting analysis.
In addition to the concern that transient transfection may result in non-physiological levels of E7, the DNA sequence of the late promoter and the E7 ORF overlap, so it was possible that the DNA in the E7 expression plasmid could bind to and titrate away a DNA-binding repressor protein from the reporter, resulting in increased promoter activity. Consistent with this latter possibility, we found that a non-translatable mutant of E7 (Stop) could increase reporter activity in our transient transfections, indicating that some promoter activation in transient transfections could be due to the DNA present in the E7 expression plasmid rather than the E7 protein alone (Figure 5b). This could explain why none of the other mutations was able to completely eliminate promoter activation by E7, and may be consistent with a modest observed effect of DNA copy number on promoter activation in the context of the complete viral genome (Spink and Laimins, 2005). However, the Stop mutant was less effective than wild type in increasing reporter activity (p<0.05), indicating that E7 protein expression is needed for full promoter activation by E7 cotransfection in our experiments.
Mitotic CDKs are not necessary for late promoter activation
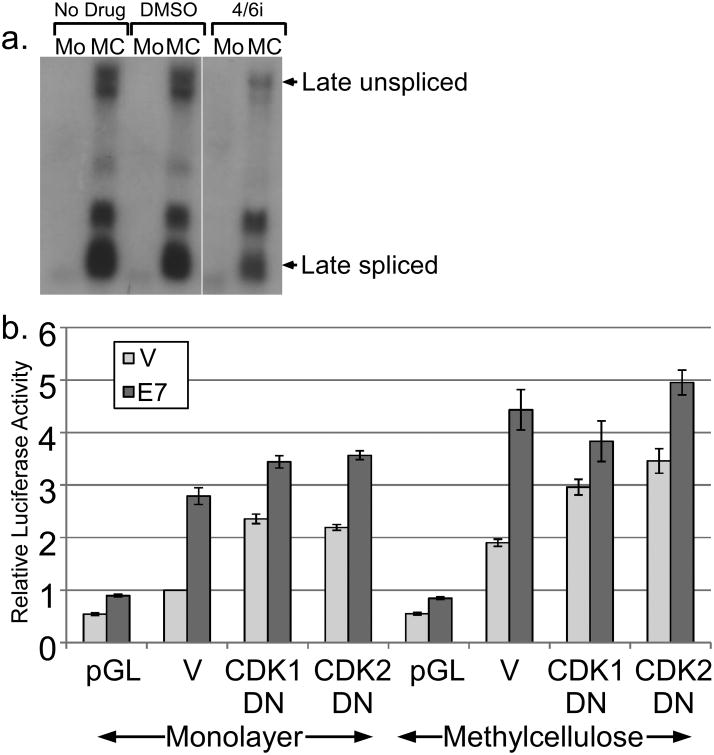
Although complicated by background levels of activation, our mutational analysis implicated the pRb family and perhaps p21CIP1 or E2F-6 as possible targets of E7 involved in late promoter activation. These factors function primarily as regulators of cell cycle progression, suggesting that cell cycling may contribute to promoter activity. In many cell contexts, differentiation and cell cycle progression are mutually exclusive. Inhibition of the cell cycle is well established to promote differentiation of keratinocytes (Freije et al., 2012; Gandarillas et al., 2000), which would be expected to increase late promoter activity. In the context of HPV infection, cell cycling coexists with differentiation, although both are modified. As we show above, and as others have also shown (McCance et al., 1988), HPV can delay or disrupt both the expression of differentiation markers and tissue morphology. Additionally, HPV-infected cells are known to arrest at G2 and thus may not be cycling in a conventional sense (Davy et al., 2005). It was therefore of interest to understand how an oncoprotein known to activate the cell cycle could also activate a differentiation-dependent promoter. We hypothesized that the late promoter has evolved to respond to both differentiation and the cell cycle, and that cell cycle activation by E7 is responsible for its activating effect. If the cell cycle were somehow necessary for promoter activation, inhibition of the cell cycle should lead to a reduction in promoter activity. Four CDKs are primarily responsible for cell cycle progression: CDK1, 2, 4, and 6. Cotransfection of p21CIP1, a cellular inhibitor of CDK2, CDK4, and 6, had no effect on either basal or E7-induced promoter activity (not shown). Treatment with a chemical inhibitor of CDK4/6 had only a slight effect on the activation of the endogenous late promoter in cells containing HPV16 episomes (Figure 6a).
Figure 6.
Role of CDKs in regulating promoter activity. (a) Keratinocytes maintaining episomal HPV16 were treated or untreated with a CDK4/6 inhibitor and total RNAs were subjected to Northern analysis using the HPV16 genome as a probe. (b) HPV16-containing keratinocytes were cotransfected with luciferase reporters for the late promoter and expression vectors for E7, DN-CDK1, or DN-CDK2. Following culture in monolayer or MC for 24 hours, luciferase activities were measured and normalized as described in Figure 1. V, pcDNA empty vector; pGL, empty luciferase reporter vector. Values represent the means of 5-9 independent experiments and bars represent ± one standard error of the mean.
We next studied the contribution of CDK1 and CDK2 by cotransfecting dominant negative (DN) mutants. We found that both DN CDKs could increase luciferase activity in monolayer and in MC (p<0.01; Figure 6b), consistent with the ability of cell cycle inhibition to promote differentiation (Freije et al., 2012; Gandarillas et al., 2000). This effect also suggests that endogenous CDK1 and CDK2 antagonize rather than activate the late promoter. We found that E7 was able to activate the promoter to high levels even in the presence of the DN-CDKs (p<0.05), suggesting that neither CDK1 nor CDK2 activity is necessary for the ability of E7 to increase promoter activity. Together, these results suggest that cell cycle progression, and mitotic CDK activity in particular, is not necessary for HPV16 late promoter activation by E7, but that the endogenous CDK1 and CDK2 antagonize promoter activity.
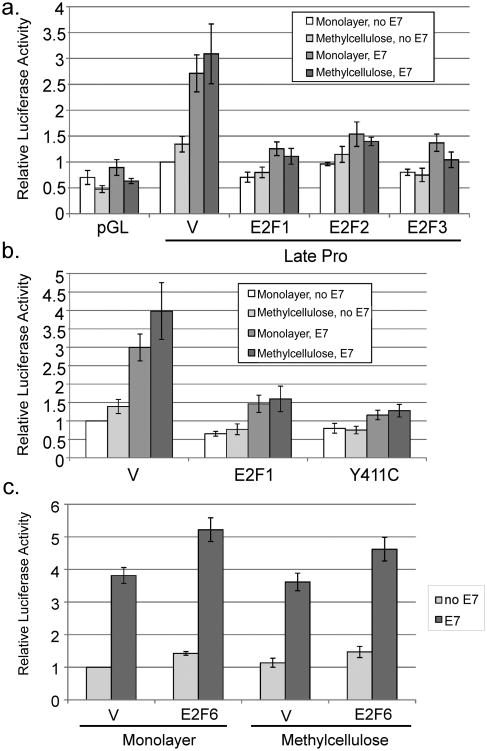
Activating E2Fs antagonize late promoter activation
To further understand the relationship between cell cycling and promoter activation, we returned to our observation that the pRb binding domain of E7 was necessary for promoter activation by E7. Given that the pRb binding domain of E7 was important for promoter activation, but pRb degradation was not, it was possible that E7's ability to disrupt E2F/pRb complexes may be important for promoter activation, since this does not require pRb degradation (Helt and Galloway, 2001). We considered the possibility that if E2F transcription factors were activate the promoter, E7 could increase promoter activity by inactivating pRb and liberating E2Fs. To test the effect of E2F transcription factors, we cotransfected expression vectors for E2F-1, -2, and -3. All three E2F factors not only failed to activate but completely eliminated E7-mediated promoter activation (p<0.02; Figure 7a). The levels of E2F expression in this experiment were not sufficient to cause significant cell death (not shown). A mutant of E2F-1 that fails to bind pRb, Y411C, also inhibited E7-mediated activation (Figure 7b), indicating that the ability of E2F1 to inhibit E7-mediated promoter activation is not dependent on its ability to bind to pRb. We conclude that the S phase-promoting E2F family members can inhibit late promoter activation by E7 and thus activation of E2F transcription factors by E7 is not likely to be responsible for promoter activation.
Figure 7.
Role of E2Fs in regulating promoter activity. HPV16-containing keratinocytes were cotransfected with luciferase reporters for the late promoter and expression vectors for E7 or the indicated E2F expression vectors. Following culture in monolayer or MC for 24 hours, luciferase activities were measured and normalized as described in Figure 1. V, pcDNA empty vector; pGL, empty luciferase reporter vector. Values represent the means of 4-8 independent experiments and bars represent ± one standard error of the mean.
A subset of E2Fs is capable of repressing rather than activating transcription and can thus interfere with cell cycle progression (Frolov and Dyson, 2004). E7 binding to one of these, E2F-6, counteracts its repressing activity (McLaughlin-Drubin et al., 2008). Because the CVQ mutant, which has reduced binding to E2F-6, is also defective for promoter activation, we were curious whether E2F-6 would have any effect on the late promoter. We found that E2F-6 did not prevent promoter activation as E2F-1 did, but that it enhanced activation by E7 by a modest but reproducible 30-40% (p<0.05; Figure 7c), consistent with the ability of E2F-6 to inhibit cell cycle progression.
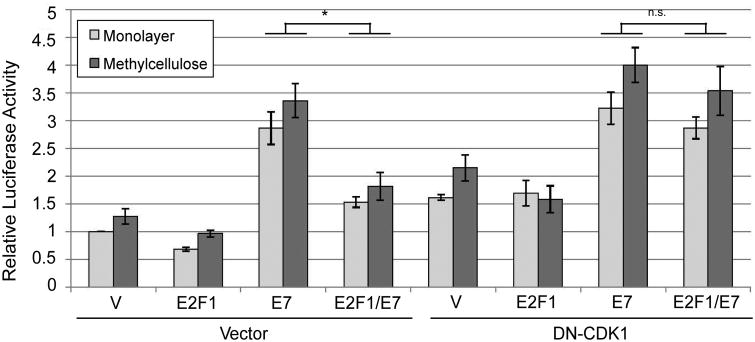
Instead of the cell cycle being necessary for promoter activity as we originally hypothesized, our data so far suggested that the cell cycle may in fact antagonize the late promoter. If so, we predicted that the inhibitory effect of E2Fs on E7-mediated promoter activation would be reversed by a factor that blocks the cell cycle. We tested this idea using DN-CDK1. As before, promoter activation by E7 was reduced the presence of additional E2F-1 (p<0.01, Figure 8a). However, the effect of E2F-1 was reversed upon addition of DN-CDK1, suggesting that the ability of E2F-1 to inhibit the promoter was due to activation of the cell cycle, and that inhibiting the cell cycle facilitated promoter activation by E7. Thus the cell cycle antagonizes late promoter activation.
Figure 8.
Promoter inhibition by E2F1 is cell cycle dependent. HPV16-containing keratinocytes were cotransfected with luciferase reporters for the late promoter and expression vectors for E7 or the indicated E2F expression vectors. Following culture in monolayer or MC for 24 hours, luciferase activities were measured and normalized as described in Figure 1. V, pcDNA empty vector; pGL, empty luciferase reporter vector. Values represent the means of 4-8 independent experiments and bars represent ± one standard error of the mean. *, p<0.01; n.s., not significant.
Discussion
We have found that an HPV early gene product, E7, can activate the viral late promoter. Late promoter activation represents a new function for E7 in the context of the normal viral life cycle. The concept of transcriptional cascades, in which late gene expression depends on expression of early genes, is hardly a novel idea in virology. For example, activation of downstream promoters to express genes involved in genome replication is a primary function of the adenovirus E1A protein (Berk, 2005). Given that E7 shares significant functional and sequence similarity with E1A, and that the genes immediately downstream of the HPV16 late promoter are involved in viral genome replication, perhaps it should not be surprising that E7 would similarly serve to activate the HPV late promoter. The HPV16 late promoter has long been known to respond to differentiation (Grassmann et al., 1996), and studies of the promoter have focused on that aspect of its regulation. Thus it has generally been assumed that the role of the virus in activation of the promoter is largely passive: that the virus lies quiescent until the cell provides the signals needed to turn on transcriptional activity. The ability of E7 to activate the promoter while at the same time inhibiting some aspects of differentiation suggests that the virus plays an active role in regulating its own late genes and that late promoter regulation is more complex and multifaceted than has been previously appreciated.
Given what we knew at the beginning of these studies about the cell cycle and promoter activation, our hypothesis was that E7 would inhibit the promoter by activating cell cycle progression. Indeed, the data presented above support our assumption that cell cycle progression would antagonize promoter activity. However, our hypothesis was incorrect. We found instead that E7 has some other activity, apparently distinct from its ability to activate the cell cycle, which serves to promote late transcription. It was possible that some combination of cell cycling and differentiation would provide the signals needed for proper promoter regulation. However, we were unable to find evidence that the cell cycle contributes positively to late promoter activity.
The cells in which the late promoter is activated are highly unusual in their combination of differentiation and cell cycling. Aside from lymphocytes, which proliferate as they differentiate, it is unusual to find cells in which both of these processes occur simultaneously. HPV-infected keratinocytes are active in the cell cycle through the functions of E6 and E7, but cell cycling is modified by the induction of G2 arrest (Davy et al., 2005). Infected cells also show many aspects of differentiation, as indicated by the upregulation of differentiation-dependent cellular markers and characteristic changes in cellular morphology. However, differentiation of infected cells is modified by the presence of viral oncogenes, and it is likely that HPV has evolved to inhibit those aspects of differentiation that are detrimental to its multiplication while retaining those that are helpful. Previous work has shown that protein products of the late promoter are high in cells expressing TGM and involucrin but not in cells expressing K10 (Ruesch et al., 1998), indicating that promoter activation correlates with some differentiation-related events but not others. Clearly those differentiation-related pathways needed for late promoter activation must be preserved in the presence of the viral oncogenes, but how HPV selects which pathways to preserve and which to inhibit will require further molecular studies.
Although withdrawal from the cell cycle is considered to be a hallmark of keratinocyte differentiation, and inhibition of the cell cycle can promote differentiation (Figure 6 and Freije et al., 2012; Gandarillas et al., 2000), the relationship between cell cycling and differentiation in keratinocytes is not entirely clear. Some reports indicate that even uninfected HFKs are capable of DNA replication without cell division as they differentiate (Freije et al., 2012; Gandarillas et al., 2000), and that a significant fraction of differentiated keratinocytes from various body sites in humans are in fact polyploid (Zanet et al., 2010). This suggests that endoreduplication is a common phenomenon in differentiating keratinocytes and that the DNA synthesis machinery may not be entirely quiescent in these cells. The dual ability of E7 to promote both the cell cycle and transcription from a differentiation-dependent promoter may be analogous to the oncogene c-Myc, which can induce cell cycling in a wide variety of cells, but is needed for both cell cycling and differentiation in keratinocytes, depending on the differentiation stage (Gandarillas et al., 2000). Although inhibitors of c-Myc had no effect on late promoter activity in our HPV-containing keratinocytes (not shown), it is possible that more detailed studies of how E7 manipulates both cell cycling and differentiation-dependent gene expression may shed light on differentiation in the absence of infection.
E7 protein levels decline upon differentiation (data not shown and Isaacson Wechsler et al., 2012). However, studies of natural lesions have shown that markers of E7 are found in the population of cells that initiates expression of E1ˆE4 (which is a major product of the late promoter; Isaacson Wechsler et al., 2012), indicating that E7 is positioned to activate the promoter in vivo, consistent with our results. We extensively used transient overexpression in the experiments reported here, which carries the risk of non-physiological effects due to high protein levels. Thus it will be important to strengthen the conclusions of this study with additional work under more stable expression conditions. In that regard, our previous work showed that CVQ mutant HPV16 genomes stably maintained in keratinocytes were less efficient at activating the late promoter than wild type, and that L67R mutant genomes were moderately capable of supporting promoter activation but defective for the production of infectious virions (Bodily et al., 2011a). Those results support our present study finding that the CVQ and L67R mutants of E7 were both deficient in promoter activation, which could account in part for their late phenotypes in the context of the complete genome. The phenotypes of these mutants are also consistent with previous work indicating that the C terminus of E7 is responsible for many of its transcriptional activities in other promoter contexts (Avvakumov et al., 2003; Brehm et al., 1999; Longworth et al., 2005; McLaughlin-Drubin et al., 2008; Rawls et al., 1990).
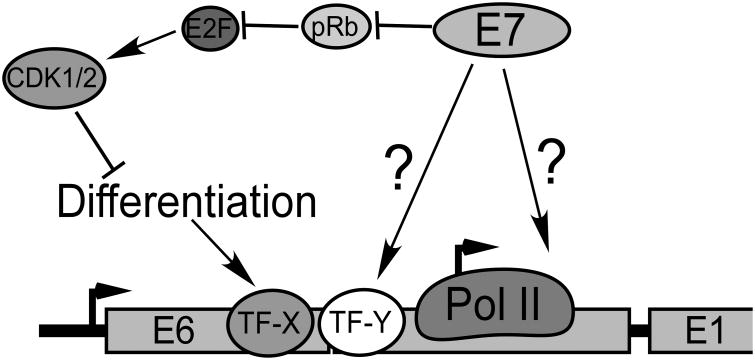
It is a conundrum that E7, which activates the cell cycle, should also activate the late promoter when the cell cycle antagonizes promoter activity. Clearly some additional E7 activity or mechanism must be involved. Although we have not yet identified the mechanism for E7-mediated promoter activation, we know several things about it: 1) It operates in spite of antagonism of the late promoter by the cell cycle, suggesting that it is a function of E7 distinct from its ability to induce unscheduled S phase. 2) It depends on sequences in both the CR2 domain and C terminus of E7. 3) It can be completely blocked by E2F-1, -2 or -3 coexpression in a cell cycle dependent manner, and inhibition by E2F1 does not depend on pRb binding to E2F1. Our working model is found in Figure 9, in which cell cycle activation by E7 is offset by a second activity which helps to activate the promoter. Whether this second activity of E7 is mediated through effects on a specific transcription factor, transcriptional coactivators, transcriptional elongation, or some other transcriptional process remains to be determined.
Figure 9.
Model. Transcription initiation from the late promoter is increased in response to cellular differentiation signals through the activity of differentiation-specific transcription factors (TF-X) such as C/EBPβ (Bodily et al., 2006; Bodily and Meyers, 2005; Gunasekharan et al., 2012; Kukimoto and Kanda, 2001; Kukimoto et al., 2006; Spink and Laimins, 2005). E7 promoter transcription through activation of a specific transcription factor or coregulator (TF-Y), or through effects on the general transcription machinery.
Acknowledgments
We thank Lindsey Hutt-Fletcher, Martin Sapp, and Laimonis Laimins for critical reading of the manuscript and helpful discussions, and William Songock, Stephen DiGiuseppe, and Haley Ruther for technical assistance. We acknowledge Frank Stubenrauch for the E8ˆE2C vector, Christine Suetterlin for the CD-CDK1 and CDK2 plasmids, and Andrew Wells for the E2F-6 expression vector. Funding for this work was provided by the LSUHSC Research Council, the Feist-Weiller Cancer Center, and the Biomedical Research Foundation of Northwest Louisiana.
References
- Avvakumov N, Torchia J, Mymryk JS. Interaction of the HPV E7 proteins with the pCAF acetyltransferase. Oncogene. 2003;22:3833–3841. doi: 10.1038/sj.onc.1206562. [DOI] [PubMed] [Google Scholar]
- Bedell MA, Hudson JB, Golub TR, Turyk ME, Hosken M, Wilbanks GD, Laimins LA. Amplification of human papillomavirus genomes in vitro is dependent on epithelial differentiation. Journal of Virology. 1991;65:2254–2260. doi: 10.1128/jvi.65.5.2254-2260.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk AJ. Recent lessons in gene expression, cell cycle control, and cell biology from adenovirus. Oncogene. 2005;24:7673–7685. doi: 10.1038/sj.onc.1209040. [DOI] [PubMed] [Google Scholar]
- Bodily J, Laimins LA. Persistence of human papillomavirus infection: keys to malignant progression. Trends in Microbiology. 2011;19:33–39. doi: 10.1016/j.tim.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodily JM, Alam S, Meyers C. Regulation of human papillomavirus type 31 late promoter activation and genome amplification by protein kinase C. Virology. 2006;348:328–340. doi: 10.1016/j.virol.2006.01.036. [DOI] [PubMed] [Google Scholar]
- Bodily JM, Mehta KP, Cruz L, Meyers C, Laimins LA. The E7 Open Reading Frame Acts in cis and in trans To Mediate Differentiation-Dependent Activities in the Human Papillomavirus Type 16 Life Cycle. Journal of Virology. 2011a;85:8852–8862. doi: 10.1128/JVI.00664-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodily JM, Mehta KP, Laimins LA. Human papillomavirus E7 enhances hypoxia-inducible factor 1-mediated transcription by inhibiting binding of histone deacetylases. Cancer Research. 2011b;71:1187–1195. doi: 10.1158/0008-5472.CAN-10-2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodily JM, Meyers C. Genetic analysis of the human papillomavirus type 31 differentiation-dependent late promoter. Journal of Virology. 2005;79:3309–3321. doi: 10.1128/JVI.79.6.3309-3321.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm A, Nielsen SJ, Miska EA, McCance DJ, Reid JL, Bannister AJ, Kouzarides T. The E7 oncoprotein associates with Mi2 and histone deacetylase activity to promote cell growth. The EMBO Journal. 1999;18:2449–2458. doi: 10.1093/emboj/18.9.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy CE, Jackson DJ, Raj K, Peh WL, Southern SA, Das P, Sorathia R, Laskey P, Middleton K, Nakahara T, Wang Q, Masterson PJ, Lambert PF, Cuthill S, Millar JB, Doorbar J. Human papillomavirus type 16 E1 E4-induced G2 arrest is associated with cytoplasmic retention of active Cdk1/cyclin B1 complexes. Journal of Virology. 2005;79:3998–4011. doi: 10.1128/JVI.79.7.3998-4011.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrmann F, Klumpp DJ, Laimins LA. Human papillomavirus type 31 E5 protein supports cell cycle progression and activates late viral functions upon epithelial differentiation. Journal of Virology. 2003;77:2819–2831. doi: 10.1128/JVI.77.5.2819-2831.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer IH. Interaction of human papillomaviruses with the host immune system: a well evolved relationship. Virology. 2009;384:410–414. doi: 10.1016/j.virol.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Freije A, Ceballos L, Coisy M, Barnes L, Rosa M, De Diego E, Blanchard JM, Gandarillas A. Cyclin E drives human keratinocyte growth into differentiation. Oncogene. 2012 doi: 10.1038/onc.2012.22.. [DOI] [PubMed] [Google Scholar]
- Frolov MV, Dyson NJ. Molecular mechanisms of E2F-dependent activation and pRB-mediated repression. Journal of Cell Science. 2004;117:2173–2181. doi: 10.1242/jcs.01227. [DOI] [PubMed] [Google Scholar]
- Gammoh N, Grm HS, Massimi P, Banks L. Regulation of Human Papillomavirus Type 16 E7 Activity through Direct Protein Interaction with the E2 Transcriptional Activator. Journal of Virology. 2006;80:1787–1797. doi: 10.1128/JVI.80.4.1787-1797.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammoh N, Isaacson E, Tomaic V, Jackson DJ, Doorbar J, Banks L. Inhibition of HPV-16 E7 oncogenic activity by HPV-16 E2. Oncogene. 2009;28:2299–2304. doi: 10.1038/onc.2009.78. [DOI] [PubMed] [Google Scholar]
- Gandarillas A, Davies D, Blanchard JM. Normal and c-Myc-promoted human keratinocyte differentiation both occur via a novel cell cycle involving cellular growth and endoreplication. Oncogene. 2000;19:3278–3289. doi: 10.1038/sj.onc.1203630. [DOI] [PubMed] [Google Scholar]
- Grassmann K, Rapp B, Maschek H, Petry KU, Iftner T. Identification of a differentiation-inducible promoter in the E7 open reading frame of human papillomavirus type 16 (HPV-16) in raft cultures of a new cell line containing high copy numbers of episomal HPV-16 DNA. Journal of Virology. 1996;70:2339–2349. doi: 10.1128/jvi.70.4.2339-2349.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunasekharan V, Hache G, Laimins L. Differentiation-dependent changes in levels of C/EBPbeta repressors and activators regulate HPV-31 late gene expression. Journal of Virology. 2012 doi: 10.1128/JVI.07239-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helt AM, Funk JO, Galloway DA. Inactivation of both the retinoblastoma tumor suppressor and p21 by the human papillomavirus type 16 E7 oncoprotein is necessary to inhibit cell cycle arrest in human epithelial cells. Journal of Virology. 2002;76:10559–10568. doi: 10.1128/JVI.76.20.10559-10568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helt AM, Galloway DA. Destabilization of the retinoblastoma tumor suppressor by human papillomavirus type 16 E7 is not sufficient to overcome cell cycle arrest in human keratinocytes. Journal of Virology. 2001;75:6737–6747. doi: 10.1128/JVI.75.15.6737-6747.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel M, Hudson JB, Laimins LA. Differentiation-induced and constitutive transcription of human papillomavirus type 31b in cell lines containing viral episomes. Journal of Virology. 1992;66:6070–6080. doi: 10.1128/jvi.66.10.6070-6080.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson Wechsler E, Wang Q, Roberts I, Pagliarulo E, Jackson D, Untersperger C, Coleman N, Griffin H, Doorbar J. Reconstruction of human papillomavirus type 16-mediated early-stage neoplasia implicates E6/E7 deregulation and the loss of contact inhibition in neoplastic progression. Journal of Virology. 2012;86:6358–6364. doi: 10.1128/JVI.07069-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingelhutz AJ, Roman A. Cellular transformation by human papillomaviruses: lessons learned by comparing high- and low-risk viruses. Virology. 2012;424:77–98. doi: 10.1016/j.virol.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukimoto I, Kanda T. Displacement of YY1 by differentiation-specific transcription factor hSkn-1a activates the P(670) promoter of human papillomavirus type 16. Journal of Virology. 2001;75:9302–9311. doi: 10.1128/JVI.75.19.9302-9311.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukimoto I, Takeuchi T, Kanda T. CCAAT/enhancer binding protein beta binds to and activates the P670 promoter of human papillomavirus type 16. Virology. 2006;346:98–107. doi: 10.1016/j.virol.2005.10.025. [DOI] [PubMed] [Google Scholar]
- Longworth MS, Wilson R, Laimins LA. HPV31 E7 facilitates replication by activating E2F2 transcription through its interaction with HDACs. The EMBO Journal. 2005;24:1821–1830. doi: 10.1038/sj.emboj.7600651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent Human Papillomavirus Vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2007;56:1–24. [PubMed] [Google Scholar]
- McCance DJ, Kopan R, Fuchs E, Laimins LA. Human papillomavirus type 16 alters human epithelial cell differentiation in vitro. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:7169–7173. doi: 10.1073/pnas.85.19.7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin-Drubin ME, Huh KW, Munger K. Human papillomavirus type 16 E7 oncoprotein associates with E2F6. Journal of Virology. 2008;82:8695–8705. doi: 10.1128/JVI.00579-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton K, Peh W, Southern S, Griffin H, Sotlar K, Nakahara T, El-Sherif A, Morris L, Seth R, Hibma M, Jenkins D, Lambert P, Coleman N, Doorbar J. Organization of human papillomavirus productive cycle during neoplastic progression provides a basis for selection of diagnostic markers. Journal of Virology. 2003;77:10186–10201. doi: 10.1128/JVI.77.19.10186-10201.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscicki AB, Schiffman M, Kjaer S, Villa LL. Chapter 5: Updating the natural history of HPV and anogenital cancer. Vaccine. 2006;24(3):S3/42–51. doi: 10.1016/j.vaccine.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Munger K, Baldwin A, Edwards KM, Hayakawa H, Nguyen CL, Owens M, Grace M, Huh K. Mechanisms of human papillomavirus-induced oncogenesis. Journal of Virology. 2004;78:11451–11460. doi: 10.1128/JVI.78.21.11451-11460.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Bodily JM, Beglin M, Kyo S, Inoue M, Laimins LA. Hypoxia-specific stabilization of HIF-1alpha by human papillomaviruses. Virology. 2009;387:442–448. doi: 10.1016/j.virol.2009.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozbun MA, Meyers C. Characterization of late gene transcripts expressed during vegetative replication of human papillomavirus type 31b. Journal of Virology. 1997;71:5161–5172. doi: 10.1128/jvi.71.7.5161-5172.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozbun MA, Meyers C. Temporal usage of multiple promoters during the life cycle of human papillomavirus type 31b. Journal of Virology. 1998;72:2715–2722. doi: 10.1128/jvi.72.4.2715-2722.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine. 2006;24(3):S11–25. doi: 10.1016/j.vaccine.2006.05.111. [DOI] [PubMed] [Google Scholar]
- Peh WL, Brandsma JL, Christensen ND, Cladel NM, Wu X, Doorbar J. The viral E4 protein is required for the completion of the cottontail rabbit papillomavirus productive cycle in vivo. Journal of Virology. 2004;78:2142–2151. doi: 10.1128/JVI.78.4.2142-2151.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peh WL, Middleton K, Christensen N, Nicholls P, Egawa K, Sotlar K, Brandsma J, Percival A, Lewis J, Liu WJ, Doorbar J. Life cycle heterogeneity in animal models of human papillomavirus-associated disease. Journal of Virology. 2002;76:10401–10416. doi: 10.1128/JVI.76.20.10401-10416.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls JA, Pusztai R, Green M. Chemical synthesis of human papillomavirus type 16 E7 oncoprotein: autonomous protein domains for induction of cellular DNA synthesis and for trans activation. Journal of Virology. 1990;64:6121–6129. doi: 10.1128/jvi.64.12.6121-6129.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson H, Kelsall G, Tellier P, Voyer H, Abrahamowicz M, Ferenczy A, Coutlee F, Franco EL. The natural history of type-specific human papillomavirus infections in female university students. Cancer Epidemiol Biomarkers Prev. 2003;12:485–490. [PubMed] [Google Scholar]
- Ruesch MN, Laimins LA. Human papillomavirus oncoproteins alter differentiation-dependent cell cycle exit on suspension in semisolid medium. Virology. 1998;250:19–29. doi: 10.1006/viro.1998.9359. [DOI] [PubMed] [Google Scholar]
- Ruesch MN, Stubenrauch F, Laimins LA. Activation of papillomavirus late gene transcription and genome amplification upon differentiation in semisolid medium is coincident with expression of involucrin and transglutaminase but not keratin-10. Journal of Virology. 1998;72:5016–5024. doi: 10.1128/jvi.72.6.5016-5024.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweiger MR, Ottinger M, You J, Howley PM. Brd4-independent transcriptional repression function of the papillomavirus e2 proteins. Journal of Virology. 2007;81:9612–9622. doi: 10.1128/JVI.00447-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson CL, Patel DM, Green KJ. Deconstructing the skin: cytoarchitectural determinants of epidermal morphogenesis. Nature reviews Molecular Cell Diology. 2011;12:565–580. doi: 10.1038/nrm3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spink KM, Laimins LA. Induction of the human papillomavirus type 31 late promoter requires differentiation but not DNA amplification. Journal of Virology. 2005;79:4918–4926. doi: 10.1128/JVI.79.8.4918-4926.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley M. Immunobiology of HPV and HPV vaccines. Gynecologic oncology. 2008;109:S15–21. doi: 10.1016/j.ygyno.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Thierry F. Transcriptional regulation of the papillomavirus oncogenes by cellular and viral transcription factors in cervical carcinoma. Virology. 2009;384:375–379. doi: 10.1016/j.virol.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Watt FM. Epidermal stem cells: markers, patterning and the control of stem cell fate. Philos Trans R Soc Lond B Biol Sci. 1998;353:831–837. doi: 10.1098/rstb.1998.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock H, Berman S, Cates W., Jr Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspectives on sexual and reproductive health. 2004;36:6–10. doi: 10.1363/psrh.36.6.04. [DOI] [PubMed] [Google Scholar]
- Wilson R, Fehrmann F, Laimins LA. Role of the E1ˆE4 Protein in the Differentiation-Dependent Life Cycle of Human Papillomavirus Type 31. Journal of Virology. 2005;79:6732–6740. doi: 10.1128/JVI.79.11.6732-6740.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R, Laimins LA. Differentiation of HPV-containing cells using organotypic “raft” culture or methylcellulose. Methods in Molecular Medicine. 2005;119:157–169. doi: 10.1385/1-59259-982-6:157. [DOI] [PubMed] [Google Scholar]
- Woodman CB, Collins S, Winter H, Bailey A, Ellis J, Prior P, Yates M, Rollason TP, Young LS. Natural history of cervical human papillomavirus infection in young women: a longitudinal cohort study. Lancet. 2001;357:1831–1836. doi: 10.1016/S0140-6736(00)04956-4. [DOI] [PubMed] [Google Scholar]
- Wu SY, Chiang CM. The double bromodomain-containing chromatin adaptor Brd4 and transcriptional regulation. The Journal of Biological Chemistry. 2007;282:13141–13145. doi: 10.1074/jbc.R700001200. [DOI] [PubMed] [Google Scholar]
- Yan J, Li Q, Lievens S, Tavernier J, You J. Abrogation of the Brd4-positive transcription elongation factor B complex by papillomavirus E2 protein contributes to viral oncogene repression. Journal of Virology. 2010;84:76–87. doi: 10.1128/JVI.01647-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanet J, Freije A, Ruiz M, Coulon V, Sanz JR, Chiesa J, Gandarillas A. A mitosis block links active cell cycle with human epidermal differentiation and results in endoreplication. PloS One. 2010;5:e15701. doi: 10.1371/journal.pone.0015701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H. Papillomaviruses in the causation of human cancers - a brief historical account. Virology. 2009;384:260–265. doi: 10.1016/j.virol.2008.11.046. [DOI] [PubMed] [Google Scholar]