Abstract
Background
This multicenter, phase II trial tested the tolerability and efficacy of lenalidomide plus rituximab in patients with previously untreated follicular lymphoma (FL).
Patients and methods
Patients with grade 1–3a FL, stage 3–4 or bulky stage 2, FL international prognostic index (FLIPI) 0–2, and no prior therapy were eligible to receive rituximab 375 mg/m2 weekly during cycle 1 and day 1 of cycles 4, 6, 8, and 10, plus lenalidomide 20–25 mg on days 1–21 for twelve 28-day cycles. The primary objectives were to evaluate response rates [complete (CR) and overall] and time to progression. Secondary objectives included toxicity, response according to polymorphisms in FcgR2A and FcgR3A, and changes in circulating pro-angiogenic cells.
Results
From October 2010 to September 2011, 66 patients were enrolled. Median age was 53 years, 34 were female, 15 had bulky disease, 21 were FLIPI 0-1, 43 FLIPI 2, and 2 FLIPI 3. One patient withdrew before receiving treatment. Fifty-one patients completed 12 cycles of lenalidomide. Reasons for discontinuation included withdrawal (n = 6), adverse events (n = 6), progression (n = 2). Grade 3–4 hematologic toxicity included neutropenia (21%), lymphopenia (9%), and thrombocytopenia (2%), infection (11%), and rash (8%). Grade 1–2 toxicity included fatigue (78%), diarrhea (37%), rash (32%), and febrile neutropenia in one patient. The overall response rate was 95%; the CR rate was 72% (95% confidence interval, 60% to 83%). With a median follow-up of 5 years, the 2- and 5-year progression-free survival were 86% and 70%, respectively, and the 5-year overall survival was 100%. There was no association between CR rate or PFS and FLIPI, histological grade, bulky disease, FcgR2A/FcgR3A polymorphism, or change in circulating endothelial cell/hematopoietic progenitor cell.
Conclusion
Lenalidomide plus rituximab was associated with low rates of grade 3–4 toxicity, yielded a CR rate and PFS similar to chemotherapy-based treatment and may represent a reasonable alternative to immunochemotherapy in previously untreated FL.
ClinicalTrials.gov Identifier
Keywords: follicular lymphoma, lenalidomide, rituximab
Introduction
Optimal frontline therapy for follicular lymphoma (FL) should balance antitumor activity with tolerability. Remission durations typically range from a few months to several years, depending on the treatment period and intensity. Although longer treatment-free intervals should be associated with better quality of life, the relative increase in treatment-related toxicity required to achieve them might detract from patient experience. Beginning in 2004, the Cancer and Leukemia Group B (CALGB; now part of the Alliance for Clinical Trials in Oncology) initiated a series of clinical trials with the aim of maximizing efficacy of rituximab-based combinations in previously untreated patients. Each trial built on the rituximab scheduled first used by the Swiss Group for Clinical Cancer Research (SAKK), in which four weekly doses was followed by four additional doses administered at the start of months 4, 6, 8, and 10 [1]. The CALGB 50402 trial evaluated the combination of extended dosing of rituximab plus the anti-CD80 antibody galiximab. The complete response rate and 3-year progression-free survival (PFS) were 32% and 29%, respectively, for patients with a high-risk FL international prognostic index (FLIPI) score and 92% and 75%, respectively, for patients with low-risk FLIPI. The CALGB 50701 trial demonstrated that the combination of rituximab plus the anti-CD22 antibody epratuzumab produced similarly promising results, with a median PFS of 3.5 years.
Lenalidomide is a potent derivative of thalidomide with immune, antiangiogenic, and direct antilymphoma effects. A randomized phase II trial of lenalidomide plus rituximab in recurrent FL demonstrated a 76.1% overall response (OR) rate [39.1% complete response (CR); 37% partial response (PR)] in patients receiving lenalidomide plus rituximab versus a 53.3% OR rate (20% CR; 33.3% PR) in patients receiving lenalidomide alone [2] with a doubling of the PFS. Based on these promising results, the Cancer and Leukemia Group B (CALGB/Alliance) 50803 trial was conducted to test the safety and efficacy of lenalidomide plus rituximab in previously untreated FL patients.
Patients and methods
This was an open-label, multicenter, phase II trial. All patients signed an institutional review board-approved, protocol-specific informed consent before enrollment.
Eligibility criteria
Patients with previously untreated, histologically confirmed FL (by central review), World Health Organisation classification grade 1, 2, or 3a, that was stage 3, 4, or bulky stage 2, based on local review were eligible for this trial. Other inclusion criteria included measurable disease, age ≥ 18 years, FLIPI 0-2, and Eastern Cooperative Oncology Group (ECOG) performance status of 0–2. Required initial laboratory values included absolute neutrophil count of ≥1000/μl, platelet count of ≥75 000/μL, estimated creatinine clearance ≥30 ml/min, and serum bilirubin ≤2× the institutional upper limit of normal. Key exclusion criteria included prior systemic therapy for non-Hodgkin’s lymphoma, including chemotherapy or immunotherapy, corticosteroids within 2 weeks of study entry, except for maintenance therapy for a non-malignant disease, known CNS involvement by lymphoma, known Human Anti-Chimeric Antibody positivity, and ongoing pregnancy (or nursing). Patients with an autoimmune disorder who required active immunosuppression and patients with history of erythema multiforme, toxic epidermal necrolysis, Stevens–Johnson syndrome, or uncontrolled seizures were not eligible.
Study treatment
Lenalidomide was administered at the starting dose of 20 mg per day orally on days 1–21, followed by 7 days of rest, every 28 days. For cycle 2 and beyond, the dose was increased to 25 mg in patients who did not experience excess toxicity. Excess toxicity was defined as grade 3 or 4 nonhematologic toxicity. Patients received a total of twelve 28-day cycles of lenalidomide, unless either rapid disease progression or unacceptable toxicity was observed. Prophylactic aspirin or low-molecular weight heparin were required for patients considered to be at high risk for a thromboembolic event, unless otherwise contraindicated.
Rituximab 375 mg/m2 per dose was administered by intravenous infusion for a total of eight doses. The first four doses (induction therapy) were administered weekly on days 1, 8, 15, and 22, starting on day 1 of lenalidomide. Subsequent rituximab doses (extended induction therapy) were administered once per week (beginning 8 weeks after the last dose of induction therapy) in weeks 13, 21, 29, and 37 (months 4, 6, 8, and 10, respectively).
Patient evaluation
Patients were restaged by positron emission tomography-computed tomography at weeks 10, 24, and 52, then every 4 months for 2 years, then every 6 months until disease progression or for a maximum of 10 years from study entry. Response to therapy was assessed per the 2007 International Working Group criteria [3]. A bone marrow biopsy was required to confirm complete response in patients with marrow involvement at baseline.
Correlative studies
Florescence-activated cell sorting analysis of circulating proangiogenic cell populations was carried out (by JR) as described previously [4, 5]. Circulating endothelial cells (CECs), defined as CD45-CD146+ CD31+ cells, circulating endothelial progenitor cells (EPCs), defined as CD45-/dimVEGFR2+CD133+ cells, and circulating hematopoietic progenitor cells (HPCs), defined as CD45+VEGFR1+CD133+ cells, were quantified by florescence-activated cell sorting analysis at baseline study entry and at week 52 following completion of study treatment. Nonviable cells were excluded from analysis with SYTOX green cell viability stain. Evaluation of levels of vascular endothelial growth factor (VEGF), VEGF-C, VEGF-D, Tie-2, Flt-1, and basic fibroblast growth factor was carried out using Meso-Scale Discovery Multiplex assays as described previously [6]. Evaluation of FCGR3A and FCGR2A polymorphisms was evaluated as described previously [7].
Statistical methods
The primary objectives of this study were to determine the response rates (OR, CR) and time to progression. The primary outcome was the rate of CR observed between trial entry and 12 months from enrollment. Based on the observed CR rates in the CALGB 50402 study, a CR rate of at least 85% for FLIPI 0-1 and 67% for FLIPI 2 would be of strong interest, whereas CR rates of <70% for FLIPI 0-1 and 47% for FLIPI 2, respectively, would be considered too low. We used a single-stage design to accrue 50 eligible and evaluable patients. The statistical testing was stratified by risk level and conditioned on the number of patients from each risk group. The conditional power is at least 85% and over 90% in the wide range of patient numbers. Based on an expected prevalence rates are 33% and 67% for low and intermediate risk groups, respectively (as observed from CALGB 50402), the marginal type I error and power were expected to be 0.078 and 0.915, respectively. The details of this method are described by Jung et al. [8].
The observed CR rate and its 95% confidence interval was estimated overall and for each FLIPI risk group using the exact method [9]. Time to event variables, PFS, and overall survival (OS), were estimated using Kaplan–Meier methods [10]. PFS was defined as the time from study entry until progression or death, whichever occurred first. Patients were censored at the last date known alive and progression free. OS was defined as the time from study entry until death from any cause. Logistic regression models were used to associate biomarkers with response after adjusting for FLIPI. All data were collected, reviewed, and analyzed by the Alliance Statistics and Data Center and study chairperson according to Alliance policies and procedures. Analyses were carried out on data collected through 8 February 2017 using SAS version 9.4 (SAS Institute, Cary, NC).
Results
From October 2010 to September 2011, 66 patients were enrolled at 21 sites in the United States; one patient never received study treatment after the baseline imaging revealed no hypermetabolic activity (although this was not an eligibility requirement). Key baseline characteristics of the patients are presented in Table 1. Twenty-one patients were FLIPI 0-1, 43 patients were FLIPI 2. Two patients were FLIPI 3 and should not have been eligible but are included in this analysis.
Table 1.
Patient characteristics (all patients)
| Characteristic | N = 66 |
|---|---|
| Age (years) | |
| Median (min, max) | 53 (32-79) |
| Sex | |
| Male | 32 (48%) |
| Female | 34 (52%) |
| Performance score | |
| ECOG/Zubrod = 0 | 49 (74%) |
| ECOG/Zubrod = 1 | 17 (26%) |
| Stage | |
| Bulky stage 2 | 5 (8%) |
| Stage 3 | 27 (41%) |
| Stage 4 | 34 (51%) |
| B-symptoms | |
| No | 59 (89%) |
| Yes | 5 (8%) |
| Unknown/not reported | 2 (3%) |
| Grade | |
| Grade 1 | 39 (59%) |
| Grade 2 | 21 (31.8%) |
| Grade 3a | 4 (6%) |
| Unknown/not reported | 2 (3%) |
| Number of nodal sites | |
| ≤4 | 33 (50%) |
| >4 | 33 (50%) |
| FLIPI risk | |
| Low | 21 (32%) |
| Intermediate/higha | 45 (68%) |
| Bulky diseaseb | |
| No | 50 (76%) |
| Yes | 15 (23%) |
| Unknown | 1 (1%) |
| FcgR polymorphismc | |
| FCGR3A 158F | 22 (37%) |
| FCGR3A 158 F/V | 27 (46%) |
| FCGR3A 158V | 10 (17%) |
| FCGR2A 131H | 18 (31%) |
| FCGR2A 131 R/H | 28 (47%) |
| FCGR2A 131R | 13 (22%) |
Two patients had FLIPI score > 2; following are their risk factors: age > 60 years, FL-Bulky stage 2, WHO class grade 3a, Hb > 12 and age > 60 years, FL-stage 4, >4 nodal sites.
Of patients with bulky disease, five were Bulky stage 2, three were stage 3 and seven were stage 4; unknown was stage 3.
Fifty-nine consented patients with FcgR results.
Disposition
A total of 65 patients started treatment (supplementary Figure S1, available at Annals of Oncology online). Fifty-one patients completed 12 cycles of lenalidomide (supplementary Figure S2, available at Annals of Oncology online). Reasons for not completing 12 cycles of lenalidomide were disease progression (n = 2; during cycle 5 and during cycle 6), adverse event (n = 6), and refused additional therapy (n = 6; 2 of these patients completed the full course of rituximab despite not completing the lenalidomide). One of the six patients listed adverse events as part of the reason for refusing further therapy. Of the six patients who voluntarily withdrew, all responded to therapy, including three with CRs. Both patients that stopped due to disease progression achieved a PR.
Adverse events
Treatment-emergent adverse events grade 3 or greater that were reported as at least possibly related are listed in Table 2. Hematologic toxicity included grade 3–4 neutropenia in 21% and grade 3–4 thrombocytopenia in 2%. Febrile neutropenia was reported in only one patient (grade 2). Eighteen grade 1–2 infections (mostly sinopulmonary), six grade 3 infections, and no grade 4 infections were reported. The most commonly reported adverse event was fatigue: grade 1–2 and grade 3 fatigue were reported in 51 and 4 patients, respectively, and was consistent throughout treatment (supplementary Figure S3, available at Annals of Oncology online). Grade 1–2 and grade 3 rash were noted in 21 and 5 patients, respectively. Other common adverse events included grade 1–2 diarrhea (24 patients), grade 1–2 constipation (16 patients), and grade 1–2 nausea (16 patients). Notably, grade 3 tumor lysis syndrome was reported in two patients and grade 3 serum sickness was reported in one patient. Three grade 1–2 thrombotic/embolic events were reported. There were no cases of other cancers reported.
Table 2.
Adverse events
| Listing of grade 1+ adverse events occurring in ≥ 10% | ||||||||
|---|---|---|---|---|---|---|---|---|
| Max grade per patient per event | ||||||||
| At least possibly related | ||||||||
| Number of evaluable patients: 65 | ||||||||
| Grade of adverse event |
||||||||
| 1—Mild |
2—Mod |
3—Severe |
4—LifeThr |
|||||
| n | (%) | n | (%) | n | (%) | n | (%) | |
| Hematologic adverse events | ||||||||
| Lymphocyte count decreased | 18 | (28) | 19 | (29) | 6 | (9) | 0 | (0) |
| Neutrophil count decreased | 17 | (26) | 5 | (8) | 10 | (15) | 4 | (6) |
| Platelet count decreased | 27 | (42) | 1 | (2) | 0 | (0) | 1 | (2) |
| Anemia | 25 | (38) | 0 | (0) | 0 | (0) | 0 | (0) |
| Nonhematologic adverse events | ||||||||
| Gastrointestinal | ||||||||
| Diarrhea | 22 | (34) | 2 | (3) | 1 | (2) | 0 | (0) |
| Constipation | 13 | (20) | 3 | (5) | 1 | (2) | 0 | (0) |
| Nausea | 11 | (17) | 5 | (8) | 0 | (0) | 0 | (0) |
| Mucositis oral | 3 | (5) | 5 | (8) | 0 | (0) | 0 | (0) |
| General | ||||||||
| Fatigue | 34 | (52) | 17 | (26) | 4 | (6) | 0 | (0) |
| Infusion-related reaction | 11 | (17) | 13 | (20) | 0 | (0) | 0 | (0) |
| Pain | 4 | (6) | 5 | (8) | 1 | (2) | 1 | (2) |
| Infections and infestations | ||||||||
| Sinus/respiratory infection | 0 | (0) | 11 | (26) | 1 | (2) | 0 | (0) |
| Other infection | 0 | (0) | 7 | (11) | 6 | (9) | (0) | |
| Investigations | ||||||||
| Alanine aminotransferase increased | 25 | (38) | 3 | (5) | 1 | (2) | 0 | (0) |
| Alkaline phosphatase increased | 13 | (20) | 1 | (2) | 0 | (0) | 0 | (0) |
| Aspartate aminotransferase increased | 18 | (28) | 1 | (2) | 1 | (2) | 0 | (0) |
| Blood bilirubin increased | 7 | (11) | 2 | (3) | 1 | (2) | 0 | (0) |
| Metabolic | ||||||||
| Hyperglycemia | 12 | (18) | 4 | (6) | 2 | (3) | 0 | (0) |
| Hypocalcemia | 7 | (11) | 1 | (2) | 1 | (2) | 0 | (0) |
| Anorexia | 6 | (9) | 1 | (2) | 0 | (0) | 0 | (0) |
| Nervous system | ||||||||
| Peripheral sensory neuropathy | 9 | 14) | 0 | (0) | 1 | (2) | 0 | (0) |
| Headache | 6 | (9) | 1 | (2) | 1 | (2) | 0 | (0) |
| Skin and subcutaneous tissue | ||||||||
| Rash maculo-papular | 10 | (15) | 11 | (17) | 5 | (8) | 0 | (0) |
| Pruritus | 10 | (15) | 1 | (2) | 0 | (0) | 0 | (0) |
| Dry skin | 8 | (12) | 1 | (2) | 0 | (0) | 0 | (0) |
Outcomes
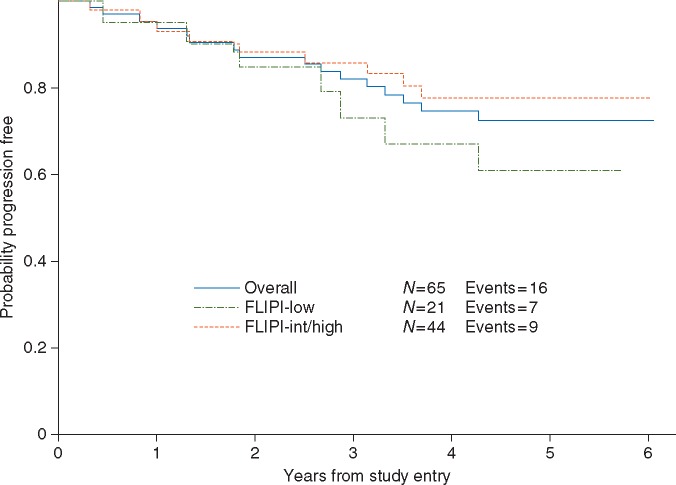
Overall, 62/65 patients (95%) responded (Table 3). The CR rate was 72% [95% confidence interval (CI), 60% to 83%; the P value for the primary objective on CR rate was 0.002] [FLIPI 0-1 CR rate was 71% (95% CI, 48% to 89%), FLIPI 2 CR rate was 73% (95% CI, 57% to 85%)]. With a median follow-up time of 5.0 years (range, <1 to 6.1 years), 16 patients have progressed (Figure 1), including seven patients with a best response of CR, eight with a best response of PR, and one with a best response of SD. The 2-, 3-, 4-, and 5-year PFS were 86% (95% CI, 75% to 93%), 81% (95% CI, 69% to 89%), 74% (95% CI, 61% to 86%), and 72% (95% CI, 58% to 82%), respectively. There was no association between FLIPI and PFS (P = 0.23). There have been no deaths reported.
Table 3.
Response
| Best response | FLIPI 0–1, n = 21 | FLIPI 2–3, n = 44a | Overall (N = 65) |
|---|---|---|---|
| CR | 15 (71%) | 32 (73%) | 47 (72%) |
| PR | 5 (23%) | 10 (23%) | 15 (23%) |
| Stable | 0 | 1 (2%) | 1 (2%) |
| Not evaluated: AE | 1 (5%) | 1 (2%) | 2 (2%) |
Two patients had FLIPI Score >2, both achieved CR. AE, adverse event; FLIPI, FL international prognostic index; CR, complete response.
Figure 1.
Progression-free survival.
Angiogenic markers
A total of 28 paired samples (baseline and end of 52-week therapy) were available for analysis of circulating pro-angiogenic cell populations. Following treatment completion at week 52, levels of both CECs and HPCs decreased significantly compared with baseline. Specifically, mean CECs decreased from 566 (range, 0–3990) to 96 (range, 0–1322) cells per ml (P = 0.02), mean HPCs decreased from 247 (range, 0–960) to 106 (range, 7–1343) cells per ml (P = 0.03), whereas mean EPCs remained stable. There was no association between decrease in CEC/HPC and CR or PFS. There were no significant changes from pre-treatment to post-treatment in the levels of VEGF, VEGF-C, VEGF-D, Tie-2, Flt-1, and basic fibroblast growth factor.
FcgR polymorphisms
Fifty-nine (91%) consented patients were included in the FCGR polymorphism analysis. The FCGR polymorphisms are reported in Table 1. The FCGR3A and FCGR2A genotypes had similar CR rates (Cochran–Armitage trend test, P = 0.55 and P = 0.31, respectively). The FCGR polymorphisms were not associated with PFS.
Discussion
In this large, multicenter phase II study with 5 years of follow-up, 12 cycles of lenalidomide plus rituximab induced a high rate of complete responses in patients with FLIPI 0-2 FL. The study met the primary endpoint, suggesting that the regimen is worthy of further evaluation. The complete response rate appeared to be independent of FLIPI, unlike prior CALGB/Alliance studies with rituximab/epratuzumab and rituximab/galiximab, suggesting that the activity of lenalidomide-rituximab may not be a regimen restricted only to low-risk patients. Additionally, lenalidomide plus rituximab induced a high rate of durable responses, with 69% of patients in complete response at 30 months (FLASH30) and a 5-year PFS of 70% [11]. These results are remarkably consistent with a previously reported single-center study in which 50 FL patients, including 14 with high FLIPI scores, achieved an 87% CR rate and a 3-year PFS of 81% with a similar 12 cycle regimen [12]. By comparison, rituximab plus chemotherapy followed by 2 years of rituximab maintenance yielded a 3-year PFS of 74.9% in the PRIMA trial although that trial included 43% of patients with FLIPI 3–5 [13]. Like other clinical trials, patients treated in this study likely represent a selected patient population. Moreover, the restriction in eligibility to low- and intermediate-risk FLIPI scores, as well as the absence of requirement for therapy in the inclusion criteria likely also biased results favorably. The results of the phase III RELEVANCE trial, which compares lenalidomide-rituximab to rituximab-chemotherapy in previously untreated FL, are eagerly awaited.
To date, no deaths have been reported, including in the 14% of patients that progressed within the first 2 years of starting treatment, and no second malignancies have been reported. Similar to the single-center study, we found that grade 3–4 toxicity was relatively modest. For example, the rate of grade 3–4 neutropenia (21%) was roughly half to one-quarter that experienced by patients treated with immunochemotherapy on a recent clinical trial [14] although the rates of grade 3–4 infections were comparable. That said, about 20% of patients that started the study did not complete all 12 cycles of planned therapy. Twelve patients either voluntarily withdrew or stopped due to adverse events. Low-grade side effects appeared particularly common with the lenalidomide-rituximab combination. Grade 1–2 fatigue, diarrhea, and rash were experienced by 78%, 37%, and 32%, compared with roughly 40%, 20%, and 20% in patients treated with immunochemotherapy. Additionally, given that the lenalidomide-rituximab was administered for 1 year, it is likely that the duration of these adverse events was longer than what might be experienced by the typical patient receiving 4–6 months of immunochemotherapy. On the other hand, the fact that patients were followed closely for a longer period of time may have resulted in detection of adverse events that might have been missed with less frequent follow-up. In the event that lenalidomide-rituximab and immunochemotherapy produce similar efficacy outcomes, the selection of therapy may depend on matching appropriate patients with toxicity profiles. In some ways, this is analogous to treatment decisions made by clinicians on a daily basis as they select different chemotherapy regimens or pursue more or less intensive therapy. In other ways, comparing the tolerability of lenalidomide-rituximab and immunochemotherapy may not be straightforward. While most investigators (us included) report the maximum severity of an adverse event, few reports contain information regarding the duration of time spent at each grade of each adverse event. For example, it might seem obvious to select a treatment that produces grade 2 arthralgia compared with grade 3 arthralgia until the time at each grade of arthralgia is factored into the decision. Future trials should focus on novel ways of evaluating the toxicity associated with chronic therapies [15].
Neo-angiogenesis is important for lymphoma pathogenesis, by promoting autocrine proliferation and survival of lymphoma tumor cells via VEGF-VEGF receptor axis, and recruiting bone marrow-derived hemangiogenesis, in the forms of proangiogenic HPCs and EPCs, to support neovascular assembly and metastasis.[16, 17] We explored the correlation between angiogenesis biomarkers and clinical responses in a subset of our study patients. Although there was no change in the levels of proangiogenic growth factors, the significant reduction of the levels of circulating CECs and HSCs in response to therapy reflects a potential mechanism of action of lenalidomide-based therapy, whereby persistent suppression of proangiogenic cells may limit angiogenesis and contribute to clinical remission. Additional tissue analysis of paired samples before and after treatment might verify changes in the tumor vascular compartment, and larger sample sizes would be needed to better define the prognostic significance of these angiogenesis biomarkers.
FCGR polymorphisms have been reported to be associated with response to single-agent rituximab in previously untreated FL [7, 18] and OS following treatment with immunochemotherapy [19] although other studies failed to reproduce the same results [20]. It is likely that multiple factors account for response to rituximab, including tumor burden, tumor microenvironment, and the combination with different agents. Our results suggest that FCGR2A and 3 A polymorphisms are unlikely to play a major role in determining response to lenalidomide-rituximab. This study also highlights the challenges of identifying predictive biomarkers for therapies with low failure rates. Future studies that focus on higher risk populations may prove more fruitful.
Twelve cycles of lenalidomide and rituximab is clearly efficacious in patients with untreated FL low/intermediate FLIPI risk, and it is associated with low rates of significant toxicity. Lenalidomide appears to have pleotropic effects, including inhibition of angiogenesis, and it may overcome the adverse prognosis associated with poor-risk FCGR2A and 3 A polymorphisms. Barring any unexpected results from the RELEVANCE trial, lenalidomide-rituximab represents a reasonable alternative to front-line immunochemotherapy in FL. In selecting a treatment, clinicians will need to weigh the differences in administration, duration of therapy, and tolerability, accepting that the comparison may not be as straightforward as comparing two chemotherapy regimens. Future trials should incorporate attempts to identify patient subgroups most likely to do well or experience significant adverse events, and adverse events should be reported in a way that better reflects the experience of patients receiving continuous therapy.
Supplementary Material
Acknowledgement
SNP analyses were carried out at The Ohio State University, Columbus, OH, in the laboratory of Dr John Byrd.
Funding
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), U10CA007968, U10CA077597, U10CA180833, U10CA180850, and U10CA180838. Also supported in part by an independent investigator award from Celgene. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The following institutional networks participated in this study:
Dana-Farber/Partners CancerCare LAPS, Boston, MA, Harold Burstein, 5U10CA180867. Delaware/Christiana Care NCI Community Oncology Research Program, Newark, DE, Gregory Masters, 5UG1CA189819. Duke University - Duke Cancer Institute LAPS, Durham, NC, Jeffrey Crawford, 5U10CA180857. Florida Hospital Orlando, Orlando, FL, Carlos Alemany. Heartland Cancer Research NCORP, Decatur, IL, James Wade, 5UG1CA189830. MedStar Georgetown University Hospital, Washington, DC, Chaitra Ujjani. Ohio State University Comprehensive Cancer Center LAPS, Columbus, OH, Clara Bloomfield, 5U10CA180850. Southeast Clinical Oncology Research (SCOR) Consortium NCORP, Winston-Salem, NC, James Atkins, 5UG1CA189858. State University of New York Upstate Medical University, Syracuse, NY, Stephen Graziano. UNC Lineberger Comprehensive Cancer Center LAPS, Chapel Hill, NC, Thomas Shea, 5U10CA180838. University of Chicago Comprehensive Cancer Center LAPS, Chicago, IL, Hedy Kindler, 5U10CA180836. University of Nebraska Medical Center, Omaha, NE, Apar Ganti. University of Vermont College of Medicine, Burlington, VT, Claire Verschraegen. VCU Massey Cancer Center Minority Underserved NCORP, Richmond, VA, Steven Grossman, UG1CA189869. Wake Forest University Health Sciences, Winston-Salem, NC, Heidi Klepin. Washington University - Siteman Cancer Center LAPS, Saint Louis, MO, Nancy Bartlett, U10CA180833. Weill Medical College of Cornell University, New York, NY, Scott Tagawa.
Disclosure
PM is a consultant for Roche-Genentech and Celgene. NLB received research funding from Roche-Genentech and Celgene. JR received research funding and consultant for Celgene. JPL is a consultant for Roche-Genentech and Celgene. BDC: Advisory Board for Celgene, Advisory board and research support for Roche-Genentech. All remaining authors have declared no conflicts of interest.
References
- 1. Ghielmini M, Schmitz S-FH, Cogliatti SB. et al. Prolonged treatment with rituximab in patients with follicular lymphoma significantly increases event-free survival and response duration compared with the standard weekly x 4 schedule. Blood 2004; 103: 4416–4423. [DOI] [PubMed] [Google Scholar]
- 2. Leonard J, Jung S, Johnson J. et al. Randomized trial of lenalidomide alone versus lenalidomide plus rituximab in patients with recurrent follicular lymphoma: CALGB 50401 (Alliance). J Clin Oncol 2015; 33: 3635–3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cheson B, Pfistner B, Juweid M. et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007; 25: 579–586. [DOI] [PubMed] [Google Scholar]
- 4. Ruan J, Martin P, Coleman M. et al. Durable responses with the metronomic regimen RT-PEPC in elderly patients with recurrent mantle cell lymphoma. Cancer 2010; 116: 2655–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bertolini F, Shaked Y, Mancuso P, Kerbel RS.. The multifaceted circulating endothelial cell in cancer: towards marker and target identification. Nat Rev Cancer 2006; 6: 835–845. [DOI] [PubMed] [Google Scholar]
- 6. Zhu AX, Finn RS, Mulcahy M. et al. A phase II and biomarker study of ramucirumab, a human monoclonal antibody targeting the VEGF receptor-2, as first-line monotherapy in patients with advanced hepatocellular cancer. Clinical Cancer Research.2013; 19: 6614.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cartron G, Dacheux L, Salles G. et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor Fcgamma RIIIa gene. Blood 2002; 99: 754–758. [DOI] [PubMed] [Google Scholar]
- 8. Jung S-H, Chang MN, Kang SJ.. Phase II cancer clinical trials with heterogeneous patient populations. J Biopharmaceut Stat 2012; 22: 312–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clopper CJ, Pearson ES.. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 1934; 26: 404–413. [Google Scholar]
- 10. Kaplan E, Meier P.. Nonparametric etimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481. [Google Scholar]
- 11. Shi Q, Flowers CR, Hiddemann W. et al. Thirty-month complete response as a surrogate end point in first-line follicular lymphoma therapy: an individual patient-level analysis of multiple randomized trials. J Clin Oncol 2016; 35: 552–560. [DOI] [PubMed] [Google Scholar]
- 12. Fowler NH, Davis RE, Rawal S. et al. Safety and activity of lenalidomide and rituximab in untreated indolent lymphoma: an open-label, phase 2 trial. Lancet Oncol 2014; 15: 1311–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Salles G, Seymour JF, Offner F. et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet 2011; 377: 42–51. [DOI] [PubMed] [Google Scholar]
- 14. Flinn IW, van der Jagt R, Kahl BS. et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood 2014; 123: 2944.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thanarajasingam G, Hubbard JM, Sloan JA, Grothey A.. the imperative for a new approach to toxicity analysis in oncology clinical trials. JNCI 2015; 107: djv216–djv216. [DOI] [PubMed] [Google Scholar]
- 16. Ruan J, Hajjar K, Rafii S, Leonard JP.. Angiogenesis and antiangiogenic therapy in non-Hodgkin's lymphoma. Ann Oncol. 2009; 20: 413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ruan J, Luo M, Wang C. et al. Imatinib disrupts lymphoma angiogenesis by targeting vascular pericytes. Blood 2013; 121: 5192.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weng WK, Levy R.. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol 2003; 21: 3940–3947. [DOI] [PubMed] [Google Scholar]
- 19. Persky DO, Dornan D, Goldman BH. et al. Fc gamma receptor 3a genotype predicts overall survival in follicular lymphoma patients treated on SWOG trials with combined monoclonal antibody plus chemotherapy but not chemotherapy alone. Haematologica 2012; 97: 937–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kenkre VP, Hong F, Cerhan JR. et al. Fc gamma receptor 3A and 2A polymorphisms do not predict response to rituximab in follicular lymphoma. Clin Cancer Res 2016; 22: 821.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.