Abstract
Background
Observational studies suggest that higher levels of 25-hydroxyvitamin D3 (25(OH)D) are associated with a reduced risk of colorectal cancer and improved survival of colorectal cancer patients. However, the influence of vitamin D status on cancer recurrence and survival of patients with stage III colon cancer is unknown.
Patients and methods
We prospectively examined the influence of post-diagnosis predicted plasma 25(OH)D on outcome among 1016 patients with stage III colon cancer who were enrolled in a National Cancer Institute-sponsored adjuvant therapy trial (CALGB 89803). Predicted 25(OH)D scores were computed using validated regression models. We examined the influence of predicted 25(OH)D scores on cancer recurrence and mortality (disease-free survival; DFS) using Cox proportional hazards.
Results
Patients in the highest quintile of predicted 25(OH)D score had an adjusted hazard ratio (HR) for colon cancer recurrence or mortality (DFS) of 0.62 (95% confidence interval [CI], 0.44–0.86), compared with those in the lowest quintile (Ptrend = 0.005). Higher predicted 25(OH)D score was also associated with a significant improvement in recurrence-free survival and overall survival (Ptrend = 0.01 and 0.0004, respectively). The benefit associated with higher predicted 25(OH)D score appeared consistent across predictors of cancer outcome and strata of molecular tumor characteristics, including microsatellite instability and KRAS, BRAF, PIK3CA, and TP53 mutation status.
Conclusion
Higher predicted 25(OH)D levels after a diagnosis of stage III colon cancer may be associated with decreased recurrence and improved survival. Clinical trials assessing the benefit of vitamin D supplementation in the adjuvant setting are warranted.
ClinicalTrials.gov Identifier
Keywords: colorectal neoplasm, vitamin D, survival analysis, prospective studies
Introduction
Increasing experimental evidence suggests that vitamin D may reduce both the development and progression of colorectal cancer (CRC). Treatment of genetically engineered mouse models of CRC (APCmin mice) with vitamin D or its synthetic analogs reduces tumor burden, whereas APCmin mice with loss of the vitamin D receptor (VDR) (APCmin/VDR-null mice) experience an increased tumor burden [1, 2].
Epidemiological studies have examined the association between vitamin D and CRC survival, using a variety of methodologies; higher vitamin D status was associated with improved CRC patient outcome in most studies, though not all [3]. The majority of these studies could not address the potential for residual confounding by other patient, dietary, and lifestyle factors, and none assessed whether the effect of vitamin D status was modified by tumor molecular characteristics. Moreover, many studies were limited by the number of available blood samples for analysis.
To evaluate the impact of vitamin D status following a diagnosis of CRC in a larger number of patients, we developed a model to predict plasma 25(OH)D levels. The 25(OH)D score takes into account the combined influence of the major determinants of vitamin D status (race as a surrogate of skin pigmentation, residential state as a surrogate of UV-B radiation exposure, leisure-time physical activity as a surrogate of sunlight exposure, adiposity, and dietary and supplementary vitamin D intake). In the current study, we assessed the influence of this vitamin D score on survival among 1016 stage III colon cancer patients participating in a National Cancer Institute (NCI)-sponsored adjuvant chemotherapy clinical trial [4].
Methods
Study population
Patients in this prospective cohort participated in the NCI-sponsored Cancer and Leukemia Group B (CALGB, now part of the Alliance for Clinical Trials in Oncology) 89803 adjuvant therapy trial for stage III colon cancer, comparing treatment with weekly 5-fluorouracil and leucovorin (FU/LV) to weekly FU/LV + irinotecan (IFL) [4]. From May 1999 to May 2001, 1264 patients were enrolled in the treatment trial. After 87 patients were enrolled, an amendment required patients to complete a self-administered questionnaire that captured diet and lifestyle habits midway through their therapy (4 months after surgery; questionnaire 1 [Q1]) and 6 months after completion of treatment (14 months after surgery; Q2). Questionnaires included validated semi-quantitative food frequency questionnaires (FFQs) that included 131 food items, vitamin and mineral supplements, and open-ended sections for other supplements and foods not specifically listed.
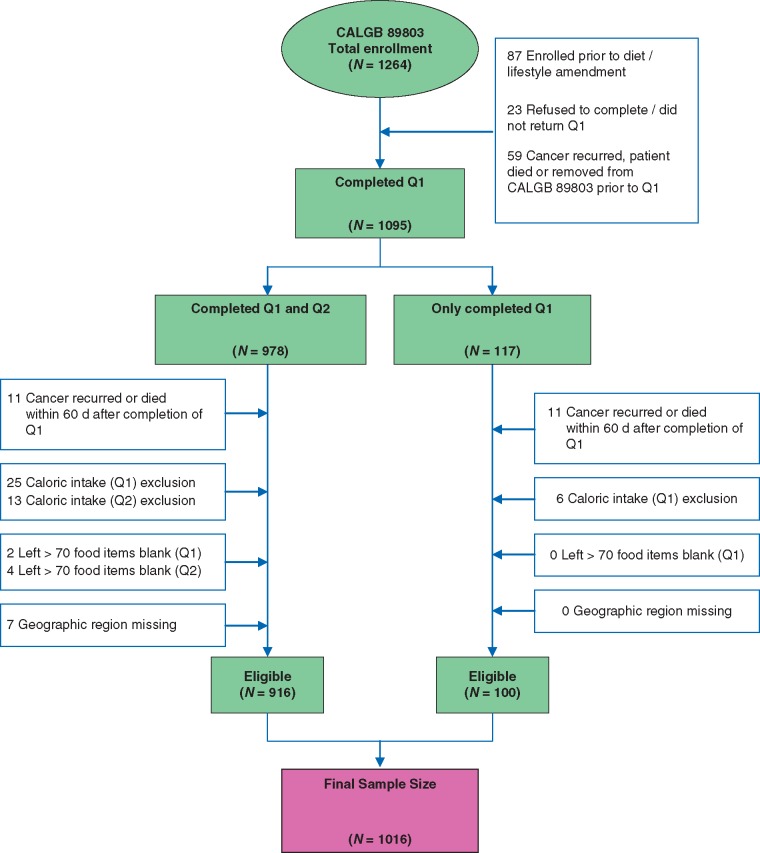
Patients were eligible if they underwent a complete surgical resection of the primary tumor within 56 days of trial entry, had regional lymph node metastases but no distant metastases, had a baseline Eastern Cooperative Oncology Group (ECOG) performance status of 0–2, and had adequate bone marrow, renal, and hepatic function. Patients were excluded if they reported significantly abnormal caloric intake (<600 or >4200 calories/day for men; <500 or >3500 calories/day for women), left >70 food items blank, or had missing zip code or address. To avoid potential biases due to declining health immediately before cancer recurrence or death, we excluded patients who recurred or died within 60 days following completion of Q1. Figure 1 illustrates the derivation of the final sample size of 1016 patients. We previously demonstrated that there were no appreciable differences in baseline characteristics between the 1016 patients eligible for this analysis and the overall CALGB 89803 population [5]. All patients signed informed consent and the study was approved by each site’s institutional review board.
Figure 1.
Derivation of the study cohort. Q1, questionnaire 1 (midway through adjuvant therapy); Q2, questionnaire 2 (6 months after completion of adjuvant therapy); caloric intake exclusion, <600 or > 4200 calories/day for men and <500 or > 3500 calories/day for women.
Exposure assessment
Derivation of the 25(OH)D prediction score has been described previously [6]. Briefly, linear regression was performed on 1095 men in the Health Professionals Follow-Up Study (HPFS) who were free of diagnosed cancer at the time of blood draw and had plasma 25(OH)D levels measured by radioimmunoassay. Race, geographic region, dietary and supplemental vitamin D intake, body mass index (BMI), and physical activity were identified as independent predictors of plasma 25(OH)D levels. Using the predictors' regression coefficients, a 25(OH)D score was calculated for each participant. The model was able to predict a wide range of 25(OH)D levels, from 9 to 36 ng ml−1. The mean actual circulating level of 25(OH)D for men in the highest decile of predicted 25(OH)D was 11 ng ml−1 higher (95% CI, 9–13) than that of men in the lowest decile.
To validate this model, a 25(OH)D score was calculated for an independent sample of 542 men in HPFS who had available plasma 25(OH)D measurements [6]. Actual plasma level rose across increasing deciles of predicted 25(OH)D (Ptrend < 0.001), and the difference in the mean actual 25(OH)D level between extreme deciles was 10 ng ml−1, similar to the difference of 11 ng ml−1 in the initial dataset. The model r2 was 28%. We also validated the prediction model among patients with metastatic CRC from the CALGB/SWOG 80405 phase III trial and observed a statistically significant Spearman correlation between the actual plasma 25(OH)D level and the predicted level (P < 0.0001). Finally, previous studies found that predicted 25(OH)D levels were associated with reduced risk of CRC among healthy men [6] and improved survival among CRC patients [7].
We calculated predicted 25(OH)D score among the 1016 stage III colon cancer patients who completed FFQs and had not recurred before completion of Q1. The median time from study entry to Q1 was 3.5 months (range 0.2–9.9 months). Race and geographic region were recorded at baseline, while values of physical activity, BMI, and total vitamin D intake (including dietary and supplement intake) were determined using cumulative averaging of values reported on Q1 and Q2, but weighted proportional to times between Q1 and Q2 and then between Q2 and disease-free survival (DFS) time.
Tumor assessments
Polymerase chain reaction (PCR) and pyrosequencing targeted for mutation hotspots in PIK3CA exons 9 and 20, BRAF codon 600, and KRAS codons 12 and 13 were performed. Mutations in TP53 exons 5–8 were determined by Sanger sequencing and sequencing by hybridization. Microsatellite instability (MSI) was assessed by PCR for 10 microsatellite markers; tumors with instability in 50% or more of the loci were classified as MSI-high; for 28 cases without PCR MSI results, those with loss of MLH1 or MSH2 were classified as MSI-high.
Study endpoints
The primary end point of our study was DFS, defined as time from completion of Q1 to tumor recurrence, occurrence of a new primary colon tumor, or death from any cause. We also assessed recurrence-free survival (RFS), defined as time from completion of Q1 to tumor recurrence or occurrence of a new primary colon tumor. For RFS, patients who died without known tumor recurrence were censored at last documented contact. Finally, overall survival (OS) was defined as the time from completion of Q1 to death from any cause.
Statistical analyses
There was no difference in outcome between the two treatment arms of the main trial [4], therefore patients were pooled for this study. The primary statistical analysis used the two-tailed linear test for trend with predicted 25(OH)D level as a continuous variable. Cox proportional hazards models were used to calculate hazard ratios (HRs), adjusted a priori for age, gender, family history of CRC, baseline performance status, depth of invasion through bowel wall, number of positive lymph nodes, grade of tumor differentiation, and treatment arm. In secondary analyses, we additionally adjusted for each determinant of vitamin D status used in the prediction model individually. The HRs were calculated according to quintiles of predicted 25(OH)D score, with the lowest quintile as the reference group. The proportionality of hazards assumption was satisfied by evaluating the cross product of predicted 25(OH)D level and time.
DFS, RFS, and OS by tertile of predicted 25(OH)D level were examined using Kaplan–Meier curves and the log-rank test. We further assessed for effect modification on the relation between predicted 25(OH)D levels and DFS by strata of other known or suspected predictors of patient outcome. For ease of graphical viewing and to conserve statistical power, respectively, we utilized tertiles of predicted 25(OH)D in these analyses. Tests of interaction were assessed by entering into the model the cross product of predicted 25(OH)D level as a continuous variable with the relevant covariable or tumoral factor as a binary variable. All analyses were performed with SAS 9.4. All P values are two-sided and considered statistically significant at the 0.05 level. Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center, which also ensured data quality per standard Alliance policies. All analyses were based on the study database frozen on 9 November 2009.
Results
Baseline characteristics
The median predicted 25(OH)D level was 27.6 ng ml−1 (range, 16.0–36.4 ng ml−1) in this cohort of 1016 stage III colon cancer patients. Baseline characteristics by level of predicted vitamin D score are shown in Table 1. Patients with higher predicted vitamin D scores were more likely to be of white race and male, were more physically active and leaner, were more likely to live in the Southern region and possess an ECOG performance status of 0, and consumed higher levels of dietary vitamin D, vitamin D supplements, and calcium.
Table 1.
Baseline characteristics of 1016 patients by quintile of predicted 25(OH)D score
| Predicted 25(OH)D Score |
|||||
|---|---|---|---|---|---|
| Quintile1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | |
| (n=207) | (n=200) | (n=204) | (n=203) | (n=202) | |
| Vitamin D score, median (range), ng/mL | 23.3 (16.0–24.7) | 25.7 (24.7–26.6) | 27.6 (26.6–28.3) | 29.2 (28.3–30.1) | 31.5 (30.1–36.4) |
| Season of questionnaire completion, No. (%) | |||||
| Summer (June, July, August) | 67 (32.4) | 62 (31.0) | 66 (32.4) | 62 (30.5) | 75 (37.1) |
| Autumn (Sept, Oct, Nov) | 25 (12.1) | 36 (18.0) | 29 (14.2) | 31 (15.3) | 31 (15.3) |
| Winter (Dec, Jan, Feb) | 42 (20.3) | 33 (16.5) | 49 (24.0) | 43 (21.2) | 37 (18.3) |
| Spring (Mar, April, May) | 73 (35.3) | 69 (34.5) | 60 (29.4) | 67 (33.0) | 59 (29.2) |
| Age, median (range), years | 59 (32–80) | 59 (21–83) | 62 (32–85) | 62 (24–85) | 60 (27–82) |
| Male, No. (%) | 79 (38.2) | 111 (55.5) | 118 (57.8) | 137 (67.5) | 127 (62.9) |
| Race, No. (%) | |||||
| White | 134 (64.7) | 184 (92.0) | 197 (96.6) | 193 (95.1) | 197 (97.5) |
| Black | 56 (27.1) | 6 (3.0) | 2 (1.0) | 1 (0.5) | 0 (0) |
| Other | 17 (8.2) | 10 (5.0) | 5 (2.5) | 9 (4.4) | 5 (2.5) |
| Residence, No. (%)a | |||||
| South | 52 (25.1) | 38 (19.0) | 61 (29.9) | 60 (29.6) | 87 (43.1) |
| Midwest/West | 70 (33.8) | 75 (37.5) | 83 (40.7) | 91 (44.8) | 85 (42.1) |
| Northeast | 85 (41.1) | 87 (43.5) | 60 (29.4) | 52 (25.6) | 30 (14.9) |
| Family history of CRC, No. (%) | |||||
| Yes | 38 (18.4) | 29 (14.5) | 38 (18.6) | 40 (19.7) | 38 (18.8) |
| No | 169 (81.6) | 171 (85.5) | 166 (81.4) | 163 (80.3) | 164 (81.2) |
| Baseline performance status, No. (%)b | |||||
| 0 | 134 (64.7) | 145 (72.5) | 157 (77.0) | 155 (76.4) | 154 (76.2) |
| 1–2 | 66 (31.9) | 47 (23.5) | 45 (22.1) | 46 (22.7) | 46 (22.8) |
| Unknown | 7 (3.4) | 8 (4.0) | 2 (1.0) | 2 (1.0) | 2 (1.0) |
| Invasion through bowel wall by T stage, No. (%)c | |||||
| T1–2 | 22 (10.6) | 24 (12.0) | 28 (13.7) | 31 (15.3) | 32 (15.8) |
| T3–4 | 175 (84.5) | 167 (83.5) | 174 (85.3) | 170 (83.7) | 169 (83.7) |
| Unknown | 10 (4.8) | 9 (4.5) | 2 (1.0) | 2 (1.0) | 1 (0.5) |
| Number of positive lymph nodes, No. (%) | |||||
| 1–3 | 131 (63.3) | 131 (65.5) | 127 (62.3) | 124 (61.1) | 126 (62.4) |
| ≥4 | 69 (33.3) | 62 (31.0) | 75 (36.8) | 77 (37.9) | 74 (36.6) |
| Unknown | 7 (3.4) | 7 (3.5) | 2 (1.0) | 2 (1.0) | 2 (1.0) |
| Grade of differentiation, No. (%) | |||||
| Well | 14 (6.8) | 16 (8.0) | 14 (6.9) | 2 (1.0) | 10 (5.0) |
| Moderate | 141 (68.1) | 131 (65.5) | 145 (71.1) | 141 (69.5) | 142 (70.3) |
| Poor/undifferentiated | 45 (21.7) | 45 (22.5) | 43 (21.1) | 56 (27.6) | 49 (24.3) |
| Unknown | 7 (3.4) | 8 (4.0) | 2 (1.0) | 4 (2.0) | 1 (0.5) |
| Clinical bowel obstruction at presentation, No. (%) | 45 (21.7) | 42 (21.0) | 41 (20.1) | 46 (22.7) | 47 (23.3) |
| Bowel perforation at presentation, No. (%) | 12 (5.8) | 6 (3.0) | 11 (5.4) | 10 (4.9) | 4 (2.0) |
| Treatment arm, No. (%) | |||||
| FU/LV | 104 (50.2) | 88 (44.0) | 109 (53.4) | 105 (51.7) | 109 (54.0) |
| IFL | 103 (49.8) | 112 (56.0) | 95 (46.6) | 98 (48.3) | 93 (46.0) |
| Body mass index, median (range), kg/m2d | 31 (19–52) | 29 (17–52) | 28 (18–42) | 27 (17–42) | 24 (17–38) |
| Physical activity, median (range), MET-h/wk4d | 1 (0–57) | 4 (0–74) | 6 (0–98) | 12 (0–103) | 24 (2–123) |
| Dietary vitamin D intake, energy-adjusted, median (range), IU/d | 100 (0–600) | 200 (0–1000) | 170 (41–530) | 197 (36–746) | 236 (48–595) |
| Vitamin D supplement intake, median (range), IU/d | 0 (0–600) | 24 (0–1100) | 200 (0–800) | 200 (0–800) | 200 (0–1200) |
| Total calcium intake, energy-adjusted, median (range), mg/d | 700 (300–2500) | 800 (400–2400) | 900 (300–3000) | 1000 (400–3400) | 1000 (300–3000) |
| KRAS mutational status | |||||
| Wildtype | 67 (64.4) | 61 (65.6) | 66 (62.3) | 79 (66.9) | 83 (70.3) |
| Mutant | 37 (35.6) | 32 (34.4) | 40 (37.7) | 39 (33.1) | 35 (29.7) |
| BRAF mutational status | |||||
| Wildtype | 84 (80.8) | 78 (84.8) | 88 (81.5) | 100 (85.5) | 107 (91.5) |
| Mutant | 20 (19.2) | 14 (15.2) | 20 (18.5) | 17 (14.5) | 10 (8.5) |
| PIK3CA mutational status | |||||
| Wildtype | 94 (91.3) | 76 (86.4) | 89 (87.3) | 97 (89.8) | 97 (85.8) |
| Mutant | 9 (8.7) | 12 (13.6) | 13 (12.7) | 11 (10.2) | 16 (14.2) |
| TP53 mutational status | |||||
| Wildtype | 47 (54.7) | 50 (59.5) | 41 (46.1) | 54 (59.3) | 51 (49.0) |
| Mutant | 39 (45.3) | 34 (40.5) | 48 (53.9) | 37 (40.7) | 53 (51.0) |
| MSI status | |||||
| MSI-high | 26 (12.6) | 24 (12.0) | 17 (8.3) | 32 (15.8) | 19 (9.4) |
| MSS/MSI-low | 181 (87.4) | 176 (88.0) | 187 (91.7) | 171 (84.2) | 183 (90.6) |
South includes OK, TX, LA, AR, MS, AL, FL, GA, SC, NC, VA, TN, KY, WV, DC, MD, DE, AZ, CA, NM, CO, UT, NV, GU, HI, PR, and VI); Midwest/West includes ND, SD, NE, KS, MN, IA, MO, WI, IL, MI, IN, OH, OR, WA, MT, ID, and WY; Northeast includes PA, NY, NJ, CT, RI, MA, NH, VT, ME, and AK.
Baseline performance status: PS 0 = fully active; PS 1 = restricted in physically strenuous activity but ambulatory and able to carry out light work; PS 2= ambulatory and capable of all self-care but unable to carry out any work activities, up and about >50% of waking hours.
T1–2 = level of invasion through the bowel wall not beyond the muscle layer; T3–4 = level of invasion through the bowel wall beyond the muscle layer.
Based on questionnaire 1.
25(OH)D, 25-hydroxyvitamin D; No., number; FU/LV, 5-fluorouracil and leucovorin; IFL, irinotecan; MET, metabolic equivalent.
Impact of predicted 25(OH)D score on cancer recurrence and death
The median follow-up from the time of completion of Q1 for subjects who were still alive as of the database lock was 7.3 years (10th and 90th percentiles: 4.6 and 8.1 years, respectively). In total, 352 of the 1016 patients included in this analysis had cancer recurrence; 274 of these 352 patients died. An additional 44 patients died without documented recurrence.
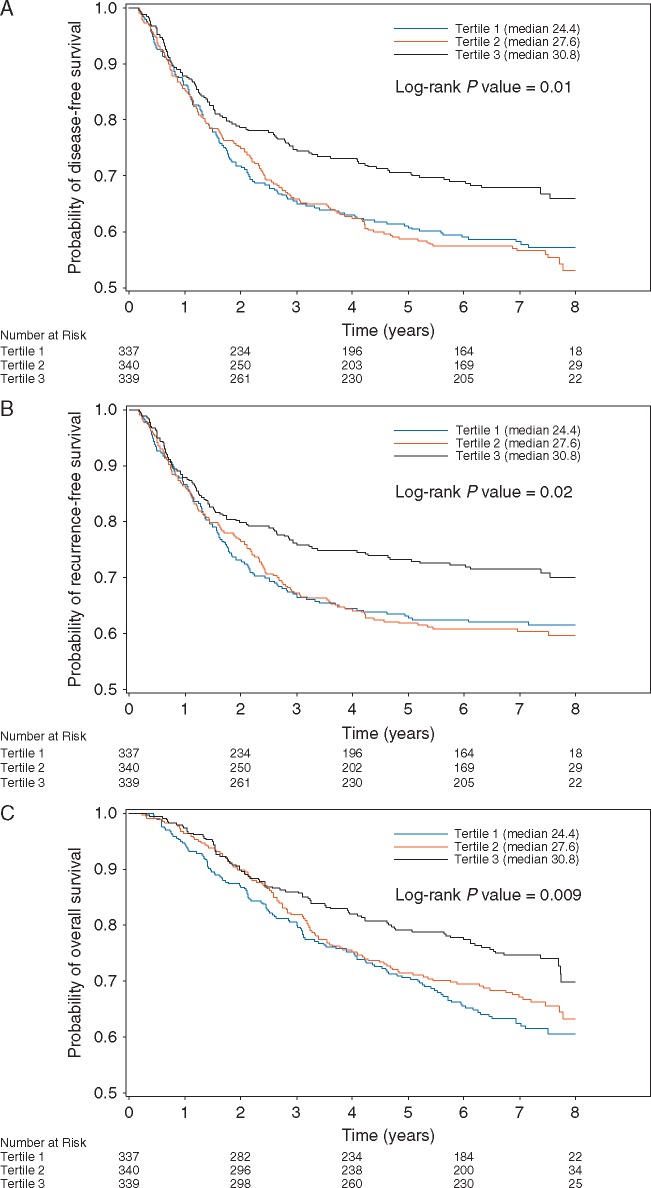
Higher predicted 25(OH)D level was associated with a significant reduction in the risk of cancer recurrence or mortality after adjusting for other predictors of outcome (Table 2 and Figure 2). Compared to patients with predicted 25(OH)D scores in the lowest quintile, those in the highest quintile had an adjusted HR for disease recurrence or mortality of 0.62 (95% CI, 0.44–0.86; Ptrend = 0.005). Increasing predicted 25(OH)D level was also associated with significantly improved OS (Ptrend = 0.0004) and RFS (Ptrend = 0.01).
Table 2.
Associations between colon cancer recurrence and mortality and predicted 25(OH)D score
| Outcome | Predicted 25(OH)D Score |
|||||
|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | Ρtrenda | |
| (n=207) | (n=200) | (n=204) | (n=203) | (n=202) | ||
| Disease-free survival | ||||||
| No. of events | 84 | 80 | 96 | 77 | 59 | |
| Unadjusted, HR (95% CI) | 1.0 | 1.00 (0.73–1.36) | 1.20 (0.90–1.61) | 0.89 (0.66–1.22) | 0.66 (0.48–0.92) | 0.02 |
| Multivariable adjusted, HR (95% CI)b | 1.0 | 0.96 (0.70–1.31) | 1.16 (0.86–1.57) | 0.84 (0.61–1.15) | 0.62 (0.44–0.86) | 0.005 |
| Recurrence-free survival | ||||||
| No. of events | 76 | 71 | 88 | 63 | 54 | |
| Unadjusted, HR (95% CI) | 1.0 | 0.98 (0.71–1.36) | 1.22 (0.90–1.66) | 0.81 (0.58–1.14) | 0.68 (0.48–0.96) | 0.02 |
| Multivariable adjusted, HR (95% CI)b | 1.0 | 0.94 (0.68–1.31) | 1.21 (0.88–1.65) | 0.77 (0.55–1.08) | 0.65 (0.46–0.93) | 0.01 |
| Overall survival | ||||||
| No. of events | 71 | 72 | 68 | 61 | 46 | |
| Unadjusted, HR (95% CI) | 1.0 | 1.08 (0.78–1.50) | 0.98 (0.71–1.37) | 0.83 (0.59–1.16) | 0.62 (0.42–0.89) | 0.004 |
| Multivariable adjusted, HR (95% CI)b | 1.0 | 1.04 (0.75–1.45) | 0.93 (0.67–1.31) | 0.72 (0.51–1.03) | 0.55 (0.38–0.80) | 0.0004 |
Two-sided P value calculated using predicted 25(OH)D score as a continuous variable.
Adjusted a priori for age (in years as a continuous variable), gender, family history of CRC (yes, no), baseline performance status (0, 1–2, unknown), depth of invasion through bowel wall (T1–2, T3–4, unknown), number of positive lymph nodes (1–3, ≥4, unknown), grade of tumor differentiation (well, moderate, poor/undifferentiated, unknown), and treatment arm (FU/LV, IFL).
HR, hazard ratio; CI, confidence interval; FU/LV, 5-fluorouracil and leucovorin; IFL, irinotecan.
Figure 2.
(A) Disease-free survival according to tertile of post-diagnosis predicted 25(OH)D levels. (B) Recurrence-free survival according to tertile of post-diagnosis predicted 25(OH)D levels. (C) Overall survival according to tertile of post-diagnosis predicted 25(OH)D levels. 25(OH)D, 25-hydroxyvitamin D.
We considered the possibility that the 25(OH)D score may be acting as a surrogate for the true causal factor, such as BMI or physical activity, which are in the prediction equation. We therefore repeated our analyses by further adjusting for individual covariables in our prediction score and our results were not substantially changed (supplementary Table S1, available at Annals of Oncology online). We additionally adjusted for total calcium intake and season of questionnaire completion, since these factors differed between quintiles of predicted 25(OH)D, and we continued to see a significant relationship between predicted 25(OH)D and DFS (Ptrend = 0.002).
Recognizing that lower levels of post-diagnosis predicted 25(OH)D could reflect the presence of occult cancer or other major illness, we excluded patients who died or recurred within 60 days following exposure assessment. When we further excluded patients who died or recurred within 180 days following exposure assessment, we continued to observe a significant relationship between predicted 25(OH)D score and disease recurrence or mortality (Ptrend = 0.01).
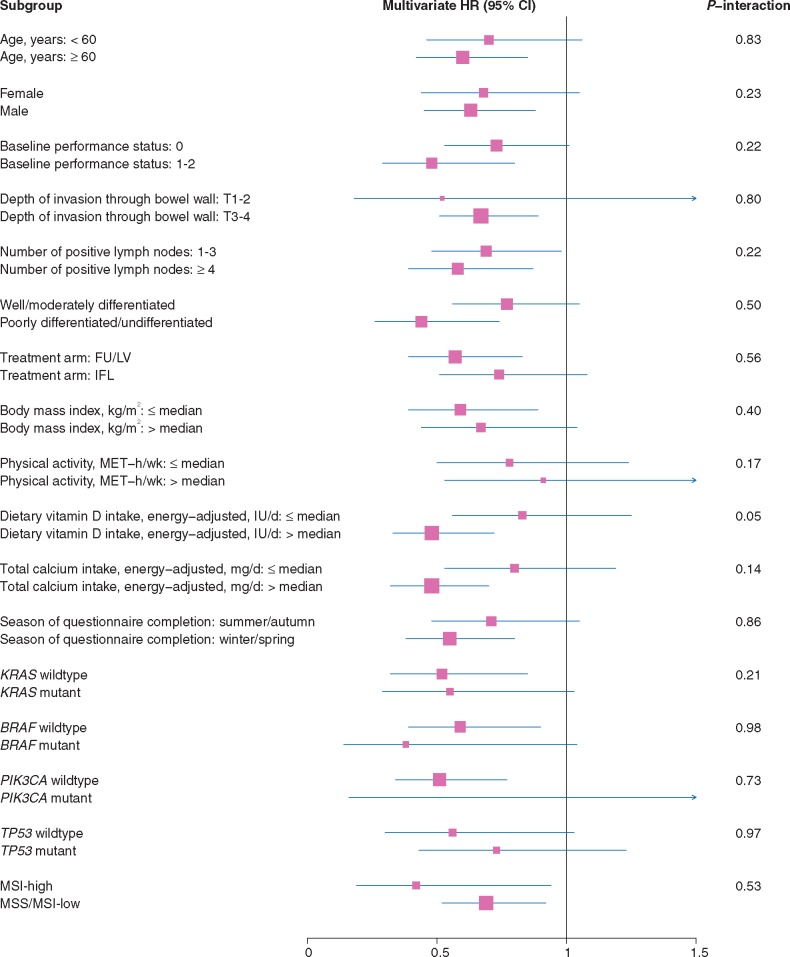
Stratified analyses
The association between predicted 25(OH)D levels and DFS appeared consistent across most strata of patient, disease, and treatment characteristics (Figure 3). The association of 25(OH)D score with improved DFS appeared slightly stronger among patients who consumed higher levels of dietary vitamin D (Pinteraction = 0.05).
Figure 3.
Adjusted hazard ratios (HR) and 95% confidence intervals (CI) comparing highest to lowest tertiles of predicted 25(OH)D level for disease-free survival across strata of predictors of patient outcome and tumor molecular features. FU/LV, 5-fluorouracil and leucovorin; IFL, irinotecan; MET, metabolic equivalent; MSI, microsatellite instability; MSS, microsatellite stable.
The association between predicted 25(OH)D levels and DFSdid not differ significantly across KRAS, BRAF, or TP53 mutational status or MSI status (Figure 3). Across strata of PIK3CA mutational status, the association between higher 25(OH)D score and improved DFS appeared limited to patients whose tumors were PIK3CA wildtype; nonetheless, the interaction test was not significant.
Discussion
In this cohort of stage III colon cancer patients enrolled in a clinical trial of postoperative chemotherapy, higher predicted 25(OH)D score was associated with a significant improvement in DFS, RFS, and OS. The benefit associated with higher predicted 25(OH)D score remained significant after adjusting for other predictors of patient outcome and was consistent across strata of clinical, pathologic, and molecular characteristics. To our knowledge, this is the first study to evaluate the association between vitamin D status and survival in a large cohort of patients with stage III colon cancer.
Our findings are consistent with prior studies in CRC supporting an improved outcome with higher vitamin D status [3]. Extensive preclinical evidence suggests vitamin D may have a preventative effect on colorectal carcinogenesis. The expression of VDR [8, 9] and 1-α-hydroyxlase [10], which converts plasma 25(OH)D into 1,25-dihydroxycholecalciferol [1,25(OH)2D], in CRC cells offers strong support for the vitamin D hypothesis. Activation of VDR by 1,25(OH)2D induces differentiation and apoptosis [11, 12], and inhibits proliferation [13], angiogenesis [14, 15], and metastatic potential [16, 17]. Vitamin D may also influence CRC biology through alternative mechanisms, including modifying systemic inflammation [18] and cellular immunity [19]. Our study has several advantages. All patients had lymph node-positive, nonmetastatic cancer at study entry, reducing the impact of heterogeneity by disease stage. Treatment and patient follow-up were carefully prescribed, and the date and nature of cancer recurrence were prospectively recorded through regular examinations. Detailed information on other potentially confounding prognostic variables was also prospectively collected at study entry. Finally, we updated dietary data to reflect changes in diet and other behaviors that may have occurred after patients had completed adjuvant therapy and recovered from treatment effects.
We considered the possibility that occult cancer recurrences or other poor prognostic characteristics may have influenced vitamin D status. To minimize this bias, we excluded recurrences or deaths within 60 days after questionnaire completion; when we excluded events within 180 days, our results remained unchanged. Moreover, because patients underwent comprehensive clinical and radiologic assessment before study entry, we would expect few patients to have occult cancer recurrences or other poor prognostic characteristics at baseline.
This study has several limitations. We used predicted 25(OH)D scores, which cannot be interpreted as direct blood measurements. However, predicted scores yielded similar results to measured plasma levels for the risks of colorectal, prostate, renal cell, and pancreatic cancers and hypertension, as well as for survival of CRC patients. Moreover, the ability to calculate predicted 25(OH)D scores on multiple questionnaires serially over time may provide better information on long-term 25(OH)D status than a single blood measure. The 25(OH)D score may act as a surrogate for other potential risk factors, such as BMI or physical activity. However, our results did not change after adjusting for these covariables, which suggests that total vitamin D status, rather than simply low BMI or physical activity, is driving the significant inverse association. Patients enrolled in clinical trials may differ from the general population. However, the distribution of dietary intake and lifestyle factors in our cohort was consistent with other U.S. cohort studies [20]. Moreover, since the study included patients from community and academic centers throughout North America, our findings should reflect the general population.
In summary, this prospective analysis suggests that higher levels of predicted 25(OH)D after diagnosis of stage III colon cancer are associated with a significant reduction in cancer recurrence and mortality. These findings are consistent with previous laboratory and epidemiologic studies and potentially offer meaningful recommendations for clinical care. Ultimately, this hypothesis needs to be examined in randomized clinical trials, and we await the results of such ongoing trials examining the role of vitamin D supplementation in CRC patients.
Funding
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821 and U10CA180882 to the Alliance for Clinical Trials in Oncology, CA31946 and CA33601 to the legacy CALGB, and CA180820 to the ECOG-ACRIN. Additional support was provided under U10CA032291, U10CA041287, U10CA045808, U10CA060138, U10CA077651, U10CA180791, U10CA180836, U10CA180838, U10CA180867, U10CA180882, UG1CA189858, and from Pharmacia & Upjohn Company, now Pfizer Oncology (no grant numbers apply). JAM, KN, and SO are supported in part by grants from the National Cancer Institute (K07 CA148894, R01 CA118553, R01 CA149222, R01 CA169141, R35 CA197735, and P50 CA127003) and the ASCO Conquer Cancer Foundation (no grant numbers apply). The sponsors did not participate in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Disclosure
KN has received research funding to the Dana-Farber Cancer Institute from Pharmavite, LLC. All remaining authors have declared no conflicts of interest.
Supplementary Material
References
- 1. Larriba MJ, Ordonez-Moran P, Chicote I. et al. Vitamin D receptor deficiency enhances Wnt/beta-catenin signaling and tumor burden in colon cancer. PLoS One 2011; 6: e23524.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zheng W, Wong KE, Zhang Z. et al. Inactivation of the vitamin D receptor in APC(min/+) mice reveals a critical role for the vitamin D receptor in intestinal tumor growth. Int J Cancer 2012; 130: 10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morales-Oyarvide V, Meyerhardt JA, Ng K.. Vitamin D and physical activity in patients with colorectal cancer: epidemiological evidence and therapeutic implications. Cancer J 2016; 22: 223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saltz LB, Niedzwiecki D, Hollis D. et al. Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: results of CALGB 89803. JCO 2007; 25: 3456–3461. [DOI] [PubMed] [Google Scholar]
- 5. Meyerhardt JA, Sato K, Niedzwiecki D. et al. Dietary glycemic load and cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Natl Cancer Inst 2012; 104: 1702–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Giovannucci E, Liu Y, Rimm EB. et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst 2006; 98: 451–459. [DOI] [PubMed] [Google Scholar]
- 7. Ng K, Wolpin BM, Meyerhardt JA. et al. Prospective study of predictors of vitamin D status and survival in patients with colorectal cancer. Br J Cancer 2009; 101: 916–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meggouh F, Lointier P, Saez S.. Sex steroid and 1,25-dihydroxyvitamin D3 receptors in human colorectal adenocarcinoma and normal mucosa. Cancer Res 1991; 51: 1227–1233. [PubMed] [Google Scholar]
- 9. Vandewalle B, Adenis A, Hornez L. et al. 1,25-dihydroxyvitamin D3 receptors in normal and malignant human colorectal tissues. Cancer Lett 1994; 86: 67–73. [DOI] [PubMed] [Google Scholar]
- 10. Zehnder D, Bland R, Williams MC. et al. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab 2001; 86: 888–894. [DOI] [PubMed] [Google Scholar]
- 11. Vandewalle B, Wattez N, Lefebvre J.. Effects of vitamin D3 derivatives on growth, differentiation and apoptosis in tumoral colonic HT 29 cells: possible implication of intracellular calcium. Cancer Lett 1995; 97: 99–106. [DOI] [PubMed] [Google Scholar]
- 12. Diaz GD, Paraskeva C, Thomas MG. et al. Apoptosis is induced by the active metabolite of vitamin D3 and its analogue EB1089 in colorectal adenoma and carcinoma cells: possible implications for prevention and therapy. Cancer Res 2000; 60: 2304–2312. [PubMed] [Google Scholar]
- 13. Scaglione-Sewell BA, Bissonnette M, Skarosi S. et al. A vitamin D3 analog induces a G1-phase arrest in CaCo-2 cells by inhibiting cdk2 and cdk6: roles of cyclin E, p21Waf1, and p27Kip1. Endocrinology 2000; 141: 3931–3939. [DOI] [PubMed] [Google Scholar]
- 14. Iseki K, Tatsuta M, Uehara H. et al. Inhibition of angiogenesis as a mechanism for inhibition by 1alpha-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 of colon carcinogenesis induced by azoxymethane in Wistar rats. Int J Cancer 1999; 81: 730–733. [DOI] [PubMed] [Google Scholar]
- 15. Fernandez-Garcia NI, Palmer HG, Garcia M. et al. 1alpha,25-Dihydroxyvitamin D3 regulates the expression of Id1 and Id2 genes and the angiogenic phenotype of human colon carcinoma cells. Oncogene 2005; 24: 6533–6544. [DOI] [PubMed] [Google Scholar]
- 16. Evans SR, Shchepotin EI, Young H. et al. 1,25-dihydroxyvitamin D3 synthetic analogs inhibit spontaneous metastases in a 1,2-dimethylhydrazine-induced colon carcinogenesis model. Int J Oncol 2000; 16: 1249–1254. [DOI] [PubMed] [Google Scholar]
- 17. Lamprecht SA, Lipkin M.. Cellular mechanisms of calcium and vitamin D in the inhibition of colorectal carcinogenesis. Ann N Y Acad Sci 2001; 952: 73–87. [DOI] [PubMed] [Google Scholar]
- 18. van Harten-Gerritsen AS, Balvers MG, Witkamp RF. et al. Vitamin D, inflammation, and colorectal cancer progression: a review of mechanistic studies and future directions for epidemiological studies. Cancer Epidemiol Biomarkers Prev 2015; 24: 1820–1828. [DOI] [PubMed] [Google Scholar]
- 19. Song M, Nishihara R, Wang M. et al. Plasma 25-hydroxyvitamin D and colorectal cancer risk according to tumour immunity status. Gut 2016; 65: 296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meyerhardt JA, Niedzwiecki D, Hollis D. et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. Jama 2007; 298: 754–764. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.