Abstract
α-Synuclein (αSyn) is an intrinsically disordered protein, the aggregation of which is highly related to the pathology of diverse α-synucleinopathies. Various hard divalent metal cations have been shown to affect αSyn aggregation. Especially, Ca2+ is suggested to be a crucial ion due to its physiological relevance to α-synucleinopathies. However, the molecular origin of αSyn aggregation mediated by the metal ions is not fully elucidated. In this study, we revealed that hard divalent metal ions had almost identical influences on αSyn aggregation. Based on these similarities, the molecular role of Ca2+ was investigated as a representative metal ion. Herein, we demonstrated that binding of multiple Ca2+ ions induces structural transition of αSyn monomers to extended conformations, which promotes rapid αSyn fibrillation. Additionally, we observed that Ca2+ induced further interfibrillar aggregation via electrostatic and hydrophobic interactions. Our results from multiple biophysical methods, including ion mobility-mass spectrometry (IM-MS), synchrotron small-angle X-ray scattering (SAXS), transmission electron microscopy (TEM), provide detailed information on the structural change of αSyn and the aggregation process mediated by Ca2+. Overall, our study would be valuable for understanding the influence of Ca2+ on the aggregation of αSyn during the pathogenesis of α-synucleinopathies.
Introduction
A number of neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease, are associated with the formation of amyloid fibrils1. During the fibrillation of amyloidogenic proteins, monomeric proteins are converted into oligomeric intermediates, and finally, to highly ordered, unbranched β-sheet structures2,3. Amyloid fibrillation is not thoroughly understood yet; however, it is considered to have a correlation with protein misfolding2,4. Therefore, understanding the mechanisms of amyloid fibrillation, which is associated with protein misfolding, is necessary for developing therapeutic strategies for amyloidosis.
α-Synuclein (αSyn) is a small amyloidogenic protein, which is abundant, particularly, at the presynaptic nerve terminals5–7. αSyn is considered to modulate the synaptic vesicle cycle, which is involved in neurotransmission7,8; however, it has received attention because of its pathological significance as the main component of Lewy bodies and Lewy neurites, observed in patients with α-synucleinopathies such as Parkinson’s disease (PD), multiple system atrophy (MSA), and Lewy body dementia (LBD)9. αSyn is an intrinsically disordered protein (IDP), and comprises 140 amino acid residues, which constitute the amphipathic domain at the N-terminal region (residues 1–60), the hydrophobic non-amyloid-β component (NAC) region (residues 61–95), and the acidic domain at the C-terminal region (residues 96–140) (Fig. 1A). The structure of the NAC region in particular, is considered important for fibrillation kinetics10,11. In water, the NAC region tends to be located towards the interior of the protein, and is shielded from contact with water12,13. This innate structure of αSyn induced by intramolecular interaction hinders intermolecular aggregation. However, once the NAC region is exposed to the outside because of the structural transitions of αSyn, the interface between water and the exposed NAC region induces intermolecular hydrophobic interactions between the NAC regions.
Figure 1.
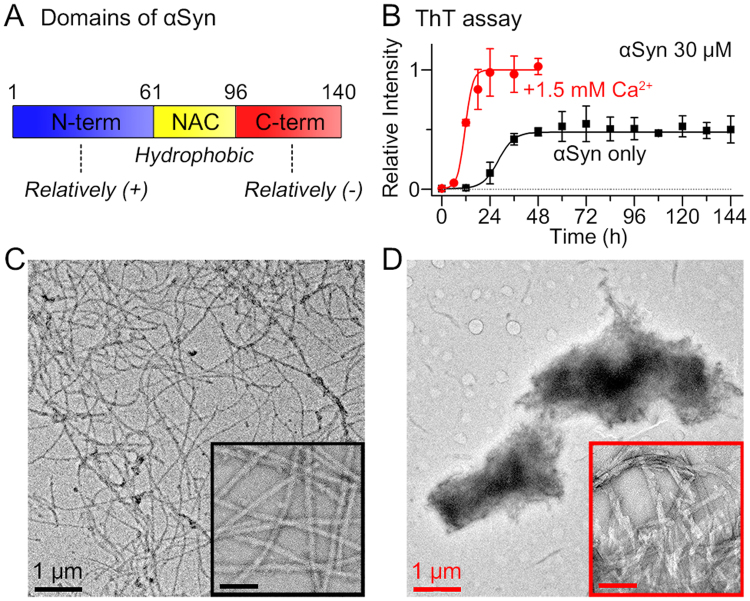
Ca2+-mediated αSyn aggregation. (A) Three domains of αSyn. (B) ThT assay for the fibrillation kinetics of αSyn (30 μM) in the presence and absence of 1.5 mM CaCl2. All αSyn samples included a low level of residual Ca2+ (~10 μM). (C) TEM images of αSyn fibrils formed in the absence of Ca2+ and (D) aggregates formed in the presence of 1.5 mM Ca2+. The scale bars in the insets of TEM images are 100 nm.
The aggregation of αSyn is believed to be associated with environmental factors, such as an imbalance in metal homeostasis14,15. Cu2+, a divalent transition metal ion, has been proposed as a potential cause of αSyn fibrillation with its high binding affinity (Kd ~10−6–10−10 M) and unique binding site (The N-terminus and His-50 are involved)16–18. Unlike Cu2+, hard divalent metal cations have been shown to promote the aggregation of αSyn15,19,20 with binding to the acidic C-terminal region (Kd ~ 10−3 M)19,21. The metal cations also induce the formation of Lewy body-like large assemblies comprising αSyn fibrils22. However, the mechanistic details on the metal-associated fast aggregation and Lewy body-like interfibrillar aggregation of αSyn have not been fully understood. Among the hard divalent metal ions, converging evidence suggests that Ca2+ is a crucial physiological factor related to αSyn aggregation. Firstly, the dysregulation of Ca2+ has been observed in aged animals23 and mice model of α-synucleinopathies24. Abnormally increased intracellular Ca2+ concentration, which is normally regulated to be ~100 nM (while its extracellular concentration is ~1.4 mM)25, can cause aggregation of αSyn26–28, and finally induce neurodegeneration26,29. Secondly, significant amount of Ca2+ has been detected in Lewy bodies of patients with PD30. This suggests the possibility of the involvement of Ca2+ in the formation of Lewy bodies. Furthermore, when αSyn is secreted to the extracellular space (secretion of αSyn is commonly observed in PD model systems31–33), αSyn can be exposed to and influenced by high level of Ca2+ (~1.4 mM). Taken together, the interaction between αSyn and Ca2+ appears to be closely related to αSyn aggregation in α-synucleinopathies.
Herein, we have reported the unique αSyn aggregations mediated by hard divalent cations to form large interfibrillar aggregates. Then, the mechanism of αSyn aggregation mediated by Ca2+, a representative hard divalent cation, was proposed by monitoring the structural transition of αSyn from the monomeric state to the large interfibrillar aggregate state. Our structural and kinetic results, which were obtained using multiple biophysical methods, including ion mobility-mass spectrometry (IM-MS), transmission electron microscopy (TEM), synchrotron small-angle X-ray scattering (SAXS), and inductively coupled plasma optical emission spectroscopy (ICP-OES) demonstrated that Ca2+ mediates the rapid formation of αSyn fibrils via the structural transition of monomeric αSyn into an extended conformation, which is prone to aggregation. We probed that direct interaction between Ca2+ and αSyn fibril induces the subsequent association of the fibrils with secondary structure changes to form large interfibrillar αSyn aggregates through electrostatic and hydrophobic interactions. Moreover, we observed that αSyn aggregates formed through Ca2+ mediation are toxic to SH-SY5Y neuroblastoma cells. We believe that the aggregation mechanism of αSyn mediated by Ca2+ provides an insight into the formation mechanism of the inclusion bodies that is commonly observed in α-synucleinopathies.
Results and Discussion
αSyn aggregation mediated by Ca2+ and other hard divalent metal cations and morphological properties
First, we investigated the fibrillation kinetics of αSyn in the presence of Ca2+ using the thioflavin T (ThT) assay (Fig. 1B). Fibrillation of αSyn was accelerated by the addition of Ca2+ (t1/2 = 11.6 h)19,27,34 compared with the control group, αSyn incubated without Ca2+ (t1/2 = 28.1 h). Furthermore, the ThT fluorescence intensity of Ca2+-mediated αSyn aggregates was almost twice as high as the control group, which implied that Ca2+ promoted the conversion of more monomers to fibrils. Then, the morphology of aggregates was observed using transmission electron microscopy (TEM). In the absence of Ca2+, normal amyloid fibrils were formed (Fig. 1C). In contrast, from αSyn incubated with Ca2+, micrometer-scale globular αSyn aggregates were formed (Fig. 1D). The inset of Fig. 1D showed that the αSyn aggregates formed through Ca2+ mediation are clusters of fibrils, as previously reported by Semerdzhiev et al.22.
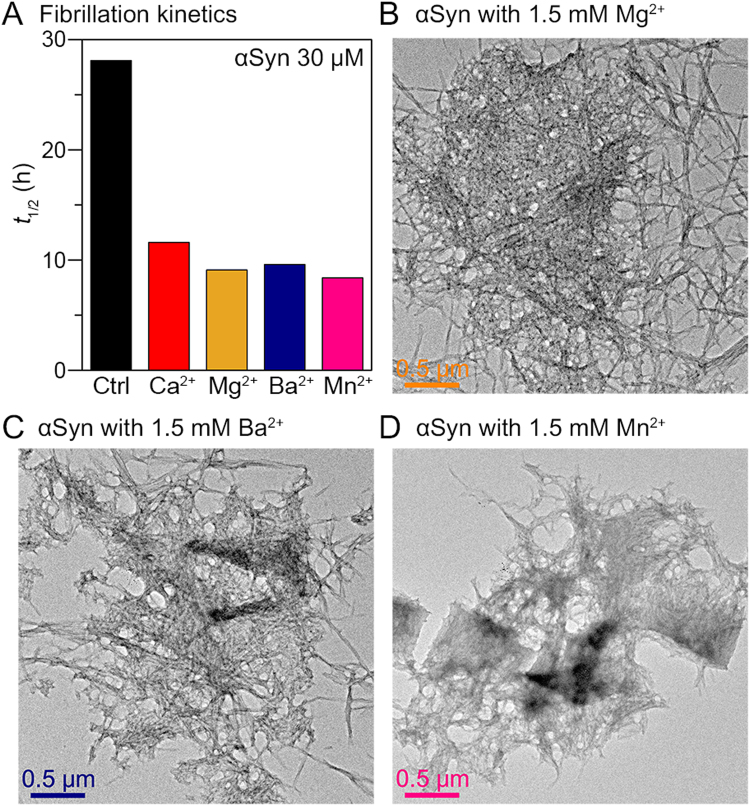
Then, we examined other hard divalent metal ions which bind to the C-terminal region of αSyn, to understand the generality of the metal charge state and the binding site on αSyn fibrillation. For the experiment, Mg2+ and Ba2+, smaller and larger alkaline-earth metals than Ca2+, respectively, and Mn2+, as an example of a transition metal, were chosen. Using ThT assay, we observed that these hard divalent metal ions also accelerated the fibrillation of αSyn (Fig. 2A and Supplementary Fig. 1). We found that the metal ions induced the formation of large aggregates comprising fibrils (Fig. 2B–D). These results indicated that hard divalent ions have similar effects to the aggregation of αSyn by their unique complexation with αSyn.
Figure 2.
αSyn aggregation mediated by hard divalent metal ions. (A) The half-time of fibrillation (t1/2) of αSyn (30 μM) in the presence of 1.5 mM hard divalent metal ions. The t1/2 values were obtained by performing ThT assay. TEM images of αSyn aggregates formed in the presence of (B) 1.5 mM Mg2+, (C) 1.5 mM Ba2+, and (D) 1.5 mM Mn2+.
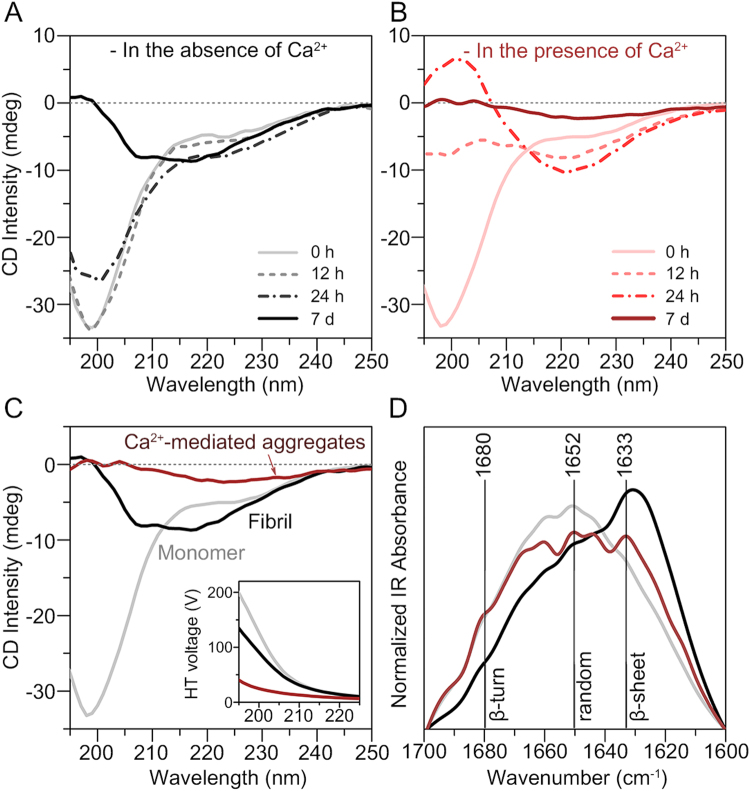
To understand the formation mechanism of αSyn aggregates by divalent metal cations, secondary structural analysis was performed on Ca2+-mediated αSyn aggregates using time-resolved circular dichroism (CD) spectroscopy. The control group without Ca2+ exhibited random coil conformation at 0 h; then, the initial conformation was gradually converted to a β-sheet conformation (negative band at 218 nm) after incubation for 7 d (Fig. 3A). In the presence of Ca2+, αSyn also exhibited random coil conformation before incubation (Fig. 3B). The conformation of αSyn, which was incubated with Ca2+, was rapidly converted to β-sheet conformation at 24 h. However, after incubation for 7 d, the spectrum of the αSyn aggregates formed through Ca2+ mediation exhibited distinctive characteristics. This pattern of events implied that Ca2+ promotes the formation of β-sheet-rich fibrillar aggregates in the early stage; however, Ca2+ mediates a different type of αSyn aggregate in the later stage.
Figure 3.
Secondary structural analysis of the αSyn aggregates formed through Ca2+ mediation. Time-resolved CD spectra of two-fold diluted αSyn samples (30 μM) incubated (A) in the absence of Ca2+ and (B) in the presence of 1.5 mM Ca2+. All incubated αSyn samples included a residual Ca2+ concentration of ~10 μM. (C) CD spectra and (D) IR spectra of the αSyn monomers (gray), fibrils (black), and aggregates formed through Ca2+ mediation (brown). The inset in the image of CD spectra shows HT voltage of the samples, which was simultaneously measured during CD measurements.
The CD spectrum of the Ca2+-mediated αSyn aggregates formed by incubation for 7 d was completely different from that of the conventional αSyn fibrils. αSyn fibrils exhibited a β-sheet-rich structure; however, the CD intensity of the Ca2+-mediated αSyn aggregates was low (Fig. 3C). In addition, the intensity of high tension (HT) voltage (Fig. 3C, inset), which is proportional to the absorbance of the samples35, was lower in the Ca2+-mediated aggregates. The absorbance of large protein aggregates is usually reduced compared with the expected absorbance as calculated using the Beer-Lambert equation, because large aggregates have lower effective cross-sectional area of chromophores than uniformly dissolved samples36. To confirm that the observed low CD intensity and HT voltage did not originate from the rapid sedimentation of αSyn aggregates, we measured the sedimentation degree of well-dispersed αSyn fibrils and Ca2+-mediated aggregates. The suspensions were left to stand for 0–30 min and the supernatants were analyzed by using ThT assay and optical density (OD) measurements (Supplementary Fig. 2). Our results showed that both rarely sedimented for 5 min, which indicated that the reason for the low CD intensity of Ca2+-mediated aggregates was their large size, as shown in Fig. 1D, which did not result from the sedimentation of aggregates during the CD measurement (~5 min).
To further reveal the secondary structure of αSyn aggregates formed through Ca2+-mediation, we performed infrared (IR) spectroscopy. The IR spectra indicated that the αSyn aggregates formed through Ca2+ mediation consisted of a low structural portion of β-sheets, compared with the conventional fibrils (Fig. 3D). Based on the different morphologies as observed by TEM and secondary structures as observed by IR spectroscopy, it is suggested that the interaction with Ca2+ stimulates the formation of distinct αSyn aggregates. Overall, our results, as shown in Figs 1 and 3, suggested that Ca2+ promotes the fibrillation of αSyn, and mediates the formation of β-sheet-rich structures in the early stage. Then, Ca2+ further induces the formation of large αSyn aggregates through interfibrillar aggregation and secondary structural change.
Rapid aggregation of αSyn with Ca2+-induced structural transition of monomers
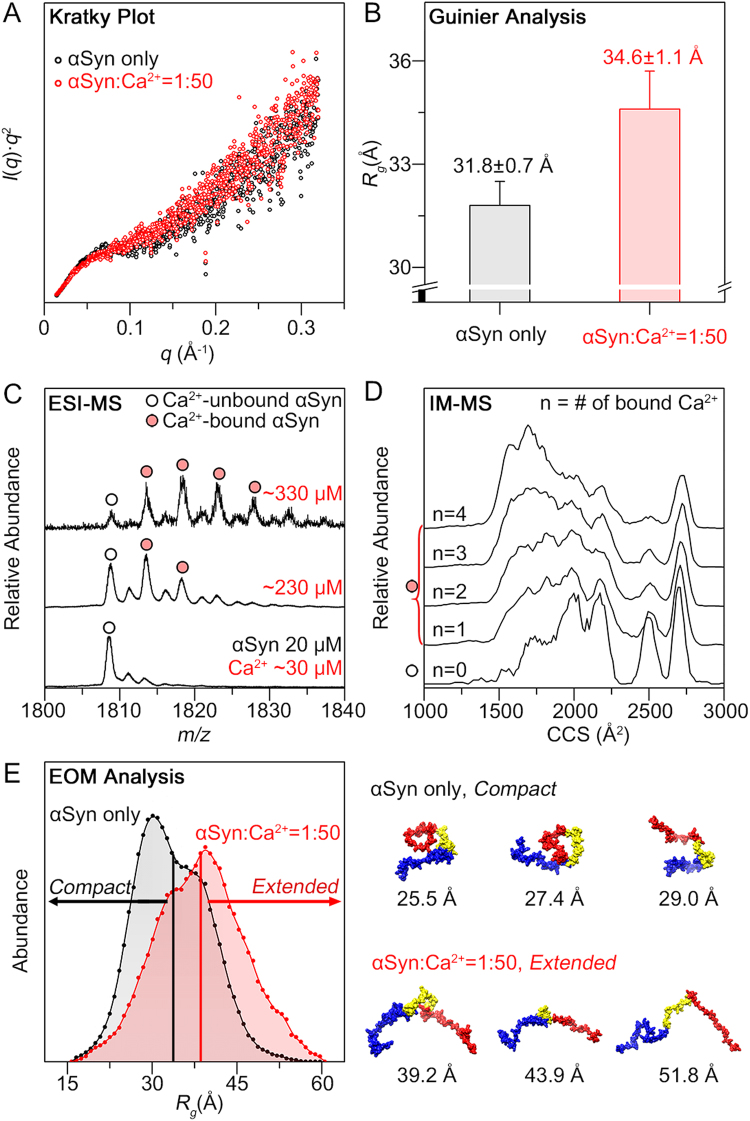
Because αSyn aggregation commonly involves structural transition of monomeric protein9,37, we utilized SAXS and IM-MS to characterize the structures of Ca2+-bound αSyn monomer. The Kratky analysis (I(q)·q2 vs q) from the SAXS profiles38 showed the typical curves of unfolded proteins (i.e. the lack of bell-shaped curves and increase in the q range)38,39 for both αSyn in the presence and absence of Ca2+ (Fig. 4A). However, the values of radius of gyration (Rg) of αSyn, which were obtained from the Guinier analysis, showed that Rg increased when Ca2+ was added. The Rg value of αSyn was measured to be 31.8 ± 0.7 Å, which was similar to the value obtained in the previous study (Fig. 4B)40. As Ca2+ concentration in the solution increased, the Rg value of αSyn tended to increase (Supplementary Fig. 3). The Rg values reached 34.6 ± 1.2 Å at 10-fold molar ratio of Ca2+ to αSyn, and a similar value was maintained at 50-fold ratio (Fig. 4B and Supplementary Fig. 3). These results indicated that Ca2+ induces αSyn monomer to form extended conformations in solution.
Figure 4.
Conformational transition of αSyn monomers induced by Ca2+. (A) Kratky plots of αSyn (200 μM) in the absence and presence of 50-equivalent Ca2+ (10 mM). (B) Rg values obtained by the Guinier analysis of SAXS scattering curves. (C) ESI-mass spectra of αSyn (20 μM) in the + 8 charge state with increasing Ca2+ concentration. The residual Ca2+ concentration of the αSyn sample without CaCl2 (bottom) was ~30 μM and all Ca2+ concentrations were the sum of the residual Ca2+ and added CaCl2. In the mass spectra, Ca2+-unbound and bound αSyn were marked with empty circles and filled circles, respectively. (D) IM-MS spectra of αSyn in the + 8 charge state with increasing number (n) of bound Ca2+ ions ([αSyn + nCa + (8–2n)H]8+). (E) Rg distribution of the structural ensemble (50 structures) obtained using EOM and some representative structures of the ensemble with the Rg values. Residues that are shown in blue, yellow, and red indicate the N-terminal, NAC, and C-terminal regions, respectively.
IM-MS coupled with electrospray ionization (ESI) detected that multiple Ca2+ ions were bound to αSyn monomer (20 μM). As Ca2+ concentration increased, the maximum number of Ca2+ bound to αSyn and the relative abundance of Ca2+-bound αSyn peaks in the mass spectrum increased (Fig. 4C and Supplementary Fig. 4). In particular, a maximum of four Ca2+ ions were bound to one αSyn molecule in the presence of 330 μM CaCl2. In solution, Ca2+ ions bind to multiple binding sites of the C-terminal region with the binding enthalpy (ΔH°) ~2 kcal/mol41 and weak affinity (Kd ~ 1 mM)19,41. Because four binding sites were previously reported41, based on Kd ~ 1 mM and the exact Ca2+ concentration, 0.71 and 0.95 Ca2+ per αSyn molecule (230 μM and 330 μM Ca2+, respectively) could bind on average. However, the electrostatic interactions between a protein and metal cations can be further enhanced in the gas phase, because solvent molecules evaporate during the ESI process42. Thus, despite the low binding affinity, a large number of bound Ca2+ ions, which were weakly associated with the C-terminal region via long range attractive interaction, were ultimately observed in the gas phase. In addition, the increased number of Ca2+ ions bound to αSyn ions in the sample with 330 μM Ca2+ than in the sample with 230 μM Ca2+ (Fig. 4C) was considered to be related to the charge saturation and location of metal ions in electrosprayed droplets. In the droplet, metal ions are likely located near the surface with higher number density as the metal concentration increases43, which can influence the complexation with IDPs, favoring it to be near the surface of the droplet44. Furthermore, we found that the total charge of the ESI was saturated at just below 330 μM (Supplementary Fig. 4) in our experimental conditions45. As a result, enhanced numbers of Ca2+ ions were most likely located at the surface of the droplet to generate αSyn ions, with a maximum of four Ca2+ from the sample of 330 μM CaCl2.
The IM-MS spectra of Ca2+-bound αSyn showed multiple ion mobility peaks with collision cross-section (CCS) values ranging between 1400~2700 Å2 for +8 charged αSyn (Fig. 4D). As the number of Ca2+ that were bound to αSyn increased, αSyn molecules tended to adopt more compact conformations in the gas phase. This pattern was opposite to the results of the SAXS measurement in solution.
To understand the relation between the structural transitions of αSyn stimulated by Ca2+ and its accelerated aggregation, molecular dynamics (MD) simulation of Ca2+-bound αSyn was performed to match our SAXS and IM-MS data. Representative structures of αSyn in solution were obtained from the structure pool of αSyn generated using replica exchange MD simulations46, based on the ensemble optimization method (EOM), which identifies the best ensemble by fitting sum of multiple theoretical SAXS profiles to the experimental SAXS profile47,48. Using the obtained representative structures as initial structures, gas-phase MD simulations were also performed to obtain gas-phase structures having theoretical CCS values (CCStheo) corresponding to the experimental values. The CCStheo value of each structure was estimated using the exact hard sphere scattering (EHSS) method49, and compared with the experimental CCS values (Supplementary Fig. 5).
The finally obtained αSyn ensembles in the absence and presence of Ca2+ in solution (50 structures for each ensemble) showed differences in Rg distributions. The αSyn ensembles that were obtained in the absence of Ca2+ and in the presence of Ca2+ had distributions ranging from 15 to 60 Å; however, the former had a distribution with an average of 33.6 ± 6.4 Å, while the latter had a distribution with an average of 38.5 ± 7.7 Å (Fig. 4E). Although the difference between averaged Rg values of αSyn is not significant, the Rg distribution of 50 αSyn structures showed the clear trend that the abundance of extended conformations was increased in the presence of Ca2+. The representative structures of the extended conformations of αSyn with Rg > 38.5 Å showed that the hydrophobic NAC region was generally exposed towards the outside (Fig. 4E). Because the exposure of the NAC region to water lowers the activation energy of intermolecular interactions, aggregation of αSyn could be triggered. Thus, we considered that the structural transition of monomeric αSyn, which was induced by Ca2+, promoted the aggregation of αSyn.
In addition, the representative structures of αSyn (8+) that were obtained using the gas-phase MD simulation (Supplementary Fig. 5) explained why Ca2+-bound αSyn tended to adopt a compact structure in the gas phase. From the various gas-phase structures of αSyn (8+), it was observed that the overall structures of the αSyn that were unbound and bound to Ca2+ (8+) were similar if their CCStheo values were similar. To understand why compact conformation was preferred in Ca2+-bound αSyn, we investigated the representative compact structure of Ca2+-bound αSyn (CCStheo = 1717.9 Å2) (Supplementary Fig. 6). The representative structure showed that the binding of Ca2+ to multiple carboxylate groups was preserved, and the electrostatic interaction between Ca2+ and the carbonyl backbone of residues in the N-terminal and NAC regions might be newly established during the ESI process (Supplementary Fig. 6). We predicted that this structural change was induced due to the absence of solvent molecules in the gas phase. Because electrostatic interaction becomes influential in the gas phase50,51, Ca2+, which had been bound only to C-terminal region, was additionally attracted to other regions of αSyn in the gas phase, thereby forming structures different from the solution structures.
Through the structural study of Ca2+-bound αSyn monomers, we observed that multiple Ca2+ ions bound to αSyn, and they could result in the formation of extended conformations of αSyn in solution (Fig. 4). We anticipated that the structural transitions of αSyn would be induced by the change in intramolecular interactions upon binding of Ca2+ to the C-terminal regions. In αSyn monomers, the N-terminal and C-terminal regions have long-range attractive interaction, because the N-terminal regions are positively charged and the C-terminal regions are negatively charged12,13. However, when Ca2+ ions were bound to the C-terminal regions, they would not attract the N-terminal regions. Thus, it was expected that the population of the compact structures reduced, which resulted in an increased average Rg value of Ca2+-bound αSyn.
From our results, it was considered that the structural changes in αSyn promoted its aggregation with the exposure of the NAC region. However, in addition to the structural aspect, charge neutralization of the C-terminal region may contribute to the induction of αSyn aggregation by reducing the repulsion between αSyn molecules, as suggested in the previous study of αSyn at low pH52. Therefore, it was considered that the aggregation of αSyn may be additionally accelerated by the change in local charge environment of the C-terminal region.
Interfibrillar aggregation of αSyn induced by Ca2+
Our TEM image of αSyn aggregates formed through Ca2+ mediation demonstrated that Ca2+ induced the formation of large αSyn aggregates through interfibrillar aggregation (Fig. 1D). In order to understand the role of Ca2+ in αSyn interfibrillar aggregation, we investigated whether Ca2+ can also induce interfibillar aggregation when it is added to mature αSyn fibrils. The mature αSyn fibrils were prepared by incubating αSyn monomer for 60 h, when the fibrillation extent was maximum (Fig. 1B). Surprisingly, we observed that the fibrils were converted to large aggregates, which were similar to the aggregates that were formed by the initial application of Ca2+ to the αSyn monomers (Fig. 5A). The inset in Fig. 5A obviously showed that the aggregates were formed through interfibrillar aggregation. We also observed that the aggregates had similar structural characteristics, such as low CD intensity and low structural portion of β-sheets as demonstrated in the IR spectrum, compared with the aggregates formed by initial Ca2+ addition (Supplementary Fig. 7).
Figure 5.
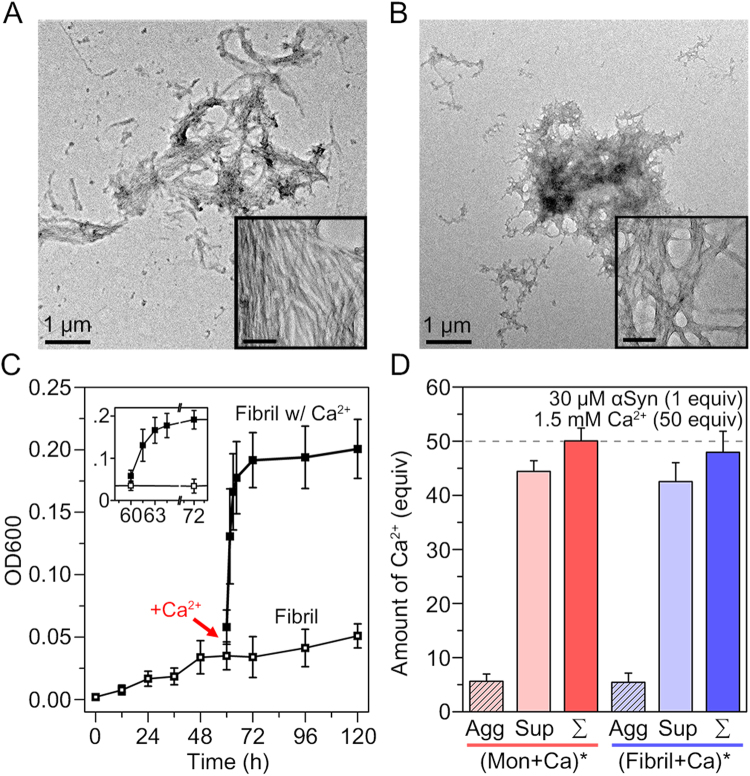
Ca2+-mediated interfibrillar aggregation of αSyn. TEM images of (A) αSyn aggregates formed by the addition of Ca2+ to mature αSyn fibrils (αSyn solution incubated for 60 h) and (B) aggregates formed by the addition of Ca2+ to αSyn, which was pre-incubated for 30 h in the absence of Ca2+ (~t1/2). For the experiments, 3.75 μL of 100 mM CaCl2 in 20 mM Tris-HCl was added to 246.25 μL of pre-incubated 30.46 μM αSyn to make solution with final concentrations of 30 μM αSyn and 1.5 mM Ca2+ (we assumed that the solution volume was conserved during the incubation). The scale bars in the insets of TEM images are 100 nm. (C) Measurement of OD600 of αSyn samples during fibril formation from monomers and interfibrillar aggregation induced by additionally added Ca2+. As a control, 30.46 μM αSyn was incubated without Ca2+. (D) Amounts of Ca2+ (50 equivalent in total, concentration of 1.5 mM) included in the αSyn aggregates (1 equivalent, concentration of 30 μM) formed through Ca2+ mediation. Agg, Sup, ∑ denote the amounts of Ca2+ in insoluble aggregates, supernatants, and the sum of the Ca2+ in aggregates and supernatants. ()* denotes the aggregates that were formed from the components enclosed within parentheses, monomers (Mon) or preformed fibrils with Ca2+.
To understand the properties of interfibrillar αSyn aggregation, we monitored the morphological changes upon the addition of Ca2+ to αSyn samples at different stages of fibrillation. As shown in Fig. 5B, large aggregates composed of fibrils were formed even when Ca2+ was added to the αSyn solution, which was incubated for 30 h (t1/2). In contrast, the αSyn samples that were incubated for 30 h and 60 h without Ca2+ showed the presence of conventional fibrils (Supplementary Fig. 8). OD measurements further showed the kinetics of Ca2+-mediated aggregation between fibrils (Fig. 5C). During the conversion of αSyn monomers to amyloid fibrils in the absence of Ca2+, the OD at 600 nm (OD600) increased gradually to approximately 0.05. However, when Ca2+ was added after 60 h of the incubation of αSyn, OD600 increased instantaneously, and reached approximately 0.20 in a few hours of incubation (Fig. 5C). This sharp increase in OD value indicated that the rates of Ca2+-mediated interfibrillar aggregation was faster than the aggregation kinetics of αSyn monomers incubated with Ca2+, which required ~20 h (Fig. 1B). This was possibly because the time required to form amyloid fibril was further included when monomers were incubated with Ca2+ in addition to the interfibrillar aggregation step. Moreover, we observed that interfibrillar aggregation subsequently commenced when fibrils started to form from αSyn incubated with Ca2+ (Supplementary Fig. 8). Therefore, it is suggested that Ca2+ promptly mediates interfibrillar aggregation at any time in the presence of αSyn fibrils.
All of the results indicate that Ca2+ directly mediates the aggregation between αSyn fibrils. However, it is not clear whether Ca2+ is incorporated in the aggregates. Therefore, we performed inductively coupled plasma optical emission spectroscopy (ICP-OES) to measure the amount of Ca2+. For the ICP-OES experiment, incubated αSyn samples were centrifuged at 18,000 × g, and the amounts of Ca2+ were measured in supernatant and insoluble αSyn aggregates. Figure 5D shows that the aggregates formed by incubating Ca2+ with αSyn monomer and fibril both include significant amount of Ca2+. Because Ca2+ enhanced the affinity between the C-terminal region of fibrils in both cases, it was considered that Ca2+ reduced the charge–charge repulsion between acidic residues of adjacent fibrils by binding to the acidic residues. In addition, we observed that the amounts of incorporated Ca2+ were similar in both aggregates (Fig. 5D). We expected that these similar amounts of Ca2+ may be due to the identical role of Ca2+ in both cases, at least with regard to interfibrillar aggregation.
The aggregation mechanism of αSyn mediated by Ca2+
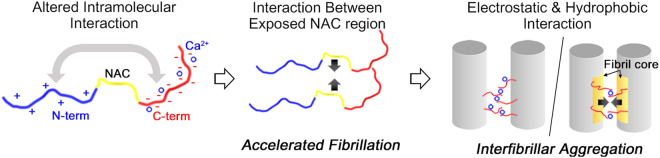
In the present study, we have shown that Ca2+ interacts with αSyn, and mediates distinct pathways of aggregation. At the early stage of aggregation, Ca2+, which binds to the C-terminal region of αSyn, induces structural transition of the protein monomer, whereby the NAC region is exposed, thereby resulting in rapid fibrillation. Then, Ca2+ triggers nonspecific interfibrillar aggregation to produce large aggregates as the final products.
When Ca2+ ions are bound to αSyn monomers, the population of αSyn conformation is changed and fibrillation rate of αSyn is increased. Thus, the attractive intramolecular interactions between the N- and C-terminal regions were likely reduced due to the positive charge of Ca2+ ions bound to the C-terminal region of αSyn monomers. This change in intramolecular interaction decreases the stability of monomeric αSyn, by inducing the hydrophobic NAC region exposed to water. Therefore, the Ca2+-bound monomers begin to undergo fibrillation to prevent exposure of their hydrophobic regions to water (Fig. 6).
Figure 6.
Mechanism of Ca2+-mediated aggregation of αSyn.
Using ThT assay, TEM, and CD spectroscopy, we found that the secondary structure of αSyn rapidly changed, becoming rich in β-sheets and forming large interfibrillar aggregates, in the presence of Ca2+. However, the secondary structures of the aggregates finally formed through Ca2+ mediation were different from those of the conventional fibrils (Fig. 3D). We examined whether this structural difference originated from the structure of fibril itself or formed during interfibrillar aggregation. Our results that were obtained upon the addition of Ca2+ to mature αSyn fibril provided a clue to resolve the issue. We observed that the secondary structures of the aggregates that were formed through Ca2+ mediation were similar regardless of whether Ca2+ was added to monomeric form or fibrillar form of αSyn (Supplementary Fig. 7). We considered that if the distinctive secondary structure of αSyn aggregates formed by initial addition of Ca2+ were merely a property of individual fibrils, the mature fibrils forming large aggregates by Ca2+ would have undergone structural change before the interfibrillar aggregation. However, based on the recently reported structure of αSyn fibril53, the binding of Ca2+ to a single strand of αSyn fibril would not be sufficient to alter the overall structure of fibril. The structure showed that the C-terminal region of αSyn fibril is located far from the N-terminal region, while the residues 30–100 in the middle form the fibril core53. This implied that the N-terminal region would not be affected by Ca2+-bound C-terminal region. Additionally, the structure showed that the fibril core region does not have a strong interaction with the C-terminal region. Thus, it was considered that the structural change of secondary structure of αSyn aggregates may occur during interfibrillar aggregation.
Semerdzhiev et al. recently reported that the enhanced ionic strength of the solution induces interfibrillar aggregation of αSyn22. They suggested that the aggregation occurs via long-ranged repulsive and short-ranged attractive interactions22. In our study, we demonstrated that the direct interaction between Ca2+ and αSyn fibrils causes interfibrillar aggregation of αSyn. The OD measurements showed that the interfibrillar aggregation of preformed αSyn fibrils is immediately initiated after Ca2+ was added (Fig. 5C). Since Ca2+ mainly binds to acidic residues in the C-terminal region and remain in the finally formed aggregates (Fig. 5D), (1) the incorporated Ca2+ may reduce the interfibrillar repulsion between negatively charged C-terminal residues, and (2) the interfibrillar interaction may be further stabilized through the chelating Ca2+ between two acidic residues (i.e. originated from each fibril). In addition, our IR spectra showed the dramatic decrease in β-sheet abundance of Ca2+-mediated fibrils (Fig. 3D and Supplementary Fig. 7B). However, changing the secondary structure cannot be explained with the only electrostatic interaction between Ca2+ and C-terminal regions, because β-sheet structure mostly originates from the fibril core region53. β-sheet is one of the most stable secondary structures and the fibril core region is composed of a large number of hydrophobic residues. Therefore, the structural change in this region implies that hydrophobic interaction was newly formed between the fibril core regions of different fibrils. Based on the structural analysis, we characterized the aggregation mechanism of αSyn mediated by Ca2+ (Fig. 6); at first, the fibrils are closely located due to the electrostatic interaction formed between divalent metal ions and C-terminal regions of fibrils; then, the hydrophobic core regions of adjacent fibrils are aggregated with a partial reorientation in the core structures of fibrils.
Cytotoxicity of αSyn fibrils and Ca2+-mediated aggregates
The fact that aggregation of both αSyn monomer and fibril are influenced by high level of Ca2+, which is similar to that of extracellular fluid, indicates that the observed Ca2+-mediated αSyn aggregation could be induced in the cells undergoing dysregulation of Ca2+ homeostasis or in the Ca2+-rich extracellular space. Because of the relationship between dysregulated Ca2+ homeostasis and α-synucleinopathies, we investigated whether aggregates formed through Ca2+ mediation have cytotoxicity, using methylthiazolyldiphenyl-tetrazolium bromide (MTT) assay with SH-SY5Y neuroblastoma cells. Compared to the control groups (buffers and monomer), the αSyn aggregates, which were formed by the initial addition of Ca2+ were as toxic as normal αSyn fibrils (cell viability of 61%), while the aggregates that were formed through Ca2+ mediation from preformed fibrils had slightly reduced cytotoxicity (cell viability of 73%) (Fig. 7). Our results showed that both Ca2+-mediated αSyn aggregates are cytotoxic regardless of when Ca2+ is added during the aggregation processes. This supports that the dysregulated Ca2+ homeostasis or secretion of αSyn to Ca2+-rich extracellular space is a potential pathogenesis of the diseases related to α-synucleinopathies.
Figure 7.
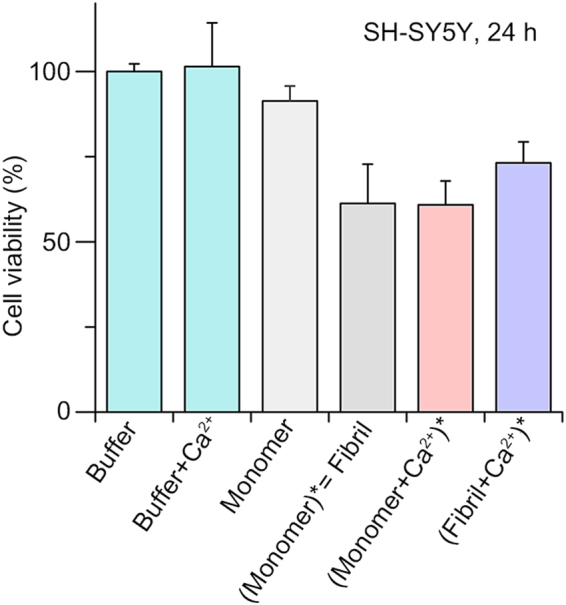
Results of the cell viability assay using SH-SY5Y cells after incubation for 24 h with each αSyn sample. ()* denotes the aggregates that were formed from the components enclosed within parentheses.
Conclusions
We have observed the formation of large interfibrillar aggregates of αSyn associated with hard divalent metal cations. Then, we characterized the pathway of αSyn aggregation mediated by Ca2+, which was a representative hard divalent metal ion, by using various biophysical techniques. Our results demonstrated that multiple Ca2+ ions bound to the C-terminal region of αSyn stimulates the structural transition of αSyn monomers that exposes the NAC region. This structural change accelerated αSyn fibrillation by lowering the activation energy for intermolecular interactions between the αSyn molecules. In addition, we observed that Ca2+ induced interfibrillar aggregation via electrostatic interaction between Ca2+ and the C-terminal regions, and hydrophobic interactions between the fibril core regions. Our cytotoxicity results suggested that the interaction between Ca2+ and αSyn accelerated the formation of toxic αSyn aggregates. As Ca2+ is the most abundant divalent metal ion in extracellular fluid (e.g., the synaptic cleft) and is a critical physiological factor for αSyn fibrillation among hard divalent ions, our results suggested the importance of the interaction between Ca2+ and αSyn in α-synucleinopathies. Furthermore, the detailed examination of the structures and the molecular interactions during αSyn aggregation would be valuable to understand the pathology of α-synucleinopathies.
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
Electronic supplementary material
Acknowledgements
This work was supported by a Basic Research Program (Grant No. NRF-2016R1A2B4013089 and 20100020209) through the National Research Foundation (NRF) of Korea, funded by the Ministry of Science, ICT, and Future Planning (MSIP), the National Research Council of Science & Technology (NST) grant by MSIP (Grant No. CAP-15-10-KRICT), and Korea University Future Research Grant. This work was also supported with supercomputing resources, including technical support (KSC-2016-C2-0021), by the National Institute of Supercomputing and Network/Korea Institute of Science and Technology Information. The synchrotron X-ray scattering measurements at the 4C SAXS II beamline of the Pohang Accelerator Laboratory were supported by the Ministry of Education and Science Technology. We acknowledge Korea Basic Science Institute (KBSI) for TEM measurements, and Agilent Technologies Inc. for support with the 6560 LC-IMS QTOFMS instrument and technical/scientific support. We also acknowledge Prof. Ho Hee Jang (Gachon University) for advices on performing cell viability assay and helpful discussion on the interpretation of the results.
Author Contributions
J.Y.H. and H.I.K. designed the experiments; J.Y.H. performed the experiments; J.Y.H. and T.S.C. analyzed the data; All authors discussed the results and wrote the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-20320-5.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat. Med. 2004;10:S10–S17. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- 2.Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 3.Knowles TP, Vendruscolo M, Dobson CM. The amyloid state and its association with protein misfolding diseases. Nat. Rev. Mol. Cell. Biol. 2014;15:384–396. doi: 10.1038/nrm3810. [DOI] [PubMed] [Google Scholar]
- 4.Hartl FU, Hayer-Hartl M. Converging concepts of protein folding in vitro and in vivo. Nat. Struct. Mol. Biol. 2009;16:574–581. doi: 10.1038/nsmb.1591. [DOI] [PubMed] [Google Scholar]
- 5.Jakes R, Spillantini MG, Goedert M. Identification of two distinct synucleins from human brain. FEBS Lett. 1994;345:27–32. doi: 10.1016/0014-5793(94)00395-5. [DOI] [PubMed] [Google Scholar]
- 6.Iwai A, et al. The precursor protein of non-Aβ component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14:467–475. doi: 10.1016/0896-6273(95)90302-X. [DOI] [PubMed] [Google Scholar]
- 7.Burré J. The synaptic function of α-synuclein. J. Parkinsons Dis. 2015;5:699–713. doi: 10.3233/JPD-150642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bendor JT, Logan TP, Edwards RH. The function of α-synuclein. Neuron. 2013;79:1044–1066. doi: 10.1016/j.neuron.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellucci A, et al. From α-synuclein to synaptic dysfunctions: new insights into the pathophysiology of Parkinson’s disease. Brain Res. Rev. 2012;1476:183–202. doi: 10.1016/j.brainres.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Bodles AM, Guthrie DJ, Harriott P, Campbell P, Irvine GB. Toxicity of non‐Aβ component of Alzheimer’s disease amyloid, and N‐terminal fragments thereof, correlates to formation of β‐sheet structure and fibrils. FEBS J. 2000;267:2186–2194. doi: 10.1046/j.1432-1327.2000.01219.x. [DOI] [PubMed] [Google Scholar]
- 11.EI-Agnaf O, Irvine G. Aggregation and neurotoxicity of α-synuclein and related peptides. Biochem. Soc. Trans. 2002;30:559–565. doi: 10.1042/bst0300559. [DOI] [PubMed] [Google Scholar]
- 12.Dedmon MM, Lindorff-Larsen K, Christodoulou J, Vendruscolo M, Dobson CM. Mapping long-range interactions in α-synuclein using spin-label NMR and ensemble molecular dynamics simulations. J. Am. Chem. Soc. 2005;127:476–477. doi: 10.1021/ja044834j. [DOI] [PubMed] [Google Scholar]
- 13.Bertoncini CW, et al. Release of long-range tertiary interactions potentiates aggregation of natively unstructured α-synuclein. Proc. Natl. Acad. Sci. USA. 2005;102:1430–1435. doi: 10.1073/pnas.0407146102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rcom-H’cheo-Gauthier A, Goodwin J, Pountney DL. Interactions between calcium and alpha-synuclein in neurodegeneration. Biomolecules. 2014;4:795–811. doi: 10.3390/biom4030795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carboni E, Lingor P. Insights on the interaction of alpha-synuclein and metals in the pathophysiology of Parkinson’s disease. Metallomics. 2015;7:395–404. doi: 10.1039/C4MT00339J. [DOI] [PubMed] [Google Scholar]
- 16.Binolfi A, Quintanar L, Bertoncini CW, Griesinger C, Fernández CO. Bioinorganic chemistry of copper coordination to alpha-synuclein: Relevance to Parkinson’s disease. Coordination Chemistry Reviews. 2012;256:2188–2201. doi: 10.1016/j.ccr.2012.05.004. [DOI] [Google Scholar]
- 17.Rasia RM, et al. Structural characterization of copper(II) binding to alpha-synuclein: Insights into the bioinorganic chemistry of Parkinson’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:4294–4299. doi: 10.1073/pnas.0407881102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villar-Piqué A, et al. Environmental and genetic factors support the dissociation between α-synuclein aggregation and toxicity. Proc. Nat. Acad. Sci. USA. 2016;113:E6506–E6515. doi: 10.1073/pnas.1606791113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nielsen MS, Vorum H, Lindersson E, Jensen PH. Ca2+ binding to α-synuclein regulates ligand binding and oligomerization. J. Biol. Chem. 2001;276:22680–22684. doi: 10.1074/jbc.M101181200. [DOI] [PubMed] [Google Scholar]
- 20.Uversky VN, Li J, Fink AL. Metal-triggered structural transformations, aggregation, and fibrillation of human α-synuclein a possible molecular link between parkinson′ s disease and heavy metal exposure. J. Biol. Chem. 2001;276:44284–44296. doi: 10.1074/jbc.M105343200. [DOI] [PubMed] [Google Scholar]
- 21.Binolfi A, et al. Interaction of α-synuclein with divalent metal ions reveals key differences: A link between structure, binding specificity and fibrillation enhancement. J. Am. Chem. Soc. 2006;128:9893–9901. doi: 10.1021/ja0618649. [DOI] [PubMed] [Google Scholar]
- 22.Semerdzhiev SA, Dekker DR, Subramaniam V, Claessens MM. Self-assembly of protein fibrils into suprafibrillar aggregates: bridging the nano-and mesoscale. ACS Nano. 2014;8:5543–5551. doi: 10.1021/nn406309c. [DOI] [PubMed] [Google Scholar]
- 23.Nikoletopoulou V, Tavernarakis N. Calcium homeostasis in aging neurons. Front. Genet. 2012;3:200. doi: 10.3389/fgene.2012.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reznichenko L, et al. In vivo alterations in calcium buffering capacity in transgenic mouse model of synucleinopathy. J. Neurosci. 2012;32:9992–9998. doi: 10.1523/JNEUROSCI.1270-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaafsma, G. In Calcium in Human Biology, 241–259 (Springer, 1988).
- 26.Fujita KA, et al. Integrating pathways of Parkinson’s disease in a molecular interaction map. Mol. Neurobiol. 2014;49:88–102. doi: 10.1007/s12035-013-8489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nath S, Goodwin J, Engelborghs Y, Pountney DL. Raised calcium promotes α-synuclein aggregate formation. Mol. Cell. Neurosci. 2011;46:516–526. doi: 10.1016/j.mcn.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Follett J, Darlow B, Wong MB, Goodwin J, Pountney DL. Potassium depolarization and raised calcium induces α-synuclein aggregates. Neurotox. Res. 2013;23:378–392. doi: 10.1007/s12640-012-9366-z. [DOI] [PubMed] [Google Scholar]
- 29.Melachroinou K, et al. Deregulation of calcium homeostasis mediates secreted α–synuclein-induced neurotoxicity. Neurobiol. Aging. 2013;34:2853–2865. doi: 10.1016/j.neurobiolaging.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Kimula Y, Utsuyama M, Yoshimura M, Tomonaga M. Element analysis of Lewy and adrenal bodies in Parkinson’s disease by electron probe microanalysis. Acta Neuropathol. 1983;59:233–236. doi: 10.1007/BF00703209. [DOI] [PubMed] [Google Scholar]
- 31.Lee H-J, Patel S, Lee S-J. Intravesicular localization and exocytosis of α-synuclein and its aggregates. J. Neurosci. 2005;25:6016–6024. doi: 10.1523/JNEUROSCI.0692-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee H-J, Bae E-J, Lee S-J. Extracellular α-synuclein—a novel and crucial factor in Lewy body diseases. Nat. Rev. Neurol. 2014;10:92–98. doi: 10.1038/nrneurol.2013.275. [DOI] [PubMed] [Google Scholar]
- 33.Luk KC, et al. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338:949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lowe R, Pountney DL, Jensen PH, Gai WP, Voelcker NH. Calcium (II) selectively induces α-synuclein annular oligomers via interaction with the C-terminal domain. Protein Sci. 2004;13:3245–3252. doi: 10.1110/ps.04879704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nordén, B. Circular dichroism and linear dichroism. Vol. 1 (Oxford University Press, USA, 1997).
- 36.Wallace B, Mao D. Circular dichroism analyses of membrane proteins: an examination of differential light scattering and absorption flattening effects in large membrane vesicles and membrane sheets. Anal. Biochem. 1984;142:317–328. doi: 10.1016/0003-2697(84)90471-8. [DOI] [PubMed] [Google Scholar]
- 37.Irwin DJ, Lee VM-Y, Trojanowski JQ. Parkinson’s disease dementia: convergence of α-synuclein, tau and amyloid-β pathologies. Nat. Rev. Neurosci. 2013;14:626–636. doi: 10.1038/nrn3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Putnam CD, Hammel M, Hura GL, Tainer JA. X-ray solution scattering (SAXS) combined with crystallography and computation: defining accurate macromolecular structures, conformations and assemblies in solution. Q. Rev. Biophys. 2007;40:191–285. doi: 10.1017/S0033583507004635. [DOI] [PubMed] [Google Scholar]
- 39.Choi TS, Lee JW, Jin KS, Kim HI. Amyloid fibrillation of insulin under water-limited conditions. Biophys. J. 2014;107:1939–1949. doi: 10.1016/j.bpj.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwalbe M, et al. Predictive atomic resolution descriptions of intrinsically disordered hTau40 and α-synuclein in solution from NMR and small angle scattering. Structure. 2014;22:238–249. doi: 10.1016/j.str.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 41.Jain MK, Bhat R. Modulation of human α-synuclein aggregation by a combined effect of calcium and dopamine. Neurobiol. Dis. 2014;63:115–128. doi: 10.1016/j.nbd.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 42.Wilm, M. Principles of electrospray ionization. Mol. Cell. Proteomics10, M111. 009407 (2011). [DOI] [PMC free article] [PubMed]
- 43.Ahadi E, Konermann L. Surface charge of electrosprayed water nanodroplets: A molecular dynamics study. J. Am. Chem. Soc. 2010;132:11270–11277. doi: 10.1021/ja1041989. [DOI] [PubMed] [Google Scholar]
- 44.Konermann L, Ahadi E, Rodriguez AD, Vahidi S. Unraveling the Mechanism of Electrospray Ionization. Anal. Chem. 2013;85:2–9. doi: 10.1021/ac302789c. [DOI] [PubMed] [Google Scholar]
- 45.Cech NB, Enke CG. Practical implications of some recent studies in electrospray ionization fundamentals. Mass Spectrom. Rev. 2001;20:362–387. doi: 10.1002/mas.10008. [DOI] [PubMed] [Google Scholar]
- 46.Sugita Y, Okamoto Y. Replica-exchange molecular dynamics method for protein folding. Chem. Phys. Lett. 1999;314:141–151. doi: 10.1016/S0009-2614(99)01123-9. [DOI] [Google Scholar]
- 47.Bernadó P, Mylonas E, Petoukhov MV, Blackledge M, Svergun DI. Structural characterization of flexible proteins using small-angle X-ray scattering. J. Am. Chem. Soc. 2007;129:5656–5664. doi: 10.1021/ja069124n. [DOI] [PubMed] [Google Scholar]
- 48.Tria G, Mertens HD, Kachala M, Svergun DI. Advanced ensemble modelling of flexible macromolecules using X-ray solution scattering. IUCrJ. 2015;2:207–217. doi: 10.1107/S205225251500202X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shvartsburg AA, Jarrold MF. An exact hard-spheres scattering model for the mobilities of polyatomic ions. Chem. Phys. Lett. 1996;261:86–91. doi: 10.1016/0009-2614(96)00941-4. [DOI] [Google Scholar]
- 50.Lee JW, Kim HI. Investigating acid-induced structural transitions of lysozyme in an electrospray ionization source. Analyst. 2015;140:661–669. doi: 10.1039/C4AN01794C. [DOI] [PubMed] [Google Scholar]
- 51.Choi TS, Lee HJ, Han JY, Lim MH, Kim HI. Molecular insights into human serum albumin as a receptor of amyloid-β in the extracellular region. J. Am. Chem. Soc. 2017;139:15437–15445. doi: 10.1021/jacs.7b08584. [DOI] [PubMed] [Google Scholar]
- 52.McClendon S, Rospigliosi CC, Eliezer D. Charge neutralization and collapse of the C‐terminal tail of alpha‐synuclein at low pH. Protein Sci. 2009;18:1531–1540. doi: 10.1002/pro.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tuttle MD, et al. Solid-state NMR structure of a pathogenic fibril of full-length human α-synuclein. Nat. Struct. Mol. Biol. 2016;23:409–415. doi: 10.1038/nsmb.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).