Significance
Schistosomiasis is an infectious disease that affects over 240 million people living in low- and middle-income countries, and is caused by parasitic worms that require snail hosts to complete its lifecycle. To improve public health control of this disease, there is growing interest in using chemical-based snail control that kills snail populations in environmental water sources, which will reduce infection rate in people. We modeled transmission of schistosomiasis and cost-effectiveness of various strategies with data from low- and high-prevalence rural Kenyan communities. Adding snail control alongside conventional mass treatment programs (instead of mass treatment programs alone) was found to be cost-effective, especially in settings with high disease burden and nonparticipation in mass treatment programs.
Keywords: mathematical modeling, parasitology, cost-effectiveness, epidemiology, environmental control
Abstract
Schistosomiasis is a parasitic disease that affects over 240 million people globally. To improve population-level disease control, there is growing interest in adding chemical-based snail control interventions to interrupt the lifecycle of Schistosoma in its snail host to reduce parasite transmission. However, this approach is not widely implemented, and given environmental concerns, the optimal conditions for when snail control is appropriate are unclear. We assessed the potential impact and cost-effectiveness of various snail control strategies. We extended previously published dynamic, age-structured transmission and cost-effectiveness models to simulate mass drug administration (MDA) and focal snail control interventions against Schistosoma haematobium across a range of low-prevalence (5–20%) and high-prevalence (25–50%) rural Kenyan communities. We simulated strategies over a 10-year period of MDA targeting school children or entire communities, snail control, and combined strategies. We measured incremental cost-effectiveness in 2016 US dollars per disability-adjusted life year and defined a strategy as optimally cost-effective when maximizing health gains (averted disability-adjusted life years) with an incremental cost-effectiveness below a Kenya-specific economic threshold. In both low- and high-prevalence settings, community-wide MDA with additional snail control reduced total disability by an additional 40% compared with school-based MDA alone. The optimally cost-effective scenario included the addition of snail control to MDA in over 95% of simulations. These results support inclusion of snail control in global guidelines and national schistosomiasis control strategies for optimal disease control, especially in settings with high prevalence, “hot spots” of transmission, and noncompliance to MDA.
Schistosomiasis is a disease caused by parasitic worms of the genus Schistosoma that affects over 240 million people in low- and middle-income countries (1, 2). Over the past decade, the global strategy for schistosomiasis has focused on control of disease morbidity through scale up of targeted mass drug administration (MDA), also known as preventive chemotherapy, to school-aged children using empiric praziquantel treatment of populations in endemic areas (3, 4). While the approach of repeated MDA with a goal of 75% coverage in school-aged children has resulted in some success in reducing long-term morbidities, transmission is not interrupted, children typically suffer from high rates of reinfection, and the broader community (preschool children, adolescents, and adult populations) remains affected by schistosomiasis (5–7). There is now growing interest in improving morbidity control through reduction or elimination of schistosomiasis (8).
In addition to MDA, one complementary approach is the local control of intermediate host snails to interrupt the nonhuman phase of the Schistosoma lifecycle (8–10). Whereas snail control is not a focus of the current global strategy, growing evidence suggests that it could play an effective role in epidemic control, especially in areas of high transmission (e.g., hot spots) (8–14). In a recent meta-analysis of observational data, snail control (mollusciding) through chemical-based method (e.g., niclosamide) was found to be effective in schistosomiasis control, with measured reductions in both prevalence and incidence of schistosomiasis (11). This conclusion was further supported by another study that found that a country-level control strategy with a focus on snail control had larger reductions in infection prevalence than countries without snail control (13). Snail control has also notably achieved widespread success in schistosomiasis control and elimination campaigns in many parts of Asia (12). Furthermore, the WHO has recently published new guidelines for field application methods for chemical-based snail control, which underscore the renewed interest in the use of snail control in some settings (10).
While there is growing support for inclusion of snail control within national strategies for schistosomiasis, the conditions under which employment of this strategy is beneficial and feasible are unclear. Chemical-based methods for snail control (e.g., niclosamide) are costly, are labor intensive, and do not prevent repopulation of snails after treatment (10, 12). Furthermore, chemical-based snail control can be toxic within the environment, which may lead to unintended ecological consequences (12). With these considerations in mind, additional guidance is needed that define the settings and conditions under which snail control could be beneficial. The key policy-relevant questions include the comparative cost-effectiveness of snail control used alone or when combined with MDA, how the decision to use snail control varies in different burden settings, and the impact of varying snail control frequencies. To address these questions, we modeled the cost-effectiveness of MDA, snail control (focal chemical-based snail control), and combined strategies against schistosomiasis using data from low- and high-burden communities in rural Kenya.
Results
Baseline Burden of Disease in Kenyan Communities.
In the low-burden 5,000-person communities with an overall mean prevalence of 12% (44% of these were heavy infection), we estimated a total of 172 disability-adjusted life years (DALYs) over 10 y without intervention (SI Appendix, Table S1). In the high-burden 5,000-person communities with mean prevalence of 38% (48% of these were heavy infections), we estimated a total of 550 DALYs over 10 y without intervention. Following the WHO guidelines (3, 4, 15), both settings would receive MDA targeting school-aged children.
Effectiveness and Cost-Effectiveness of MDA, Snail Control, and Combined Strategies.
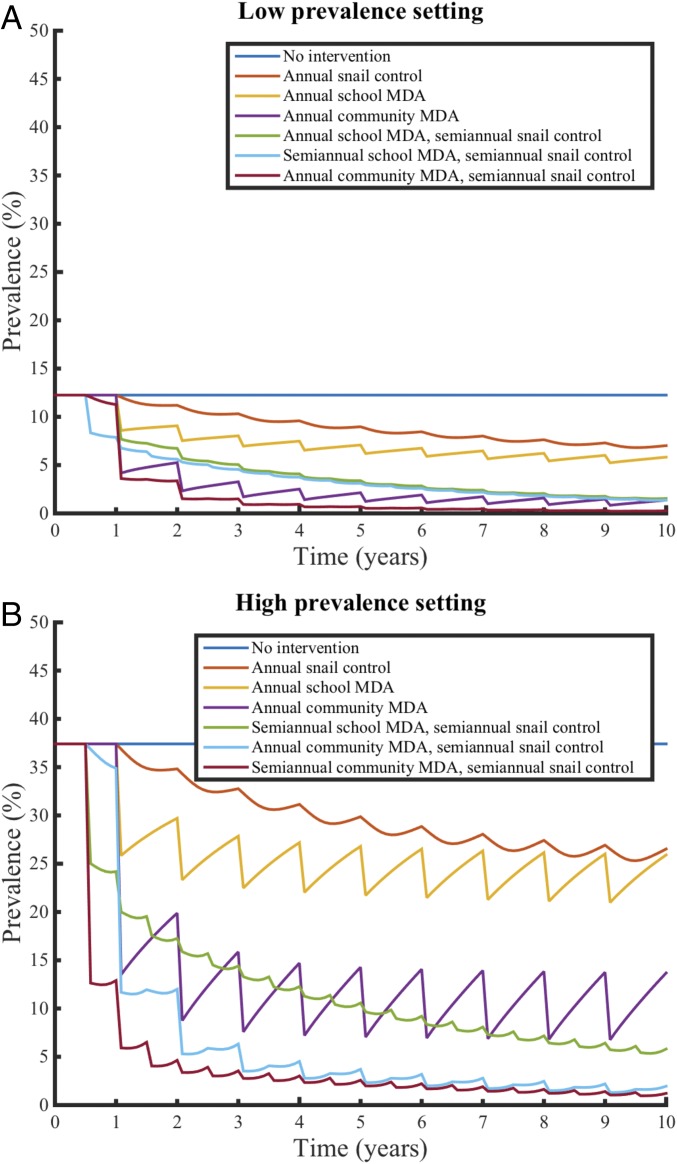
We found that expanded MDA (community-wide compared with school-based), more frequent intervention, and the additional of snail control would substantially reduce infection prevalence and infection intensity beyond the effect of the standard WHO intervention of school-based MDA alone (Fig. 1 and SI Appendix, Fig. S1). We found that use of snail control interventions alone projected prevalence reductions similar but less than those achieved by school-based MDA alone. Community-wide MDA provided greater prevalence reductions than school-based MDA or snail control alone. The more aggressive combined strategies (community-wide MDA and snail control) were the most effective to reduce both prevalence and infection intensity. Notably, there was a nonlinearity in effectiveness (averted DALYs) with more aggressive strategies, where additional intervention yielded smaller gains in effectiveness (Fig. 2).
Fig. 1.
Effectiveness of selected MDA, snail control, and combined interventions for schistosomiasis in low- and high-burden Kenyan communities. We simulated interventions of MDA, snail control, and combined approaches in an age-stratified population of preschool-aged children, school-aged children, and adults in (A) low-prevalence Kenyan communities and (B) high-prevalence Kenyan communities with 75% coverage for MDA. The figure displays selected interventions for visualization purposes; plots for all tested interventions are available in SI Appendix.
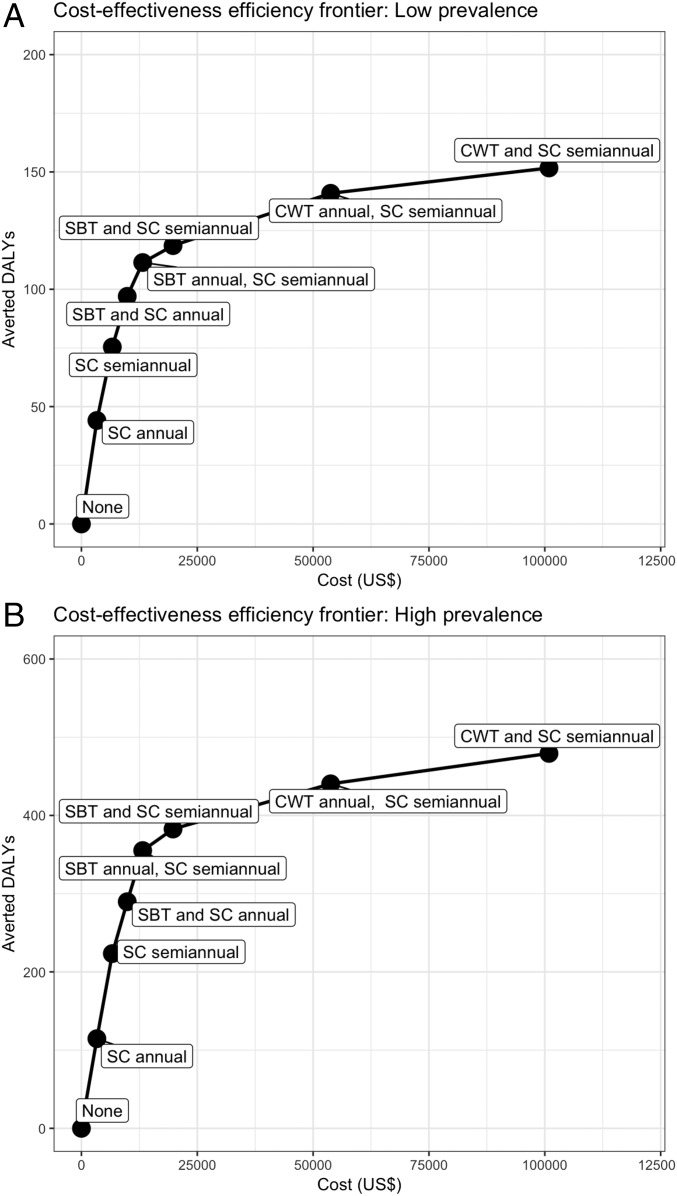
Fig. 2.
Cost-effectiveness efficiency frontier for selected MDA, snail control, and combined interventions for schistosomiasis in low- and high-burden Kenyan communities. We computed the costs (US dollars) and averted DALYs for nondominated interventions of MDA, snail control, and combined approaches in the (A) low-prevalence Kenyan communities and (B) high-prevalence Kenyan communities. Dominated strategies are not shown, and full results are available in SI Appendix, Table S1. The cost-effectiveness of each strategy is measured with the ICER. The ICER is computed in reference with the next best strategy in terms of averted DALYs (corresponding to the strategy directly to the left on the frontier). The ICER is computed as the difference in cost divided by the difference in DALYs, which is shown as the slope between strategies. A steeper slope indicates a lower ICER (more cost-effective), while a flatter slope suggests a higher ICER (less cost-effective). Notably, there is strong nonlinearity in effectiveness (averted DALYs), whereby additional intervention yields smaller gains and high ICER. CWT, community-wide treatment with MDA; SBT, school-based treatment with MDA; SC, snail control.
Under the base case scenario with 10% systematic noncompliance (SI Appendix, Table S1), we found that, in low-burden settings, semiannual school-based MDA with semiannual snail control was highly cost-effective [incremental cost-effectiveness ratio (ICER): $904 US per DALY], and annual community-wide MDA with semiannual snail control was bordering on being highly cost-effective (ICER: $1,531 US per DALY). In high-burden settings, annual community-wide MDA with semiannual snail control was highly cost-effective (ICER: $588 US per DALY), and semiannual community-wide MDA with semiannual snail control was also highly cost-effective (ICER: $1,213 US per DALY). The cost-effectiveness efficiency frontier showed the incremental costs and averted disability of key strategies (Fig. 2). The addition of snail control had a lower ICER (more cost-effective) in the high-burden setting, suggesting the prioritization of snail control in high-prevalence regions rather than lower-prevalence areas.
Sensitivity, Scenario, and Uncertainty Analyses.
We performed one-way sensitivity analyses for our primary cost-effective strategies and found that transmission uncertainty (which varied environmental and behavioral conditions and influenced snail control effectiveness), MDA delivery cost, schistosomiasis-associated disability weights, environmental conditions (e.g., snail and human density, “hot spot” populations), and systematic noncompliance were influential model parameters (Fig. 3). In scenario analysis, we simulated spatial connectivity with neighboring environments or hot spots by introducing a constant migration of snails and infected humans into the environment and a proportion of the force of infection from an external source; we found these factors to improve the cost-effectiveness (lower ICER) of key strategies (Fig. 3). In scenario analyses, we found that addition of snail control was most cost-effective (lower ICER) in settings with higher disease burden and higher snail and human population density (Figs. 2 and 3 and SI Appendix, Tables S2 and S3). We repeated our base case analysis with differing levels of systematic noncompliance for participation in MDA. We found that increased systematic noncompliance improved the cost-effectiveness of adding snail control in many settings, while reducing the impact and cost-effectiveness of MDA; however, the overall cost-effectiveness of combined strategies in systematically noncompliant populations were lower (higher ICER), since MDA strategies had less impact. We varied intervention effectiveness (e.g., snail control, praziquantel efficacy for worm reduction), which often improved the cost-effectiveness (lower ICER) of more aggressive strategies, since the incremental benefit became larger when considering less effective interventions. We characterized the programmatic parameter values where these primary strategies were no longer robust (SI Appendix).
Fig. 3.
One-way sensitivity analysis of key model parameters. This analysis tested the effect of changing a single model input on the ICER of the highly cost-effective interventions from the primary analysis: (A) semiannual school-based MDA with semiannual snail control in low-prevalence settings, (B) annual community-wide MDA with semiannual snail control in low-prevalence settings, (C) annual community-wide MDA with semiannual snail control in high-prevalence settings, and (D) semiannual community-wide MDA with semiannual snail control in high-prevalence settings. We varied values for model inputs related to transmission dynamics, costs, and intervention effectiveness, including sampling from the posterior distribution generated during model calibration, which affects transmission projections and snail control effectiveness. The horizontal axis represents the ICER values (US dollars per DALY averted), while the vertical axis includes tested parameters with respective ranges of values. A lower ICER can be interpreted as a more cost-effective intervention, and we considered all strategies left of the $1,377 US per DALY averted to be highly cost-effective, although the full axis is provided to relax reliance on a single threshold. *The 95% credible interval of the transmission projection incorporates the full range of values for the effectiveness snail control and in some cases, was dominated by extension in the lower ranges. **Snail control effectiveness on schistosomiasis was calibrated based on empirical data and is a function of multiple parameters (including snail control efficacy); the lower MDA coverage range still simulated 75% coverage for school-based MDA.
In the uncertainty analysis (SI Appendix, Figs. S2 and S3), for low-burden settings, we found that school-based MDA with snail control was the optimal cost-effective strategy in 50% of simulations, while community-wide MDA with snail control was optimal in 46% (Table 1). In high-prevalence settings, community-wide MDA with snail control was the optimal cost-effective strategy in 95% of simulations. In 99% of simulations, the optimally cost-effective scenario included snail control; over 97% included both snail control and MDA.
Table 1.
Proportion of simulations from multiway uncertainty analysis, where the control strategy is the optimal cost-effective strategy
| MDA | Snail control | Low-prevalence communities | High-prevalence communities |
| None | None | 0 | 0 |
| None | Annual | 0 | 0 |
| None | Semiannual | 2.8 | 0 |
| SBT annual | None | 0 | 0 |
| SBT semiannual | None | 0 | 0 |
| SBT annual | Annual | 0.1 | 0 |
| SBT annual | Semiannual | 20.6 | 0 |
| SBT semiannual | Annual | 0 | 0 |
| SBT semiannual | Semiannual | 30.2 | 5.1 |
| CWT, annual | None | 0 | 0 |
| CWT semiannual | None | 0.1 | 0.3 |
| CWT annual | Annual | 3.5 | 0.4 |
| CWT annual | Semiannual | 42 | 23.5 |
| CWT semiannual | Annual | 0 | 0.3 |
| CWT semiannual | Semiannual | 0.7 | 70.5 |
The strategy that is the optimal cost-effective choice has the highest averted DALYs, with an ICER below the threshold of $1,377 US per DALY. CWT, community-wide treatment with MDA; SBT, school-based treatment with MDA.
In low-prevalence communities, we found the strategy of semiannual school-based MDA with semiannual snail control to be highly cost-effective in 67% of the simulations and optimally cost-effective in 30% [ICER, 95% uncertainty interval (95% UI): $351–$3,884 US per DALY]; annual community-wide MDA with semiannual snail control was highly cost-effective in 43% of the simulations and optimally cost-effective in 42% (ICER, 95% UI: $495–$4,867 US per DALY). In high-prevalence settings, we found the strategy of semiannual community-wide MDA with semiannual snail control to be highly cost-effective in 71% of simulations and optimally cost-effective in 71% (ICER, 95% UI: $511–$2,773 US per DALY).
Discussion
In this modeling study, we found that inclusion of snail control alongside MDA is a highly cost-effective strategy for targeting schistosomiasis. In low-burden settings, we estimated that school-based MDA with snail control and possibly, community-wide MDA with snail control could be highly cost-effective. In high-burden communities, which may be recalcitrant to school-based MDA alone, community-wide MDA with snail control was robust in being highly cost-effective. In over 95% of simulations, the optimally cost-effective strategy included snail control. Importantly, setting-specific differences could inform implementation of more refined snail control strategies (10, 11). Overall, these findings support the inclusion of snail control in global guidelines and national schistosomiasis control strategies to achieve optimal disease control, especially in settings with high-disease burden, hot spots of transmission, and high systematic noncompliance to MDA.
Over the past decade, MDA programs have scaled up across many low- and middle-income countries for control of schistosomiasis, and great progress has been made to decrease the burden of this helminthiasis (16). The primary public health strategy has been MDA targeting school-aged children, and while this strategy has reduced disease burden in this demographic group, the approach of school-based MDA often results in reinfection, does not address broader age groups, and has not led to elimination of transmission (5–7). Recent modeling and health economic studies have found that expanded community-wide MDA that includes preschool-aged children, adolescents, and adults would better reduce overall disease burden and reinfection at a rate greater than that for school-based control alone and would be highly cost-effective (17–20). As the global strategy changes and broader revisions of guidelines are considered, complementary strategies are important to evaluate. For these reasons, we tested the projected cost-effectiveness of snail control implemented alongside MDA strategies to understand the optimal epidemiologic conditions for implementation to support national and global strategies. Importantly, while MDA strategies aim to reduce infection prevalence and avert disability by treating human infections and reducing transmission, snail control works solely by reducing future transmission.
Using data from low- and high-prevalence settings in rural Kenya, we generated a range of hypothetical communities for low- and high-prevalence conditions that shared similar baseline disease burden but had varied snail environment and behavioral conditions. We found that addition of snail control alongside MDA was highly cost-effective in almost all simulations. These results suggest a need for addition of snail control with inclusion of expanded community-wide MDA in many settings, especially high-burden settings, where there are higher rates of reinfection and disease burden cannot be reduced without additional “vector control.” Overall, the study conclusions align with a past analysis that examined costs and snail mortality in China, which found that focal snail control can be an efficient strategy for schistosomiasis control (21). These main study findings suggest that the current global strategy of school-based MDA alone is too restrictive for optimal morbidity control and support the recent publication of the WHO guidelines on field use of snail control in hot spot regions and expanded community-wide MDA in some settings (10).
The cost-effectiveness of adding snail control alongside community-wide MDA was robust in high-burden settings but less so in some low-burden settings, where semiannual school-based MDA with snail control may be sufficient. To assess the predicting factors, we performed sensitivity and uncertainty analyses that tested a broad range of plausible values for model inputs across Schistosoma epidemiology, cost, and intervention parameters, which may improve the generalizability of our study findings to other settings. There is likely a wide range of setting-specific responses to snail control in terms of effectiveness and coverage, and therefore, we provided sensitivity analyses to characterize the distribution of possible cost-effectiveness results. Interestingly, sensitivity analyses found improved cost-effectiveness for more aggressive strategies (e.g., frequent community-wide MDA with snail control) when snail control and praziquantel were less effective, since these strategies yielded larger incremental benefits and suffered less from diminishing returns with more aggressive strategies. Importantly, setting-specific analyses that incorporate local data (e.g., epidemiologic inputs, willingness-to-pay threshold) can allow for a more tailored cost-effectiveness analysis. In these cases, N.C.L. may be contacted to create context-specific findings on cost-effectiveness of snail control and MDA.
Notably, increasing levels of systematic noncompliance to MDA and hot spots of transmission improved the cost-effectiveness of adding snail control strategies. Systematic noncompliance has been documented in many MDA programs (22) and reduces the impact of MDA on transmission, whereas snail control depends less on sustained community participation. Systematic noncompliance may be particularly high when sensitization is not included before MDA campaigns and may also vary by Schistosoma spp. depending on clinical presentation (e.g., hematuria from Schistosoma haematobium). We simulated hot spots and potential spatial connectivity with neighboring communities and found more aggressive strategies that combined MDA and snail control to be more cost-effective in these locations. This is likely because the incremental benefit of more aggressive interventions (e.g., community MDA, frequent snail control) relative to other less intensive strategies is increased in hot spots because of the high force of infection and reinfection.
We focused on evaluating the addition of chemical-based methods for snail control because of its historical use and greater strength of observational evidence for its effectiveness (11, 23). However, other forms of snail control may be relevant, including biological control through introduction of competitor snail species or prawns that feed on host snail populations (24–27). In our analysis, we did not account for potential unintended environmental or ecological consequences of chemical-based snail control and their associated cost, which should be considered during intervention planning, although ecological costs from snail control could be conceptualized as a higher cost of the intervention as examined in the sensitivity analysis. Evidence suggests that the commonly used chemical (niclosamide) has minimal risk to humans, livestock, poultry, plants, and environment when applied appropriately, especially given its short half-life in the water; however, the chemical is directly toxic to fish and amphibians (e.g., frogs), which could have larger ecological ramifications (9, 10, 28). For this reason, snail control should be limited to regions with known schistosomiasis disease burden, use recommended doses to mitigate ecological consequences, and be implemented in discussion with the local community to balance public health needs and environmental concerns (9, 10). Furthermore, focal snail control strategies modeled in this study likely have reduced ecological impact compared with the alternative of blanket application.
The study results should be interpreted within the context of its limitations, model assumptions, and available data. There is uncertainty around many aspects of cost, intervention effectiveness, and disability for schistosomiasis. We addressed this by constructing a 95% UI that provided a range of plausible outcomes for cost-effectiveness, which was driven by both uncertainty and setting-specific differences. There is debate around the measurement of disability for schistosomiasis (29, 30). We used conservative estimates that focus on acute morbidities that are reversible through intervention (20, 31), although this approach may underestimate the chronic sequelae from long-term infection and exposure to Schistosoma eggs (29, 30, 32). Future work should include modeling “cumulative person-level worm-years” to account for reductions in chronic morbidity from sustained public health interventions, which would provide increased cost-effectiveness for an intervention over time (33, 34). While the effectiveness estimates for snail control were informed by a recent meta-analysis of observational studies (11), there are uncertainties and setting-specific differences (e.g., number of water bodies, distance to travel to water, and volume of water) that will affect both the costs and benefits of snail control. To address this, we tested a range of possible costs and effectiveness estimates informed by data from observational studies and found our results to be broadly robust. Importantly, structural and parameter assumptions can influence an intervention's impact on transmission (35). We made a number of simplifying assumptions as per common modeling practice: we modeled the effect of treatment to be instantaneous; we assumed perfect mixing within each subpopulation; we did not account for the effect of transmission superspreaders; we modeled transmission dynamics, including that infection of snails (force of infection) saturated with human infectivity, which can have significant affect on control outcomes; and we explicitly accounted for the effect of snail density on transmission (19, 36–38). We assumed stationary transmission environment and snail biology without seasonality. In reality, the snail component can vary from “near stationary” (river, lakeshore, irrigation scheme) to highly dynamic with dramatic changes of snail abundance (seasonal ponds). Seasonality could potentially be leveraged for more efficient design of control interventions, and future work should address these topics by utilizing resource-driven models of snail population biology and control (39, 40). The model does not include a maintenance cost for snails or crowding effect from snail density, although these snail resource effects on overall transmission (mediated by snail cercariae production) are likely to be minimal based on empirical data in realistic environmental conditions (11). We did not model any resistance to praziquantel or to chemical-based snail control because of limited evidence for their existence, although rigorous monitoring for efficacy would be necessary as these interventions are scaled up and treatment pressure is increased. Finally, future work should evaluate the effects of supplemental water, sanitation, and hygiene interventions, which are likely to prove necessary to fully eliminate transmission, and include development of an interactive tool for programmatic decision-making that incorporates model-based results for schistosomiasis programming alongside other diseases and health programs.
In summary, our study results support the inclusion of snail control in global and national strategies against schistosomiasis, especially in high-burden settings, hot spot regions, or areas with systematic noncompliance to MDA, since snail control can mitigate risk of reinfection in endemic ecosystems and does so irrespective of person-level compliance. The analysis also supports previous work that finds that community-wide MDA is highly cost-effective relative to school-based MDA alone. The disparity between current WHO recommendations for school-based MDA alone and the cost-effectiveness of expanded community-wide MDA with addition of snail control in many settings supports calls to consider strengthened guidelines and strategy to reduce the global disease burden of schistosomiasis.
Materials and Methods
Methods Overview.
We adapted a mathematical model for transmission of S. haematobium and modeled cost-effectiveness of various interventions over a 10-y period in 5,000-person communities (18, 19, 37, 41). We chose the time horizon based on the duration necessary to capture long-term differences between strategies and timelines for country-level programmatic planning. We calibrated the model to age-structured empirical data on prevalence and mean infection intensity (measured in eggs per 10 mL of urine) from a simulated set of low-burden (5–20% prevalence; mean: 12%) and high-burden (25–50% prevalence; mean: 38%) rural settings in the southeastern coastal regions of Kenya to model different epidemiologic settings (37, 41–43) (Tables 2 and 3). Notably, we simulated many sets of hypothetical communities for both low- and high-prevalence settings, which shared similar baseline disease burden but had varying snail environment and behavioral conditions. We simulated school-based and community-based MDA with praziquantel, focal chemical-based snail control using niclosamide (an intervention that concentrates on known freshwater bodies where transmission may be occurring), and a combined approach that included both strategies. We tested both annual and semiannual (twice per year) interventions.
Table 2.
Baseline cohort characteristics
| Parameter | Base case value | Source |
| Preschool children, % | 18 | —* |
| School-aged children, % | 28 | —* |
| Adults, % | 54 | —* |
| Women, % | 50 | —* |
| Community population | 5,000 | Assumption |
| Adult male mean Hb (SD), g/L | 134 (19) | Refs. 19 and 20 |
| Adult female mean Hb (SD), g/L | 111 (16) | Refs. 19 and 20 |
| Child mean Hb (SD), g/L | 112 (15) | Refs. 19 and 20 |
| Systematic noncompliance, % | 10 | Ref. 22 |
Table 3.
Baseline cohort epidemiology
| Parameter | Mean prevalence, % | Mean prevalence, heavy intensity, % |
| Kenyan communities, low prevalence | ||
| Preschool-aged child prevalence | 4.5 | 1.8 |
| School-aged child prevalence | 20.6 | 10.0 |
| Adult prevalence | 10.6 | 4.2 |
| Overall | 12.3 | 5.4 |
| Kenyan communities, high prevalence | ||
| Preschool-aged child prevalence | 16.4 | 7.8 |
| School-aged child prevalence | 69.0 | 40.1 |
| Adult prevalence | 28.5 | 10.5 |
| Overall | 37.7 | 18 |
Model of Helminth Transmission and Interventions.
We adapted the dynamic, demographically structured, and deterministic stratified worm burden model to simulate transmission of S. haematobium (37, 41) (SI Appendix). This compartmental model stratified a human population by age group and worm burden and dynamically tracked each worm stratum over time to represent changing prevalence and infection intensity. This transmission structure included many aspects of the biology of transmission, including human release of eggs from urine into the environment, the snail intermediate host that allows for maturation of the parasite in the environment, and eventual infection of humans through direct contact with fresh water. We included important epidemiologic factors and intrahost biology, such as worm mating, age-specific mortality, and random overdispersed egg release by infected human hosts (38, 44, 45). The local snail community was explicitly modeled and coupled to the human population, which allowed for simulation of interventions for both MDA (affecting humans) and snail control (affecting the snail population). We assumed a 10% prevalence of systematic noncompliance to MDA in the base case analysis to account for a proportion of the population that was repeatedly missed by treatment based on estimates from the literature (22). The transmission model was calibrated with a Bayesian Monte Carlo procedure to estimate a joint distribution of model parameters using observed cross-sectional surveys on prevalence and infection intensity in children and adults from rural Kenyan communities (37, 41–43) (SI Appendix).
We modeled MDA as an instantaneous reduction in human worm burden after treatment with praziquantel using data from clinical trials on drug efficacy (46) and similarly assumed an immediate reduction in snail density after focal chemical snail control (11) (SI Appendix). There is substantial heterogeneity in the observed effectiveness of snail control (measured in relative incidence reduction and prevalence reduction in the human population) (11). We modeled the effect of snail control using a combination of model parameters to generate a distribution for the effect size of snail control for the base case analysis (SI Appendix). We calibrated this distribution to broadly align with the range of observed prevalence and incidence reductions after snail control interventions using data from a recent meta-analysis (11) (SI Appendix, Fig. A5).
Cost-Effectiveness Model and Assumptions.
We adapted a cost-effectiveness model that included cost and disability estimates for implementation of various MDA and snail control strategies against schistosomiasis (19, 20). We estimated direct programmatic costs (2016 US dollars) from the perspective of a national disease control program. We estimated costs for MDA and snail control based on published literature and programmatic data from the Schistosomiasis Consortium for Operational Research and Evaluation (SCORE) Trial (3, 19, 47–51) (SI Appendix). The cost of MDA was estimated as the per-person price of drugs (not assumed to be donated) and delivery (e.g., staff salaries, transportation, and administrative fees) for either school- or community-based programs (3, 19, 47, 48). We estimated a per-community (e.g., small 5,000-person community) cost for snail control that included both capital cost of equipment and variable costs per community (i.e., chemical for snail control, staff salaries, transportation), which was informed by experience from historical literature and the recent SCORE Trial that implemented chemical-based snail control (49–52). We annualized equipment over the expected lifetime of utility and work schedule. The estimated costs are presented in Table 4.
Table 4.
Estimated intervention costs and disability of schistosomiasis
| Category | Description | Units | Quantity | Cost per unit, US$ |
| MDA costs (3, 19, 47, 48) | ||||
| Drug | Praziquantel | Per person | —* | 0.21 |
| Delivery | School-based delivery | Per delivery | —* | 0.50 |
| Delivery | Community-based delivery | Per delivery | —* | 1.50 |
| Total cost, school-based program | Per person | — | 0.71 | |
| Total cost, community-based program | Per person | — | 1.71 | |
| Snail control costs (49–51) | ||||
| Capital costs† | ||||
| Equipment | Spraying unit, Hudson | Per unit | 1 | 400 |
| Equipment | Spraying unit, Petrol | Per unit | 1 | 300 |
| Equipment | Protective clothing | Per team | 4 | 50 |
| Equipment | GPS | Per unit | 1 | 200 |
| Equipment | pH meter | Per unit | 1 | 250 |
| Variable costs | ||||
| Snail control chemical | Niclosamide chemical | Per kilogram | 3 | 40 |
| Personnel | Personal compensation | Per day | 5 | 25 |
| Transportation | Transportation (car, fuel) | Per day | 1 | 100 |
| Equipment | Laboratory consumables | Per day | 1 | 25 |
| Total cost, snail control‡ | Per community | — | 379.43 |
Quantity is computed based on age targeting and coverage.
Capital costs are annualized using an equivalent annual cost over 3 y at 3% discounting, with 65 working days per year (number of weekdays during the 3 rainy months of the year).
Snail control costs are estimated for intervention in a small (5,000-person) community. This estimation includes both capital and variable costs. It assumes that one team can treat one community per day with 3 kg of chemical snail control per community.
We modeled disability with the DALY using published sequelae and disability weights (which measures disease disability for 1 y of a human life, where zero is perfect health and one is death) following convention for cost-effectiveness analyses (20, 53, 54). We distributed the infection-associated disability weights based on observed egg counts in urine as an indicator of infection intensity using the WHO thresholds for egg counts (>50 eggs per 10 mL of urine as a heavy infection) and for anemia (Table 5). The cost-effectiveness of a strategy was computed as the ICER (US dollars per DALY averted), which is a relative ratio that compares two strategies and is the difference in cost divided by the difference in DALYs. The computation procedure for the ICER ranks all strategies by increasing health gains (averted DALYs), and each strategy’s ICER is then computed in reference to the next most effective strategy (defined as the control intervention with next highest averted DALYs that is nondominated on the cost-effectiveness frontier). This incremental calculation using the “next best comparator” (rather than a single comparator) follows convention, accounts for all available and mutually exclusive choices, and is necessary to maximize the objective function for cost-effectiveness. We used a base case strategy of no intervention. Strategies were defined as highly cost-effective if the ICER was below a willingness-to-pay threshold of the gross domestic product (GDP) per capita ($1,377 US per DALY for Kenya) following common practice, although we tested alternative values to relax reliance on a single threshold. We defined the optimal cost-effective strategy as the choice with highest averted DALYs and ICER below the willingness-to-pay threshold. For further conceptualization of the willingness-to-pay threshold used to interpret the ICER, some common global health interventions and their associated ICERs include malaria bed nets ($5–$17 US per DALY), childhood vaccination ($10–$30 US per DALY), antiretroviral treatment ($300–$500 US per DALY), improvements in water and sanitation ($1,100–$15,000 US per DALY), and latent TB treatment ($4,000–$25,000 US per DALY) (55). We defined strategies as strictly dominated when they had lower effectiveness and higher cost than another choice and dominated by extension when the strategy was less effective and had a higher ICER relative to another choice. We computed the cost-effectiveness efficiency frontier, which plots costs and effectiveness (averted DALYs) of all strategies to understand comparison and tradeoff of cost and health gains among many control strategies to support decision-making, especially in cases where resources may be limited. On the frontier, the optimal strategy maximized averted DALYs while maintaining an ICER (slope of line between strategy and next best comparator; measured in US dollars per averted DALYs) below a defined willingness-to-pay threshold. Total costs and disability were discounted at 3% annually, and undiscounted results were also calculated (56) (SI Appendix).
Table 5.
Estimated intervention costs and disability of schistosomiasis
| Source | Sequelae | Infection intensity | Disability weights | Refs. |
| Disability structure | ||||
| Schistosoma infection | Infection | Light | 0.014 | 19, 20, 29, 48, 53, 54 |
| Schistosoma infection | Infection | Heavy | 0.05 | 19, 20, 29, 48, 53, 54 |
| Anemia | Mild anemia | All | 0.0041 | 19, 20, 53 |
| Anemia | Moderate anemia | All | 0.0056 | 19, 20, 53 |
| Anemia | Severe anemia | All | 0.1615 | 19, 20, 53 |
Sensitivity, Scenario, and Uncertainty Analyses.
We tested the robustness of our primary study findings with one-way sensitivity and multiway uncertainty analyses that varied key model parameters across a range of possible values. We conducted one-way sensitivity analyses on inputs related to Schistosoma natural history, transmission, environmental conditions (e.g., snail population, spatial connectivity), intervention effectiveness, compliance (MDA and snail control), and cost. We accounted for uncertainty in disease transmission by sampling from the posterior distribution, which provided multiple sets of fitted transmission parameters to create a range of model projections, and applied this in the one-way and multiway sensitivity analyses. This sampling process also captured heterogeneity in snail control effectiveness by creating a distribution for the effectiveness of snail control (measured in relative incidence and prevalence reduction), which helped estimate the robustness of the model conclusions for varying effectiveness levels for snail control (full distribution of effect size for snail control is in SI Appendix, Fig. A5). We also explicitly varied the effectiveness of snail control, which could also be conceptualized as regional coverage. To understand the impact of environmental differences, we incorporated snail migration and a proportion of the force of infection from external human sources to simulate spatial connectivity with neighboring environments, which could represent hot spot communities. We also included a formal characterization of costing values where primary findings are no longer robust and alternative assumptions on systematic noncompliance to MDA (22). Scenario analyses of key combination of model inputs were also computed.
We performed a multiway uncertainty analysis on helminth transmission and cost-effectiveness, where we varied many model inputs simultaneously to generate a 95% UI. In these analyses, we sampled from the posterior distribution of the transmission parameters to propagate uncertainty in disease transmission and also varied the model inputs for the cost-effectiveness analysis, including the school-based and community-wide MDA cost, snail control cost, disability weights, and noncompliance (SI Appendix, Table S4). In each uncertainty analysis simulation, we constructed a cost-effectiveness frontier and computed the ICER of our key strategies relative to the next best option in terms of averted disability, and we reported the proportion of simulations where each strategy was optimally cost-effective. The data and model code are available online (57). This study was not human subject research and relied on published data.
Supplementary Material
Acknowledgments
We thank the health, education, and local authorities; study participants; and laboratory technicians who supported the original epidemiological studies in Kenya. N.C.L. is supported by the Medical Scientist Training Program (MSTP) at Stanford University School of Medicine. Additional support was provided by the Schistosomiasis Consortium for Operational Research and Evaluation funded by the University of Georgia Research Foundation through a grant from the Bill & Melinda Gates Foundation (D.G., N.Y., and C.H.K.). The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The model code reported in this paper is available online at https://github.com/NathanLo3/Publication-codes.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1708729114/-/DCSupplemental.
References
- 1.Lai YS, et al. Spatial distribution of schistosomiasis and treatment needs in sub-Saharan Africa: A systematic review and geostatistical analysis. Lancet Infect Dis. 2015;15:927–940. doi: 10.1016/S1473-3099(15)00066-3. [DOI] [PubMed] [Google Scholar]
- 2.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: Systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6:411–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 3.WHO . Preventive Chemotherapy in Human Helminthiasis. Coordinated Use of Anthelminthic Drugs in Control Interventions: A Manual for Health Professionals and Programme Managers. WHO; Geneva: 2006. [Google Scholar]
- 4.WHO . Helminth Control in School-Age Children: A Guide for Managers of Control Programmes. WHO; Geneva: 2011. [Google Scholar]
- 5.Leenstra T, et al. Schistosoma japonicum reinfection after praziquantel treatment causes anemia associated with inflammation. Infect Immun. 2006;74:6398–6407. doi: 10.1128/IAI.00757-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Satayathum SA, Muchiri EM, Ouma JH, Whalen CC, King CH. Factors affecting infection or reinfection with Schistosoma haematobium in coastal Kenya: Survival analysis during a nine-year, school-based treatment program. Am J Trop Med Hyg. 2006;75:83–92. [PMC free article] [PubMed] [Google Scholar]
- 7.Lelo AE, et al. No apparent reduction in schistosome burden or genetic diversity following four years of school-based mass drug administration in mwea, central kenya, a heavy transmission area. PLoS Negl Trop Dis. 2014;8:e3221. doi: 10.1371/journal.pntd.0003221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lo NC, et al. A call to strengthen the global strategy against schistosomiasis and soil-transmitted helminthiasis: The time is now. Lancet Infect Dis. 2017;17:e64–e69. doi: 10.1016/S1473-3099(16)30535-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.King CH, Bertsch D. Historical perspective: Snail control to prevent schistosomiasis. PLoS Negl Trop Dis. 2015;9:e0003657. doi: 10.1371/journal.pntd.0003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO . Field Use of Molluscicides in Schistosomiasis Control Programmes: An Operational Manual for Programme Managers. WHO; Geneva: 2017. [Google Scholar]
- 11.King CH, Sutherland LJ, Bertsch D. Systematic review and meta-analysis of the impact of chemical-based mollusciciding for control of Schistosoma mansoni and S. haematobium transmission. PLoS Negl Trop Dis. 2015;9:e0004290. doi: 10.1371/journal.pntd.0004290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rollinson D, et al. Time to set the agenda for schistosomiasis elimination. Acta Trop. 2013;128:423–440. doi: 10.1016/j.actatropica.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Sokolow SH, et al. Global assessment of schistosomiasis control over the past century shows targeting the snail intermediate host works best. PLoS Negl Trop Dis. 2016;10:e0004794. doi: 10.1371/journal.pntd.0004794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sokolow SH, et al. To reduce the global burden of human schistosomiasis, use ‘old fashioned’ snail control. Trends Parasitol. 2017 doi: 10.1016/j.pt.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO 2002. Prevention and control of schistosomiasis and soil-transmitted helminthiasis: Report of a WHO Expert Committee (WHO, Geneva), Technical Report Series 912.
- 16.WHO . Weekly Epidemiological Record. WHO; Geneva: 2015. [Google Scholar]
- 17.Anderson R, Truscott J, Hollingsworth TD. The coverage and frequency of mass drug administration required to eliminate persistent transmission of soil-transmitted helminths. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130435. doi: 10.1098/rstb.2013.0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson RM, Turner HC, Truscott JE, Hollingsworth TD, Brooker SJ. Should the goal for the treatment of soil transmitted helminth (STH) infections be changed from morbidity control in children to community-wide transmission elimination? PLoS Negl Trop Dis. 2015;9:e0003897. doi: 10.1371/journal.pntd.0003897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lo NC, et al. Comparison of community-wide, integrated mass drug administration strategies for schistosomiasis and soil-transmitted helminthiasis: A cost-effectiveness modelling study. Lancet Glob Health. 2015;3:e629–e638. doi: 10.1016/S2214-109X(15)00047-9. [DOI] [PubMed] [Google Scholar]
- 20.Lo NC, et al. Assessment of global guidelines for preventive chemotherapy against schistosomiasis and soil-transmitted helminthiasis: A cost-effectiveness modelling study. Lancet Infect Dis. 2016;16:1065–1075. doi: 10.1016/S1473-3099(16)30073-1. [DOI] [PubMed] [Google Scholar]
- 21.Yang GJ, et al. Optimizing molluscicide treatment strategies in different control stages of schistosomiasis in the People’s Republic of China. Parasit Vectors. 2012;5:260. doi: 10.1186/1756-3305-5-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dyson L, Stolk WA, Farrell SH, Hollingsworth TD. Measuring and modelling the effects of systematic non-adherence to mass drug administration. Epidemics. 2017;18:56–66. doi: 10.1016/j.epidem.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Utzinger J, Zhou XN, Chen MG, Bergquist R. Conquering schistosomiasis in China: The long march. Acta Trop. 2005;96:69–96. doi: 10.1016/j.actatropica.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Sokolow SH, et al. Reduced transmission of human schistosomiasis after restoration of a native river prawn that preys on the snail intermediate host. Proc Natl Acad Sci USA. 2015;112:9650–9655. doi: 10.1073/pnas.1502651112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jobin WR, Laracuente A. Biological control of schistosome transmission in flowing water habitats. Am J Trop Med Hyg. 1979;28:916–917. [PubMed] [Google Scholar]
- 26.Jordan P, Christie JD, Unrau GO. Schistosomiasis transmission with particular reference to possible ecological and biological methods of control. A review. Acta Trop. 1980;37:95–135. [PubMed] [Google Scholar]
- 27.Pointier JP, Giboda M. The case for biological control of snail intermediate hosts of Schistosoma mansoni. Parasitol Today. 1999;15:395–397. doi: 10.1016/s0169-4758(99)01517-3. [DOI] [PubMed] [Google Scholar]
- 28.Dawson VK. Environmental fate and effects of the lampricide bayluscide: A review. J Great Lakes Res. 2003;29:475–492. [Google Scholar]
- 29.King CH. It’s time to dispel the myth of “asymptomatic” schistosomiasis. PLoS Negl Trop Dis. 2015;9:e0003504. doi: 10.1371/journal.pntd.0003504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.King CH, Dangerfield-Cha M. The unacknowledged impact of chronic schistosomiasis. Chronic Illn. 2008;4:65–79. doi: 10.1177/1742395307084407. [DOI] [PubMed] [Google Scholar]
- 31.Andrade G, Bertsch DJ, Gazzinelli A, King CH. Decline in infection-related morbidities following drug-mediated reductions in the intensity of Schistosoma infection: A systematic review and meta-analysis. PLoS Negl Trop Dis. 2017;11:e0005372. doi: 10.1371/journal.pntd.0005372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giboda M, Bergquist NR. Post-transmission schistosomiasis. Parasitol Today. 1999;15:307–308. doi: 10.1016/s0169-4758(99)01487-8. [DOI] [PubMed] [Google Scholar]
- 33.Gurarie D, King CH. Age- and risk-targeted control of schistosomiasis-associated morbidity among children and adult age groups. Open Trop Med J. 2008;1:21–30. [Google Scholar]
- 34.Gurarie D, Wang X, Bustinduy AL, King CH. Modeling the effect of chronic schistosomiasis on childhood development and the potential for catch-up growth with different drug treatment strategies promoted for control of endemic schistosomiasis. Am J Trop Med Hyg. 2011;84:773–781. doi: 10.4269/ajtmh.2011.10-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Truscott JE, et al. A comparison of two mathematical models of the impact of mass drug administration on the transmission and control of schistosomiasis. Epidemics. 2017;18:29–37. doi: 10.1016/j.epidem.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson RM, Truscott JE, Pullan RL, Brooker SJ, Hollingsworth TD. How effective is school-based deworming for the community-wide control of soil-transmitted helminths? PLoS Negl Trop Dis. 2013;7:e2027. doi: 10.1371/journal.pntd.0002027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gurarie D, King CH, Yoon N, Li E. Refined stratified-worm-burden models that incorporate specific biological features of human and snail hosts provide better estimates of Schistosoma diagnosis, transmission, and control. Parasit Vectors. 2016;9:428. doi: 10.1186/s13071-016-1681-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gurarie D, et al. Modelling control of Schistosoma haematobium infection: Predictions of the long-term impact of mass drug administration in Africa. Parasit Vectors. 2015;8:529. doi: 10.1186/s13071-015-1144-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perez-Saez J, et al. Hydrology and density feedbacks control the ecology of intermediate hosts of schistosomiasis across habitats in seasonal climates. Proc Natl Acad Sci USA. 2016;113:6427–6432. doi: 10.1073/pnas.1602251113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gurarie D, King CH, Yoon N, Wang X, Alsallaq R. Seasonal dynamics of snail populations in coastal Kenya: Model calibration and snail control. Adv Water Resour. 2017;108:397–405. [Google Scholar]
- 41.Gurarie D, King CH, Wang X. A new approach to modelling schistosomiasis transmission based on stratified worm burden. Parasitology. 2010;137:1951–1965. doi: 10.1017/S0031182010000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muchiri EM, Ouma JH, King CH. Dynamics and control of Schistosoma haematobium transmission in Kenya: An overview of the Msambweni Project. Am J Trop Med Hyg. 1996;55(Suppl 5):127–134. doi: 10.4269/ajtmh.1996.55.127. [DOI] [PubMed] [Google Scholar]
- 43.Bisanzio D, et al. Cross-sectional study of the burden of vector-borne and soil-transmitted polyparasitism in rural communities of Coast Province, Kenya. PLoS Negl Trop Dis. 2014;8:e2992. doi: 10.1371/journal.pntd.0002992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gryseels B, De Vlas SJ. Worm burdens in schistosome infections. Parasitol Today. 1996;12:115–119. doi: 10.1016/0169-4758(96)80671-5. [DOI] [PubMed] [Google Scholar]
- 45.Hubbard A, et al. Estimating the distribution of worm burden and egg excretion of Schistosoma japonicum by risk group in Sichuan Province, China. Parasitology. 2002;125:221–231. doi: 10.1017/s003118200200207x. [DOI] [PubMed] [Google Scholar]
- 46.Zwang J, Olliaro PL. Clinical efficacy and tolerability of praziquantel for intestinal and urinary schistosomiasis-a meta-analysis of comparative and non-comparative clinical trials. PLoS Negl Trop Dis. 2014;8:e3286. doi: 10.1371/journal.pntd.0003286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guyatt H. The cost of delivering and sustaining a control programme for schistosomiasis and soil-transmitted helminthiasis. Acta Trop. 2003;86:267–274. doi: 10.1016/s0001-706x(03)00047-0. [DOI] [PubMed] [Google Scholar]
- 48.King CH, et al. Utility of repeated praziquantel dosing in the treatment of schistosomiasis in high-risk communities in Africa: A systematic review. PLoS Negl Trop Dis. 2011;5:e1321. doi: 10.1371/journal.pntd.0001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barnish G. Evaluation of chemotherapy in the control of Schistosoma mansoni in Marquis Valley, Saint Lucia. II. Biological results. Am J Trop Med Hyg. 1982;31:111–115. doi: 10.4269/ajtmh.1982.31.111. [DOI] [PubMed] [Google Scholar]
- 50.Barnish G, Christie JD, Prentice MA. Schistosoma mansoni control in Cul de Sac Valley, Saint Lucia. I. A two-year focal surveillance-mollusciciding programme for the control of Biomphalaria glabrata. Trans R Soc Trop Med Hyg. 1980;74:488–492. doi: 10.1016/0035-9203(80)90064-4. [DOI] [PubMed] [Google Scholar]
- 51.Barnish G, Jordan P, Bartholomew RK, Grist E. Routine focal mollusciciding after chemotherapy to control Schistosoma mansoni in Cul de Sac valley, Saint Lucia. Trans R Soc Trop Med Hyg. 1982;76:602–609. doi: 10.1016/0035-9203(82)90220-6. [DOI] [PubMed] [Google Scholar]
- 52.Knopp S, et al. Study and implementation of urogenital schistosomiasis elimination in Zanzibar (Unguja and Pemba islands) using an integrated multidisciplinary approach. BMC Public Health. 2012;12:930. doi: 10.1186/1471-2458-12-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salomon JA, et al. Common values in assessing health outcomes from disease and injury: Disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380:2129–2143, and erratum (2013) 381:628. doi: 10.1016/S0140-6736(12)61680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.King CH, Dickman K, Tisch DJ. Reassessment of the cost of chronic helmintic infection: A meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet. 2005;365:1561–1569. doi: 10.1016/S0140-6736(05)66457-4. [DOI] [PubMed] [Google Scholar]
- 55.Laxminarayan R, Chow J, Shahid-Salles SA. Intervention cost-effectiveness: Overview of main messages. In: Jamison DT, et al., editors. Disease Control Priorities in Developing Countries. 2nd Ed. World Bank; Washington, DC: 2006. [PubMed] [Google Scholar]
- 56.Tan-Torres Edejer T, et al. WHO Guide to Cost-Effectiveness Analysis. WHO; Geneva: 2003. [Google Scholar]
- 57.Lo NC. 2017 Github repository. Available at https://github.com/NathanLo3/Publication-codes. Accessed November 17, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.