Significance
Salinization and alkalinization impact water quality, but these processes have been studied separately, except in arid regions. Globally, salinization has been largely attributed to agriculture, resource extraction, and land clearing. Alkalinization has been attributed to recovery from acidification, with less recognition as an environmental issue. We show that salinization and alkalinization are linked, and trends in these processes impact most of the drainage area of the United States. Increases in salinity and alkalinity are caused by inputs of salts containing strong bases and carbonates that originate from anthropogenic sources and accelerated weathering. We develop a conceptual model unifying our understanding of salinization and alkalinization and its drivers and impacts on fresh water in North America over the past century.
Keywords: emerging contaminants, drinking water, land use, anthropocene, carbon cycle
Abstract
Salt pollution and human-accelerated weathering are shifting the chemical composition of major ions in fresh water and increasing salinization and alkalinization across North America. We propose a concept, the freshwater salinization syndrome, which links salinization and alkalinization processes. This syndrome manifests as concurrent trends in specific conductance, pH, alkalinity, and base cations. Although individual trends can vary in strength, changes in salinization and alkalinization have affected 37% and 90%, respectively, of the drainage area of the contiguous United States over the past century. Across 232 United States Geological Survey (USGS) monitoring sites, 66% of stream and river sites showed a statistical increase in pH, which often began decades before acid rain regulations. The syndrome is most prominent in the densely populated eastern and midwestern United States, where salinity and alkalinity have increased most rapidly. The syndrome is caused by salt pollution (e.g., road deicers, irrigation runoff, sewage, potash), accelerated weathering and soil cation exchange, mining and resource extraction, and the presence of easily weathered minerals used in agriculture (lime) and urbanization (concrete). Increasing salts with strong bases and carbonates elevate acid neutralizing capacity and pH, and increasing sodium from salt pollution eventually displaces base cations on soil exchange sites, which further increases pH and alkalinization. Symptoms of the syndrome can include: infrastructure corrosion, contaminant mobilization, and variations in coastal ocean acidification caused by increasingly alkaline river inputs. Unless regulated and managed, the freshwater salinization syndrome can have significant impacts on ecosystem services such as safe drinking water, contaminant retention, and biodiversity.
The abundance, integrity, and distribution of Earth’s fresh water are critical for human welfare. Only 2.5% of Earth’s water is fresh, and dissolved salts within this water determine the degree to which it can be used for drinking, industry, agriculture, and energy production (1, 2). The primary causes of freshwater salinization around the world are agriculture, resource extraction, and land clearing (3, 4). Relatively recently, human salt inputs are increasingly becoming recognized as important over large geographic scales (5, 6). Alkalinization has received relatively less recognition as a related environmental issue, but increases in alkalinity are also generated by salt pollution, accelerated weathering, and microbial processes that influence water chemistry (7). Salinization and alkalinization of fresh water can be interconnected processes that deteriorate water quality over a range of climates, but it is typically assumed that these processes are related only in arid regions (6–9). In particular, increasing concentrations of dissolved salts with strong bases and carbonates can increase the pH of fresh water over time, linking salinization to alkalinization.
We propose a concept, the freshwater salinization syndrome, which links salinization and alkalinization processes along hydrologic flow paths from small watersheds to coastal waters. The freshwater salinization syndrome manifests to varying degrees as concurrent trends in specific conductance, pH, alkalinity, and base cations (i.e., sodium, calcium, magnesium, and potassium). There are at least three primary categories of salt sources driving the freshwater salinization syndrome: (i) anthropogenic salt inputs (e.g., road salts, sewage, brines, sodic/saline irrigation runoff); (ii) accelerated weathering of natural geologic materials by strong acids (e.g., acid rain, fertilizers, and acid mine drainage); and (iii) human uses of easily weathered resource materials (e.g., concrete, lime), which cause increases in salts with strong bases and carbonates (6, 9–13). Although previous work has focused primarily on sodium chloride as a dominant form of salt pollution, increases in different mixtures of salt ions such as sodium, bicarbonate, magnesium, sulfate, etc., as part of the freshwater salinization syndrome produce differential toxicity to aquatic life (14, 15), and further supports the need for studying the dynamics of dissolved salts holistically. Although environmental impacts of the freshwater salinization syndrome are still poorly understood, symptoms can include: changes in biodiversity due to osmotic stress and desiccation, corrosion of infrastructure, increased contaminant mobilization, enhanced river carbonate transport, and impacts on coastal ocean acidification caused by increasingly alkaline river inputs.
Over geologic time, salinization and alkalinization of water have naturally influenced the distribution and abundance of Earth’s life (16). However, rates of salinization and alkalinization in the Anthropocene are strongly influenced by three primary factors: atmospheric deposition, geology, and land use (7). Atmospheric acid deposition accelerates chemical weathering over large geographic scales due to chemical dissolution and ion exchange in rocks and soils (7, 12, 17). Accelerated weathering of geological substrates can contribute to increased concentrations of major ions in water including bicarbonate, calcium, magnesium, and potassium (7, 12, 18–21). Finally, land-use activities enhance the anthropogenic inputs of easily weathered materials, including agricultural lime and impervious surfaces made of concrete, which can impact chemistry of drainage waters (13, 22, 23). Road salt and brines from fracking fluids further contribute to ion exchange in soils and sediments, and can increase salinity and alkalinity via changes in base cations and bicarbonate concentrations (11). Mining and resource extraction contribute to accelerated weathering of rocks and soils and the release of major ions and salts (21, 24–26). Finally, wastewater, water reuse, and irrigation practices associated with urban and agricultural land use can increase salinity and alkalinity in fresh water (7, 10, 27). All of these factors synergistically contribute to the freshwater salinization syndrome. The directionality and severity of trends for different salt ions, alkalinity, and pH are influenced on a regional scale by dilution capacity of rivers due to water demand, changes in river runoff due to climate variability, and sea level rise and saltwater intrusion into freshwater sections of rivers and estuaries (6, 28, 29). A holistic understanding of concurrent patterns in salinization and alkalinization on a continental scale is important for identifying and prioritizing regional management needs, given that interactions between different salts, specific conductance, and pH can influence aquatic life, ecosystem functioning, and services (14, 15, 19, 30).
In this study, we (i) quantify concurrent trends in salinization rates, alkalinization rates, and base cation concentrations in fresh water on a continental scale and (ii) present a conceptual framework, called the freshwater salinization syndrome, which illustrates and explains how coupled salinization and alkalinization can occur. We quantified long-term trends in specific conductance, base cations, alkalinity, and pH in 232 stream and river sites sampled by the USGS throughout North America. Although these sites include both streams and rivers, we will mainly refer to them as rivers throughout. Watersheds drained areas primarily in the United States, but also extended into Canada, and spanned diverse regions and climates. Watersheds also sometimes drained major reservoirs. Surprisingly, we found that increasing trends in the pH of rivers occurred decades before US Clean Air Act Amendments in 1990, which specifically targeted reductions in acid rain (17). After 1990, a decrease in acidic precipitation and acid mine drainage may be expected to contribute to an increase in alkalinity and pH in the latter part of the twentieth century, but such reductions in acidification would not adequately explain earlier concurrent increases in specific conductance, base cations, alkalinity, and pH beginning in the early and middle twentieth century (31). The freshwater salinization syndrome illustrates how anthropogenic salts and human-accelerated weathering of natural and anthropogenic substrates synergistically drive salinization and alkalinization due to a plethora of salts containing strong bases and carbonates with potential for acid neutralization. In addition, increased sodium from salt pollution displaces other base cations on soil exchange sites, which further increases pH and contributes to alkalinization. From a biological perspective, in-stream microbial processes such as denitrification, sulfate reduction, etc., can further increase pH due to biochemical reactions that generate alkalinity. Finally, photosynthesis and gross primary production in eutrophic fresh waters in the Anthropocene can raise pH through depletion of total dissolved carbonate relative to equilibrium with CO2. The freshwater salinization syndrome can impact ecosystem services related to drinking water quality (6, 7, 9), economic costs related to switching water treatment and sources (9, 10), impacts on aquatic biodiversity (8), pipe corrosion and leaching of metals (7, 9), and shifting rates of coastal ocean acidification (32, 33).
Results
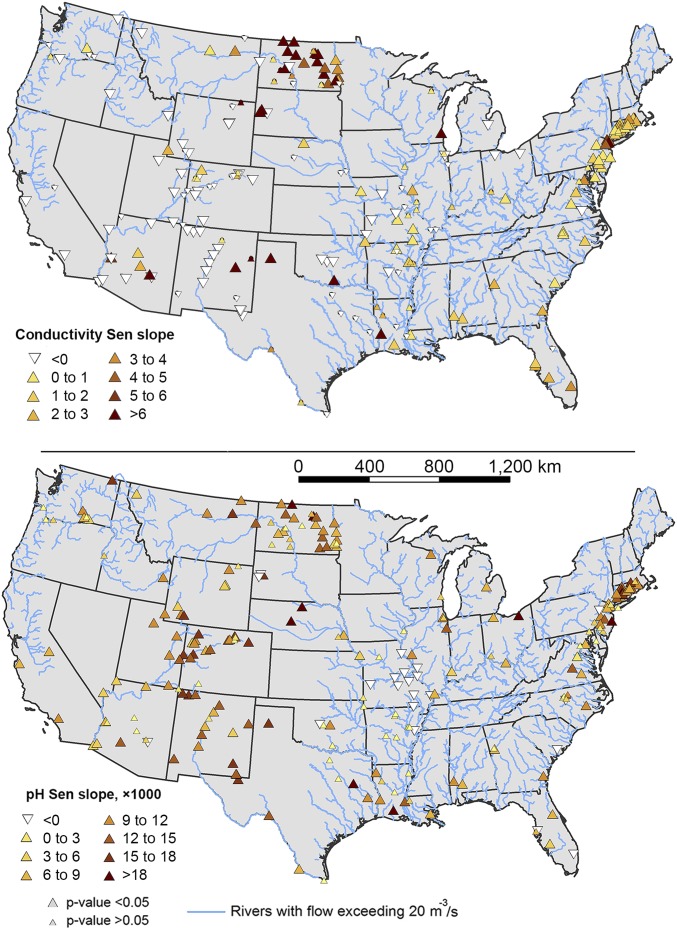
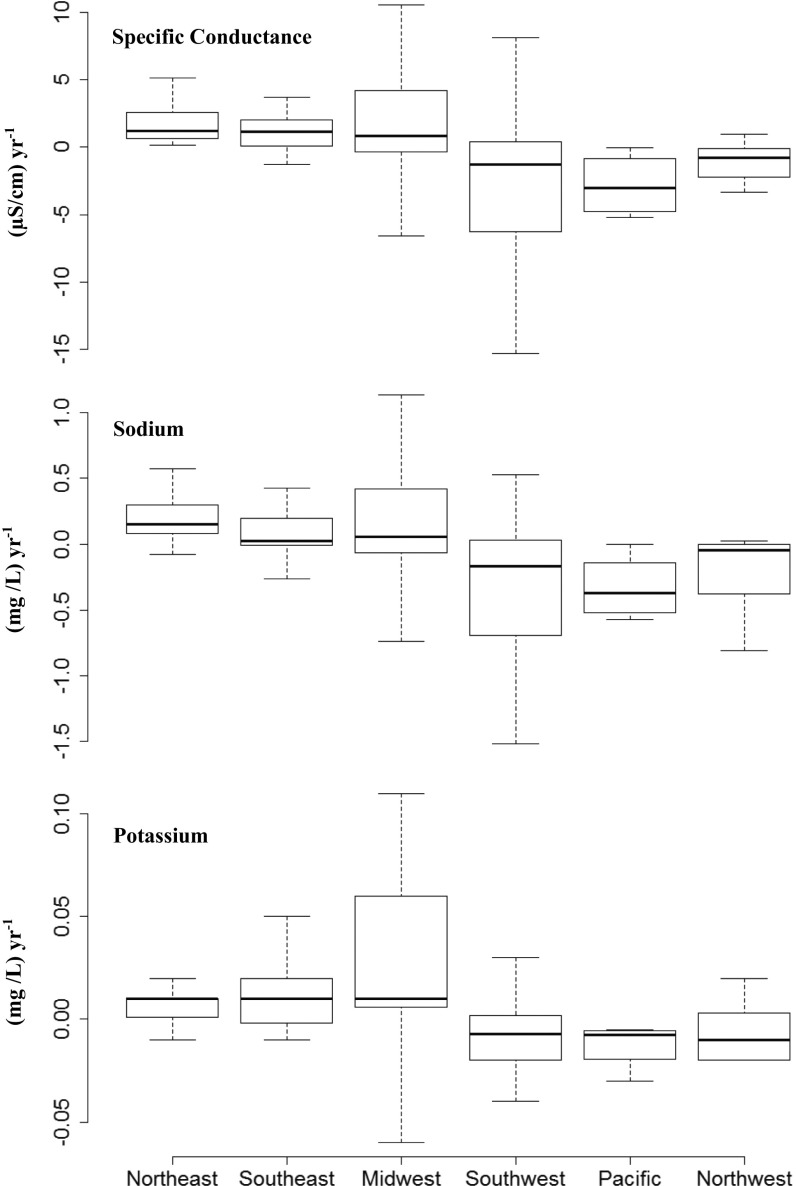
A large proportion of the streams and rivers in the contiguous United States have increasing trends in pH and specific conductance impacting most of the stream flow draining humid regions, such as the eastern United States (Figs. 1–3; Supporting Information). We estimate that alkalinization (significantly increased pH over time) and salinization (significantly increased specific conductance over time) have impacted 90% and 37% of the drainage area of the contiguous United States, respectively. Increasing trends in specific conductance were typically highest in the northeastern United States (Figs. 1 and 2), and trends tended to be negative in the arid western United States (Figs. 1 and 4), which receives significantly less precipitation (Supporting Information). Statistically increasing trends in pH were typical throughout all regions of the contiguous United States (Figs. 1 and 3). At some sites, the pH of river water actually declined following the Clean Air Act Amendments in 1990, which specifically targeted acid rain; this may have been due to a reduction in weathering rates in some watersheds (discussed further below). Of 232 sites, statistically increasing trends were observed at 66% of sites for pH, 39% of sites for specific conductance, 34% of sites for sodium, 29% for calcium, 33% for magnesium, and 36% for potassium concentrations. In contrast, there was a geographic cluster of decreasing trends in specific conductance and major cations in the arid western United States (Figs. 1 and 4). Thus, the fastest rates of increase for most dissolved salts occurred from west to east on a regional scale with the eastern United States showing the fastest rates. For example, the fastest median rates of increase in specific conductance and sodium were in the northeastern and southeastern United States, respectively (Fig. 4). There were exceptions for some salts. For example, the fastest increase in median potassium concentrations was in the midwestern United States likely due to potash from fertilizers (Fig. 4). Interestingly, there were decreasing median trends for specific conductance, and sodium and potassium concentrations in the Pacific Northwest, Southwest, and Pacific regions (Fig. 4). Decreasing trends were observed for 9% of sites for pH, 18% of sites for specific conductance, 18% of sites for sodium, 25% for calcium, 17% for magnesium, and 21% for potassium concentrations primarily in the arid western United States (e.g., west of the 100th meridian).
Fig. 1.
Maps showing locations of increasing, decreasing, and/or no trends in specific conductance and pH in stream water throughout the continental United States. Streamlines represent all conterminous US rivers with mean annual discharge exceeding 20 m3/s (47).
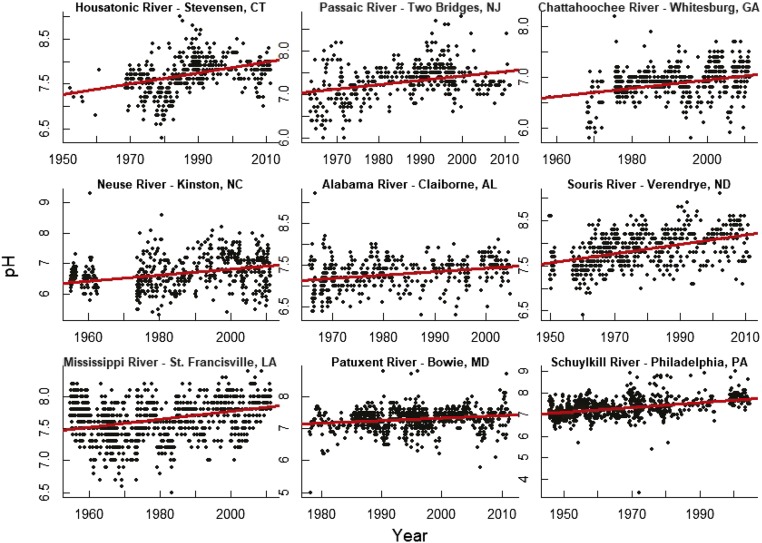
Fig. 3.
Examples of increasing trends in pH in stream water throughout the continental United States. The pH decreased at some sites following US Clean Air Act Amendments in 1990, which targeted reductions in acidic deposition. Please note that vertical axes differ.
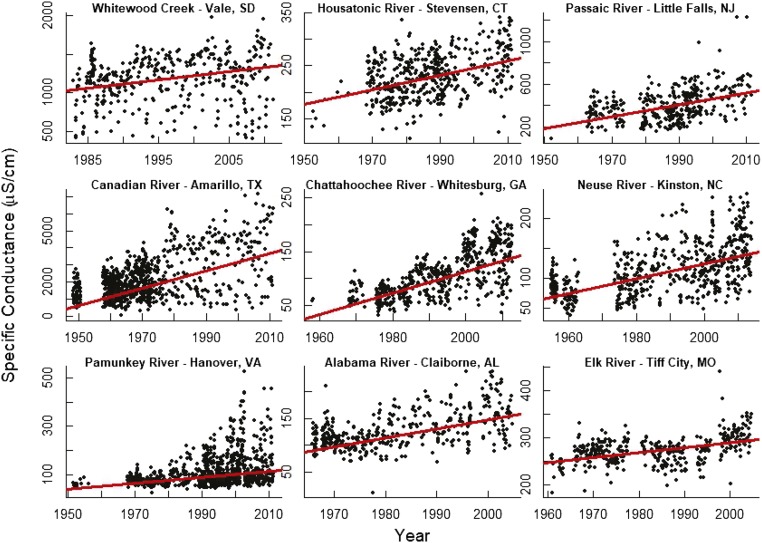
Fig. 2.
Examples of increasing trends in conductance in stream water throughout the continental United States, which are characteristic of ranges in their respective regions. Please note that vertical axes differ.
Fig. 4.
Differences in trends in specific conductance and sodium and potassium concentrations in stream and river sites across different regions of the United States. The center vertical lines of the box and whisker plots indicate the median of the sample. The length of each whisker shows the range within which the central 50% of the values fall. Box edges indicate the first and third quartiles. Outliers were excluded and were primarily in the southwestern US region.
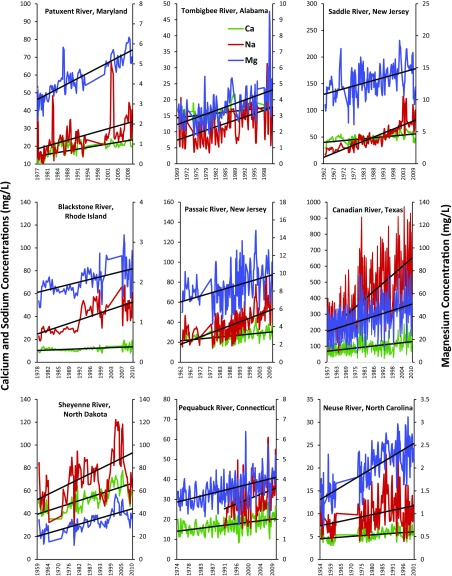
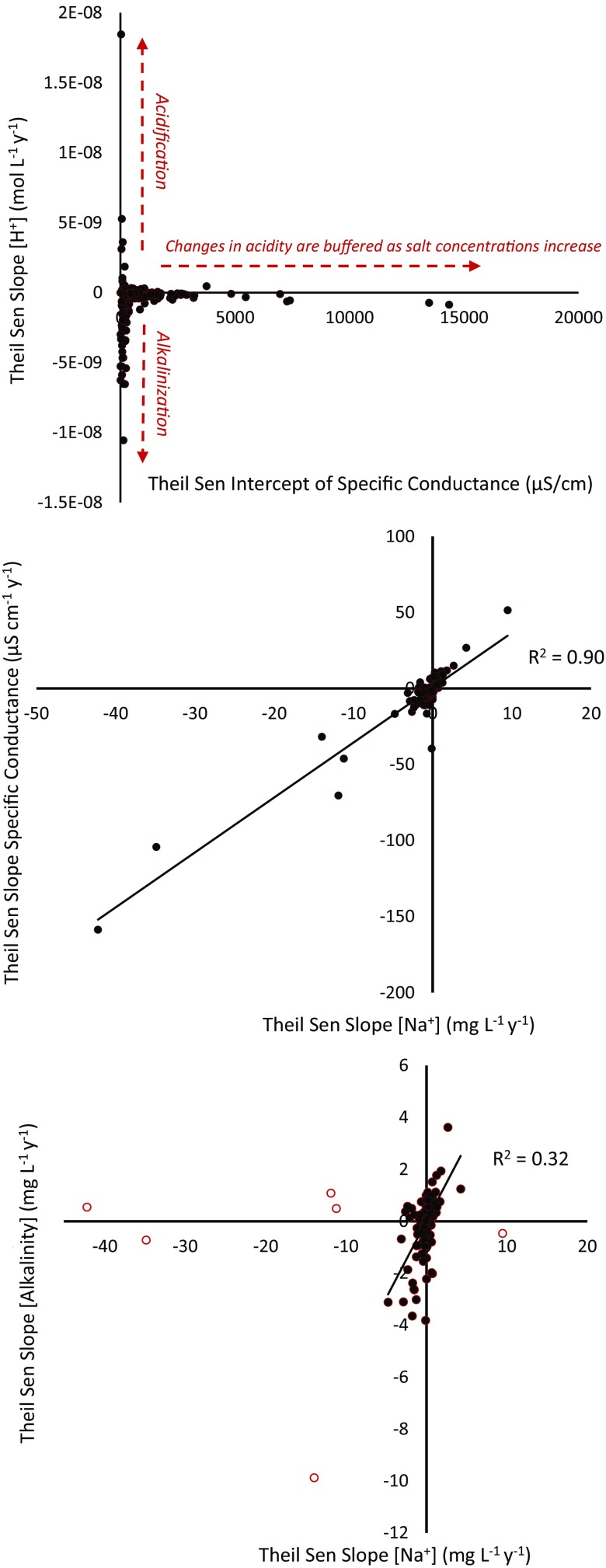
Across all 232 sites, there were interrelationships between trends in pH, specific conductance, and base cations. There were concurrently increasing or decreasing trends in base cations at some sites (Fig. 5 shows examples of increasing trends, but there were also decreasing trends primarily in the arid western United States as indicated by Figs. 1 and 4). Although the increase in sodium concentrations may be expected, it was surprising that increases in magnesium concentrations could exceed increases in calcium concentrations at some sites (Fig. 5). Magnesium concentrations were considerably lower than calcium concentrations in rivers, and therefore may have been more sensitive to anthropogenic disturbances over time at some sites (Fig. 5). Across all sites, variability in the rate of change of pH (whether increasing or decreasing) declined as the initial specific conductance of stream and river water increased (Fig. 6). The most rapid changes in long-term pH trends occurred in rivers with the lowest specific conductance (<250 µs/cm at 25 °C), whereas those with the highest salinity and buffering capacity had relatively minor or insignificant rates of change in pH (Fig. 6). Thresholds in the initial specific conductance of rivers and buffering influenced whether there were significant long-term increasing or decreasing pH trends. Rates of change in both specific conductance and alkalinity were positively and linearly related to rates of change in sodium concentrations across all sites (Fig. 6).
Fig. 5.
Examples of increasing trends in base cations (sodium, calcium, and magnesium) in stream water throughout the continental United States. Time series were smoothed as moving averages over every three data points/observations. Please note that vertical axes differ.
Fig. 6.
Rates of change in acidity of stream water (Theil–Sen slopes) are related to initial specific conductance of stream water (y intercept of slopes) throughout the continental United States. Rates of change in sodium concentrations (Theil–Sen slopes) over time are also strongly related to rates of changes in specific conductance (Theil–Sen slopes) over time. Rates of change in sodium concentrations (Theil–Sen slopes) are related to rates of change in alkalinity concentrations (Theil–Sen slopes) except for 6 out of 232 sites with the most extreme rates of change in sodium concentrations (open circles).
Discussion
Based on long-term river chemistry data, positive trends in salinization and alkalinization of fresh water were observed across large geographic areas of North America, which include watersheds draining the United States and Canada. Such trends were common in humid regions of the eastern United States, where acidic precipitation and human population density are the greatest (6, 7, 17). Our analysis demonstrates that salinization and alkalinization of inland waters are not restricted to arid regions as once assumed, and it suggests that these processes are coupled throughout many humid regions in the United States. Human activities have increased salt inputs and accelerated chemical weathering and cation exchange in watersheds, which contribute to dissolved salts containing strong bases and alkali metals and alkaline earth metals (Na+, Ca2+, Mg2+, K+) and carbonates (7, 13, 23). Geochemical and biogeochemical relationships between these salts and acid-neutralizing capacity lead to interrelated increases in specific conductance, alkalinity, base cations, and pH (6, 7, 34, 35). Our results suggest that these coupled changes in specific conductance, major ions, and pH related to the freshwater salinization syndrome have influenced the water quality of most of the stream flow in the eastern United States (Fig. 1; Supporting Information). The causes and consequences of this freshwater salinization syndrome have implications for ecosystem services of water including availability of high-quality drinking water, scaling and corrosion of pipes, toxicity of metals and ammonia, and buffering of coastal ocean acidification.
Variations in the Freshwater Salinization Syndrome Across a Continental Scale.
As mentioned earlier, regional patterns in the freshwater salinization syndrome are influenced by geographic variations in atmospheric deposition, geology, and land use (35–38). It is well known that there are regional differences in sodic and saline waters based on pH, electrical conductivity, and sodium absorption ratio throughout North America. Typically, these regional patterns represent: (i) fresh water with low salinity/alkalinity in humid regions draining bedrock resistant to weathering; (ii) fresh water with moderate salinity/alkalinity in humid regions draining bedrock prone to weathering; and (iii) fresh water with high salinity/alkalinity in arid regions prone to high evaporation rates. However, regional differences in salinization and alkalinization rates and changes in major ions over time on a continental scale are less known, and we discuss patterns and processes of the freshwater salinization syndrome below.
In the northeastern United States, watersheds draining humid, developed land cover with low to moderate salinity/alkalinity typically had the fastest increasing trends in salinization and alkalinization (Fig. 1). These watersheds also had the fastest median rates of increasing sodium concentrations. In general, this region represented the fastest increases in specific conductance, major cations, and pH due to anthropogenic salts and human-accelerated weathering (6, 7). Regional recovery from acid rain and acid mine drainage can contribute to increases in pH in the latter part of the record for some rivers, but do not adequately explain concurrent and widespread increases in specific conductance and base cations during the early and middle twentieth century before the Clean Air Act (31). Road salt is a dominant source of salinization of fresh water in colder, humid regions of the northeastern United States, and has contributed to long-term, increasing trends in sodium and chloride in surface water and ground water (5, 6, 34, 39). Although less considered, road salts can also influence pH and long-term alkalinization of fresh water, and this is discussed further below in a subsequent section regarding fast weathering (23).
Despite no or minimal use of road salts in the region, the freshwater salinization syndrome also impacts the southeastern United States, which suggests additional mechanisms. The freshwater salinization syndrome is also strongly influenced by the chemical weathering of soils, bedrock, and easily weathered anthropogenic substrates (e.g., agricultural lime, urban impervious surfaces) across all regions (13, 18, 22, 23). It is well known that alkalinity, base cations, and pH in rivers of humid regions are related to underlying lithology, land cover/land use, and weathering rates (7, 37). The southeastern United States has elevated rates of acid deposition and urban impervious surfaces similar to the northeastern United States (17, 38), which further contribute to the freshwater salinization syndrome along with salt pollution and accelerated weathering.
Agricultural activities further contribute to the freshwater salinization syndrome in the midwestern United States. The fastest median rates of increasing potassium concentrations were observed in midwestern rivers. This increase was likely due to potassium in potash in agricultural fertilizers, and the variability in rates was likely due to K limitation in response to excess nitrogen and phosphorus from fertilizers (40). Agricultural liming to improve crop yields can also contribute to long-term alkalinization and increased dissolved salts in rivers of the midwestern United States (13). Finally, fertilizer-enhanced nitrification can also synergistically accelerate chemical weathering of cations from bedrock and soils via leaching and enhanced ion exchange reactions (17, 38, 41, 42). Thus, agricultural activities in combination with high levels of road salt pollution from impervious surfaces and human-accelerated weathering can further contribute to the freshwater salinization syndrome in the midwestern United States.
Interestingly, specific conductance and base cation concentrations have decreased in some streams and rivers throughout the Northwest, Pacific, and Southwest (particularly in arid regions with high baseline salinity and alkalinity in surface waters). Despite decreasing trends in specific conductance, there were still long-term increasing trends in pH in fresh water throughout the western United States. Previous work has also documented decreasing trends in total dissolved solids and increasing trends in pH in the southwestern United States (35, 43). Concentrations of alkalinity and major ions have decreased throughout the western United States due to a combination of dilution and retention of weathering products behind dams in reservoirs (e.g., calcite precipitation) (31). For example, decreasing trends in total dissolved solids have been attributable to complex water diversions (mostly for agriculture) and decreased irrigation return flows of salts to river systems (35). Efficiency of agricultural and irrigation practices has also increased over time, which could also decrease salt accumulation rates in some drainages (44, 45).
Furthermore, return flow from urban wastewater has increased while return flow from irrigation has decreased in some areas throughout the western United States (46, 47). Although urban wastewater contains dissolved salts, its salinity can be significantly lower than irrigation return flow and contribute to dilution over time (46). Wastewater discharges represent a significant fraction of stream flow and nutrients in some rivers of the western United States (47, 48). Regional wastewater treatment has improved with reductions in organic carbon and oxygen deficit, which has contributed to long-term increases in pH in western rivers (46, 47). Nutrients in wastewater can increase pH of stream water further by stimulating primary production in the receiving streams (48, 49). Long-term effects of dilution and retention of weathering products due to combined effects of hydrologic modifications, land-use change, and urban wastewater treatment on decreasing symptoms of the freshwater salinization syndrome warrant further investigation throughout the western United States.
Acid Rain and Road Salt Pollution Increases Ion Exchange: Fast Weathering.
From a regional and continental perspective, a historical legacy of acid deposition still exerts a major influence on the acid/alkaline chemistry of fresh water throughout much of the United States, despite regulations reducing acid deposition (7, 17, 38). Long-term monitoring shows that acid rain accelerates chemical weathering via ion exchange and leaching processes such as displacement of base cations by hydrogen ions and forms of nitrogen (17, 20, 38). Increased base cations transported from watersheds can eventually contribute to increased acid buffering capacity and long-term increases in pH of fresh water (50). Acid deposition has decreased during recent decades in some regions due to regulations, and atmospheric inputs of acidity can be neutralized; both factors contribute to increasing acid neutralizing capacity and elevated pH in some rivers (38, 51). However, declining acid deposition can also decrease weathering rates, and this decrease can actually lead to decreases in pH at other sites. For example, we observed a decline in pH at some sites following Clean Air Act Amendments in 1990, which may have been due to decreased weathering rates and a corresponding decrease in bicarbonate alkalinity from weathering of rocks and soils (7). Long-term effects of acid deposition may be particularly evident in larger watersheds with longer hydrologic residence times due to potential storage and release of weathering products in soils and ground water (7). Accelerated cation exchange from acid rain may be particularly important in soils that already have high base cation saturation (50). Furthermore, acid rain may interact with other anthropogenic salts (e.g., road salt) to accelerate chemical weathering and base cation exchange in watersheds (46).
Similar to acid deposition, road salts can significantly mobilize transport of base cations from soil exchange sites to streams via ion exchange (41, 52, 53). For example, road salts increase leaching of calcium and magnesium from soil cation exchange sites (53), which are primarily responsible for maintaining soil aggregate structure. Cation exchange driven by sodium in road salt can release up to 40% to 80% of calcium and magnesium from soil exchange sites to ground water and streams (52, 53). Other related work has documented regionally increasing trends in base cations in lakes affected by road salt applications (41). Similarly, there can be long-term increases in pH in lakes due to increased acid neutralizing capacity and base cation inputs (50, 54). Road salts accumulate in soils and ground water contributing to enhanced cation exchange and mobilization of major ions over time (6, 23). Therefore, acid rain, road salt, and other forms of salt pollution (such as sodic/saline irrigation runoff) can synergistically contribute to the freshwater salinization syndrome over decades by enhancing fast weathering and ion exchange.
The Freshwater Salinization Syndrome: A Conceptual Model.
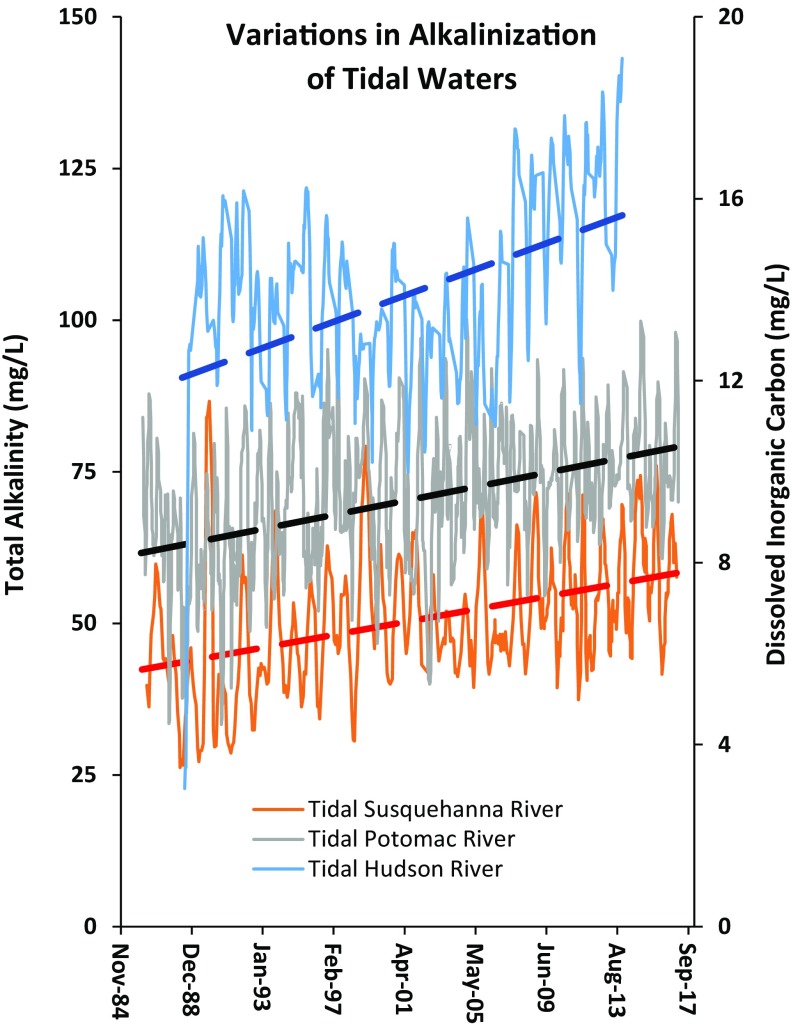
The freshwater salinization syndrome can be influenced by cumulative weathering reactions across hydrologic flow paths extending from soils, ground water, and headwater streams to larger rivers. Although the freshwater salinization syndrome originates in headwaters, the terminus along rivers and receiving waters is still poorly understood, but may actually extend into tidal waters (Fig. 7) (55–57). For example, there have been long-term increases in alkalinity in some tidal waters of tributaries in the Chesapeake Bay and more recent increases in dissolved inorganic carbon (primarily bicarbonate) in the tidal Hudson River estuary (Fig. 7). Changes in weathering and cation exchange may explain alkalinization in some tidal waters of the Chesapeake Bay (7, 24), whereas increasing dissolved inorganic carbon in the Hudson River estuary may be due to a combination of changes in primary production, land development, changes in salt pollution, and/or responses to changes in acid rain. In fact, a wide array of potential mechanisms has been proposed to explain recent observations of increased river alkalinization (7, 13, 31). However, there are currently no conceptual models linking salinization and alkalinization directly from headwaters to receiving waters and explicitly recognizing the synergistic importance of atmospheric inputs, geochemical processes, and anthropogenic salt pollution. Such conceptual models are needed to drive our understanding, research, and management of these complicated interactions.
Fig. 7.
Examples of significant increasing trends (P < 0.05) in alkalinity and dissolved inorganic carbon in tidal waters of the US East Coast. Time series were smoothed as moving averages over every three data points/observations. Alkalinity concentrations are shown for the tidal Potomac and Susquehanna Rivers, and dissolved inorganic carbon concentrations are shown for the tidal Hudson River. Please note that vertical axes differ in scale.
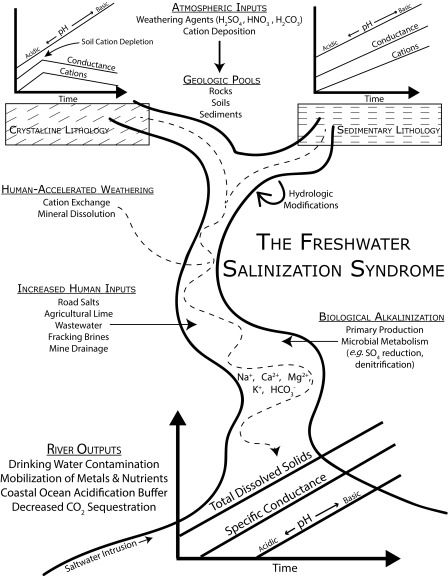
The freshwater salinization syndrome recognizes that acid deposition, anthropogenic salts, and fertilizers have differential impacts on weathering of watersheds draining different lithologies (51, 54, 58) (Fig. 8). Along drainage networks, weathering products accumulate in ground and surface waters across hydrologic flow paths due to acid neutralization and ion exchange reactions (18, 51, 58) (Fig. 8). Buffering capacity increases as base cation concentrations increase (23). Calcium, magnesium, potassium, and sodium are highly soluble and increase in concentrations downstream relative to other less soluble elements that may be adsorbed onto soils and sediments (18, 58) (Fig. 8). The pH in streams increases along hydrologic flow paths due to the accumulation of weathering products and increased buffering capacity (51, 54, 58) (Fig. 8). Thus, differences in lithology and surface and subsurface groundwater flow paths along the drainage network influence the potential for acidification vs. alkalinization of fresh water (38). For example, headwaters are prone to acidification depending on underlying lithology (sedimentary vs. crystalline) (50, 51). Larger rivers are prone to alkalinization downstream due to cumulative effects of weathering reactions and biological processes, which can also increase alkalinity and pH along flow paths (e.g., primary production and anaerobic metabolism) (23, 49, 50) (Fig. 8).
Fig. 8.
A conceptual model of the freshwater salinization syndrome, illustrating potential drivers and changes in increased salinization and alkalinization of fresh water. At least three sets of process contribute to the freshwater salinization syndrome, and they are listed in a hypothetical order from upstream to downstream including: (i) accelerated weathering in headwaters and throughout the drainage network; (ii) human salt inputs from developed landscapes; and (iii) increased biological alkalinization in larger-order streams with increased light and nutrient availability. Crystalline and sedimentary lithology results in different starting points in headwaters based on the potential for chemical weathering. Weathering process such as dissolution and cation exchange in soils and sediments generate alkalinity, bicarbonate, and base cations along drainage networks (accelerated weathering is represented by the dashed line extending throughout the drainage network). The relative importance of accelerated weathering may change with increasing disturbances along stream order, as salt and nutrient pollution increases further downstream and/or in response to hydrologic modifications. The terminus of the freshwater salinization syndrome varies based on climate, land use and management, saltwater intrusion, and underlying geology but can potentially extend into tidal waters.
In addition to human-accelerated weathering, anthropogenic salts and fertilizers have greatly contributed to salinization and alkalinization, and their relative importance can accumulate along increasing stream order (Fig. 8). There are inputs of different mixtures of salts due to urbanization, agriculture, mining, and resource extraction (6, 8) (Fig. 8). Densities of urban and agricultural land within a watershed can be strong predictors of base cations and pH in streams and rivers (22, 23, 54, 59). In residential and urban areas with colder climates, road deicers including sodium chloride, calcium chloride, and magnesium chloride are an important source of salinization in the United States and globally (6, 34). Fertilizers can stimulate aquatic primary production, which increases pH and alkalinity (49, 50, 54). Anaerobic metabolism and decomposition can further increase dissolved inorganic carbon production, alkalinity, and pH in urbanized and agricultural watersheds (22, 23, 50). Agriculture can contribute significant bicarbonate and base cations, including calcium, magnesium, and potassium, from liming, potash, and fertilizer applications (in addition to those ions generated from agriculturally enhanced weathering) (13, 60). Fertilizers can stimulate nitrification and accelerated weathering of agricultural soils, leading to further increases in concentrations of base cations in water (20, 42). Mine drainage can produce strong acids from sulfide oxidation, which contributes to accelerated weathering and increased concentrations of base cations downstream (13, 25). Brines from energy extraction activities, including hydrofracking, may also further contribute to salinization and alkalinization of fresh water (11). Overall, anthropogenic salt inputs can interact with and accelerate chemical weathering processes throughout the entire drainage network (Fig. 8). The net result of accelerated weathering and cation exchange and salt pollution is to produce the freshwater salinization syndrome on a continental scale with regional- and watershed-scale variations related to atmospheric deposition, land and water use, and underlying geology.
Environmental Implications of the Freshwater Salinization Syndrome.
The freshwater salinization syndrome currently poses direct and indirect concerns for ecosystem services and may influence rates of coastal ocean acidification. Elevated pH can contribute to ammonia toxicity and mortality for aquatic organisms (49, 60). Different salts influence toxicity to aquatic organisms leading to recent calls for regulating salt composition and concentrations in fresh water by government agencies (8). Elevated pH and base cations, such as calcium and magnesium, may reduce the bioavailability and toxicity of trace metals (60), but salinization may also enhance ion exchange and mobilization of trace metals from soils to streams (61). Increased sodium concentrations decrease soil aggregate stability and soil fertility through leaching of calcium and magnesium (62). Salinization from road salts can increase leaching and mobilization of carbon, nitrogen, and phosphorus from soils and sediments to streams (63). However, detailed work has also shown that alkalinization or rather sodium absorption ratio or exchangeable sodium percentage is responsible for increasing dissolved organic carbon and not salinization (64). Salinization also may directly influence the quality of different fractions of organic matter released from soils to streams (65). Elevated alkalinity can also stimulate production of nitrate by microbial nitrification, and salinization can alter the urban evolution of ecosystems and aquatic communities (22). Increased temperatures in rivers throughout the United States (66) may further influence weathering and other geochemical reactions, and interact with impacts of freshwater salinization.
Furthermore, increased pH, alkalinity, and salinity influence the amount and quality of inorganic and organic carbon transported by rivers, which can influence aquatic species and rates of coastal ocean acidification (32, 33, 56, 57). Changes in the pH and alkalinization of rivers also influence rates of CO2 evasion, which are important for global carbon budgets (67). From a coastal perspective, increased alkalinity can contribute to increased shell thickness of calcareous organisms such as found in zebra mussels in the Hudson River estuary (57), where there has been an increase in alkalinity over the past decade (Fig. 8). Estuarine waters of the Chesapeake Bay also have long-term positive trends in pH and alkalinity near tributaries (Fig. 8) (56). Overall, the effects of the freshwater salinization syndrome on the riverine carbon cycle and variations in coastal ocean acidification due to riverine inputs warrant further investigation (12, 32, 33).
The freshwater salinization syndrome can increase risks to the safety of drinking water and infrastructure. Elevated salt levels in drinking water can contribute to hypertension in people on sodium-restricted diets and is of concern to people requiring kidney dialysis (9). Salinization and alkalinization influence the corrosivity of water, and this can affect leaching of metals from pipes carrying drinking water (9, 68). Salinization increases corrosion of transportation infrastructure with United States economic costs estimated in the billions of dollars (69). Given increasing impacts on ecosystems and human welfare, increased salinization and alkalinization of fresh water is now a pervasive water quality issue, which may require aggressive management in both arid and humid climates across latitudes.
Methods
Long-term time series of specific conductance, pH, alkalinity, and base cation concentrations (calcium, magnesium, potassium, and sodium) were analyzed from 232 different stream and river sites located throughout the continental United States (7). Some rivers were sampled at multiple locations. All records were compiled from water quality measurements made by the USGS. Time series included in our analyses were based on at least 25 y of data and more than 156 observations for at least one parameter. We retained all series for pH, alkalinity, and specific conductance with less than six consecutive years of missing data and a period of monitoring until at least the year 2000. Some datasets had longer temporal discontinuities for base cations (e.g., Fig. 4). Time series for all parameters included in our analysis ranged from 25 to almost 80 y in length. Further information on related methods for analyses can be found elsewhere (7) and in Supporting Information. All data are publicly available through the USGS website.
Theil–Sen slopes were estimated to characterize average rates of change in specific conductance, pH, and base cation concentrations per year at each site. The Theil–Sen regression estimates the slope of change in a parameter across time by calculating the median slope of all pairwise points in a series (70), providing robust slope estimates despite missing data, nonnormal distributions, and occasional outliers (7). We considered slopes to be statistically significant if P values, estimated via nonparametric Wilcoxon tests as applied to the Theil–Sen slope (71), were <0.05. Because of the large number of sites that had irregular sampling periods, seasonal average values were used to detect long-term trends. Seasonal averages were defined as winter (December January, February), spring (March, April, May), summer (June, July, August), and fall (September, October, November). A bootstrap resampling procedure was used to generate confidence intervals and estimate P values. Theil–Sen slopes were calculated using R package mblm (R 3.2.1, mblm version 0.12). One limitation to the approach used here is that concentration data were not flow normalized. Flow normalization describes the changing state of the system over time by removing the influence of variations in concentration caused by variations in discharge. Discharge can vary considerably over time at the study sites and can influence trends. One way to deal with this would be to test for trends in discharge. Higher discharge would cause dilution reducing salinity and alkalinity. For example, the Hudson River showed an increase in dissolved inorganic carbon concentrations, which could be due to lower discharge, but the opposite was actually the case—discharge has increased. Therefore, our analysis presents estimates of what was actually observed at the sites over time (and the interrelationships between those trends). This approach can make teasing apart land use and lithology more complicated for some individual sites.
We applied an interpolation and precipitation-weighting procedure to determine the total drainage area and amount of surface water subjected to either decreasing or increasing slopes at the continental scale (Supporting Information). The estimated slope of all areas within the continental United States was determined using ordinary kriging (72) to interpolate within the 232 analyzed sites to generate a raster of Theil–Sen slope estimates for the entire continental United States. Kriging was achieved using a spherical semivariogram model with a 12-point, variable distance search radius and an output cell resolution of 10 km2. The kriging approach represents an oversimplification of watershed processes in that broad, 2D interpolation will neglect entities that affect water quality such as point sources. However, our goal was to broadly illustrate and estimate trends occurring at the continental scale, thus our interpolated estimates are meant to reflect patterns associated with large-scale geologic attributes, land-cover patterns, and climatic variables but should not be used as precise estimates for a specific waterway. We also determined the volume of water for every 1 km2 pixel in the same area by subtracting the estimated total mean annual evapotranspiration (73) from mean annual precipitation (74) (Supporting Information). The two rasters were merged to a common spatial scale (10 km2), which allowed for subsequent calculation of the total amount of water that becomes groundwater and surface runoff subjected to increasing or decreasing alkalization (Supporting Information).
Supplementary Material
Acknowledgments
This was work primarily supported by National Science Foundation Grant EAR 1521224 with partial support from Grants DEB 1027188 and CBET 105850. Long-term data were provided by the USGS. Data from the Hudson River were provided by the Hudson River group at the Cary Institute of Ecosystem Studies and supported by National Science Foundation Grant DEB-1119739 and the Hudson River Foundation. Data from the tidal Potomac River and the tidal Susquehanna River were provided by the Chesapeake Bay Program and Maryland Department of Natural Resources.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1711234115/-/DCSupplemental.
References
- 1.Jackson RB, et al. Water in a changing world. Ecol Appl. 2001;11:1027–1045. [Google Scholar]
- 2.Oki T, Kanae S. Global hydrological cycles and world water resources. Science. 2006;313:1068–1072. doi: 10.1126/science.1128845. [DOI] [PubMed] [Google Scholar]
- 3.Williams WD. Anthropogenic salinization of inland waters. Hydrobiologia. 2001;466:329–337. [Google Scholar]
- 4.Cañedo-Argüelles M, et al. Salinisation of rivers: An urgent ecological issue. Environ Pollut. 2013;173:157–167. doi: 10.1016/j.envpol.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Dugan HA, et al. Salting our freshwater lakes. Proc Natl Acad Sci USA. 2017;114:4453–4458. doi: 10.1073/pnas.1620211114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaushal SS, et al. Increased salinization of fresh water in the northeastern United States. Proc Natl Acad Sci USA. 2005;102:13517–13520. doi: 10.1073/pnas.0506414102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaushal SS, et al. Increased river alkalinization in the Eastern U.S. Environ Sci Technol. 2013;47:10302–10311. doi: 10.1021/es401046s. [DOI] [PubMed] [Google Scholar]
- 8.Cañedo-Argüelles M, et al. WATER. Saving freshwater from salts. Science. 2016;351:914–916. doi: 10.1126/science.aad3488. [DOI] [PubMed] [Google Scholar]
- 9.Kaushal SS. Increased salinization decreases safe drinking water. Environ Sci Technol. 2016;50:2765–2766. doi: 10.1021/acs.est.6b00679. [DOI] [PubMed] [Google Scholar]
- 10.Rengasamy P. World salinization with emphasis on Australia. J Exp Bot. 2006;57:1017–1023. doi: 10.1093/jxb/erj108. [DOI] [PubMed] [Google Scholar]
- 11.Vidic RD, Brantley SL, Vandenbossche JM, Yoxtheimer D, Abad JD. Impact of shale gas development on regional water quality. Science. 2013;340:1235009. doi: 10.1126/science.1235009. [DOI] [PubMed] [Google Scholar]
- 12.Guo J, Wang F, Vogt RD, Zhang Y, Liu CQ. Anthropogenically enhanced chemical weathering and carbon evasion in the Yangtze Basin. Sci Rep. 2015;5:11941. doi: 10.1038/srep11941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raymond PA, Oh N-H, Turner RE, Broussard W. Anthropogenically enhanced fluxes of water and carbon from the Mississippi River. Nature. 2008;451:449–452. doi: 10.1038/nature06505. [DOI] [PubMed] [Google Scholar]
- 14.Kunz JL, et al. Use of reconstituted waters to evaluate effects of elevated major ions associated with mountaintop coal mining on freshwater invertebrates. Environ Toxicol Chem. 2013;32:2826–2835. doi: 10.1002/etc.2391. [DOI] [PubMed] [Google Scholar]
- 15.Hintz WD, Relyea RA. Impacts of road deicing salts on the early-life growth and development of a stream salmonid: Salt type matters. Environ Pollut. 2017;223:409–415. doi: 10.1016/j.envpol.2017.01.040. [DOI] [PubMed] [Google Scholar]
- 16.Kempe S, Kazmierczak J. Biogenesis and early life on Earth and Europa: Favored by an alkaline ocean? Astrobiology. 2002;2:123–130. doi: 10.1089/153110702753621394. [DOI] [PubMed] [Google Scholar]
- 17.Likens GE, Driscoll CT, Buso DC. Long-term effects of acid rain: Response and recovery of a forest ecosystem. Science. 1996;272:244–246. [Google Scholar]
- 18.Meybeck M. Global chemical-weathering of surficial rocks estimated from river dissolved loads. Am J Sci. 1987;287:401–428. [Google Scholar]
- 19.Cañedo-Argüelles M, et al. Effects of potash mining on river ecosystems: An experimental study. Environ Pollut. 2017;224:759–770. doi: 10.1016/j.envpol.2016.12.072. [DOI] [PubMed] [Google Scholar]
- 20.Aquilina L, et al. Long-term effects of high nitrogen loads on cation and carbon riverine export in agricultural catchments. Environ Sci Technol. 2012;46:9447–9455. doi: 10.1021/es301715t. [DOI] [PubMed] [Google Scholar]
- 21.Palmer MA, et al. Science and regulation. Mountaintop mining consequences. Science. 2010;327:148–149. doi: 10.1126/science.1180543. [DOI] [PubMed] [Google Scholar]
- 22.Kaushal SS, McDowell WH, Wollheim WM. Tracking evolution of urban biogeochemical cycles: Past, present, and future. Biogeochemistry. 2014;121:1–21. [Google Scholar]
- 23.Kaushal SS, et al. Human-accelerated weathering increases salinization, major ions, and alkalinization in fresh water across land use. Appl Geochem. 2017;83:121–135. doi: 10.1016/j.apgeochem.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raymond PA, Oh N-H. Long term changes of chemical weathering products in rivers heavily impacted from acid mine drainage: Insights on the impact of coal mining on regional and global carbon and sulfur budgets. Earth Planet Sci Lett. 2009;284:50–56. [Google Scholar]
- 25.Lindberg TT, et al. Cumulative impacts of mountaintop mining on an Appalachian watershed. Proc Natl Acad Sci USA. 2011;108:20929–20934. doi: 10.1073/pnas.1112381108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKnight DM, Bencala KE. The chemistry of iron, aluminum, and dissolved organic material in three acidic, metal-enriched, mountain streams, as controlled by watershed and in-stream processes. Water Resour Res. 1990;26:3087–3100. [Google Scholar]
- 27.Steele MK, Aitkenhead-Peterson JA. Long-term sodium and chloride surface water exports from the Dallas/Fort Worth region. Sci Total Environ. 2011;409:3021–3032. doi: 10.1016/j.scitotenv.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 28.Jeppesen E, et al. Ecological impacts of global warming and water abstraction on lakes and reservoirs due to changes in water level and related changes in salinity. Hydrobiologia. 2015;750:201–227. [Google Scholar]
- 29.Mahmuduzzaman M, Ahmed ZU, Nuruzzaman AKM, Ahmed FRS. Causes of salinity intrusion in coastal belt of Bangladesh. Int J Plant Res. 2014;4:8–13. [Google Scholar]
- 30.Cormier SM, Suter GW, 2nd, Zheng L, Pond GJ. Assessing causation of the extirpation of stream macroinvertebrates by a mixture of ions. Environ Toxicol Chem. 2013;32:277–287. doi: 10.1002/etc.2059. [DOI] [PubMed] [Google Scholar]
- 31.Stets EG, Kelly VJ, Crawford CG. Long-term trends in alkalinity in large rivers of the conterminous US in relation to acidification, agriculture, and hydrologic modification. Sci Total Environ. 2014;488-489:280–289. doi: 10.1016/j.scitotenv.2014.04.054. [DOI] [PubMed] [Google Scholar]
- 32.Mackenzie FT, Andersson AJ, Arvidson RS, Guidry MW, Lerman A. Land-sea carbon and nutrient fluxes and coastal ocean CO2 exchange and acidification: Past, present, and future. Appl Geochem. 2011;26:S298–S302. [Google Scholar]
- 33.Pfister CA, et al. Detecting the unexpected: A research framework for ocean acidification. Environ Sci Technol. 2014;48:9982–9994. doi: 10.1021/es501936p. [DOI] [PubMed] [Google Scholar]
- 34.Corsi SR, Graczyk DJ, Geis SW, Booth NL, Richards KD. A fresh look at road salt: Aquatic toxicity and water-quality impacts on local, regional, and national scales. Environ Sci Technol. 2010;44:7376–7382. doi: 10.1021/es101333u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anning DW, Flynn ME. Dissolved-solids sources, loads, yields, and concentrations in streams of the conterminous United States. US Geol Surv Sci Investig Rep. 2014 doi: 10.3133/sir20145012. [DOI] [Google Scholar]
- 36.Gibbs RJ. Mechanisms controlling world water chemistry. Science. 1970;170:1088–1090. doi: 10.1126/science.170.3962.1088. [DOI] [PubMed] [Google Scholar]
- 37.Thornton GJP, Dise NB. The influence of catchment characteristics, agricultural activities and atmospheric deposition on the chemistry of small streams in the English Lake District. Sci Total Environ. 1998;216:63–75. [Google Scholar]
- 38.Likens GE. Biogeochemistry of a Forested Ecosystem. 3rd Ed Springer; New York: 2013. [Google Scholar]
- 39.Dailey KR, Welch KA, Lyons WB. Evaluating the influence of road salt on water quality of Ohio rivers overt time. Appl Geochem. 2014;47:25–35. [Google Scholar]
- 40.Tripler CE, Kaushal SS, Likens GE, Walter MT. Patterns in potassium dynamics in forest ecosystems. Ecol Lett. 2006;9:451–466. doi: 10.1111/j.1461-0248.2006.00891.x. [DOI] [PubMed] [Google Scholar]
- 41.Rosfjord CH, et al. Anthropogenically driven changes in chloride complicate interpretation of base cation trends in lakes recovering from acidic deposition. Environ Sci Technol. 2007;41:7688–7693. doi: 10.1021/es062334f. [DOI] [PubMed] [Google Scholar]
- 42.Fortner SK, et al. Silicate weathering and CO2 consumption within agricultural landscapes, the Ohio-Tennessee River Basin, USA. Biogeosciences. 2012;9:941–955. [Google Scholar]
- 43.Lettenmaier DP, Hooper ER, Wagoner C, Faris KB. Trends in stream quality in the continental United States, 1978-1987. Water Resour Res. 1991;27:327–339. [Google Scholar]
- 44.Miller MP, Buto SG, Lambert PM, Rumsey CA. 2017. Enhanced and updated spatially referenced statistical assessment of dissolved-solids load sources and transport in streams of the Upper Colorado River Basin (USGS, Reston, VA), Scientific Investigations Report 2017-5009.
- 45.Anning DW. Modeled sources, transport, and accumulation of dissolved solids in water resources of the Southwestern United States. J Am Water Resour Assoc. 2011;47:1087–1109. doi: 10.1111/j.1752-1688.2011.00579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Passell HD, Dahm CN, Bedrick EJ. Hydrological and geochemical trends and patterns in the Upper Rio Grande, 1975 to 1999. JAWRA. 2004;40:111–127. [Google Scholar]
- 47.Passell HD, Dahm CN, Bedrick EJ. Nutrient and organic carbon trends and patterns in the upper Rio Grande, 1975-1999. Sci Total Environ. 2005;345:239–260. doi: 10.1016/j.scitotenv.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 48.Kaushal SS, Lewis WM, Jr, McCutchan JH., Jr Land use change and nitrogen enrichment of a Rocky Mountain watershed. Ecol Appl. 2006;16:299–312. doi: 10.1890/05-0134. [DOI] [PubMed] [Google Scholar]
- 49.O’Brien WJ, Noyelles F. Photosynthetically elevated pH as a factor in zooplankton mortality in nutrient enriched ponds. Ecology. 1972;53:605–614. [Google Scholar]
- 50.Kilham P. Acid precipitation–Its role in the alkalinization of a lake in Michigan. Limnol Oceanogr. 1982;27:856–867. [Google Scholar]
- 51.Johnson NM. Acid rain: Neutralization within the Hubbard Brook ecosystem and regional implications. Science. 1979;204:497–499. doi: 10.1126/science.204.4392.497. [DOI] [PubMed] [Google Scholar]
- 52.Ostendorf DW, Xing B, Kallergis N. Cation exchange in a glacial till drumlin at a road salt storage facility. J Contam Hydrol. 2009;106:118–130. doi: 10.1016/j.jconhyd.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 53.Shanley JB. Effects of ion-exchange on stream solute fluxes in a basin receiving highway deicing salts. J Environ Qual. 1994;23:977–986. doi: 10.2134/jeq1994.00472425002300050019x. [DOI] [PubMed] [Google Scholar]
- 54.Whitmore TJ, et al. Inadvertent alkalinization of a Florida lake caused by increased ionic and nutrient loading to its watershed. J Paleolimnol. 2006;36:353–370. [Google Scholar]
- 55.Muller JD, Schneider B, Rehder G. Long-term alkalinity trends in the Baltic Sea and their implications for CO2 induced acidification. Limnol Oceanogr. 2016;61:1984–2002. [Google Scholar]
- 56.Prasad MBK, Kaushal SS, Murtugudde R. Long-term pCO2 dynamics in rivers in the Chesapeake Bay watershed. Appl Geochem. 2013;31:209–215. [Google Scholar]
- 57.Natesan S, Strayer DL. Long-term increases in shell thickness of zebra mussels (Dreissena polymorpha) in the Hudson River. Fundam Appl Limnol. 2016;188:245–248. [Google Scholar]
- 58.Chiarenzelli J, Lock R, Cady C, Bregani A, Whitney B. Variation in river multi-element chemistry related to bedrock buffering: An example from the Adirondack region of northern New York, USA. Environ Earth Sci. 2012;67:189–204. [Google Scholar]
- 59.Zampella RA, Procopio NA, Lathrop RG, Dow CL. Relationship of land-use/land-cover patterns and surface-water quality in the Mullica River Basin. J Am Water Resour Assoc. 2007;43:594–604. [Google Scholar]
- 60.Boyd CE, Tucker CS, Somridhivej B. Alkalinity and hardness: Critical but elusive concepts in aquaculture. J World Aquacult Soc. 2016;47:6–41. [Google Scholar]
- 61.Lofgren S. The chemical effects of deicing salt on soil and stream water of five catchments in southeast Sweden. Water Air Soil Pollut. 2001;130:863–868. [Google Scholar]
- 62.Rosenberry DO, et al. Movement of road salt to a small New Hampshire lake. Water Air Soil Pollut. 1999;109:179–206. [Google Scholar]
- 63.Duan S, Kaushal SS. Salinization alters fluxes of bioreactive elements from stream ecosystems across land use. Biogeosciences. 2015;12:7331–7347. [Google Scholar]
- 64.Steele MK, Aitkenhead-Peterson JA. Salt impacts on organic carbon and nitrogen leaching from senesced vegetation. Biogeochemistry. 2013;112:245–259. [Google Scholar]
- 65.Gabor RS, et al. Influence of leaching solution and catchment location on the fluorescence of water-soluble organic matter. Environ Sci Technol. 2015;49:4425–4432. doi: 10.1021/es504881t. [DOI] [PubMed] [Google Scholar]
- 66.Kaushal SS, Likens GE, Jaworski N, Pace ML. Rising stream and river temperatures in the United States. Front Ecol Environ. 2010;8:461–466. [Google Scholar]
- 67.Raymond PA, et al. Global carbon dioxide emissions from inland waters. Nature. 2013;503:355–359. doi: 10.1038/nature12760. [DOI] [PubMed] [Google Scholar]
- 68.Stets EG, Lee CJ, Lytle DA, Schock MR. Increasing chloride in rivers of the conterminous U.S. and linkages to potential corrosivity and lead action level exceedances in drinking water. Sci Total Environ. 2017 doi: 10.1016/j.scitotenv.2017.07.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koch GH, Brongers MH, Thompson MG, Virmani YP, Payer JH. 2002. Corrosion cost and preventative strategies in the United States (Federal Highway Administration, Washington, DC), Report FHWA-RD-01-156.
- 70.US Geological Survey 2013 National hydrography geodatabase: The national map viewer available on the World Wide Web. Available at https://nhd.usgs.gov/NHD_High_Resolution.html. Accessed December 1, 2016.
- 71.Siegel AF. Robust regression using repeated medians. Biometrika. 1982;69:242–244. [Google Scholar]
- 72.Oliver MA. Kriging: A method of interpolation for geographical information systems. Int J Geogr Inf Syst. 1990;4:313–332. [Google Scholar]
- 73.Drapek RJ, Kim JB, Neilson RP. 2015. The dynamic general vegetation model MC1 over the United States and Canada at a 5-arc-minute resolution: Model inputs and outputs (US Department of Agriculture Forest Service Pacific Northwest Research Station, Portland, OR), General Technical Report PNW-GTR-904.
- 74.Daly C, et al. Physiographically-sensitive mapping of temperature and precipitation across the conterminous United States. Int J Climatol. 2008;28:2031–2064. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.