Significance
Chemical cues are essential to marine life, particularly for detecting predators. Despite decades of research, almost nothing is known of the molecular nature of these waterborne cues. This prevents us from assessing environmental variation and impacts of these cues and from understanding and manipulating predator–prey signaling pathways. Leveraging natural chemical variation in the urine of a predatory crab using metabolomics, the chemical profiles of urine from crabs fed different diets were revealed to be predictive of their fear-inducing potency. This pattern led us to identify the major constituents of the chemical cue used by mud crab prey to detect and avoid their predator. This investigation serves as a blueprint for investigating the molecular nature of these community-structuring waterborne cues.
Keywords: blue crab, chemical ecology, metabolomics, nonconsumptive effects, predation
Abstract
An effective strategy for prey to survive in habitats rich in predators is to avoid being noticed. Thus, prey are under selection pressure to recognize predators and adjust their behavior, which can impact numerous community-wide interactions. Many animals in murky and turbulent aquatic environments rely on waterborne chemical cues. Previous research showed that the mud crab, Panopeus herbstii, recognizes the predatory blue crab, Callinectus sapidus, via a cue in blue crab urine. This cue is strongest if blue crabs recently preyed upon mud crabs. Subsequently, mud crabs suppress their foraging activity, reducing predation by blue crabs. Using NMR spectroscopy- and mass spectrometry-based metabolomics, chemical variation in urine from blue crabs fed different diets was related to prey behavior. We identified the urinary metabolites trigonelline and homarine as components of the cue that mud crabs use to detect blue crabs, with concentrations of each metabolite dependent on the blue crab’s diet. At concentrations found naturally in blue crab urine, trigonelline and homarine, alone as well as in a mixture, alerted mud crabs to the presence of blue crabs, leading to decreased foraging by mud crabs. Risk perception by waterborne cues has been widely observed by ecologists, but the molecular nature of these cues has not been previously identified. Metabolomics provides an opportunity to study waterborne cues where other approaches have historically failed, advancing our understanding of the chemical nature of a wide range of ecological interactions.
Gathering and interpreting information from the environment is imperative to organisms’ ability to recognize food, mates, predators, and appropriate habitat. Many aquatic species rely on chemical cues in environments where auditory, visual, and mechanosensory mechanisms are often compromised (1–3). Significant efforts have been made to understand chemical defenses and feeding deterrents (4–7) as many have potential medicinal applications (8–10); however, waterborne cues remain almost completely unidentified.
The ability to sense and recognize predators remotely is particularly important for organisms because it allows for the production of behavioral, morphological, or life historical adjustments to minimize predation (11). Although significant evidence exists for widespread chemical detection of predators and alarm cues among conspecifics in the marine environment (12–14), little is known about the molecular nature of the cues involved. These cues are of particular importance as they routinely produce ecologically significant nonconsumptive effects, that is, altered species interactions beyond the effects of lost prey by cause of consumption due to changes in the morphology, behavior, or life history of prey (15). Nonconsumptive effects have been demonstrated in aquatic (16–18) and terrestrial environments (19) and are suggested to have even greater capacity to structure communities than the direct effects of consuming prey (15). Due to the lack of a molecular understanding of these cue systems, little is known about how cues disperse in aquatic systems, the receptors that mediate chemoreception and recognition of these cues, and the specificity to these cues, necessitating further research.
One explanation for the lack of characterized waterborne cues is that purification methodologies for highly water-soluble compounds have been unsatisfactory, especially when working with seawater samples that contain substantial quantities of inorganic salts. Standard sampling methods for airborne chemical cues such as headspace analysis for volatile insect pheromones (20) are unavailable for nonvolatile waterborne cues. Many waterborne cues are present in very low concentrations, unstable to handling in the laboratory, and produced by organisms that either are not abundant or not readily cultivated in the laboratory. It may be difficult to identify in which tissues of an organism these cues are produced or stored, and when this is possible the signaling molecules may occur as part of a complex mixture with many other, irrelevant metabolites.
Traditionally, chemists have applied a process of bioassay-guided fractionation to purify and then characterize biologically active compounds. However, this multistep approach often leads to decomposition of labile cues and exclusion of multicomponent cues, while simultaneously requiring substantial quantities of the chemical cue mixture for biological testing after each chemical separation step (21). Recent advances in NMR spectroscopy and MS metabolomics allow for fast, efficient, and cost-effective profiling of complex mixtures containing waterborne cues, whose chemical variation can be leveraged to correlate the presence or abundance of particular compounds within the mixture with biological potency of the mixture. Recently, a novel microalgal mate attraction pheromone was identified using MS-based metabolomics despite pheromone concentrations in the nanomolar range (22).
Previous research showed that the mud crab, Panopeus herbstii, detects its predator, the blue crab, Callinectus sapidus, using unknown metabolites released in the urine of blue crabs (23). Mud crabs reacted differently to urine from blue crabs fed mud crabs vs. other prey. Using NMR- and MS-based metabolomics we aimed to leverage the chemical variation in urine from blue crabs fed different diets to identify the component(s) of the cue mixture that mud crabs use to recognize blue crabs. This study provides a roadmap to identify complex waterborne cues that can be used to further our understanding of chemically mediated interactions in the marine environment.
Results
Mud Crabs Perceive Risk via Concentration Differences in Predator Urinary Metabolites.
When we employed 1H NMR-based metabolomics comparing the chemical profiles of urine from blue crabs fed mud crabs vs. oysters, principal component analysis (PCA) revealed a single principal component accounting for 19.5% of the total variation among urine samples which differentiated urine of blue crabs fed the two diets (Fig. 1A). An orthogonalized partial least squares discriminant analysis (oPLS-DA) model also differentiated urine from blue crabs fed these two diets; however, the first latent variable only accounted for 10.4% of the total chemical variation (SI Appendix, Fig. S1). A complementary MS-based PCA model similarly distinguished urine from blue crabs fed different diets using a single principal component, accounting for 89.3% of the total variation among samples (Fig. 1B).
Fig. 1.
Blue crab diet affects urine metabolite profile. PCA of metabolomics data from (A) 1H NMR spectra and (B) MS differentiate urine of blue crabs fed mud crabs and from urine of blue crabs fed oysters along the first principal component (variance captured along PC1 is stated in parentheses; P = 0.0074 for NMR, P = 0.0003 for MS). Crab symbols represent data from urine from blue crabs fed mud crabs (n = 9 for NMR, n = 3 for MS); oyster symbols represent data from urine from blue crabs fed oysters (n = 10 for NMR, n = 3 for MS).
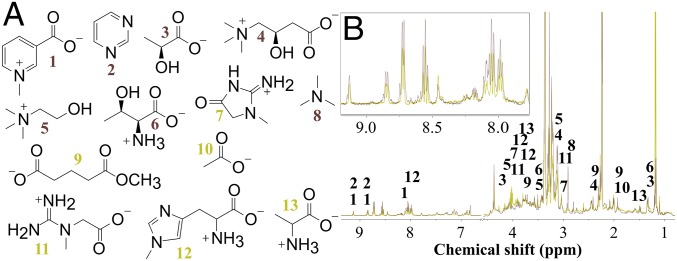
Analysis of the NMR-based PCA loadings (SI Appendix, Fig. S2) which highlighted spectral features that were more abundant in urine from blue crabs fed one diet vs. the other, along with 2D NMR spectroscopic data of blue crab urine (SI Appendix, Figs. S3–S5 and Table S1) which determined the connectivity of atoms within each molecule, led to the putative identification of a subset of urinary metabolites, 13 in total, that we hypothesized mud crabs use to differentiate among predators that impose different degrees of risk (Table 1). Mass spectrometric data confirmed these identifications (SI Appendix, Fig. S6 and Table S1). Thus, whereas individual metabolites pinpointed via PCA were not purified from blue crab urine, their structures were confidently assigned, as components of a complex urine matrix, by a combination of NMR and MS approaches. Analysis of the MS-based PCA model highlighted 28 unique metabolites of the 661 total detected metabolites (SI Appendix, Tables S3–S5) whose concentrations were enhanced in the urine of blue crabs fed mud crabs, which act as a discriminating panel to differentiate between urine from blue crabs fed different diets. Carnitine (4) was identified through both NMR- and MS-based metabolomics analyses as more abundant when blue crabs ate mud crabs, but most relevant molecules were revealed through statistical modeling by only one of the approaches, highlighting the complementary nature of the two techniques. Additionally, acetylcholine and propionylcarnitine were identified via MS profiling while choline and carnitine were identified via NMR spectroscopic profiling, further accentuating useful complementarity of the two spectroscopic techniques.
Table 1.
Metabolites identified from blue crab urine by NMR spectroscopy-based PCA model (n = 9–10 urine samples for each diet)
| Metabolite | Concentration in urine of blue crabs fed mud crabs | |
| Absolute, µM | Relative to oyster diet | |
| Trigonelline (1) | 90 | 1.8× |
| Pyrimidine (2) | 72 | 1.6× |
| Lactate (3) | 250 | 1.3× |
| Carnitine (4) | 160 | 1.2× |
| Choline (5) | 110 | 1.2× |
| Threonine (6) | 160 | 1.1× |
| Creatinine (7) | 62 | 0.72× |
| Trimethylamine (8) | —* | >1.0× |
| Methyl glutarate (9) | —* | <1.0× |
| Acetate (10) | —* | <1.0× |
| Creatine (11) | —* | <1.0× |
| N-methylhistidine (12) | 99 | 0.75× |
| Alanine (13) | 180 | 0.50× |
Concentration could not be determined due to NMR spectral overlap of diagnostic protons.
Different diets did not lead to different identities of metabolites in blue crab urine relevant to prey behavior but rather resulted in altered concentrations of the same metabolites perceived by prey via exposure to urine (Fig. 2). Variable concentrations of individual metabolites were evident from the differential integration of common 1H NMR spectroscopic features (Fig. 2B, Inset). These metabolites included those involved in amino acid metabolism, niacin metabolism, energy metabolism, energy shuttling, and choline metabolism (24) (Table 1 and SI Appendix, Table S1). Collectively, these data predicted that the fear-inducing cue in blue crab urine consists of relatively simple, primary metabolites that vary in concentration depending on diet.
Fig. 2.
Blue crab diet affects concentration of specific urinary metabolites. (A) Compounds identified by 1H NMR metabolomics PCA model that distinguish urine of blue crabs fed mud crab vs. oyster diets. Annotated metabolites consisted of trigonelline (1), pyrimidine (2), lactate (3), carnitine (4), choline (5), threonine (6), creatinine (7), trimethylamine (8), methyl glutarate (9), acetate (10), creatine (11), N-methylhistidine (12), and alanine (13). (B) Overlay of 1H NMR spectra of urine from blue crabs fed mud crabs (brown) or oyster (yellow) averaged across 12 urine samples for each diet. Peak intensity was normalized to an internal standard whose concentration was identical in all samples. Numbers above 1H NMR signals in B refer to numbered compounds in A. Only protons used to calculate concentration of metabolites are labeled. (Inset) An expansion of the downfield region of overlaid 1H NMR spectra.
A Subset of Metabolites Account for Fear-Inducing Properties of Blue Crab Urine.
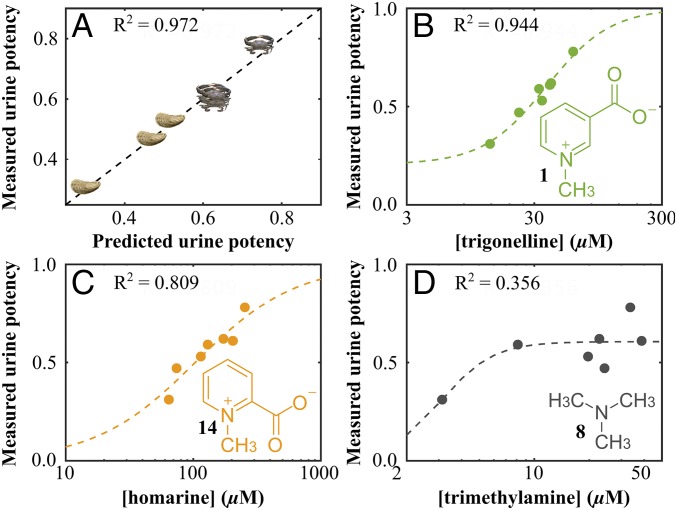
To provide further evidence of the role of metabolites found in blue crab urine we applied partial least-squares regression (PLS-R) analysis to the 1H NMR spectroscopic data to test for correlation between the measured potency of blue crab urine samples and the chemical variation among urine samples as revealed by PCA. A near-perfect linear fit was observed based on analysis of seven blue crab urine samples, four from blue crabs fed mud crabs and three from blue crabs fed oysters (Fig. 3A). Upon inspection of the variable importance parameters (VIP; SI Appendix, Fig. S7A) and the PLS-R loadings (SI Appendix, Fig. S7B), trigonelline (1) was the only urinary metabolite identified by the PLS-R model that was also a critical component of the loadings via PCA (Table 1 and SI Appendix, Fig. S7).
Fig. 3.
PLS-R model of 1H NMR metabolomics data suggests that trigonelline (1) and homarine (14), but not trimethylamine (8), are components of the fear-inducing cue of blue crab urine. (A) PLS-R model highlights the linear relationship between the measured and model-predicted potency of fear-inducing behavior of blue crab urine. A potency value of 1 would indicate complete suppression of mud crab feeding in the presence of the urine sample, whereas a potency value of zero represents no feeding suppression. Crab symbols represent data from urine from blue crabs fed mud crabs; oyster symbols represent data from urine from blue crabs fed oysters (n = 4 for urine from blue crabs fed mud crabs, n = 3 for urine from blue crabs fed oysters). Dashed lines represent sigmoidal curve fitted to experimental data using MATLAB curve-fitting toolbox with adjusted R2 values for (B) trigonelline (1), (C) homarine (14), and (D) trimethylamine (8) at the highest possible concentration (based on all protons resonating at 2.89 ppm belonging to trimethylamine and no other metabolites), as indicated by square brackets around metabolite name. n = 7 urine samples.
In addition to trigonelline’s being predicted as a constituent of the fear-inducing cue the VIP and the loadings of the PLS-R model suggested that additional unidentified metabolites were constituents of the cue mixture. Inspection of the aromatic spectral region of the PLS-R loadings (SI Appendix, Fig. S7), coupled with total correlation spectroscopy data (SI Appendix, Fig. S8), pointed to a second fear-inducing metabolite differing from trigonelline only in the pattern of substituents around the aromatic ring. Homarine (14), a constitutional isomer of trigonelline, was isolated from blue crab urine, characterized by MS and NMR spectroscopy (SI Appendix, Fig. S8), and synthesized to confirm its molecular structure.
The relationship between measured potency of blue crab urine and the measured concentrations of trigonelline and homarine indicated sigmoidal patterns for both, with greater fear-inducing potency for urine samples with higher concentrations of trigonelline and homarine, as expected (Fig. 3). In contrast, the fear-inducing potency of urine poorly correlated with the concentration of trimethylamine. Although trimethylamine was identified by the PCA model as being significantly more concentrated in urine from blue crabs fed mud crabs vs. oysters (Table 1), it did not emerge from the PLS-R model as a likely candidate for the fear-inducing cue, and as such it acted as a further calibrant for our model. Overall, these findings led us to conclude that both trigonelline and homarine are likely constituents of the fear-inducing cue in blue crab urine but that trimethylamine is not.
Trigonelline and Homarine Induce Fear in Mud Crabs.
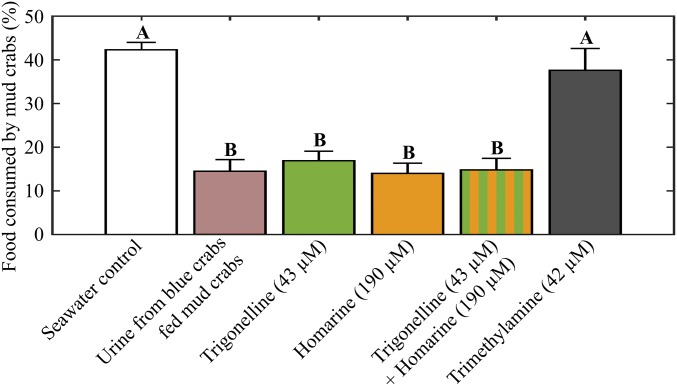
To explicitly test the functions of trigonelline and homarine as components of the fear-inducing cue in blue crab urine, mud crabs were exposed to each compound, alone and together, at concentrations found naturally in urine of blue crabs fed mud crabs (43 µM for trigonelline and 190 µM for homarine). Trigonelline (purchased commercially and analyzed spectroscopically to confirm its identity) and homarine (synthesized and characterized) suppressed foraging by 60% and 67%, respectively, when each compound was presented to mud crabs. Mud crabs reduced their feeding behavior by 65% when these two compounds were tested as a mixture, similar to the effect of blue crab urine itself (Fig. 4).
Fig. 4.
At natural concentrations found in blue crab urine, trigonelline (1) and homarine (14), but not trimethylamine (8), induce fear, evidenced by reduced foraging among mud crabs. Urinary metabolites were formulated to match concentrations observed in blue crab urine (n = 10 for all treatments). Letters indicate significant grouping of treatments (one-way ANOVA with Tukey post hoc test). Error bars represent ± 1 SEM.
In contrast, and as expected given the poor predictive relationship between trimethylamine (8) and urine (Fig. 3D), trimethylamine did not significantly affect mud crab behavior (Fig. 4). The exact concentration of trimethylamine in blue crab urine could not be determined by 1H NMR spectroscopy due to spectral overlap and the nondiagnostic nature of the only unique proton signal associated with trimethylamine. The concentration (42 µM) used in the mud crab behavior assay was the highest possible concentration of trimethylamine (if all protons resonating at 2.89 ppm belonged to trimethylamine and not to other metabolites), yet no significant behavioral response indicative of fear was observed in mud crabs exposed to trimethylamine at this high concentration (Fig. 4).
Although the PLS-R model predicted that additional unidentified blue crab urine metabolites are likely components of the cue mixture, trigonelline and homarine appear to be the major constituents of the fear-inducing cue. The suppressive effects that these compounds induced on mud crab behavior were statistically indistinguishable from the effects of blue crab urine itself (Fig. 4), revealing that other compounds were not necessary to recapitulate the activity of whole urine.
To predict the source of the components of the fear-inducing cue we quantified trigonelline, homarine, and trimethylamine in the flesh of each food source (mud crab, oyster, and shrimp; SI Appendix, Table S2). Trimethylamine and homarine were found in tissues of all three food sources, whereas trigonelline was only detected in mud crab and shrimp tissues. The concentrations of all three metabolites were higher in mud crab tissue than in other tissues, indicating that it is possible that blue crab urine derives at least a portion of these three metabolites directly from the diets of blue crabs. However, it is unlikely that all of the components of the fear-inducing cue come from blue crab food sources, since trigonelline is present the urine of blue crabs fed oysters but was not detected in oyster tissue.
Trigonelline and Homarine Are Constitutional Isomers with Different Electron Delocalization.
The two critical components of the fear-inducing cue in blue crab urine have similar biological activities and appear nearly structurally identical; however, they have different electronic structures. Although each contains a methylated pyridinium ring and a carboxylate group, their connectivity differs. Through molecular modeling, the attachment of the carboxylate to the pyridinium ring of trigonelline allows for a greater delocalization of electron density, as evidenced by increased electron density around the carboxylate (SI Appendix, Fig. S9). In contrast, homarine does not exhibit such delocalization, since its carboxylate is rotated 64° relative to the ring, preventing orbital overlap. When similar models were applied to related molecules picolinic acid (15) and o-toluic acid (16), the steric implications on torsional angle of the carboxylate relative to delocalization of electron density became apparent. For picolinic acid, the torsional angle between the carboxylate and the ring is 0.0°, allowing electron density from the aromatic system to be pulled toward the carboxylate (SI Appendix, Fig. S9). However, in o-toluic acid, the carboxylate rotates out of the plane of the ring with a torsional angle of 37° to decrease steric interactions with the bulkier methyl, preventing extended conjugation because the p orbitals of the carboxylate cannot sufficiently interact with those of the aromatic system, as evidenced by most of the electron density remaining around the aromatic system (SI Appendix, Fig. S9). Thus, connectivity of the carboxylate to the pyridinium ring at carbon 2 in homarine leads to steric interactions with the N-methyl, forcing the carboxylate to rotate out of the plane of the ring, thus preventing further electron delocalization. While not directly tested, the differences in electronic structure could lead to differential interaction with a mud crab receptor, thus possibly explaining the similar levels of potency of trigonelline and homarine at different concentrations (Fig. 4).
Discussion
We identified the two major chemical components of a waterborne cue by leveraging the variable fear-inducing potencies of urine from blue crabs fed different diets, resulting in the conclusion that trigonelline and homarine strongly affect risk perception in prey (Fig. 4). These cues, when released into seawater via the urine of blue crabs, allow mud crab prey to recognize the presence of a predator and adjust their behavior to avoid detection by that predator. The relative concentrations of these metabolites in predator urine reveals whether it has recently consumed mud crabs, which mud crabs then interpret as additional risk. Mud crabs subsequently respond with reduced foraging activity compared with when they are exposed to urine with lower concentrations of trigonelline and homarine (Fig. 3). Although trigonelline and homarine, when tested alone or in combination, induced fear in prey crabs similarly to intact blue crab urine (Fig. 4), these two metabolites are not the only components of the fear-inducing cue. Both 1H NMR and MS metabolomics suggested multiple additional components. However, the lack of diagnostic protons with VIP scores greater than those of trigonelline and homarine (SI Appendix, Fig. S7A) and the stand-alone potencies of trigonelline and homarine (Fig. 4) indicate that while additional metabolites are predicted to act as components of the fear-inducing cue their importance is limited.
Waterborne Cues for Predator Detection Have Been Infrequently Characterized.
The current system represents one of the few waterborne cues that has been chemically identified, among those used for predator detection and risk perception by prey. However, there is significant evidence that such cues are sensed widely in invertebrates (25, 26) and vertebrates (27, 28). More importantly, the current study is one of the first in which nonconsumptive effects of predators, mediated by waterborne predator chemistry, have been revealed at the molecular level. Nonconsumptive effects on prey are increasingly predicted to be more important than consumptive effects (removal of prey through consumption) in systems with a top predator, intermediate predator, and basal resource, such as the current system (15). The potential for these interactions to broadly structure communities emphasizes the need to understand the chemical nature of the cues, which would provide insight into what confers cue specificity, and therefore how organisms may respond in the presence of many cues with different degrees of salience or risk. Since prey in natural communities will be simultaneously exposed to many different cues, developing a framework to predict and understand the effects of multiple cues is essential if we are to progress beyond understanding prey response in single predator–prey pairs.
A rare case of chemically mediated nonconsumptive effect in which the chemical cue has been identified involves the copepodamides, a class of taurine-containing polar lipids released into seawater by copepods, leading to an increase in toxicity in their dinoflagellate prey (29). A second example is that of the use of aliphatic sulfates and sulfamates by the freshwater green alga Scenedesmus to detect predatory Daphnia pulex, inducing colony formation in Scenedesmus as a defense mechanism (30). A significant contrast between these two identified cues is that the compounds involved, like many terrestrial cues, appear to be more specific to certain taxonomic groups than the fear-inducing metabolites identified in the current study, trigonelline and homarine, yet many may be waste products. The unavoidable exudation of waste compounds by predators may therefore be important in many systems enabling prey to recognize predators, sometimes in species-specific or even diet-specific manners.
Trigonelline and Homarine Are Common Invertebrate Signaling Molecules.
The two critical components of the fear-inducing cue in blue crab urine, trigonelline and homarine, are well documented in marine invertebrate tissues (31–37). Homarine and trigonelline, present in marine hydroid oocytes at ∼25 nM and 8 nM, respectively, prevent the metamorphosis of larvae to adults as well as limiting formation of the head and stolon in adults (38, 39). Homarine defends an Antarctic gastropod against its sea star predator, which flees from its prey when exposed to high concentrations of the compound (40). Homarine in gorgonian corals repels fouling by a surface-associated diatom (41). When nicotinic acid (the demethylated analog of homarine), picolinic acid (the demethylated analog of trigonelline), and pyridine (neither methylated nor possessing a carboxyl side chain as do trigonelline or homarine) were tested for their antifouling properties the methylation state of the compound was found to be unimportant, whereas the presence of the carboxyl group at position two of the aromatic ring (as found in homarine) was crucial for the antifouling nature of the compounds (41). These last findings run counter to those of the current study in which trigonelline appears to be slightly more potent than homarine based on the similar fear-inducing effects at lower concentration (Fig. 4). This suggests different molecular mechanisms of action for these molecules when involved in fear induction in prey compared with fouling deterrence. Additionally, trigonelline and homarine were found to act as waterborne cues in the current study whereas previously reported systems considered the roles of trigonelline and homarine as components in animal tissue, further contrasting the systems.
Trigonelline and Homarine Appear Structurally Similar but Differ in Biosynthetic Origins.
The multiple biological functions of trigonelline and homarine argue for seeking a better understanding of their biosynthetic origins. Although trigonelline and homarine appear to have very similar molecular structures, their biosynthetic origins differ in organisms for which this has been studied. Homarine biosynthesis in marine invertebrates is predominately known from isotope incorporation experiments of shrimp in which glycine and succinyl CoA form N-succinylglycine which, through a series of modifications, is transformed to homarine (42, 43). Tryptophan was not incorporated into homarine in feeding experiments and as such a hypothesized route to homarine through that amino acid was rejected (42); acetate was incorporated to form homarine through quinolinate (44). Although no study has investigated trigonelline biosynthesis in marine invertebrates, it is known that plants produce trigonelline from tryptophan and aspartate via quinolinic acid, which is converted to nicotinic acid and methylated to form trigonelline (45). Despite the incorporation of quinolinic acid into both trigonelline and homarine, the lack of tryptophan incorporation in homarine synthesis suggests that either trigonelline and homarine are derived from divergent biosynthetic pathways in invertebrates or that invertebrates utilize multiple pathways toward the formation of quinolinic acid.
Metabolomics as a Promising Tool for Chemical Ecology.
For decades, the principal platform for isolating biologically active compounds in chemical ecology research has been bioassay-guided fractionation. This approach has failed to facilitate the discovery of waterborne cues, despite the obvious importance of such cues in aquatic systems, due in part to the lack of sensitivity and resolution in analytical technologies used to elucidate the molecular structures of compounds accessible only in low quantities. In the current study we avoided working with a low-concentration cue by collecting the cue at its source (urine collection via catheterization) rather than concentrating compounds from seawater after release and dispersal of the cue. Additionally, bioassay-guided fractionation requires a destructive bioassay after each separation step to pinpoint those fractions containing bioactive molecules to proceed to the next step of chemical separation. These separations can lead to loss of biological activity as multicomponent cues are separated into multiple fractions and as protectant molecules, such as antioxidants, are removed from mixtures containing bioactive molecules and degradation processes take over. With increasing NMR sensitivity, MS resolving power, and big data analysis tools, metabolomics has become a useful approach for chemical ecology and natural product discovery (46, 47). The complementary nature of MS metabolomics, which is highly sensitive and allows simultaneous analysis of many compounds, and NMR metabolomics, which is quantitative and can detect compounds with poor MS ionization properties, makes the combination of these two spectroscopic approaches desirable when the identities of relevant molecules are completely unknown. The advantages of metabolomics for identifying waterborne cues will continue to increase as chemical libraries become more accessible and profiling technologies become more widespread.
Conclusions
A combined NMR-MS metabolomics approach led to the successful characterization of chemical cues in a complex behavioral interaction between predator and prey, in which prey recognize and respond to predators via exuded metabolites. By identifying the major components of this fear-inducing cue we can now better address important questions about the impacts of nonconsumptive effects by predators on prey, the ecological importance of these interactions on community structure, and the possible ubiquity of chemically mediated nonconsumptive interactions in the marine environment. More generally, we posit that this study serves as a roadmap for further studies aimed at identifying and understanding the many diverse systems that ecologists hypothesize are mediated by waterborne cues but in which the chemistry is yet unknown. Despite noted examples where metabolomics has previously identified important cues, the cues were either not fully characterized, were terrestrial in origin, or were highly specific pheromones instead of more ubiquitous cues such as in the current study. Only with a more thorough understanding of the chemistry of these systems can we begin to address other important aspects, such as cue dispersal, dose–response relationships, structure–activity relationships, chemoreception and signal transduction, potential effects of climate change on cue longevity in the water column, and potential effects of anthropogenic pollutants in the water.
Ecologists have been long interested in the role of chemical compounds in mediating interactions among species. The natural variation inherent in these systems has often been seen as an inconvenience to be minimized or overcome toward defining the function of individual compounds. This approach misses the opportunity to leverage naturally occurring variation—whether it be in the behavior or chemistry of an organism, or both—toward understanding how chemistry shapes biology. Wherever a correlation between chemical composition and a biological outcome can be gleaned, hypotheses can be constructed to explicitly test the function of such chemistry. In the current study, the chemical fingerprint of blue crab urine was found to be diverse, both in terms of the identity of metabolites and their relative concentrations. Rather than ignoring or minimizing this variation (for example, by pooling urine samples into one large batch) we capitalized on this variation. The fact that urine chemistry could be manipulated by adjusting diet, with concomitant change to the potency of urine in inducing fear among prey, provided the opportunity to determine which urinary metabolites caused fear. The dose dependency of individual metabolites, acting together to enable prey to assess relative risk, confirms that such environmental cues do not act as on/off switches but rather produce a subtle range of biological outcomes predictable by compound concentration gradients. This is the great power of metabolomics in chemical ecology: to go beyond a false determination of “yes” (the organism responds) or “no” (the organism does not respond), toward making the most of existing variation and generating a deeper understanding of these responses at the chemical and biological level.
Materials and Methods
Urine Collection.
Urine was collected from blue crabs via catheter twice weekly, by gentle suction (<35 kPa) via a 22-gauge needle inserted into each nephropore of a blue crab. Urine contaminated with hemolymph or particulate matter was rejected. Urine from blue crabs of each diet treatment was collected approximately every other day, pooled, filtered by 0.22-µm Teflon syringe filter, and stored at −20 °C at Georgia Institute of Technology (GT). A total of 12 unique urine samples (each from two to six different crabs) per diet treatment were collected. Urine collected at Skidaway Institute of Oceanography was transported to GT frozen and stored at −20 °C. Urine was either assayed or profiled within 2 wk of collection.
Behavioral Assay.
The mud crab behavioral assay was performed following the previously reported protocol (23). In short, four starved mud crabs were placed in 20-L aquaria with 2.0 L of artificial seawater of salinity (25–30 ppt; see SI Appendix, SI Materials and Methods for animal maintenance and animal selection for bioassay). Crabs acclimated to their enclosure for 2 h, after which raw shrimp (3.8–4.2 g) cut into 6–10 pieces was added along with urine, cue solution, or artificial seawater as control and the water was agitated to ensure mixing (see SI Appendix, SI Materials and Methods for bioassay treatments). Mud crabs were allowed to eat undisturbed for 4 h, after which the remaining shrimp was collected and weighed to determine mass loss due to consumption. The amount of food consumed as a proportion of the total provided was analyzed using one-way analysis of variance with a Tukey post hoc test using PRISM version 10.0.
Urine Profiling, Metabolite Analysis, and Annotation.
Urine samples from blue crabs fed both diets were prepared and profiled using 1H NMR spectroscopy and ultrahigh-performance liquid chromatography-MS (see SI Appendix, SI Materials and Methods for sample preparation, procedures, and analytical method parameters). Spectral data (NMR and MS) were preprocessed before the generation of PCA and oPLS-DA models to investigate differences in urine metabolomes (MATLAB, version 8.1.0.604, with PLS_Toolbox, version 7.9.1; Eigenvector Research). Metabolites with discriminatory power were annotated using the Chenomx Profiler, ChemSpider, the Human Metabolome Database, KEGG database, and MzCloud. Representative urine samples were used to collect 2D NMR spectral data to aid in annotation. A PLS-R model was used to relate urine potency and chemical differences of urine from blue crabs fed different diets to identify possible cue components.
Identification of Blue Crab Urine Cue Constituents.
Trigonelline (1) and trimethylamine (8) were purchased commercially, whereas homarine (14) was isolated from blue crab urine as well as synthesized (SI Appendix, SI Materials and Methods). All three compounds—commercial (1) and (8) and synthesized (14)—were tested alone and in combination in the behavioral assay to assess their roles in the fear-inducing cue. A one-way ANOVA with Tukey post hoc test was run to test statistical significance of foraging suppression.
Supplementary Material
Acknowledgments
We thank L. Connolly, N. Ochs, M. Phillips, L. Krause, K. Syhapanha, K. Martin, J. Pruett, J. Beauvais, D. Brumley, Dr. S. Mascuch, A. Draper, F. Nagau-Lavoie, and C. Poulin for animal collection and husbandry; Drs. L. Gelbaum and J. Leisen for assistance with NMR spectroscopy; Dr. V. Agarwal for use of LC/MS; and D. Bostwick for assistance with MS data collection and analysis for characterization of metabolites. This work was supported by NSF Grant OCE-1234449.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Metabolomic data for this project are publicly available on the Biological and Chemical Oceanography Data Management Office database (https://www.bco-dmo.org/project/565703).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1713901115/-/DCSupplemental.
References
- 1.Dobretsov S, Teplitski M, Paul V. Mini-review: Quorum sensing in the marine environment and its relationship to biofouling. Biofouling. 2009;25:413–427. doi: 10.1080/08927010902853516. [DOI] [PubMed] [Google Scholar]
- 2.Kats LB, Dill LM. The scent of death: Chemosensory assessment of predation risk by prey animals. Ecoscience. 1998;5:361–394. [Google Scholar]
- 3.Zimmer RK, Butman CA. Chemical signaling processes in the marine environment. Biol Bull. 2000;198:168–187. doi: 10.2307/1542522. [DOI] [PubMed] [Google Scholar]
- 4.Blunt JW, Copp BR, Keyzers RA, Munro MHG, Prinsep MR. Marine natural products. Nat Prod Rep. 2017;34:235–294. doi: 10.1039/c6np00124f. [DOI] [PubMed] [Google Scholar]
- 5.Hay ME, Fenical W. Marine plant-herbivore interactions: The ecology of chemical defense. Annu Rev Ecol Syst. 1988;19:111–145. [Google Scholar]
- 6.Puglisi MP, Sneed JM, Sharp KH, Ritson-Williams R, Paul VJ. Marine chemical ecology in benthic environments. Nat Prod Rep. 2014;31:1510–1553. doi: 10.1039/c4np00017j. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz ER, Poulin RX, Mojib N, Kubanek J. Chemical ecology of marine plankton. Nat Prod Rep. 2016;33:843–860. doi: 10.1039/c6np00015k. [DOI] [PubMed] [Google Scholar]
- 8.Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 9.Harvey AL, Edrada-Ebel R, Quinn RJ. The re-emergence of natural products for drug discovery in the genomics era. Nat Rev Drug Discov. 2015;14:111–129. doi: 10.1038/nrd4510. [DOI] [PubMed] [Google Scholar]
- 10.Lane AL, et al. Desorption electrospray ionization mass spectrometry reveals surface-mediated antifungal chemical defense of a tropical seaweed. Proc Natl Acad Sci USA. 2009;106:7314–7319. doi: 10.1073/pnas.0812020106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lima SL. Nonlethal effects in the ecology of predator-prey interactions. Bioscience. 1998;48:25–34. [Google Scholar]
- 12.Bjærke O, Andersen T, Titelman J. Predator chemical cues increase growth and alter development in nauplii of a marine copepod. Mar Ecol Prog Ser. 2014;510:15–24. [Google Scholar]
- 13.Castorani MCN, Hovel KA. Native predator chemical cues induce anti-predation behaviors in an invasive marine bivalve. Biol Invasions. 2016;18:169–181. [Google Scholar]
- 14.Chivers DP, Smith RJF. Chemical alarm signalling in aquatic predator-prey systems: A review and prospectus. Ecoscience. 1998;5:338–352. [Google Scholar]
- 15.Preisser EL, Bolnick DI, Benard MF. Scared to death? The effects of intimidation and consumption in predator–prey interactions. Ecology. 2005;86:501–509. [Google Scholar]
- 16.Grabowski JH, Powers SP. Habitat complexity mitigates trophic transfer on oyster reefs. Mar Ecol Prog Ser. 2004;277:291–295. [Google Scholar]
- 17.Trussell GC, Ewanchuk PJ, Bertness MD. Trait-mediated effects in rocky intertidal food chains: Predator risk cues alter prey feeding rates. Ecology. 2003;84:629–640. [Google Scholar]
- 18.Turner AM, Montgomery SL. Spatial and temporal scales of predator avoidance: Experiments with fish and snails. Ecology. 2003;84:616–622. [Google Scholar]
- 19.Peckarsky BL, et al. Revisiting the classics: Considering nonconsumptive effects in textbook examples of predator-prey interactions. Ecology. 2008;89:2416–2425. doi: 10.1890/07-1131.1. [DOI] [PubMed] [Google Scholar]
- 20.Agelopoulos NG, Hooper AM, Maniar SP, Pickett JA, Wadhams LJ. A novel approach for isolation of volatile chemicals released by individual leaves of a plant in situ. J Chem Ecol. 1999;25:1411–1425. [Google Scholar]
- 21.Prince EK, Poulson KL, Myers TL, Sieg RD, Kubanek J. Characterization of allelopathic compounds from the red tide dinoflagellate Karenia brevis. Harmful Algae. 2010;10:39–48. [Google Scholar]
- 22.Gillard J, et al. Metabolomics enables the structure elucidation of a diatom sex pheromone. Angew Chem Int Ed Engl. 2013;52:854–857. doi: 10.1002/anie.201208175. [DOI] [PubMed] [Google Scholar]
- 23.Weissburg M, Poulin RX, Kubanek J. You are what you eat: A metabolomics approach to understanding prey responses to diet-dependent chemical cues released by predators. J Chem Ecol. 2016;42:1037–1046. doi: 10.1007/s10886-016-0771-2. [DOI] [PubMed] [Google Scholar]
- 24.Wishart DS, et al. HMDB 3.0–The Human Metabolome Database in 2013. Nucleic Acids Res. 2013;41:D801–D807. doi: 10.1093/nar/gks1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keppel E, Scrosati R. Chemically mediated avoidance of Hemigrapsus nudus (Crustacea) by Littorina scutulata (Gastropoda): Effects of species coexistence and variable cues. Anim Behav. 2004;68:915–920. [Google Scholar]
- 26.Smee DL, Weissburg MJ. Hard clams (Mercenaria mercenaria) evaluate predation risk using chemical signals from predators and injured conspecifics. J Chem Ecol. 2006;32:605–619. doi: 10.1007/s10886-005-9021-8. [DOI] [PubMed] [Google Scholar]
- 27.Laurila A, Kujasalo J, Ranta E. Different antipredator behaviour in two anuran tadpoles: Effects of predator diet. Behav Ecol Sociobiol. 1997;40:329–336. [Google Scholar]
- 28.Martel G, Dill LM. Feeding and aggressive behaviours in juvenile coho salmon (Oncorhynchus kisutch) under chemically-mediated risk of predation. Behav Ecol Sociobiol. 1993;32:365–370. [Google Scholar]
- 29.Selander E, et al. Predator lipids induce paralytic shellfish toxins in bloom-forming algae. Proc Natl Acad Sci USA. 2015;112:6395–6400. doi: 10.1073/pnas.1420154112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yasumoto K, et al. Isolation of new aliphatic sulfates and sulfamate as the Daphnia kairomones inducing morphological change of a phytoplankton Scenedesmus gutwinskii. Chem Pharm Bull (Tokyo) 2008;56:133–136. doi: 10.1248/cpb.56.133. [DOI] [PubMed] [Google Scholar]
- 31.Gasteiger EL, Haake PC, Gergen JA. An investigation of the distribution and function of homarine (N-methyl picolinic acid) Ann N Y Acad Sci. 1960;90:622–636. doi: 10.1111/j.1749-6632.1960.tb26410.x. [DOI] [PubMed] [Google Scholar]
- 32.Hayashi T, Konosu S. Quaternary ammonium bases in the adductor muscle of fan-mussel. Nippon Suisan Gakkaishi. 1977;43:343–348. [Google Scholar]
- 33.Hiltz DF. Occurrence of trigonelline (N-methyl nicotinic acid) in the adductor muscle of a lamellibranch, the sea scallop (Placopecten magellanicus) J Fish Res Board Can. 1970;27:604–606. [Google Scholar]
- 34.Hirano T. On the distribution and seasonal variation of homarine in some marine invertebrates. Nippon Suisan Gakkaishi. 1975;41:1047–1051. [Google Scholar]
- 35.Núñez-Pons L, Avila C. Natural products mediating ecological interactions in Antarctic benthic communities: A mini-review of the known molecules. Nat Prod Rep. 2015;32:1114–1130. doi: 10.1039/c4np00150h. [DOI] [PubMed] [Google Scholar]
- 36.Suwetja IK, Hori K, Miyazawa K, Ito K. Changes in content of ATP-related compounds, homarine, and trigonelline in marine-invertebrates during ice storage. Nippon Suisan Gakkaishi. 1989;55:559–566. [Google Scholar]
- 37.Yasumoto T, Kawagishi N, Asano H. Identification of N-methyl-alpha-picolinium and other quaternary ammonium compounds from the oyster. Nippon Suisan Gakkaishi. 1978;44:529–529. [Google Scholar]
- 38.Berking S. Is homarine a morphogen in the marine hydroid Hydractinia? Rouxs Arch Dev Biol. 1986;195:33–38. doi: 10.1007/BF00444039. [DOI] [PubMed] [Google Scholar]
- 39.Berking S. Homarine (N-methylpicolinic acid) and trigonelline (N-methylnicotinic acid) appear to be involved in pattern control in a marine hydroid. Development. 1987;99:211–220. doi: 10.1242/dev.99.2.211. [DOI] [PubMed] [Google Scholar]
- 40.McClintock JB, et al. Homarine as a feeding deterrent in common shallow-water antarctic lamellarian gastropod Marseniopsis mollis: A rare example of chemical defense in a marine prosobranch. J Chem Ecol. 1994;20:2539–2549. doi: 10.1007/BF02036190. [DOI] [PubMed] [Google Scholar]
- 41.Targett NM, Bishop SS, McConnell OJ, Yoder JA. Antifouling agents against the benthic marine diatom, Navicula salinicola Homarine from the gorgonians Leptogorgia virgulata and L. setacea and analogs. J Chem Ecol. 1983;9:817–829. doi: 10.1007/BF00987807. [DOI] [PubMed] [Google Scholar]
- 42.Netherton JC, 3rd, Gurin S. Biosynthesis in vitro of homarine and pyridine carboxylic acids in marine shrimp. J Biol Chem. 1980;255:9549–9551. [PubMed] [Google Scholar]
- 43.Netherton JC, 3rd, Gurin S. Biosynthesis and physiological role of homarine in marine shrimp. J Biol Chem. 1982;257:11971–11975. [PubMed] [Google Scholar]
- 44.Hall ER, Gurin S. Experiments in marine biochemistry. Homarine metabolism in Penaeus duorarum. J Biol Chem. 1975;250:6943–6946. [PubMed] [Google Scholar]
- 45.Ashihara H. Metabolism of alkaloids in coffee plants. Braz J Plant Physiol. 2006;18:1–8. [Google Scholar]
- 46.Kuhlisch C, Pohnert G. Metabolomics in chemical ecology. Nat Prod Rep. 2015;32:937–955. doi: 10.1039/c5np00003c. [DOI] [PubMed] [Google Scholar]
- 47.Prince EK, Pohnert G. Searching for signals in the noise: Metabolomics in chemical ecology. Anal Bioanal Chem. 2010;396:193–197. doi: 10.1007/s00216-009-3162-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.