Significance
Mice can reduce their body temperature and metabolic rate and enter torpor when they are subjected to cold, calorie deprivation, or administration of some metabolic inhibitors. Here we show that administration of pyruvate, a key metabolic intermediate and substrate for gluconeogenesis, induces torpor in obese mice, resulting in marked hypothermia, decreased activity, and decreased metabolic rate. This is dependent on adenosine signaling and on changes in key brain metabolites, including GABA. Lean mice are protected from pyruvate-induced torpor by activating their brown adipose tissue. Understanding this pathway can help provide new approaches for inducing a hypometabolic state in mammals, which could be beneficial in states of food deprivation or ischemia–reperfusion injury and for organ preservation in surgical situations.
Keywords: torpor, body temperature regulation, mass spectroscopic brain imaging, brown fat, GABA
Abstract
Mice subjected to cold or caloric deprivation can reduce body temperature and metabolic rate and enter a state of torpor. Here we show that administration of pyruvate, an energy-rich metabolic intermediate, can induce torpor in mice with diet-induced or genetic obesity. This is associated with marked hypothermia, decreased activity, and decreased metabolic rate. The drop in body temperature correlates with the degree of obesity and is blunted by housing mice at thermoneutrality. Induction of torpor by pyruvate in obese mice relies on adenosine signaling and is accompanied by changes in brain levels of hexose bisphosphate and GABA as detected by mass spectroscopy-based imaging. Pyruvate does not induce torpor in lean mice but results in the activation of brown adipose tissue (BAT) with an increase in the level of uncoupling protein-1 (UCP1). Denervation of BAT in lean mice blocks this increase in UCP1 and allows the pyruvate-induced torpor phenotype. Thus, pyruvate administration induces torpor in obese mice by pathways involving adenosine and GABA signaling and a failure of normal activation of BAT.
Thermoregulation and metabolism are well-regulated mechanisms in mammals and are essential for survival and reproduction. When mammals are subjected to cold temperature and/or diminished calories, energy-saving thermoregulation strategies become critical. Many mammals can save energy by inducing a state of hypometabolism (1), which, depending on its duration, is called either “hibernation” or “torpor.” Hibernation consists of consecutive multiday bouts of torpor with a low metabolic rate and low body temperature (Tb) (although the variation among species is large), can last months, and is often interrupted by episodes of arousal (2). Torpor is a state in which core Tb drops to less than 32 °C and there is markedly reduced activity (3). Torpor usually lasts less than 12–14 h. Mice undergo torpor when confronted with food deprivation or a combination of food deprivation and cold (4, 5). Torpor can also be induced by administration of 2-deoxy-D-glucose, which blocks cellular uptake of glucose (6), or hydrogen sulfide, which inhibits oxidative phosphorylation (7).
The biochemical events underlying torpor are poorly understood. In mammals, the fall in leptin (an anorexigenic hormone) during fasting has been suggested to play a role, and administration of leptin prevents deep bouts of fasting-induced torpor (8, 9). In addition, leptin-deficient ob/ob mice undergo longer episodes of torpor when calorie-deprived than lean mice (9). On the other hand, administration of ghrelin, an orexigenic peptide, deepens torpor bouts by acting on neuropeptide Y neurons in the arcuate nucleus of the hypothalamus (10). This brain region is a key player in the homeostatic regulation of food intake and metabolism and also is important in fasting-induced torpor, as fasted mice with lesions in the arcuate nucleus do not enter torpor (10).
Mammals generate heat by two major mechanisms: shivering thermogenesis involving skeletal muscle and nonshivering thermogenesis via activation of brown and beige adipose tissue (BAT). Nonshivering thermogenesis is primarily under the control of the sympathetic nervous system (SNS), which can activate BAT-specific mitochondrial uncoupling protein-1 (UCP1). UCP1 activity is modulated during hibernation and torpor. For example, in the ground squirrel the thermogenic capacity of BAT increases before hibernation as a result of increased BAT mass, increased abundance of mitochondria in BAT, and increased expression of genes involved in thermogenesis in BAT, including UCP1 (11). Conversely, during entrance into fasting-induced torpor, sympathetic activation of BAT in mice is shut down, and the thermoneutral zone is shifted to a lower ambient temperature to compensate for the energy deficit (12). However, the exact links between BAT and metabolic factors triggering torpor are still poorly understood.
In the present study, we show that systemic administration of pyruvate induces a full-blown state of torpor with hypothermia and markedly decreased activity and respiratory rate in both diet-induced obese (DIO) mice and in mice with genetic obesity (db/db and ob/ob) but not in lean mice. UCP1 activation in BAT is key in preventing pyruvate induced-torpor in lean mice, and denervation of BAT allows pyruvate to induce the torpor phenotype in these mice. In obese mice, torpor relies on adenosine signaling, and MALDI-MS imaging shows a critical role for brain metabolites, including GABA, in the induction of torpor. Our data show that the energy-rich substrate pyruvate is a metabolic trigger of torpor in obese mice, and prevention of this hypometabolic state in lean mice relies on BAT activation, adenosine signaling, and GABAergic brain circuits.
Results
Pyruvate Induces Torpor in DIO Mice.
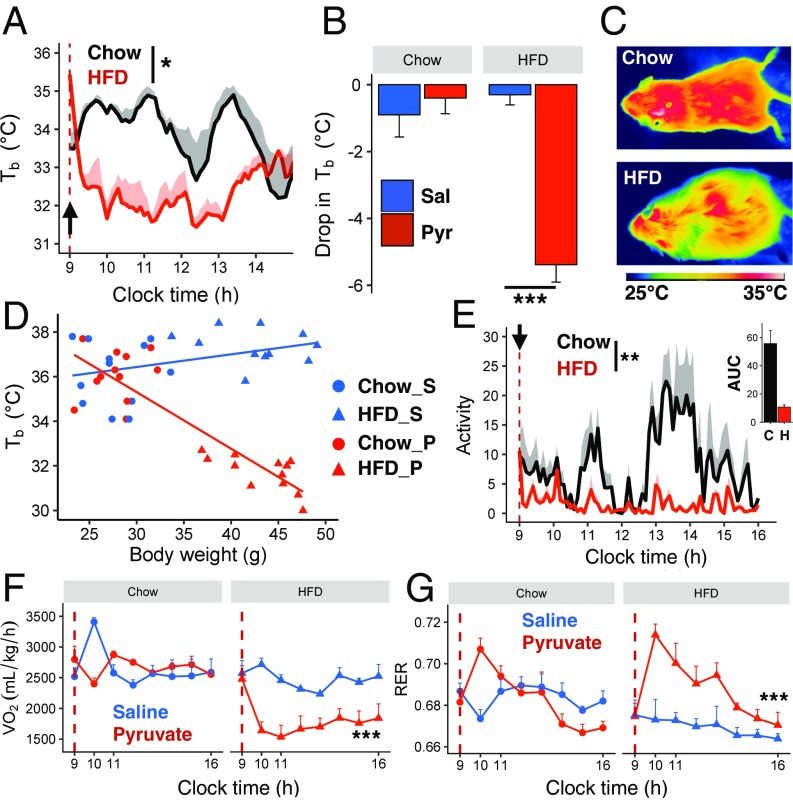
Pyruvate is an energy-rich intermediate in cellular metabolism and a precursor for glucose production in the liver. Pyruvate supplements enhance weight loss during dietary treatment of obesity and improve hypercholesterolemia (13). To investigate the effect of pyruvate in obesity, C57BL/6J mice were placed on a high-fat diet (HFD) for 13 wk and then were injected with pyruvate (2 g/kg) in the fasted state. At that time, the prefasting mean body weight was ∼46 g for the HFD-obese mice and ∼31 g for age-matched, chow-fed lean controls. In the lean controls, overnight fasting induced an acute drop in Tb of ∼3–4 °C, as measured by an s.c.-implanted intrascapular sensor (Fig. S1A). In the DIO mice, Tb also tended to drift down during the overnight fast, but the fall did not reach statistical significance (Fig. S1A). However, pyruvate injection induced a rapid and dramatic loss of Tb in fasted DIO mice, which reached an average of 31.7 °C at 1 h after injection; this was not seen in lean controls (diet effect: P = 0.01) (Fig. 1A). These differences were confirmed by a measurement of Tb using a rectal probe, which showed a ΔTb of approximately −6 °C in HFD-fed mice versus no change in lean mice (Fig. 1B and Fig. S1B), and by surface thermal imaging (Fig. 1C). The extent of the drop in Tb in pyruvate-injected mice negatively correlated with body weight (P < 0.001, R2 = 0.78) (Fig. 1D) and was accompanied by a 50% decrease in heat production in DIO mice (Fig. S1C). Pyruvate-injected DIO mice also showed an 81% decrease in spontaneous activity compared with lean mice (P = 0.003) (Fig. 1E). The combination of the fall in Tb below 33 °C for two or more consecutive hours and the decrease in activity defines the state as torpor (14).
Fig. 1.
Pyruvate induces a torpor-like state in HFD-obese mice but not in lean mice. (A) Intrascapular Tb in mice fed chow (n = 4) or HFD (n = 3) for 13 wk after i.p. injection of pyruvate (dashed line and black arrow mark time of injection). (B and C) Change in rectal Tb (B) and thermal images showing surface temperature (C) in fasted chow-fed and HFD-obese mice 2 h after saline or pyruvate injections (n = 6 per group). (D) Correlation between Tb and the body weight of chow-fed (Chow) and HFD-obese mice 2 h after pyruvate (P) or saline (S) injection. (E–G) Activity levels (E), O2 consumption (F), and RER (G) after injection of pyruvate or saline (n = 4 per group). *P < 0.05, **P < 0.01, ***P < 0.001.
To determine whether pyruvate decreases Tb through effects on metabolism, we analyzed O2 consumption (VO2), CO2 production (VCO2), and the respiratory exchange ratio (RER), using metabolic cages. In fasted lean mice, VO2 and VCO2 increased transiently after saline injection, but there was no significant effect of injection over time (Fig. 1F and Fig. S1D). In fasted DIO mice, VO2 decreased by over 40% (from 2,600 to ∼1,500 mL⋅kg−1⋅h−1) within 10 min after pyruvate injection (Fig. 1F), and this was maintained for the duration of the study (7 h). Likewise, VCO2 dropped from 1,700 mL⋅kg−1⋅h−1 to ∼1,000 mL⋅kg−1⋅h−1 over a similar timeframe (Fig. S1D), reflecting a fall in the metabolic rate. The RER increased after pyruvate injection in both control and DIO mice but increased significantly more in the latter (P = 0.0002) (Fig. 1G). This is consistent with the conversion of pyruvate to acetyl CoA, which enters the TCA cycle and mirrors an increase in carbohydrate versus lipid oxidation. Thus, in DIO mice, pyruvate produces a profound state of torpor with a major suppression of activity, metabolic rate, and Tb.
Pyruvate Injection Induces Changes in Circulating Pyruvate, Glucose, and Lactate.
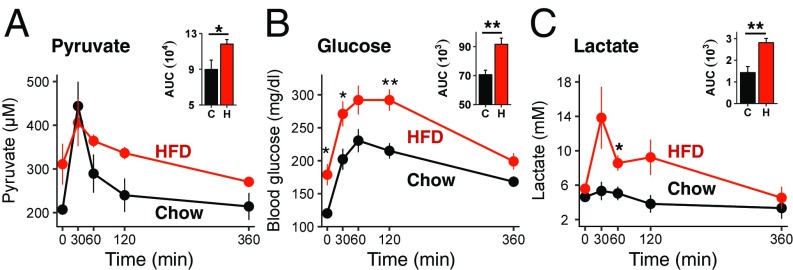
Pyruvate is converted into glucose by gluconeogenesis in the liver, and gluconeogenesis is impaired in obese insulin-resistant mice (15). We hypothesized that this difference in pyruvate metabolism between lean and obese mice might affect circulating molecules of downstream signaling pathways. Following i.p. injection of pyruvate, plasma levels of pyruvate increased in lean mice for 30 min and then declined to basal levels by 6 h after injection (Fig. 2A). DIO mice tended to have higher fasting pyruvate levels (P = 0.08), but the kinetics of increase and decline followed a similar time course, although the area under the curve (AUC) was significantly higher. As expected, pyruvate injection increased blood glucose levels in both lean and DIO mice (Fig. 2B), with a greater effect in the latter, consistent with their insulin-resistant state and increased gluconeogenesis (P = 0.0002) (Fig. 2B). Insulin levels, which were markedly increased in HFD mice, decreased after fasting and were not changed 2 h after pyruvate administration (Fig. S1E). Since pyruvate can be converted into lactate, plasma lactate increased acutely by 2.6-fold in HFD-obese mice after pyruvate administration. In lean mice, lactate increased by less than 20% (Fig. 2C), despite starting with similar fasting levels. Plasma leptin was 20-fold higher in DIO mice than in lean mice, reflecting the increased fat mass and leptin resistance. However, leptin and FGF21 levels were not changed 2 h after the injection of pyruvate in either chow-fed or DIO mice (Fig. S1 F and G). We also ruled out the possibility that pyruvate induced lactic acidosis, as blood pH did not decrease but instead increased rather significantly at 2 h after pyruvate injection in both lean and DIO mice (Fig. S1H).
Fig. 2.
Pyruvate injection induces changes in hormones and metabolites in chow-fed and HFD-obese mice. (A–C) Plasma pyruvate (A), glucose (B), and lactate (C) levels in mice fed chow or an HFD for 13 wk following acute injection of pyruvate (n = 3–6 per group). *P < 0.05, **P < 0.01.
Pyruvate Induces Torpor in ob/ob and db/db Mice.
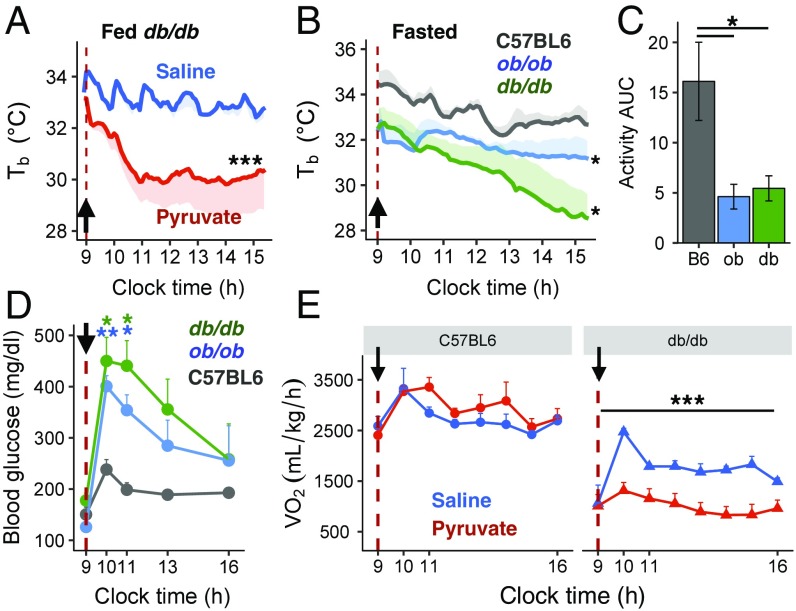
Mice with homozygous deletion of leptin (ob/ob) or leptin receptor (db/db) are hyperphagic and develop marked obesity and glucose intolerance. As in HFD-obese mice, db/db and ob/ob mice exhibited a profound decrease in intrascapular s.c. Tb following the injection of pyruvate compared with saline. This occurred in both the fed (Fig. 3A) and fasted states (Fig. 3B), although the fall in Tb was more rapid in the fed state than in the fasted state. The hypothermia observed in both states was more prolonged than in DIO mice. The pyruvate-induced hypothermia in db/db mice was accompanied by decreased heat production (Fig. S2A) and decreased skin temperature (Fig. S2B). As in DIO mice, pyruvate-induced torpor in ob/ob and db/db mice was associated with an ∼70% drop in activity levels in both the fasted (Fig. 3C) and the fed (Fig. S2C) state and by greater increases in blood glucose levels than in lean C57BL/6 mice (Fig. 3D). Furthermore, in db/db mice, but not in lean mice, both VO2 and VCO2 were significantly lower after pyruvate administration than after saline administration (Fig. 3E and Fig. S2D), reflecting a lower metabolic rate. As in DIO mice, the RER increased markedly after pyruvate injection in db/db mice (Fig. S2E). Thus, pyruvate-induced hypothermia in db/db mice was more severe and was accompanied by reduced heat production and a lower metabolic rate.
Fig. 3.
Pyruvate induces a torpor-like state in ob/ob and db/db mice. (A) Intrascapular Tb in random-fed db/db mice after i.p. injection of saline (n = 5) or pyruvate (n = 8) (dashed line and black arrow mark time of injection). (B) Intrascapular Tb in fasted C57BL/6, ob/ob and db/db mice after i.p. injection of saline or pyruvate (n = 3–6 per group). (C) Activity AUC levels calculated from the time of pyruvate injection until 2 h postinjection in fasted C57BL/6, ob/ob, and db/db mice (n = 3–6 per group). (D) Blood glucose levels in fasted C57BL/6, ob/ob, and db/db mice after pyruvate injection (n = 4–7 per group). (E) O2 consumption following injection of pyruvate in fasted C57BL/6 and db/db mice (n = 4–7 per group). *P < 0.05, **P < 0.01, ***P < 0.001.
Role of BAT in Pyruvate-Induced Torpor.
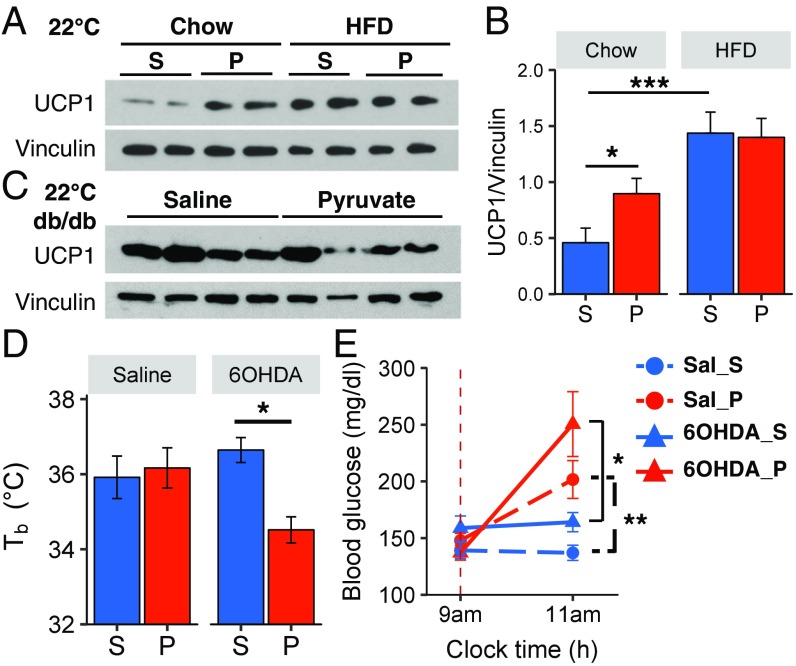
To determine the role of BAT thermogenesis in pyruvate-induced torpor, we measured the levels of UCP1 in the BAT of lean and DIO mice after pyruvate administration. UCP1 protein levels increased by twofold in the BAT of chow-fed mice by 2 h after pyruvate injection compared with saline-injected mice (P < 0.05, four independent experiments) (Fig. 4 A and B). This increase in UCP1 protein occurred despite a small but significant decrease in UCP1 mRNA (Fig. S3A), suggesting that the increase in UCP1 was due to an increase in translation or a decrease in UCP1 degradation. Consistent with previous studies (16), DIO mice had approximately threefold higher levels of UCP1 than lean mice, but in these mice pyruvate did not increase UCP1 at either the protein (Fig. 4 A and B) or mRNA (Fig. S3A) level. On the other hand, db/db mice had lower levels of UCP1 in BAT than lean mice, consistent with previous studies (17), and this also did not change following pyruvate injection (Fig. 4C).
Fig. 4.
Pyruvate-induced torpor is dependent on BAT function. (A and B) Representative Western blots (A) and densitometry analysis (B) of UCP1 protein levels in BAT samples from chow-fed or HFD-obese mice injected with saline or pyruvate; n = 8 per group. Vinculin served as a loading control. (C) Representative Western blots of UCP1 protein levels in BAT samples from db/db mice injected with saline or pyruvate. (D and E) Rectal Tb (D) and blood glucose levels (E) of fasted C57BL/6 mice injected with saline or pyruvate, after treatment with saline or 6-OHDA into BAT fat pads (n = 6 or 7 per group). P, pyruvate; S, saline. *P < 0.05, **P < 0.01, ***P < 0.001.
To determine the role of sympathetic innervation of BAT in the ability of lean mice to avoid pyruvate-induced torpor, we chemically denervated BAT in lean mice by the injection of 6-hydroxydopamine (6-OHDA) into the tissue to destroy dopaminergic and noradrenergic nerve fibers. Indeed, 2 wk after injection, there was a >95% reduction of norepinephrine (NE) content and an 80% reduction in UCP1 protein levels in the BAT of 6-OHDA–injected mice (Fig. S3 B and C). In contrast to the lack of effect in controls, administration of pyruvate in 6-OHDA BAT-denervated lean mice led to a significant decrease in Tb (Fig. 4D) and to torpor-like behavior with a marked decrease in activity level (Movie S1). Interestingly, BAT-denervated lean mice also failed to increase UCP1 levels in response to pyruvate as observed in normal lean mice (Fig. S3D). Chemical denervation of BAT, on the other hand, had no effect on the ability of pyruvate to increase blood glucose (Fig. 4E). Taken together, these data show that SNS-mediated induction of UCP1 in the BAT of lean mice is an important defense mechanism to maintain thermogenesis and prevent torpor.
Thermoneutrality Blunts Pyruvate-Induced Torpor.
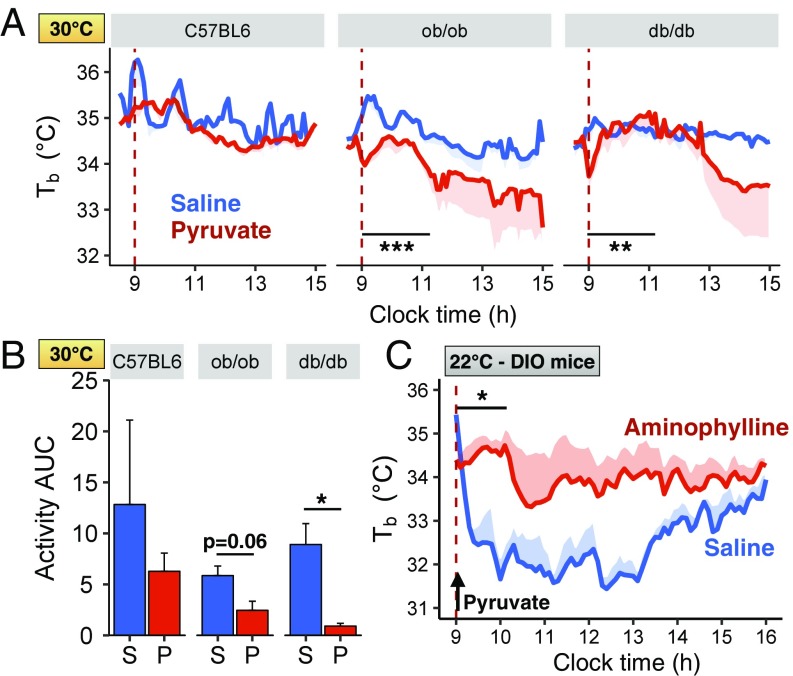
In mice housed at thermoneutral conditions (30 °C), pyruvate induced a drop in Tb and activity in genetically obese db/db and ob/ob mice (Fig. 5 A and B) but not in lean mice; however, the decrease was dampened compared with that observed in mice housed at 22 °C (1.5 vs. 4 °C, respectively, 6 h after pyruvate administration). Pyruvate induced a small increase in blood glucose in lean, ob/ob, and db/db mice (Fig. S3E), and this glucose response was also blunted compared with the increased glucose levels observed when pyruvate was administered at 22 °C. As expected, housing both lean C57BL/6 and obese db/db mice at thermoneutrality for 5 d before the pyruvate challenge resulted in decreased levels of UCP1 in BAT (Fig. S3F). UCP1 levels were not changed by pyruvate administration in C57BL/6, db/db, or ob/ob mice housed at thermoneutrality (Fig. S3G).
Fig. 5.
Pyruvate-induced torpor in obese mice is blunted at thermoneutrality and relies on adenosine receptors. (A) Intrascapular Tb of C57BL/6, ob/ob, and db/db mice after i.p. injection of pyruvate or saline (dashed line) at 30 °C (n = 2–5 per group). (B) Activity AUC levels at 30 °C measured by telemetry and calculated for 2 h from the time of saline (S) or pyruvate (P) injection (n = 2–5 per group). (C) Intrascapular Tb of fasted HFD-obese mice after i.p. injection of pyruvate. Mice were preinjected i.p. with either saline (blue line, n = 3) or aminophylline (20 mg/kg, red line, n = 4), an adenosine receptor antagonist, 10 min before pyruvate injection. *P < 0.05, **P < 0.01, ***P < 0.001.
Pyruvate-Induced Torpor in DIO Mice Relies on Adenosine Receptors but Does Not Depend on Central Pyruvate or Brain Insulin Resistance.
Adenosine signaling has been shown to play a role in fasting-induced torpor. Indeed, administration of aminophylline, an adenosine receptor antagonist, blocks the induction of fasting-induced torpor in lean mice (18) and stimulates thermogenesis in rats (19). To determine whether pyruvate-induced torpor in obese mice might be mediated via adenosine receptors, lean and HFD-obese mice were pretreated with saline or aminophylline before pyruvate administration. In HFD-obese mice, aminophylline significantly blunted pyruvate-induced hypothermia (Fig. 5C). This occurred without affecting the rise in blood glucose, i.e., increased gluconeogenesis (Fig. S4A). By contrast, aminophylline had no effect on Tb in lean mice injected with pyruvate (Fig. S4B) or on UCP1 activation in lean or DIO mice receiving pyruvate (Fig. S4C).
Exogenous pyruvate can cross the blood–brain barrier and is a potent energy substrate for neurons in vivo (20). To determine if the effect of pyruvate was due to a direct action in the central nervous system, we injected pyruvate into the lateral ventricle and monitored intrascapular Tb. Direct intracerebroventricular (i.c.v.) injection of pyruvate did not significantly affect Tb or activity levels in chow-fed or DIO mice (although there was a nonsignificant difference in basal temperature levels between the control and pyruvate groups) (Fig. S5 A and B). Interestingly, i.c.v. injection of pyruvate did increase blood glucose levels in DIO mice (Fig. S5C), suggesting that increasing pyruvate levels in the CNS could affect control of hepatic glucose output, possibly by agouti-related protein (AgRP) neurons in the hypothalamus (21). Since both HFD and genetically obese mice have been shown to have insulin resistance in the brain (22, 23), we tested whether the induction of torpor was dependent on brain insulin signaling by examining the effect of peripheral pyruvate injection in NIRKO mice, i.e., mice in which the insulin receptor is knocked out on both neuronal and glial cells in brain (24). Pyruvate injection in NIRKO mice increased blood glucose levels to a similar degree as observed in lean mice (Fig. S6A). Pyruvate did not induce hypothermia or affect activity in these mice (Fig. S6 B and C), although there was a difference in basal temperature between mice injected with saline and pyruvate.
Pyruvate-Induced Torpor Affects Brain Metabolite Levels.
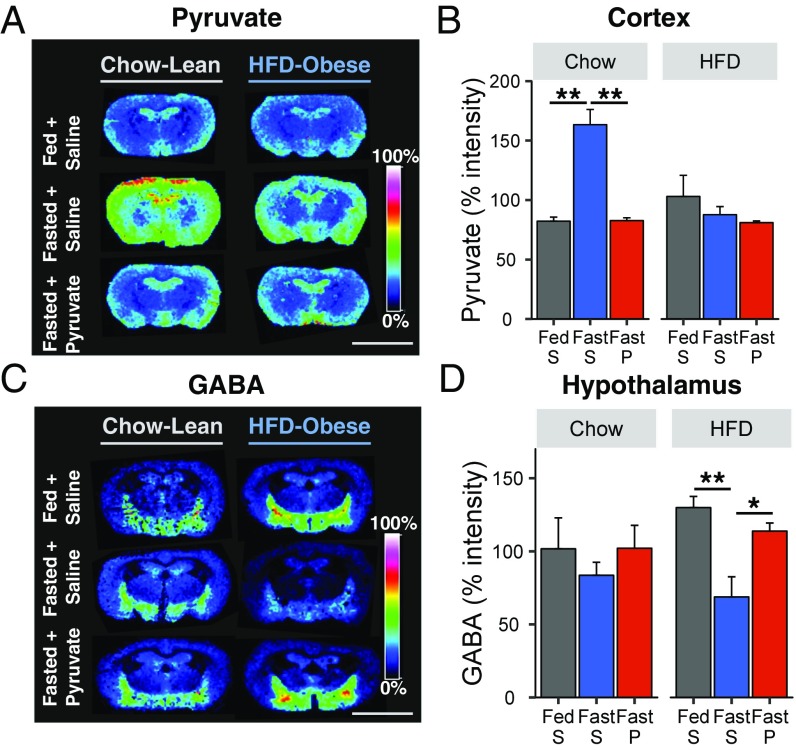
To better understand the role of brain metabolism during pyruvate-induced torpor, we used MALDI-MS imaging to assess metabolite levels in brain regions important for energy homeostasis, metabolism, and regulation of vital functions, including the hypothalamus, hippocampus, amygdala, cortex, and thalamus. This revealed that overnight fasting alone induced an increase in both pyruvate and lactate levels in the brain of lean mice, especially in the cortex (Fig. 6 A and B and Fig. S7 A and B). Surprisingly, 2 h after pyruvate administration to fasting animals, there was a significant decrease in CNS levels of pyruvate and lactate to levels equal to those in random-fed lean mice, indicating some peripheral–central counterregulation mechanism. This was confirmed by direct measurement in the cortex of chow-fed mice, which showed decreases of pyruvate and lactate levels at both 5 min and 2 h after pyruvate administration (Fig. S7 C and D). By contrast, levels of hexose bisphosphate and GABA, a major inhibitory neurotransmitter in the CNS, were not altered by fasting or pyruvate administration in chow-fed mice (Fig. 6 C and D and Fig. S7 E and F).
Fig. 6.
Effect of fasting and pyruvate administration on brain levels of pyruvate, hexose-bisphosphate, and GABA. (A and C) Distribution of pyruvate (A) and GABA (C) from chow- or HFD-fed mice 2 h after injection of saline (fed or fasted mice) or pyruvate (fasted mice). (Scale bars, 5 mm.) (B and D) Quantification of pyruvate and GABA levels in the cortex (B) or hypothalamus (D) relative to the mean intensity of each run (n = 5 or 6 per group). P, pyruvate; S, saline. *P < 0.05, **P < 0.01.
In DIO mice, a different pattern of central metabolism was observed. Thus, neither fasting nor pyruvate injection altered CNS pyruvate and lactate levels (Fig. 6 A and B and Fig. S7 A and B), but hexose bisphosphate levels were significantly decreased after pyruvate injection, both in the whole brain and in specific areas such as the cortex (Fig. S7 E and F). In addition, fasting significantly decreased GABA in the hypothalamus of obese mice, and this was restored to fed levels following pyruvate administration (Fig. 6 C and D). These changes were confirmed by direct measurement of GABA in hypothalamic samples of DIO mice 5 min and 2 h after pyruvate administration (Fig. S8A). GABA has been shown to modulate the expression of major hypothalamic neurotransmitters, such as AgRP, neuropeptide Y (NPY), pro-opiomelanocortin (POMC), and the cocaine- and amphetamine-regulated transcript (CART) (25). As expected, in DIO mice, mRNA levels of POMC and CART were increased, and those of AgRP and NPY were decreased, but these did not change further following pyruvate injection (Fig. S8B).
Discussion
Many vertebrate animals, including mammals, respond to harsh environmental conditions by entering into states in which metabolic rate and Tb drop to conserve energy for survival. Depending on their depth and duration, these states are referred to as “torpor” or “hibernation” (2). In the present study, we show that pyruvate induces a state of torpor with profound hypothermia and markedly decreased activity and metabolism in mice with either HFD- or genetically induced obesity. The effect of pyruvate depends on the degree of obesity, with the heaviest mice having the lowest core Tb. While most DIO mice endure the hypothermia and low respiratory rate and recover spontaneously, in db/db and ob/ob mice, which are even more obese and metabolically unhealthy, torpor can persist for more than 7 h and lead to death. The induction of torpor in obese mice is due to a loss of BAT-mediated thermogenesis, which protects lean mice from pyruvate-induced torpor. Consistent with this, pyruvate can induce torpor in lean mice in which BAT has been denervated, whereas housing at thermoneutrality blunts the phenotype in obese mice. Mechanistically, pyruvate-induced torpor is mediated by pathways involving adenosine and GABA signaling in the central nervous system.
One striking finding of this study is the dissociation between energy balance and induction of torpor by pyruvate. Thus, torpor is usually observed in states of low energy availability. Indeed, in lean mice, torpor is induced by an overnight fast (present study and ref. 9) but not by pyruvate administration. Other small molecules previously shown to induce torpor in lean mice are metabolic inhibitors, such as 2-deoxyglucose, a nonmetabolizable glucose analog that mimics hypoglycemia (6), and hydrogen sulfide, which blocks mitochondrial complex IV activity and inhibits oxidative phosphorylation in mice (7). By contrast, HFD-obese mice do not undergo torpor following an overnight fast (present study and ref. 26) but do go into a state of profound torpor after injection of pyruvate, an energy-rich substrate. Finally, pyruvate induces torpor in genetically obese db/db and ob/ob mice, but, in contrast to DIO mice and like lean mice, these mice also enter torpor upon fasting (even more severe than in lean mice). Hence, the effects of pyruvate and fasting in inducing torpor in mice depend on both the presence or absence of obesity and the nature of the obesity.
While pyruvate is a known precursor for glucose production, our data show that pyruvate-induced torpor is discrete from the actions of pyruvate as a substrate for hepatic gluconeogenesis. Consistent with our findings, previous studies have suggested an interaction between obesity and torpor/hibernation. For instance, mammals actively terminate the torpor state by increasing internal heat production, and body mass has been shown to be inversely related to rewarming following hibernation or torpor in a range of mammalian species (27). The role played by body fat in the mechanisms underlying torpor is unknown but has been suggested to involve leptin or FGF21. In the present study, neither leptin nor FGF21 was affected by pyruvate, so they are not likely mediators. It is possible that other signaling molecules produced in fat facilitate the induction of torpor; however, more research will be required to identify such signals.
Pyruvate-induced torpor in obese mice is associated with a deficit in BAT thermogenesis. Indeed, in lean mice, which do not undergo torpor after pyruvate administration, pyruvate induces an increase of UCP1 protein, the major regulator of BAT-driven thermogenesis. In contrast to adrenergic regulation of UCP1, pyruvate induction appears to occur primarily at a posttranscriptional level. Nonetheless, sympathetic denervation of BAT decreases basal UCP1 levels in lean mice and blunts the pyruvate-induced increased in UCP1 protein; as a result, pyruvate induces a fall in core Tb in these mice. This is accompanied by a drop in spontaneous activity, which is likely a consequence of the drop in Tb rather than a separate function, as UCP1-knockout mice have normal physical activity (28). By contrast to lean mice, pyruvate administration does not change UCP1 protein levels in obese mice, which already have a defective BAT function. Indeed, both DIO and genetically obese mice have an impaired thermogenic response to cold (17) as well as mitochondrial alterations, inflammation, and insulin resistance in BAT (29, 30). In addition, housing obese mice at thermoneutrality, which minimizes BAT thermogenic capacity and turns off sympathetic nervous tone, blunts pyruvate-induced torpor, although pyruvate still induces a modest decrease in Tb and activity. Pyruvate-induced torpor in obese mice also relies on adenosine signaling, as shown by the protective effect of aminophylline, an adenosine receptor antagonist. This appears to be independent of UCP1, although under some conditions adenosine can directly activate BAT (31). Consistent with this, peripheral stimulation of the A3 adenosine receptor (32) and central stimulation of the A1 adenosine receptor (33) trigger hypothermia in mice. This mechanism is different from the one underlying fasting-induced torpor in lean mice, which does not rely on A1 or A3 adenosine receptor signaling (33).
DIO mice have increased circulating pyruvate levels, and this pyruvate can be metabolized into lactate and glucose. In addition, injection of exogenous pyruvate leads to higher levels of pyruvate, lactate, and glucose in DIO mice than in lean mice. This can be explained by the reduction of pyruvate metabolism in obesity and diabetes because of the inhibition of pyruvate dehydrogenase by pyruvate dehydrogenase kinase (34). As both lean and obese mice exhibit rises in blood glucose, but only the obese mice go into torpor, the induction of torpor is not dependent on hepatic glucose release or diminished glucose storage. However, central pyruvate injection does not induce torpor in DIO mice, indicating its peripheral metabolism in the induction of torpor. Interestingly, pyruvate injected centrally does produce a rise in blood glucose in DIO mice, suggesting that part of the glycemic response following pyruvate administration is due to an effect in the CNS to regulate hepatic glucose output, possibly through activation of AgRP neurons (21). This is not surprising, as lactate metabolized centrally also affects peripheral glucose homeostasis (35). MALDI-MS imaging data show that brain pyruvate and lactate levels increase with fasting in lean mice, consistent with previous findings suggesting that pyruvate is an excellent substrate for neuronal TCA cycle activity (20) and that fasting increases pyruvate uptake in the brain (20). In addition, hexose bisphosphate levels are significantly decreased after pyruvate injection in HFD-obese mice, suggesting decreased glycolysis in brain.
Pyruvate-induced torpor in DIO mice is paralleled with increased levels of GABA in the hypothalamus. Other studies have shown that about 20% of brain GABA is labeled following i.v. injection of 13C-labeled pyruvate, indicating that pyruvate can serve as a precursor for this major inhibitory neurotransmitter (20). In the preoptic area of the hypothalamus, there is a set of warm-sensitive GABAergic neurons that have a robust effect on Tb (36), and optogenetic activation (36) or injection of a GABAA receptor agonist into this area induces a torpor-like state in rats (37). Thus, it is likely that the pyruvate-induced increase in GABA in DIO mice results in an increased firing rate of these neurons, which would provoke the inhibition of adaptive thermogenesis in the BAT and induce hypothermia. Many neuropeptides can affect GABA signaling at post- or presynaptic sites (38), but the major hypothalamic neuropeptides (CART, POMC, NPY, AgRP) are not affected by pyruvate in DIO mice, at least at the gene-expression level. This suggests that other hypothalamic nuclei, such as those in the paraventricular nucleus or the dorsomedial hypothalamus, have an important role in the modulation of thermogenic responses (39). The specific targets of GABA signaling and which metabolites affect its activity remain to be determined. MALDI-MS imaging provides the levels of many other identified metabolites, such as IMP, AMP, cAMP, ATP, UMP, and glutamate, which do not change significantly with pyruvate injection.
In summary, systemic administration of pyruvate induces torpor, with a marked reduction of Tb, activity, and metabolism, in diet-induced and genetically obese mice. This is dependent upon a lack of normal BAT activation, which occurs in lean animals. Our data show that adenosine and GABA signaling in brain are involved in the effects of pyruvate on thermogenesis and metabolism. Targeting the mechanisms underlying this regulated induction of torpor could provide a new approach for inducing a hypometabolic state, which could be beneficial in reducing ischemia–reperfusion injuries (40) and for organ preservation in surgical situations (41).
Methods
Mice.
Male C57BL/6J, ob/ob, and db/db mice (6–12 wk old) were purchased from Jackson Laboratory. NIRKO mice were bred as described previously (24). All mice were housed at 22 °C on a 12-h light/dark cycle. For the thermoneutrality experiment, mice were housed at 30 °C for 5 d before injections. Experiments complied with the regulations of the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of the Joslin Diabetes Center (no. 97-05).
Injection of Pyruvate and Aminophylline.
Pyruvate (Sigma) dissolved in sterile saline was administered i.p. (2 g/kg body weight). All injections took place around 9 AM, either after an overnight fast (∼18 h) or in a random-fed state. Aminophylline [20 mg/kg (42)] was injected 10 min before pyruvate or saline administration.
Metabolic Measurements.
Intrascapular Tb was measured using a telemetry system, and core Tb was measured using a RET-3 rectal probe. Glucose levels were measured in tail vein blood using infinity glucose monitors (US Diagnostics). Blood pH was measured in whole blood using a pH microelectrode. Whole-body energy expenditure was measured at ambient temperature (∼22 °C) using a Comprehensive Lab Animal Monitoring System (Columbus Instruments).
Diets, telemetric measurements, chemical sympathectomy, Western blot, qPCR analysis, analytical procedures, brain i.c.v. injection, MALDI-MS brain imaging, and statistics are detailed in SI Methods.
Supplementary Material
Acknowledgments
We thank Christie Penniman, Samir Softic, Michael D. Tuck, and David Pober for their assistance. This work was supported by NIH Grants R01DK082659 and R01D031036 (to C.R.K.) and Joslin Diabetes and Endocrinology Research Center Grants P30DK36836 and 5P41 GM103391-07 (to R.M.C.). L.O. was supported by the École Normale Supérieure de Cachan.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1717507115/-/DCSupplemental.
References
- 1.Geiser F, Stawski C. Hibernation and torpor in tropical and subtropical bats in relation to energetics, extinctions, and the evolution of endothermy. Integr Comp Biol. 2011;51:337–348. doi: 10.1093/icb/icr042. [DOI] [PubMed] [Google Scholar]
- 2.Ruf T, Geiser F. Daily torpor and hibernation in birds and mammals. Biol Rev Camb Philos Soc. 2015;90:891–926. doi: 10.1111/brv.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geiser F, Currie SE, O’Shea KA, Hiebert SM. Torpor and hypothermia: Reversed hysteresis of metabolic rate and body temperature. Am J Physiol Regul Integr Comp Physiol. 2014;307:R1324–R1329. doi: 10.1152/ajpregu.00214.2014. [DOI] [PubMed] [Google Scholar]
- 4.Brown JC, Staples JF. Mitochondrial metabolism during fasting-induced daily torpor in mice. Biochim Biophys Acta. 2010;1797:476–486. doi: 10.1016/j.bbabio.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Oelkrug R, Heldmaier G, Meyer CW. Torpor patterns, arousal rates, and temporal organization of torpor entry in wildtype and UCP1-ablated mice. J Comp Physiol B. 2011;181:137–145. doi: 10.1007/s00360-010-0503-9. [DOI] [PubMed] [Google Scholar]
- 6.Bechtold DA, et al. A role for the melatonin-related receptor GPR50 in leptin signaling, adaptive thermogenesis, and torpor. Curr Biol. 2012;22:70–77. doi: 10.1016/j.cub.2011.11.043. [DOI] [PubMed] [Google Scholar]
- 7.Blackstone E, Morrison M, Roth MB. H2S induces a suspended animation-like state in mice. Science. 2005;308:518. doi: 10.1126/science.1108581. [DOI] [PubMed] [Google Scholar]
- 8.Freeman DA, Lewis DA, Kauffman AS, Blum RM, Dark J. Reduced leptin concentrations are permissive for display of torpor in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol. 2004;287:R97–R103. doi: 10.1152/ajpregu.00716.2003. [DOI] [PubMed] [Google Scholar]
- 9.Gavrilova O, et al. Torpor in mice is induced by both leptin-dependent and -independent mechanisms. Proc Natl Acad Sci USA. 1999;96:14623–14628. doi: 10.1073/pnas.96.25.14623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gluck EF, Stephens N, Swoap SJ. Peripheral ghrelin deepens torpor bouts in mice through the arcuate nucleus neuropeptide Y signaling pathway. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1303–R1309. doi: 10.1152/ajpregu.00232.2006. [DOI] [PubMed] [Google Scholar]
- 11.Hampton M, Melvin RG, Andrews MT. Transcriptomic analysis of brown adipose tissue across the physiological extremes of natural hibernation. PLoS One. 2013;8:e85157. doi: 10.1371/journal.pone.0085157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sunagawa GA, Takahashi M. Hypometabolism during daily torpor in mice is dominated by reduction in the sensitivity of the thermoregulatory system. Sci Rep. 2016;6:37011. doi: 10.1038/srep37011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalman D, Colker CM, Wilets I, Roufs JB, Antonio J. The effects of pyruvate supplementation on body composition in overweight individuals. Nutrition. 1999;15:337–340. doi: 10.1016/s0899-9007(99)00034-9. [DOI] [PubMed] [Google Scholar]
- 14.Willis CK. An energy-based body temperature threshold between torpor and normothermia for small mammals. Physiol Biochem Zool. 2007;80:643–651. doi: 10.1086/521085. [DOI] [PubMed] [Google Scholar]
- 15.Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschöp MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci USA. 2008;105:9793–9798. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fromme T, Klingenspor M. Uncoupling protein 1 expression and high-fat diets. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1–R8. doi: 10.1152/ajpregu.00411.2010. [DOI] [PubMed] [Google Scholar]
- 17.Martins FF, Bargut TCL, Aguila MB, Mandarim-de-Lacerda CA. Thermogenesis, fatty acid synthesis with oxidation, and inflammation in the brown adipose tissue of ob/ob (-/-) mice. Ann Anat. 2017;210:44–51. doi: 10.1016/j.aanat.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Iliff BW, Swoap SJ. Central adenosine receptor signaling is necessary for daily torpor in mice. Am J Physiol Regul Integr Comp Physiol. 2012;303:R477–R484. doi: 10.1152/ajpregu.00081.2012. [DOI] [PubMed] [Google Scholar]
- 19.Wang LC, Anholt EC. Elicitation of supramaximal thermogenesis by aminophylline in the rat. J Appl Physiol. 1982;53:16–20. doi: 10.1152/jappl.1982.53.1.16. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez SV, Nguyen NH, Rise F, Hassel B. Brain metabolism of exogenous pyruvate. J Neurochem. 2005;95:284–293. doi: 10.1111/j.1471-4159.2005.03365.x. [DOI] [PubMed] [Google Scholar]
- 21.Könner AC, et al. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 2007;5:438–449. doi: 10.1016/j.cmet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Arnold SE, et al. High fat diet produces brain insulin resistance, synaptodendritic abnormalities and altered behavior in mice. Neurobiol Dis. 2014;67:79–87. doi: 10.1016/j.nbd.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koch C, et al. Leptin rapidly improves glucose homeostasis in obese mice by increasing hypothalamic insulin sensitivity. J Neurosci. 2010;30:16180–16187. doi: 10.1523/JNEUROSCI.3202-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brüning JC, et al. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- 25.Ito Y, et al. GABA type B receptor signaling in proopiomelanocortin neurons protects against obesity, insulin resistance, and hypothalamic inflammation in male mice on a high-fat diet. J Neurosci. 2013;33:17166–17173. doi: 10.1523/JNEUROSCI.0897-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solymár M, Pétervári E, Balaskó M, Szelényi Z. The onset of daily torpor is regulated by the same low body mass in lean mice and in mice with diet-induced obesity. Temperature (Austin) 2015;2:129–134. doi: 10.1080/23328940.2015.1014250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geiser F, Baudinette RV. The relationship between body mass and rate of rewarming from hibernation and daily torpor in mammals. J Exp Biol. 1990;151:349–359. doi: 10.1242/jeb.151.1.349. [DOI] [PubMed] [Google Scholar]
- 28.Liu X, et al. Paradoxical resistance to diet-induced obesity in UCP1-deficient mice. J Clin Invest. 2003;111:399–407. doi: 10.1172/JCI15737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohtomo T, et al. Chronic high-fat feeding impairs adaptive induction of mitochondrial fatty acid combustion-associated proteins in brown adipose tissue of mice. Biochem Biophys Rep. 2017;10:32–38. doi: 10.1016/j.bbrep.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberts-Toler C, O’Neill BT, Cypess AM. Diet-induced obesity causes insulin resistance in mouse brown adipose tissue. Obesity (Silver Spring) 2015;23:1765–1770. doi: 10.1002/oby.21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gnad T, et al. Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature. 2014;516:395–399. doi: 10.1038/nature13816. [DOI] [PubMed] [Google Scholar]
- 32.Carlin JL, et al. Peripheral adenosine A3 receptor activation causes regulated hypothermia in mice that is dependent on central histamine H1 receptors. J Pharmacol Exp Ther. 2016;356:474–482. doi: 10.1124/jpet.115.229872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carlin JL, et al. Hypothermia in mouse is caused by adenosine A1 and A3 receptor agonists and AMP via three distinct mechanisms. Neuropharmacology. 2017;114:101–113. doi: 10.1016/j.neuropharm.2016.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gray LR, Tompkins SC, Taylor EB. Regulation of pyruvate metabolism and human disease. Cell Mol Life Sci. 2014;71:2577–2604. doi: 10.1007/s00018-013-1539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borg MA, Tamborlane WV, Shulman GI, Sherwin RS. Local lactate perfusion of the ventromedial hypothalamus suppresses hypoglycemic counterregulation. Diabetes. 2003;52:663–666. doi: 10.2337/diabetes.52.3.663. [DOI] [PubMed] [Google Scholar]
- 36.Tan CL, et al. Warm-sensitive neurons that control body temperature. Cell. 2016;167:47–59.e15. doi: 10.1016/j.cell.2016.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cerri M, et al. The inhibition of neurons in the central nervous pathways for thermoregulatory cold defense induces a suspended animation state in the rat. J Neurosci. 2013;33:2984–2993. doi: 10.1523/JNEUROSCI.3596-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dicken MS, Tooker RE, Hentges ST. Regulation of GABA and glutamate release from proopiomelanocortin neuron terminals in intact hypothalamic networks. J Neurosci. 2012;32:4042–4048. doi: 10.1523/JNEUROSCI.6032-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cano G, et al. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J Comp Neurol. 2003;460:303–326. doi: 10.1002/cne.10643. [DOI] [PubMed] [Google Scholar]
- 40.Drew KL, Rice ME, Kuhn TB, Smith MA. Neuroprotective adaptations in hibernation: Therapeutic implications for ischemia-reperfusion, traumatic brain injury and neurodegenerative diseases. Free Radic Biol Med. 2001;31:563–573. doi: 10.1016/s0891-5849(01)00628-1. [DOI] [PubMed] [Google Scholar]
- 41.Carey HV, Andrews MT, Martin SL. Mammalian hibernation: Cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev. 2003;83:1153–1181. doi: 10.1152/physrev.00008.2003. [DOI] [PubMed] [Google Scholar]
- 42.Swoap SJ, Rathvon M, Gutilla M. AMP does not induce torpor. Am J Physiol Regul Integr Comp Physiol. 2007;293:R468–R473. doi: 10.1152/ajpregu.00888.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.