Significance
Global biodiversity consists not only of the sum of taxonomic units such as species, but also of their ecological or functional variety. These two components of biodiversity might be expected to rise or fall in tandem, but we find they are capable of strikingly independent behavior. In three major declines in taxonomic diversity—spatially from equator to poles today and temporally in the Permian–Triassic and Cretaceous–Paleogene extinctions—only the first one shows a concomitant drop in the number of functional groups, whereas virtually all functional categories survived the extinction events. We present a conceptual framework for understanding this contrast, and we suggest that the differing behavior of these two biodiversity components will be important in anticipating the impacts of impending losses in today’s biota.
Keywords: functional diversity, taxonomic diversity, mass extinction, latitudinal diversity gradient
Abstract
Taxonomic diversity of benthic marine invertebrate shelf species declines at present by nearly an order of magnitude from the tropics to the poles in each hemisphere along the latitudinal diversity gradient (LDG), most steeply along the western Pacific where shallow-sea diversity is at its tropical maximum. In the Bivalvia, a model system for macroevolution and macroecology, this taxonomic trend is accompanied by a decline in the number of functional groups and an increase in the evenness of taxa distributed among those groups, with maximum functional evenness (FE) in polar waters of both hemispheres. In contrast, analyses of this model system across the two era-defining events of the Phanerozoic, the Permian–Triassic and Cretaceous–Paleogene mass extinctions, show only minor declines in functional richness despite high extinction intensities, resulting in a rise in FE owing to the persistence of functional groups. We hypothesize that the spatial decline of taxonomic diversity and increase in FE along the present-day LDG primarily reflect diversity-dependent factors, whereas retention of almost all functional groups through the two mass extinctions suggests the operation of diversity-independent factors. Comparative analyses of different aspects of biodiversity thus reveal strongly contrasting biological consequences of similarly severe declines in taxonomic diversity and can help predict the consequences for functional diversity among different drivers of past, present, and future biodiversity loss.
Biodiversity has many dimensions or currencies (1). Taxonomic richness at the level of species or genus is the most common currency, but analyses of its relation to other aspects of diversity—e.g., morphological, functional, and phylogenetic—can provide novel insight into the origin and maintenance of biodiversity over time and space. Of these different aspects, functional diversity can be defined as “the value and range of those species and organismal traits that influence ecosystem functioning” (ref. 3, p. 742 paraphrases ref. 2, p. 109). Thus, major changes in functional diversity can have far-reaching macroecological and macroevolutionary implications (for example, refs. 4–6).
Here we compare declines in taxonomic and functional diversity in the most dramatic spatial pattern in taxonomic richness today, the latitudinal diversity gradient (LDG), to those seen in two of the most severe temporal drops in taxonomic diversity in the fossil record, the Permian–Triassic mass extinction (PT)—the largest extinction event of the Phanerozoic Eon (7)—and the Cretaceous–Paleogene mass extinction (KPg). While the extinctions and the LDG show equally severe diversity reductions, the patterns of functional diversity losses differ significantly and illuminate the differences in causes and outcomes between the two types of taxonomic declines.
Comparative Analyses of Functional Diversity
Comparative analyses of large-scale spatial and temporal dynamics of functional diversity can profit from the integration of extant and fossil organisms into a common framework of ecological function. Continuous performance or trait variables are often emphasized for extant plants and vertebrates [e.g., metabolic and growth rates (3, 5, 8)]. However, these approaches are difficult to apply in the fossil record, where organisms are instead classified into lower-resolution, discrete categories termed “functional groups” (FGs). Here, we use an ecospace (9) comprising separate axes for tiering, motility, fixation, and feeding mechanisms, which generate 567 possible functional states, of which 52 are realized in our study intervals (Materials and Methods).
These broad functional categories capture approximate ecological equivalencies among even distantly related taxa and can thus detect stability in ecosystem functioning at macroecological and macroevolutionary scales, e.g., compositional turnover of communities with latitude or over time (10, 11). In parallel with taxonomic diversity, functional diversity can be decomposed into functional richness (FR), the number of FGs occupied by a biota or clade, and functional evenness (FE), the distribution of taxa among FGs (see Materials and Methods for definition of FR and FE metrics). The relationships among temporal and spatial trends in FR and FE with taxonomic richness have been little studied, but evaluating the coupling of these different macroevolutionary currencies will improve our predictions of diversity dynamics under different modes of environmental and biological change.
Modern Ocean: Taxonomic and Functional Diversity
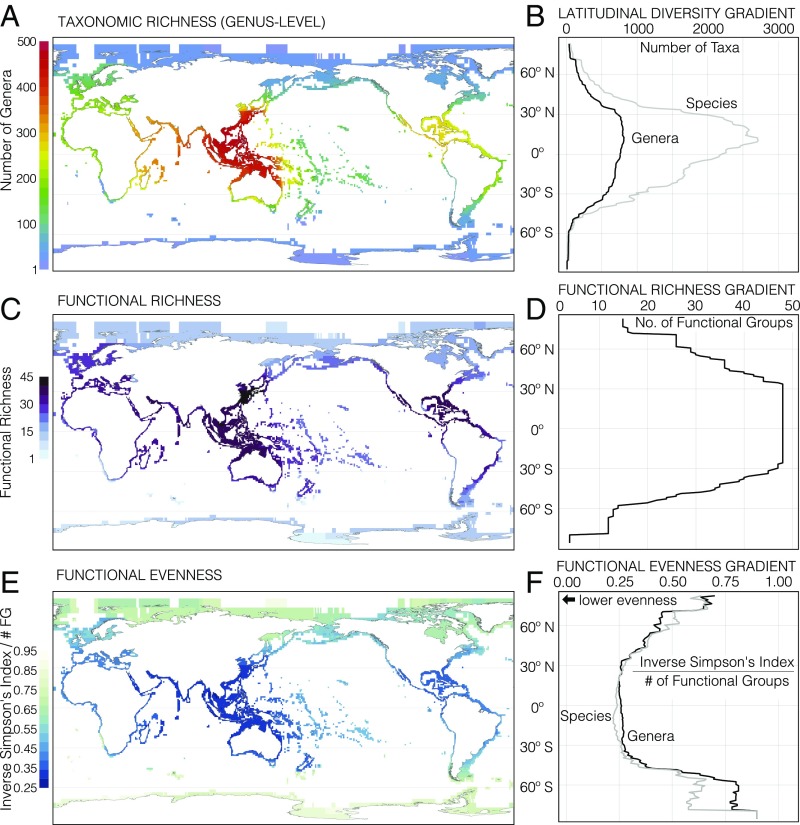
Both taxonomic diversity and functional diversity currently show strong latitudinal trends in most biological groups (12); these trends have steepened and shallowed through geologic time as global climate warmed and cooled (13, 14). Marine bivalves, which have become a model system for the study of macroevolution and macroecology (13, 15, 16), today exhibit an 81–98% decline in bivalve species richness from the tropics to the poles along major coastlines in the shallow sea, depending on the tropical starting point (genera show a similar 80–95% decline; Fig. 1 A and B). Major coastlines show a range of declines in FR from 45% to 70%, and only 19 and 16 of the 48 tropical FGs persist into the Arctic and Antarctic, respectively (Fig. 1 C and D and Fig. S1). This latitudinal drop in the two currencies, which is seen in other marine groups (17), produces a rise in FE at both poles (Fig. 1 E and F and Fig. S1). These patterns differ strikingly from the taxonomic and functional patterns associated with mass extinctions, as discussed below.
Fig. 1.
Present-day taxonomic and functional diversity patterns for marine bivalves along the continental shelf (water depths <200 m). Details of the recent marine bivalve dataset are provided in Recent Marine Bivalve Dataset. (A) Taxonomic richness of marine bivalve genera binned into 111-km2 equal-area grid cells (∼1° of latitude at the equator). Global genus richness peaks in the Indo-West Pacific and is the location of the strongest latitudinal gradient across north–south coastlines. (B) Integrated occurrences of distinct genera across ° latitudinal bands reveal a broad richness peak within the tropics; species show a steeper gradient peaking at ∼10° N. (C) Functional richness, the number of distinct FGs, of marine bivalves per equal-area grid cell (binned as in A) peaks in the Indo-West Pacific, similar to genus richness in A. (D) Integrated occurrences of FGs across ° latitudinal bands show a global increase in FR from the tropics to the poles, with a fully saturated richness spanning nearly the entire tropics and warm-temperate zones. (E) Functional evenness of bivalve genera per equal-area grid cell measured as the inverse Simpson index and normalized by the number of FGs per cell (Materials and Methods; binned as in A). The lowest evenness occurs in the Indo-West Pacific, the region of highest taxonomic and functional diversity. While tropical regions of other coastlines are less even than the Indo-West Pacific, each coastline exhibits an increase in FE from the tropics to the poles. (F) FE increases globally from the tropics to the poles across ° latitudinal bands at both the genus and species levels.
The roster of factors hypothesized to shape spatial gradients in diversity is long (12, 18), involving such multifactorial features as environmental heterogeneities at many scales, thermal and trophic settings, stability of many environmental factors, sizes of habitat areas, the sizes and structures of ecosystems themselves, in situ evolution, and geographic range shifts into and out of a focal region—almost anything capable of affecting patterns in the numbers of taxa or the numbers of their attributes that can be counted. Nevertheless, selection for metabolism-based adaptation to latitudinal differences in temperature and for promoting adaptive breadth in more seasonal climates appears to be particularly important (18–20). Thus, species in higher latitudes, which must endure seasons of low light and primary productivity, must access a larger proportion of available resource types than populations in lower latitudes and so tend to be more generalized. A reasonable model is that the number of species that can be supported declines with increasing latitude as environmental conditions—particularly lower temperatures and higher seasonality—increasingly favor the evolution and maintenance of a few gluttons over many epicures.
Within this general framework relating richness to seasonality and its correlates, the poleward decline in the number of FGs and the overall drop in FE values are consistent with the “out-of-the-tropics” dynamic observed for bivalve clades over the past 12 My (21). The highly uneven FR in the tropics reflects variation in taxonomic origination rates among lineages in the different FGs (21), a “supply-side” effect that is increasingly damped as taxonomic richness declines toward the poles. Operating in tandem with this effect was extinction among the FGs that were present at high latitudes before refrigeration of the poles during the late Cenozoic (22, 23). The independent or combinatory action of these processes as they unfold within the environmental template described above will tend to decrease FR and increase FE from the tropics to the poles.
Era Boundaries: Taxonomic and Functional Diversity
The most extreme losses in taxonomic richness in marine invertebrates since the Cambrian diversification of complex animals occurred during major extinctions, most notably in the events that are responsible for the faunal changes that separate the Phanerozoic Eras, which we analyze here using the taxonomic and functional framework applied to the bivalves of the present-day LDG. Global analyses of mass extinctions are complicated by sampling issues, illustrated by the “Lazarus effect,” with numerous taxa at the genus and even family level disappearing from the stratigraphic record for several My around extinction events (24); this effect is particularly severe around the PT (7, 24, 25) (15 of 50 families in this study) but also occurs around the KPg (26) (12 of 78 families in this study). Regardless of whether the Lazarus effect derives from preservation or sampling failure, depleted local populations of survivors, or restriction of many survivors to localized refugia (or more likely all of these), a simple inventory of taxa recorded from the immediate postextinction time interval will tend to overestimate extinction intensity measured in any currency. Accordingly, we analyze the faunas of the last well-sampled intervals before each event from a phylogenetic perspective, relative to the faunas of the postextinction eras, rather than in a narrow comparison with the first postextinction time bin.
PT.
The most severe Phanerozoic event was the PT about 252 Ma, which removed 76% of marine bivalve genera (Permian–Triassic Extinction) and 61–64% of all marine genera (27). Species losses are estimated to be 81–85% (27), and even these estimates slightly underestimate the proportion of the fauna lost near the boundary because they aim to exclude “background” extinctions just before the event. This was a sweeping, unprecedented change in the taxonomic composition of the marine biota—and was comparable in magnitude to the species-level LDG observed today.
Because of severe sampling problems, different scenarios have been framed for the PT, ranging from a single major extinction event preceded by a geologically brief (28) or prolonged (29, 30) decline to a pattern of two distinct extinction pulses, with an earlier severe event at the end of the Guadalupian Epoch about 260 Ma (31, 32). Whether the Late Permian global biota was poorly sampled or already suffering substantial losses, or both, virtually all authors agree that our last good picture of the relatively unperturbed Paleozoic fauna was in the Guadalupian Epoch, at least 8 My before the final PT event. Accordingly, we consider the functional biology of the PT from the standpoint of the Guadalupian bivalve fauna. Analyses restricted to the latest Permian do not change results, except for reducing the total number of FGs being considered owing to smaller sample sizes.
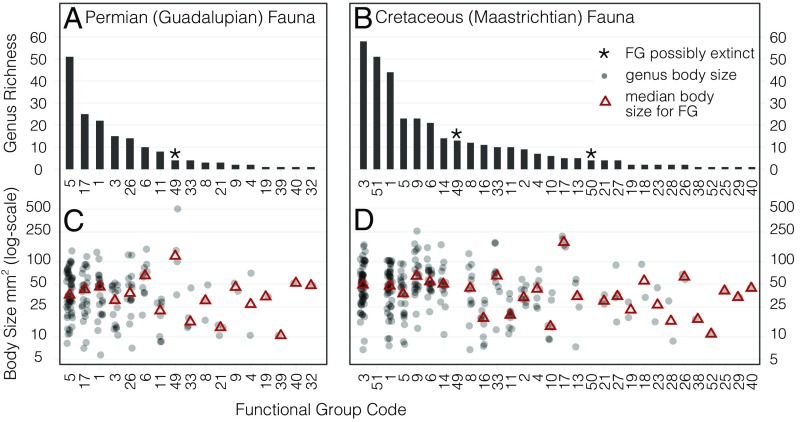
All, or possibly all but one, of the bivalve FGs in the Guadalupian Epoch survive the PT (Fig. 2A). Poor preservation and sampling in the Early Triassic undermine the calculation of a robust evenness value for the bivalve fauna surviving the PT, but the 76% genus extinction estimate across the PT, combined with the survival of all, or all but one FG would have increased FE under virtually any realistic scenario (Fig. S2 B and C). The ambiguous persistence or loss of one FG reflects the difficulty in inferring photosymbiosis in two extinct tropical clades, the Alatoconchidae, lost in the Permian, and the Megalodontidae, surviving well into the Mesozoic (33, 34). Reassigning these taxa as immobile epifaunal and mobile semiinfaunal suspension feeders, respectively (35), does not significantly alter the results.
Fig. 2.
Genus richness of FGs and maximum recovered body sizes of genera from the Guadalupian epoch of the Permian (272–260 Ma) and the Maastrichtian stage of the Cretaceous (72–66 Ma). See Table S1 for FG codes plotted along the x axis. Details of the fossil marine bivalve datasets are provided in Era Boundaries Marine Bivalve Dataset. (A) The FE of the Guadalupian fauna was 0.38, similar to the temperate latitudes in today’s diversity gradient. The FG 49 marked as possibly extinct reflects the potential photosymbiosis in the Alatoconchidae (33). (B) The Maastrichtian fauna had 13 more FGs than the Guadalupian, but the FE was very similar at 0.39. FGs 49 and 50 marked as possibly extinct reflects uncertainty on photosymbiosis among “rudists” (order Hippuritida) (34, 42). (C) Low-richness Guadalupian FGs contained genera with similar body sizes to the median values of the high-richness genera. (D) As in the Guadalupian, low-richness Maastrichtian FGs were of a similar large-body size to those genera in the high-richness FGs. We did not include body sizes of the FGs composed of exclusively rudist taxa (FGs 49–51), which were known to range in size from a few cm to >1 m in length (42).
The stability of FR and inferred increase in FE of the surviving fauna are largely consistent with previous studies of the PT (35, 36). For the entire marine biota, 9 of 25 FGs drop out of the fossil record, but all, or all but one, persisted through the crisis when phylogenetic continuity across the boundary is taken into account (37). More substantial declines in FR were seen in other studies (38–40). However, one analysis (38) involved a single Permian fauna and four Triassic faunas of different ages and used a more finely subdivided functional classification (10), two attributes that would tend to elevate estimates of FR loss owing to both genuine spatial variation and sampling effects. The other analyses (39, 40) used a similar functional classification to that used here, but did not take into account phylogeny or Lazarus effects, which elevated their estimates of FR loss above ours and that of ref. 37. The few studies addressing FE also find an increase across the PT (37, 38, 41), with the functional status of postextinction ecosystems likened to that of a skeleton crew manning a ship: “each post was occupied, but only by a few individual taxa” (ref. 37, p. 235).
Among all of these studies, the differences in spatial, temporal, and taxonomic resolution; in how the FGs were defined; and in the measures used to assess effects of the extinction and rediversification intervals created ambiguity in the response of functional diversity across the PT and its relation to present-day diversity trends. Focusing on a single major group present in both settings permitted us to apply a consistent functional and taxonomic framework that allowed direct comparisons among temporal and spatial patterns.
KPg.
The KPg was less severe than the PT for marine bivalves (64% genus extinction; Cretaceous–Paleogene Extinction), but there were more FGs (n = 30) (Fig. 2B). This mass extinction thus provides a useful additional test for the generality of FG survival in the face of large-scale taxonomic loss. Here we use the bivalve fauna of the final Cretaceous stage (the Maastrichtian), which is relatively well sampled globally (43); the earliest Paleogene tends to be more poorly sampled (26), creating a Lazarus effect and impeding direct calculations of FR and FE changes across the boundary, and so we estimate losses relative to the entire Cenozoic.
As in the PT, the number of FGs lost depends on the interpretation of a large-bodied, potentially photosymbiotic clade, the rudists (order Hippuritida) (34). Even assuming that this clade was photosymbiotic [and discounting a Paleocene record in Crimea (44)], only 7% (2 of 30) of the FGs are lost, and FE likely increased significantly, although not to the level of the PT (Fig. S2F). Using this single framework, we conclude that FGs were remarkably difficult to eradicate completely across the PT and KPg compared with the LDG, even for groups that contained few taxa.
Contrasting Responses of Functional Diversity Across the LDG and Era Boundaries
The patterns documented here show that the different currencies of biological diversity are capable of strikingly independent behavior when analyzed in a common taxonomic and functional framework. Functional groups are lost in concert with the great reduction in taxonomic richness along the modern LDG, whereas all, or nearly all, of the FGs in the bivalve fauna survived two of the greatest mass extinctions of the past 0.5 billion years. These contrasting patterns suggest that similar magnitudes of taxonomic loss can have radically different functional consequences and that FE can increase for more than one reason. We hypothesize that these patterns are directly related to differential effects of the factors that regulate taxonomic and functional diversity in marine systems.
From the tropics to the poles, the bivalve fauna responds to environmental gradients in seasonality, climate, and primary productivity by adapting to conditions that require more generalized ecological function, which accommodates fewer taxa (16, 20). The LDG in taxonomic richness and FR can thus be attributed to diversity-dependent factors, i.e., those arising from resource limitation. When conditions disfavor resource partitioning, e.g., under highly variable or seasonal resources, taxonomic diversity is relatively low, whereas greater resource stability accordingly permits greater partitioning and thus higher taxonomic diversity (9, 45). Of the 26 FGs that occur in the tropics but are absent at the poles today, 2 are photosymbiotic and thus probably not viable there given the annual solar cycle, and 17 belong to a single trophic category, phytoplankton suspension feeding, and so are subject to resource limitation in the highly seasonal Arctic and circum-Antarctic waters. If the trophic dimension were the key diversity-dependent factor, we would expect the exclusion of taxa, as in the Arctic, to focus on the feeding axis and to be unselective with respect to the other functional attributes, i.e., mobility and substratum relationships, and this appears to be the case (21).
In contrast, the PT and KPg events were remarkably indifferent to the ecological adaptations encoded in our FGs. Taxonomic diversity was severely reduced but all or nearly all of the functional categories persisted. Such discordance between taxonomic and FR is inconsistent with the factors implicated in the LDG such as seasonality and levels of trophic resources (reviews in refs. 18 and 20). Instead, the biotic patterns associated with the mass extinctions suggest the operation of diversity-independent factors—that is, factors such as temperature that are not partitioned by individuals, populations, or taxa (9), although they can partially govern other factors, such as productivity in the case of temperature, that are diversity dependent. Diversity-independent factors have been hypothesized as key drivers for the PT and KPg events, albeit with different triggers, such as sudden temperature perturbations, elevated pCO2, and ocean acidification (40, 46–48).
Although the operation of diversity-independent factors can explain why FGs were not selected against during the PT and KPg, it does not necessarily account fully for the survival of low-richness FGs in the face of such high overall extinction intensities. We can list four hypotheses and potential tests for the seemingly disproportionate survival of FGs. Testing any of these scenarios, or combinations thereof, for the two era-defining events of the Phanerozoic—and in other situations—will be a significant step toward understanding how specific diversity-independent factors can decouple taxonomic and functional extinction.
“Preadaptation.”
The survival of all FGs could reflect the existence, within each FG, of taxa that can tolerate the combination of adverse conditions that drove the extinctions. We then might expect the low-richness FGs to occur, before the extinction, in marine settings where they were “preadapted” to the extinction drivers. That is, few species could tolerate the environmental conditions of the PT or KPg, but those conditions did not impose selection on the biota along the functional categories used here. For example, perhaps each FG contained taxa that were relatively robust in pH buffering capabilities or had relatively efficient respiratory physiology. To test this interpretation, a more direct window into the paleophysiology of fossil invertebrates is needed to go beyond the broad-brush inference from extant members at the level of taxonomic class applied to date (46, 47).
Asymmetric Functional Selectivity.
The mass extinctions might have directly disfavored the richest FGs and favored or were neutral to taxon-poor FGs. For both extinctions, the low-richness bivalve FGs overlap along all four functional axes with the high-richness groups (both sets include burrowers, suspension feeders, attached forms, etc.; Fig. 2 A and B). Thus, the selectivity in this scenario would have to operate on biotic attributes not incorporated in the scheme used here or on specific combinations in a trade-off effect. For example, the eight FGs that are taxon poor in the Guadalupian, and thus are most likely to have been lost entirely under adverse conditions, include relatively energy-intensive lifestyles such as boring into hard substrata or short-burst swimming and body sizes comparable to those seen in the taxon-rich FGs (Fig. 2 A and C) (35). The same characteristics apply to the Maastrictian fauna as well, where the low-richness FGs include large-bodied burrowers and suspension feeders (Fig. 2 B and D). Thus, FG survival is not obviously associated with small body sizes or low-energy lifestyles.
Special Case of Diversity Dependence.
The PT and KPg drivers were actually diversity dependent, but only within FGs. That is, every FG remained viable under extinction conditions, but each could support only a few taxa owing to limited or unstable resources; this mode of diversity limitation, tightly focused by FG, is not evident along the modern LDG (22) or along modern bathymetric gradients, where entire FGs disappear at depth (49). Resource abundance may have dropped considerably in the PT and KPg intervals, contributing to the overall decline in taxonomic richness, but the types of resources, from phytoplankton to chemosymbionts, necessary to maintain at least one species in each FG apparently persisted, despite scenarios for the inhibition of photosynthesis at the KPg (48). Tests for similar nonanalog patterns could be performed at other episodes of global warming, intensifying ocean acidification and/or changing atmospheric composition, but the most obvious case, the Paleocene–Eocene Thermal Maximum (50), shows a remarkably modest extinction among marine macrobenthos (51).
Hitchhiking on Refugia or Range Size.
FGs might have survived because they hitchhiked on other extinction-resistant factors. The existence of geographical or environmental refugia harboring a full range of FGs from their global biotas would be consistent with the Lazarus effect observed for both extinction events (24), but no such refugia have yet been identified. Severe low-latitude heating has been hypothesized for the earliest Triassic (47, 52, but see ref. 53), and observed FR may be more evenly distributed latitudinally following both mass extinctions (37). Whether this pattern represents spatial variation in extinction intensity, a more general spatial homogenization of the global biota (54), a phylogenetic focus of intense extinction as perhaps within the potentially photosymbiotic Alataconchidae, or sampling effects is still unclear. Although sampling issues require caution and hamper robust analyses of spatial distributions, the Permian record currently gives no support for an extinction filter that harbored the full range of FGs at high latitudes (47).
For the KPg, where ranges sizes are better known, no refugia have been detected for marine bivalves at the regional (55) or provincial (56) scale. One analysis argued for lesser extinction near the KPg poles (57), but this study defined biogeographic units extending from Australia to northwestern Europe and from northern South America to western Greenland and so is difficult to interpret biologically. Alternatively, broad geographic ranges increase the probability of encountering smaller or FG-specific refugia and are often associated with taxon survival (56, 58). If all FGs contained a few wide-ranging taxa, they would tend to persist despite some losses. As with the Permian alataconchids, the loss of two FGs across the KPg represents the total or near extirpation of a single clade, the rudists (order Hippuritida), owing to any or all of these factors: near-exclusive tropicality, near-exclusive narrow ranges, and phylogenetic selectivity.
Conclusions
The similarities and differences in responses of the two components of functional diversity to major declines in taxonomic diversity raise broader questions about the interpretation of spatial and temporal dynamics in FR and FE. The most dramatic contrast is FR constancy across the mass extinctions vs FR decline with climate change [i.e., the climate shifts that shaped the current marine LDG via a combination of extinction, damped origination, and damped immigration (15, 16, 22)]. We also find unexpected patterns in FE. In the short term and on local scales, as in pollution events, FE is generally expected to decline in perturbed ecosystems as taxonomic diversity drops and a few stress-tolerant or opportunistic species predominate (59, 60). However, the opposite appears to be true for the mass extinctions and along the LDG, indicating that exploration of these apparent consequences of scale is needed. Further, although space can proxy for time in some situations (61–63), the bivalve patterns show that such extrapolation weakens with increasing spatial and temporal scale, so that its application can lead to strongly misleading inferences (61, 64, 65).
Finally, a deeper understanding of when and why losses in taxonomic and functional diversity can be decoupled has urgency now. Pressures on today’s marine biota (66, 67) appear to involve both diversity-dependent and diversity-independent factors, and as seemingly irreversible alterations in habitats and biotas accumulate, approaches to conservation, remediation, and recovery are expanding beyond the numbers and identities of taxa toward functional attributes (68–71). Comparative analyses of spatial and temporal patterns within a single taxonomic and functional framework, as provided here, are essential for developing the next generation of models relating the different currencies of biological diversity.
Materials and Methods
Marine Bivalve Ecospace.
We assigned bivalve genera to single states across four functional axes following the methodology of ref. 21: (i) mobility (immobile, mobile, swimming); (ii) tiering (infaunal asiphonate, deep-infaunal siphonate, shallow-infaunal siphonate, deep/shallow infaunal siphonate, borer, commensal, semiinfaunal, nestler, epifaunal); (iii) feeding (suspension, subsurface deposit, surface deposit, photosymbiotic, chemosymbiotic, carnivore, mixed deposit/suspension); and (iv) fixation (unattached, cemented, byssate). For genera lacking previously published functional information, we applied the most common set of functional attributes for the family or order, depending on the extent of missing information across the taxonomic hierarchy. Functional assignments for the Permian and Maastrichtian genera are available in Dataset S1. Functional codes used in the figures are defined in Table S1.
Estimating FR and FE.
We estimated FR as the number of distinct FGs in a given time or place. Given the entirely discrete nature of our functional data, we characterized FE as the distribution of taxa within FGs, using “Simpson’s measure of evenness” (inverse Simpson’s index normalized by the number of FGs in a sample) because it provides an “intuitive gradient in evenness” (72) and maps discernable changes in the distribution of taxa among FGs to a single metric (compare Fig. S1 and Fig. 1). This approach to FE differs from that of ref. 21 but the observed latitudinal patterns of FE are similar.
Supplementary Material
Acknowledgments
We thank the joint D.J.–T. D. Price laboratory for comments and suggestions, M. Foote and S. M. Kidwell for reviews, D. H. Erwin and H. Zeng for access to scarce Chinese sources, P. B. Wignall for advice on Svalbard stratigraphy, and M. Clapham for entry into the Permian invertebrate literature via the Paleobiology Database. We thank the National Aeronautics and Space Administration (EXOB08-0089) and the National Science Foundation (NSF) (EAR-0922156) (to D.J.) and NSF Graduate Research Fellowship Program and NSF Doctoral Dissertation Improvement Grant (DEB-1501880) (to S.M.E.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1717636115/-/DCSupplemental.
References
- 1.Jablonski D. Biotic interactions and macroevolution: Extensions and mismatches across scales and levels. Evolution. 2008;62:715–739. doi: 10.1111/j.1558-5646.2008.00317.x. [DOI] [PubMed] [Google Scholar]
- 2.Tilman D. Functional diversity. In: Levin S, editor. Encyclopedia of Biodiversity. Academic; San Diego: 2001. pp. 109–120. [Google Scholar]
- 3.Petchey OL, Gaston KJ. Functional diversity: Back to basics and looking forward. Ecol Lett. 2006;9:741–758. doi: 10.1111/j.1461-0248.2006.00924.x. [DOI] [PubMed] [Google Scholar]
- 4.Villéger S, Novack-Gottshall PM, Mouillot D. The multidimensionality of the niche reveals functional diversity changes in benthic marine biotas across geological time. Ecol Lett. 2011;14:561–568. doi: 10.1111/j.1461-0248.2011.01618.x. [DOI] [PubMed] [Google Scholar]
- 5.Pigot AL, Trisos CH, Tobias JA. Functional traits reveal the expansion and packing of ecological niche space underlying an elevational diversity gradient in passerine birds. Proc R Soc B. 2016;283:20152013. doi: 10.1098/rspb.2015.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swenson NG, et al. Constancy in functional space across a species richness anomaly. Am Nat. 2016;187:E83–E92. doi: 10.1086/685083. [DOI] [PubMed] [Google Scholar]
- 7.Erwin DH. Extinction: How Life on Earth Nearly Ended 250 Million Years Ago. Princeton Univ Press; Princeton: 2006. [Google Scholar]
- 8.Lamanna CA, et al. Functional trait space and the latitudinal diversity gradient. Proc Natl Acad Sci USA. 2014;111:13745–13750. doi: 10.1073/pnas.1317722111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valentine JW. Evolutionary Paleoecology of the Marine Biosphere. Prentice-Hall; Englewood Cliffs, NJ: 1973. [Google Scholar]
- 10.Novack-Gottshall PM. Using a theoretical ecospace to quantify the ecological diversity of Paleozoic and modern marine biotas. Paleobiology. 2007;33:273–294. [Google Scholar]
- 11.Bush AM, Novack-Gottshall PM. Modelling the ecological-functional diversification of marine Metazoa on geological time scales. Biol Lett. 2012;8:151–155. doi: 10.1098/rsbl.2011.0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mittelbach G, et al. Evolution and the latitudinal diversity gradient: Speciation, extinction and biogeography. Ecol Lett. 2007;10:315–331. doi: 10.1111/j.1461-0248.2007.01020.x. [DOI] [PubMed] [Google Scholar]
- 13.Crame JA. Evolution of taxonomic diversity gradients in the marine realm: Evidence from the composition of recent bivalve faunas. Paleobiology. 2000;26:188–214. [Google Scholar]
- 14.Powell MG, Moore BR, Smith TJ. Origination, extinction, invasion, and extirpation components of the brachiopod latitudinal biodiversity gradient through the Phanerozoic Eon. Paleobiology. 2015;41:330–341. [Google Scholar]
- 15.Jablonski D, Roy K, Valentine J. Out of the tropics: Evolutionary dynamics of the latitudinal diversity gradient. Science. 2006;314:102–106. doi: 10.1126/science.1130880. [DOI] [PubMed] [Google Scholar]
- 16.Jablonski D, Huang S, Roy K, Valentine JW. Shaping the latitudinal diversity gradient: New perspectives from a synthesis of paleobiology and biogeography. Am Nat. 2017;189:1–12. doi: 10.1086/689739. [DOI] [PubMed] [Google Scholar]
- 17.Stuart-Smith RD, et al. Integrating abundance and functional traits reveals new global hotspots of fish diversity. Nature. 2013;501:539–542. doi: 10.1038/nature12529. [DOI] [PubMed] [Google Scholar]
- 18.Fine P. Ecological and evolutionary drivers of geographic variation in species diversity. Annu Rev Ecol Evol Syst. 2015;46:369–392. [Google Scholar]
- 19.Brown JH. Why are there so many species in the tropics? J Biogeogr. 2014;41:8–22. doi: 10.1111/jbi.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valentine JW, Jablonski D. A twofold role for global energy gradients in marine biodiversity trends. J Biogeogr. 2015;42:997–1005. [Google Scholar]
- 21.Berke SK, Jablonski D, Krug AZ, Valentine JW. Origination and immigration drive latitudinal gradients in marine functional diversity. PLoS One. 2014;9:e101494. doi: 10.1371/journal.pone.0101494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krug AZ, Jablonski D, Roy K, Beu AG. Differential extinction and the contrasting structure of polar marine faunas. PLoS One. 2010;5:e15362. doi: 10.1371/journal.pone.0015362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crame JA, et al. The early origin of the Antarctic marine fauna and its evolutionary implications. PLoS One. 2014;9:e114743. doi: 10.1371/journal.pone.0114743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jablonski D. Causes and consequences of mass extinctions: A comparative approach. In: Elliott DK, editor. Dynamics of Extinction. Wiley; New York: 1986. pp. 183–229. [Google Scholar]
- 25.Fraiser ML, Clapham ME, Bottjer DJ. 2011. Mass extinctions and changing taphonomic processes fidelity of the Guadalupian, Lopingian, and early Triassic fossil records. Taphonomy: Process and Bias Through Time, eds Allison PA, Bottjer DJ (Springer, Berlin), Vol 32, 2nd Ed, pp 569–590.
- 26.Sessa JA, Patzkowsky ME, Bralower TJ. The impact of lithification on the diversity, size distribution, & recovery dynamics of marine invertebrate assemblages. Geology. 2009;37:115–118. [Google Scholar]
- 27.Stanley SM. Estimates of the magnitudes of major marine mass extinctions in Earth history. Proc Natl Acad Sci USA. 2016;113:E6325–E6334. doi: 10.1073/pnas.1613094113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, et al. Quantifying the process and abruptness of the end-Permian mass extinction. Paleobiology. 2014;40:113–129. [Google Scholar]
- 29.Clapham ME, Bottjer DJ. Prolonged Permian-Triassic ecological crisis recorded by molluscan dominance in late Permian offshore assemblages. Proc Natl Acad Sci USA. 2007;104:12971–12975. doi: 10.1073/pnas.0705280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clapham ME, Shen S, Bottjer DJ. The double mass extinction revisited: Reassessing the severity, selectivity, and causes of the end-Guadalupian biotic crisis (late Permian) Paleobiology. 2009;35:32–50. [Google Scholar]
- 31.Stanley SM, Yang X. A double mass extinction at the end of the Paleozoic Era. Science. 1994;266:1340–1344. doi: 10.1126/science.266.5189.1340. [DOI] [PubMed] [Google Scholar]
- 32.Bond DPG, et al. An abrupt extinction in the middle Permian (Capitanian) of the boreal realm (Spitsbergen) and its link to anoxia and acidification. Bull Geol Soc Am. 2015;127:1411–1421. [Google Scholar]
- 33.Asato K, Kase T, Ono T, Sashida K, Agematsu S. Morphology, systematics and paleoecology of Shikamaia, aberrant Permian bivalves (Alatoconchidae: Ambonychioidea) from Japan. Paleontol Res. 2017;21:358–379. [Google Scholar]
- 34.Vermeij GJ. The evolution of molluscan photosymbioses: A critical appraisal. Biol J Linn Soc. 2013;109:497–511. [Google Scholar]
- 35.Mondal S, Harries PJ. Phanerozoic trends in ecospace utilization: The bivalve perspective. Earth-Sci Rev. 2016;152:106–118. [Google Scholar]
- 36.Erwin DH, Valentine JW, Sepkoski JJ. A comparative study of diversification events: The early Paleozoic versus the Mesozoic. Evolution. 1987;41:1177–1186. [PubMed] [Google Scholar]
- 37.Foster WJ, Twitchett RJ. Functional diversity of marine ecosystems after the late Permian mass extinction event. Nat Geosci. 2014;7:233–238. [Google Scholar]
- 38.Dineen AA, Fraiser ML, Sheehan PM. Quantifying functional diversity in pre- and post-extinction paleocommunities: A test of ecological restructuring after the end-Permian mass extinction. Earth-Sci Rev. 2014;136:339–349. [Google Scholar]
- 39.Knope ML, Heim NA, Frishkoff LO, Payne JL. Limited role of functional differentiation in early diversification of animals. Nat Commun. 2015;6:1–6. doi: 10.1038/ncomms7455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petsios E, Thompson JR, Pietsch C, Bottjer DJ. Biotic impacts of temperature before, during, and after the end-Permian extinction: A multi-metric and multi-scale approach to modeling extinction and recovery dynamics. Palaeogeogr Palaeoclimatol Palaeoecol. September 12, 2017 doi: 10.1016/j.palaeo.2017.08.038. [DOI] [Google Scholar]
- 41.Dineen AA, Fraiser ML, Tong J. Low functional evenness in a post-extinction Anisian (Middle Triassic) paleocommunity: A case study of the Leidapo member (Qingyan formation), South China. Glob Planet Change. 2015;133:79–86. [Google Scholar]
- 42.Ross DJ, Skelton PW. Rudist formations of the Cretaceous: A palaeoecological, sedimentological and stratigraphical review. In: VP Wright., editor. Sedimentology Review. Blackwell; Oxford: 1993. pp. 73–91. [Google Scholar]
- 43.Jablonski D. Extinction and the spatial dynamics of biodiversity. Proc Natl Acad Sci USA. 2008;105:11528–11535. doi: 10.1073/pnas.0801919105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skelton P, Benton M. Mollusca: Rostroconchia, Scaphopoda and Bivalvia. In: Benton MJ, editor. The Fossil Record. Vol 2. Chapman & Hall; London: 1993. pp. 237–263. [Google Scholar]
- 45.Valentine JW, Jablonski D, Krug AZ, Roy K. Incumbency, diversity, and latitudinal gradients. Paleobiology. 2008;34:169–178. [Google Scholar]
- 46.Knoll AH, Bambach RK, Payne JL, Pruss S, Fischer WW. Paleophysiology and end-Permian mass extinction. Earth Planet Sci Lett. 2007;256:295–313. [Google Scholar]
- 47.Payne JL, Clapham ME. End-Permian mass extinction in the oceans: An ancient analog for the twenty-first century? Annu Rev Earth Planet Sci. 2012;40:89–111. [Google Scholar]
- 48.Vellekoop J, et al. Rapid short-term cooling following the Chicxulub impact at the Cretaceous-Paleogene boundary. Proc Natl Acad Sci USA. 2014;111:7537–7541. doi: 10.1073/pnas.1319253111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rex MA, Etter RJ. Deep-Sea Biodiversity. Harvard Univ Press; Cambridge, MA: 2010. [Google Scholar]
- 50.McInerney FA, Wing SL. The Paleocene-Eocene thermal maximum: A perturbation of carbon cycle, climate, and biosphere with implications for the future. Annu Rev Earth Planet Sci. 2011;39:489–516. [Google Scholar]
- 51.Melott AL, Bambach R. Analysis of periodicity of extinction using the 2012 geological timescale. Paleobiology. 2014;40:177–196. [Google Scholar]
- 52.Sun YD, et al. Lethally hot temperatures during the early Triassic greenhouse. Science. 2012;338:366–370. doi: 10.1126/science.1224126. [DOI] [PubMed] [Google Scholar]
- 53.Ramono C, et al. Marine Early Triassic Actinopterygii from Elko County (Nevada, USA): Implications for the Smithian equatorial vertebrate eclipse. J Paleontol. 2017;91:1025–1046. [Google Scholar]
- 54.Jablonski D. Geographic variation in the molluscan recovery from the end-Cretaceous extinction. Science. 1998;279:1327–1330. doi: 10.1126/science.279.5355.1327. [DOI] [PubMed] [Google Scholar]
- 55.Raup DM, Jablonski D. Geography of end-Cretaceous marine bivalve extinctions. Science. 1993;260:971–973. doi: 10.1126/science.11537491. [DOI] [PubMed] [Google Scholar]
- 56.Jablonski D. Scale and hierarchy in macroevolution. Palaeontology. 2007;50:87–109. [Google Scholar]
- 57.Vilhena DA, et al. Bivalve network reveals latitudinal selectivity gradient at the end-Cretaceous mass extinction. Sci Rep. 2013;3:1790. [Google Scholar]
- 58.Jablonski D. Mass extinctions and macroevolution. Paleobiology. 2005;31:192–210. [Google Scholar]
- 59.Gusmao JB, Brauko KM, Eriksson BK, Lana PC. Functional diversity of macrobenthic assemblages decreases in response to sewage discharges. Ecol Indic. 2016;66:65–75. [Google Scholar]
- 60.Mouillot D, Graham NA, Villéger S, Mason NW, Bellwood DR. A functional approach reveals community responses to disturbances. Trends Ecol Evol. 2013;28:167–177. doi: 10.1016/j.tree.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 61.Pickett STA. Space-for-time substitution as an alternative to long-term studies. In: Likens G, editor. Long-Term Studies in Ecology. Springer; New York: 1989. pp. 110–135. [Google Scholar]
- 62.Habeeb RL, Trebilco J, Wotherspoon S, Johnson CR. Determining natural scales of ecological systems. Ecol Monogr. 2005;75:467–487. [Google Scholar]
- 63.Blois JL, Williams JW, Fitzpatrick MC, Jackson ST, Ferrier S. Space can substitute for time in predicting climate-change effects on biodiversity. Proc Natl Acad Sci USA. 2013;110:9374–9379. doi: 10.1073/pnas.1220228110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dobson LL, La Sorte FA, Manne LL, Hawkins BA. The diversity and abundance of North American bird assemblages fail to track changing productivity. Ecology. 2015;96:1105–1114. doi: 10.1890/14-0057.1. [DOI] [PubMed] [Google Scholar]
- 65.Levy O, Buckley LB, Keitt TH, Angilletta MJ. Ontogeny constrains phenology: Opportunities for activity and reproduction interact to dictate potential phenologies in a changing climate. Ecol Lett. 2016;19:620–628. doi: 10.1111/ele.12595. [DOI] [PubMed] [Google Scholar]
- 66.Halpern BS, et al. Spatial and temporal changes in cumulative human impacts on the world’s ocean. Nat Commun. 2015;6:7615. doi: 10.1038/ncomms8615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harnik PG, et al. Extinctions in ancient and modern seas. Trends Ecol Evol. 2012;27:608–617. doi: 10.1016/j.tree.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 68.Cadotte MW, Carscadden K, Mirotchnick N. Beyond species: Functional diversity and the maintenance of ecological processes and services. J Appl Ecol. 2011;48:1079–1087. [Google Scholar]
- 69.Naeem S, Duffy JE, Zavaleta E. The functions of biological diversity in an age of extinction. Science. 2012;336:1401–1406. doi: 10.1126/science.1215855. [DOI] [PubMed] [Google Scholar]
- 70.Tomašových A, et al. Unifying latitudinal gradients in range size and richness across marine and terrestrial systems. Proc R Soc B. 2016;283:20153027. doi: 10.1098/rspb.2015.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Perring MP, et al. Advances in restoration ecology: Rising to the challenges of the coming decades. Ecosphere. 2015;6:1–25. [Google Scholar]
- 72.Magurran A. Measuring Biological Diversity. Blackwell, Malden; MA: 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.