Significance
This study provides important insight into how the human brain regulates fluid intake in response to changes in hydration status. The findings presented here reveal that activity in the anterior midcingulate cortex (aMCC) is associated with drinking responses during a state of thirst, and that this region is likely to contribute to the facilitation of drinking during this state. These results are consistent with a reduction in the influence of the aMCC contributing to the conclusion of drinking during a state of satiation. Because drinking stops before changes in blood volume and chemistry signal the restoration of fluid balance, these results implicate the aMCC in the regulation of drinking behavior before these changes manifest within the circulatory system.
Keywords: thirst, hydration, drinking, cingulate, fMRI
Abstract
In humans, activity in the anterior midcingulate cortex (aMCC) is associated with both subjective thirst and swallowing. This region is therefore likely to play a prominent role in the regulation of drinking in response to dehydration. Using functional MRI, we investigated this possibility during a period of “drinking behavior” represented by a conjunction of preswallow and swallowing events. These events were examined in the context of a thirsty condition and an “oversated” condition, the latter induced by compliant ingestion of excess fluid. Brain regions associated with swallowing showed increased activity for drinking behavior in the thirsty condition relative to the oversated condition. These regions included the cingulate cortex, premotor areas, primary sensorimotor cortices, the parietal operculum, and the supplementary motor area. Psychophysical interaction analyses revealed increased functional connectivity between the same regions and the aMCC during drinking behavior in the thirsty condition. Functional connectivity during drinking behavior was also greater for the thirsty condition relative to the oversated condition between the aMCC and two subcortical regions, the cerebellum and the rostroventral medulla, the latter containing nuclei responsible for the swallowing reflex. Finally, during drinking behavior in the oversated condition, ratings of swallowing effort showed a negative association with functional connectivity between the aMCC and two cortical regions, the sensorimotor cortex and the supramarginal gyrus. The results of this study provide evidence that the aMCC helps facilitate swallowing during a state of thirst and is therefore likely to contribute to the regulation of drinking after dehydration.
In humans, drinking is commonly motivated by the state of thirst that accompanies dehydration. This motivation needs to be tightly regulated, as underdrinking or overdrinking can lead to serious health effects and potentially even death (1–4). This suggests an intimate relationship exists between the state of thirst and a volitional drinking response. Both the satiation of thirst and the conclusion of drinking also occur before changes in blood volume and osmolality signal the restoration of fluid balance. The volume of fluid drunk nevertheless approximates the fluid deficit, raising the possibility that a reduction in subjective thirst could help regulate the drinking response in the absence of this interoceptive signal from the circulatory system.
Valuable insight into the relationship between thirst and drinking has been provided by the results of recent animal studies. These studies have demonstrated neurons in the subfornical organ, the median preoptic nucleus, and organum vasculosum of the lamina terminalis integrate information regarding a thirst stimulus and drinking responses (5–7). This integration represents a potential allostasis mechanism (8) that allows these neurons to anticipate the correction of fluid balance in advance and adjust drinking behavior accordingly (6). Such “anticipatory osmoregulation” could thus allow an animal to rehydrate efficiently without depending upon delayed changes to blood chemistry and volume. This mechanism appears to operate automatically, as illustrated in a recent study that optogenetically activated taste receptors for water to produce a swallowing response in water-deprived mice (9).
The findings in these animal studies are limited, however, by an inability to investigate the subjective motivation underlying the urge to drink. Humans, in comparison, can report their experience of thirst and the brain regions underlying this experience can be identified using neuroimaging modalities, which include functional MRI (fMRI) and positron emission tomography (PET). In these studies, subjective thirst has consistently been associated with activity in the anterior midcingulate cortex (aMCC). This region (10) is activated in response to reports of maximum thirst (11–13), along with changes in plasma sodium levels (14), and shows reduced activation in response to reports of satiation (11). Regional cerebral blood flow in this region has also been shown to correlate with thirst ratings in young and older male participants (15) and increased functional connectivity during a state of thirst has been demonstrated between the adjacent anterior cingulate cortex (ACC) and the lamina terminalis (16), the region containing the nuclei identified in the animal studies discussed above (5–7). Moreover, along with the primary sensorimotor cortex and the insula, the cingulate cortex is one of the most consistently activated regions in human fMRI studies of swallowing (17–19). Activity in the aMCC/ACC has been specifically related to tongue movements (20–22), preparing to swallow and clearing the throat (21), volitional swallowing (23, 24), and sensory stimulation, motor planning, and motor execution related to swallowing (25). The aMCC therefore appears to be involved in both the subjective state of thirst and the cortical control of swallowing, and thus represents a prime candidate for a region capable of integrating both processes.
Other evidence implicating the aMCC in motivated drinking is provided by studies that have investigated the influence of hydration status on subjective ratings and brain activity during drinking-related behavior. In these studies, decreases in aMCC activity due to progressive intake of water during a state of thirst have been shown to correlate with decreases in ratings of thirst (26) and pleasantness (26, 27). In addition, swallowing is experienced as unpleasant (27, 28) and becomes more difficult (28) if drinking continues after satiation. These findings are consistent with the human aMCC processing dynamic changes in incentive value relating to fluid ingestion (26). They imply that hydration status, represented subjectively by the states of thirst and satiation, produces dynamic changes in motivation that are processed within the aMCC, which could subsequently influence the probability of drinking. These changes are thus compatible with a proposed role for the aMCC in reward-based response selection (29–31).
To explicitly investigate whether activity in the aMCC is associated with drinking responses during a state of thirst, we analyzed data from one of our earlier fMRI studies (28) in which participants were scanned while drinking during a thirsty condition and during an “oversated” condition produced by excess compliant drinking. In each condition, participants held 5-mL volumes of liquid in their mouths before swallowing and subsequently rating how pleasant the liquid tasted, along with the effort required to swallow it. The preswallow and swallow components of this protocol incorporate the oral, pharyngeal, and esophageal phases of swallowing (32, 33), which are all likely to contribute to the regulation of drinking (34–36). The oral and pharyngeal phases, in addition, are likely to contribute to the satiation of thirst (36–38). We consequently analyzed the imaging data using a conjunction analysis that incorporated a 7-s liquid in the mouth event, representing the oral phase, and a 3-s swallowing event, representing the pharyngeal and esophageal phases. For simplicity, the resulting 10-s interval is referred to hereafter as the period of “drinking behavior.”
Using the conjunction analysis, we were able to provide unique insight into brain activity related to swallowing by identifying brain regions specifically involved in all three phases of swallowing. In comparison, previous studies have investigated the combined brain activity associated with each of the three phases (23, 24, 27, 39, 40), along with the activity associated with the oral preparatory phase (26, 28, 41, 42), the pharyngeal and esophageal phases (20), and behavior related to swallowing (21, 22, 25). Without examining regions that are active during all three phases, these earlier studies were unable to identify potential higher-order “supervisory” regions that could integrate sensory information about thirst with motor information associated with each phase of swallowing.
As the evidence presented above indicates, the aMCC is likely to fulfill the role of such a supervisory region; we expected this area to be active during the period of drinking behavior. By contrasting the thirsty and oversated conditions during this period, we could therefore examine whether this region showed a greater drinking-related response during the thirsty state. Psychophysical interaction (PPI) analyses (43, 44) were then performed for each of the conditions to examine whether correlated activity (functional connectivity) increased between an aMCC “seed” region and other brain areas during the period of drinking behavior, relative to a nondrinking baseline. Finally, the thirsty and oversated conditions were contrasted to identify, again for the period of drinking behavior, any brain regions that showed a greater increase in functional connectivity with the aMCC for the thirsty condition compared with the oversated condition.
Results
Brain Activity Between Thirsty and Oversated Conditions.
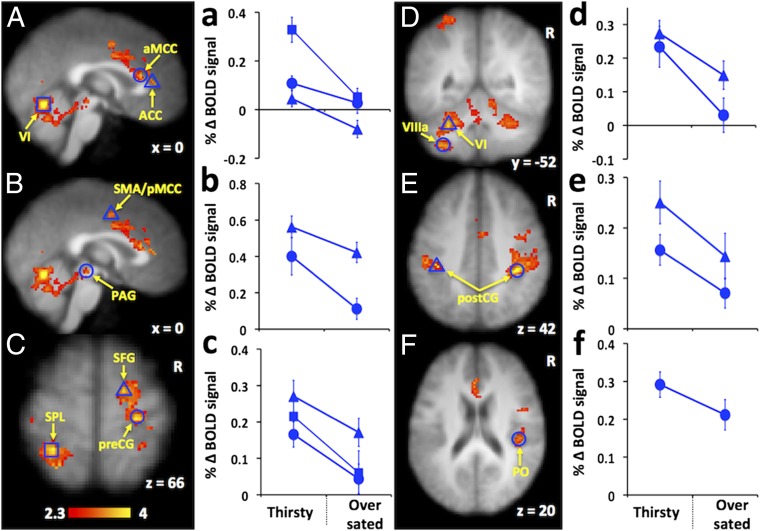
During drinking behavior extensive activation in cortical and subcortical areas in both the thirsty (Table S1) and oversated conditions (Table S2) was revealed. The cingulate cortex, cerebellum, supplementary motor area (SMA), parietal cortex, postcentral gyrus (postCG), precentral gyrus (preCG), prefrontal cortex, parietal operculum (PO), and a cluster of regions in the midbrain showed greater activity during the thirsty condition compared with the oversated condition (Fig. 1). No brain regions showed activity during the oversated condition that was greater than activity during the thirsty condition.
Fig. 1.
Brain regions showing increased activation during drinking behavior in the thirsty condition compared with the oversated condition. Regions of interest (ROI) are represented by open blue shapes in brain images (A–F). All ROIs were selected according to two criteria: z statistic > 3.0 and >50% gray matter (based on probabilities provided by the Harvard–Oxford Cortical Structural Atlas, an analytic tool provided by FSL to interrogate fMRI data). Shapes in graphs (a–f) represent the average blood oxygen level-dependent (BOLD) signal percentage change from the thirsty condition to the oversated condition for the ROIs possessing the same shape in adjacent brain images. All ROIs show an increase in BOLD activity during the thirsty condition relative to the oversated condition. Abbreviations: ACC, anterior cingulate cortex; aMCC, anterior midcingulate cortex; PAG, periaqueductal gray; pMCC, posterior midcingulate cortex; PO, parietal operculum; postCG, postcentral gyrus; preCG, precentral gyrus; SFG, superior frontal gyrus; SMA, supplementary motor cortex; SPL, superior parietal lobule; VI, cerebellar lobule VI (49); VIIIa, cerebellar lobule VIIIa (49).
PPI During Thirsty and Oversated Conditions.
For brevity, whenever “functional connectivity” is mentioned in text and figures hereafter, it refers to the degree of correlated activity between the aMCC seed and other brain areas during the period of drinking behavior, relative to a baseline period of nondrinking behavior. In both the thirsty (Table S4) and oversated conditions (Table S5), an increase in functional connectivity was revealed between the aMCC and a distributed network of brain regions, including the brainstem, cerebellum, and extended regions of both the lateral and medial posterior cortex. Many of the regions identified in the PPI analysis for the thirsty condition also corresponded to those regions that showed greater activity during the thirsty condition relative to the oversated region (Fig. S2). These included the midcingulate cortex (MCC), postCG, preCG, PO, superior frontal gyrus (SFG), and cerebellum.
PPI Contrast Between Thirsty and Oversated Conditions.
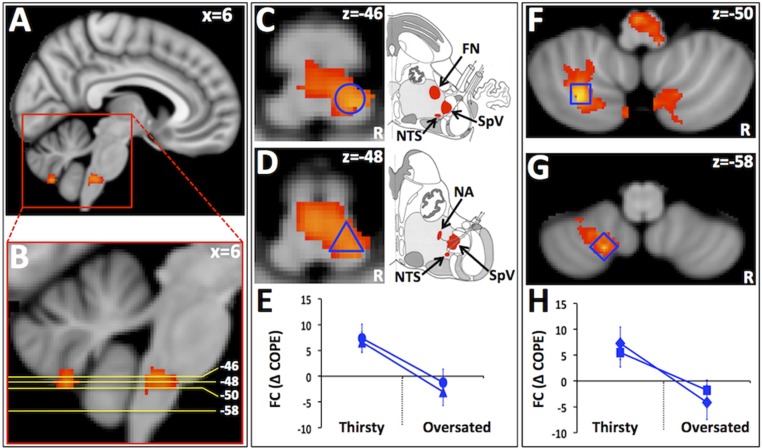
When the thirsty and oversated conditions were contrasted (see Fig. S1 for details), several loci in the cerebellum and midbrain showed a greater increase in functional connectivity with the aMCC seed during the thirsty condition compared with the oversated condition (Fig. 2). These included regions within the rostral medulla (RM) likely to represent the facial nucleus (FN), nucleus of the solitary tract (NTS), the oral part of the spinal trigeminal nucleus (SpV), and the nucleus ambiguus (NA). The location of these regions corresponds to areas that were significantly activated during drinking behavior in the thirsty condition and with areas identified in the PPI analysis for the thirsty condition (Fig. S3). No regions showed a greater increase in functional connectivity with the aMCC seed for the oversated condition compared with the thirsty condition.
Fig. 2.
Brain regions showing increased functional connectivity with the aMCC seed in the thirsty condition relative to the oversated condition. (A) Sagittal view of the brain showing location of significant clusters in RM and cerebellum. (B) Position of axial slices shown in C, D, F, and G. (C and D) RM clusters likely to include: (C) FN, NTS, and the SpV; (D) NTS, SpV, and the NA. (F and G) Cerebellum clusters: (F) cerebellar lobule VIIIa, (G) cerebellar lobule VIIIb (49). (E and H) Blue shapes in graphs correspond to blue shapes in brain images that represent ROIs; circle and triangle in E represent ROI’s located in the RM in C and D, while square and diamond in H represent ROI’s located in the cerebellum in F and G. The graphs show the average change in functional connectivity between the two conditions for the ROIs as indicated by the contrast of parameter estimates (COPEs) derived from the PPI analysis. ROIs in C and D were selected according to the criteria: z statistic > 3.2 and >50% gray matter (see Fig. 1 legend for details). ROIs in F and G were selected according to the criteria: z statistic > 3.5 and >50% gray matter (see Fig. 1 legend for details).
PPI Correlation with Ratings of Swallowing Effort During Oversated Condition.
When functional connectivity in the oversated condition was correlated with ratings of swallowing effort during the same condition, the supramarginal gyrus (SMG) and primary sensorimotor cortex showed a negative association between ratings and functional connectivity with the aMCC (Fig. S4). These cortical regions also showed greater activation during drinking behavior in the thirsty condition compared with the oversated condition and were identified in the PPI analysis for the thirsty condition (Fig. S5). No regions showed a positive association between effort ratings and functional connectivity during the oversated condition. Similarly, neither a positive nor a negative association was found when functional connectivity for the thirsty condition was correlated with ratings of swallowing effort during the thirsty condition.
Discussion
The aim of this study was to investigate whether the aMCC is associated with drinking responses during a state of thirst. Four results provide converging evidence for this hypothesis. First, the aMCC and regions previously associated with swallowing, including premotor areas, primary sensorimotor cortices, cingulate motor regions, and the PO, showed an increase in activation for the thirsty condition compared with the oversated condition. Second, during the thirsty condition, increased functional connectivity between the aMCC and the same regions was revealed. Third, when PPI analyses for the thirsty and oversated conditions were contrasted the RM, a region that contains nuclei involved in coordinating the swallowing reflex, showed a greater increase in functional connectivity with the aMCC seed during the thirsty condition. Finally, a negative association between functional connectivity during the oversated condition and ratings of swallowing effort in the same condition was shown in the most consistently activated region in relation to voluntary swallowing, the primary sensorimotor cortex. These four findings reveal activity in the aMCC is associated with drinking responses during a state of thirst and that this region is likely to contribute to a facilitation of drinking during this state. Because pleasantness ratings reveal the process of drinking during dehydration is associated with reward in humans (26–28), the findings of this study provide corroborating evidence that the aMCC plays a general role in reward-based response selection (29–31). The drinking response is likely to be “rewarding,” and thus selected, on the basis that it alleviates the aversive stimulus represented by thirst (45).
Increased Brain Activity During the Thirsty Condition.
Many of the cortical areas that showed an increase in activation for the thirsty condition compared with the oversated condition during drinking behavior correspond to regions identified in previous studies that have examined brain activity related to swallowing. Activity in the lateral primary sensorimotor cortex has been associated with sensory stimulation during swallowing (25), throat clearing, and tongue movements (21, 46), along with automatic and voluntary swallowing (23) and reflexive swallowing (20). Activity in the premotor cortex is associated with both swallowing (23, 39, 47) and tongue movements (21, 22), as is activity in the superior parietal lobule (SPL) (21) and the SMA (22, 46). The PO has been implicated in the voluntary control of facial, pharyngeal, lingual, and masticatory muscles (48). Activity in the aMCC/ACC is related to tongue movements (20–22), preparing to swallow and clearing the throat (21), as well as voluntary but not automatic swallowing (23, 24). The latter region has also been associated with sensory stimulation, motor planning, and motor execution related to swallowing (25). The correspondence between areas identified in the present study and those reported above affirms that, in the present work, regions involved in swallowing show activation in relation to drinking behavior that is dependent upon hydration status. Furthermore, as subjective ratings of swallowing effort increase following satiation (28), the finding that these regions showed greater activation for the thirsty condition compared with the oversated condition suggests these areas facilitate drinking behavior during a state of thirst. Finally, the inclusion of the aMCC as one of the regions involved in this putative facilitation supports the hypothesis that the aMCC is associated with drinking responses during this state.
Several subcortical regions also showed increased activation for the thirsty condition compared with the oversated condition, with the most pronounced activity observed in two cerebellar lobules, VI and VIIIa (49). Activation of the cerebellum has previously been associated with the subjective state of thirst (50), the taste of water (42), voluntary swallowing (40, 51), and tongue movements (40). The activation of lobule VI in the present study is of particular interest given this region has strong representations of the face and mouth, which suggests it may contain a potential representation of swallowing (52). Given its association with thirst and swallowing, along with its established role in the coordination and timing of motor behavior (53), it seems likely that the relative increase in cerebellar activity for the thirsty condition reflects improved coordination and timing of motor events associated with swallowing during a state of thirst. Such activity could therefore also contribute to the facilitation of drinking during this condition.
PPI During the Thirsty Condition.
Many of the cortical and subcortical regions discussed above showed an increase in functional connectivity with the aMCC seed during drinking behavior for both the thirsty and oversated conditions. The identification of a functionally connected network during drinking behavior that shows increased activation during the thirsty condition therefore consolidates the evidence that the implicated regions contribute to the facilitation of drinking during the thirsty condition. In addition, the identification of this network using a seed located in the aMCC implies a central role for this region in the facilitation of drinking in response to dehydration.
PPI Between Thirsty and Oversated Conditions.
The regions in the medulla that showed a greater increase in functional connectivity during the thirsty condition are likely to contain premotor or motor neurons that control the oral, pharyngeal, and esophageal phases of swallowing. These include the FN, NTS, SpV, and NA (32, 54). In animal studies, it has been demonstrated that these nuclei receive input from all of the cranial nerves involved in initiating or facilitating swallowing (55, 56), including those from the superior laryngeal nerve (57), which consistently elicits swallowing when stimulated (58, 59). While swallowing is induced by chemical or tactile stimulation of the regions innervated by these nerves (60), which include the tongue, epiglottis, and posterior pharynx, stimulation of the medulla nuclei themselves can also elicit a basic swallowing pattern in the absence of any input (58, 61). This suggests that these nuclei are the preeminent structures involved in swallowing. In the present study, increased functional connectivity between the aMCC and these regions during the thirsty condition is therefore consistent, with the aMCC influencing swallowing during a state of thirst. Given swallowing effort is also reduced in the thirsty condition relative to the oversated condition (28), the aMCC may thus facilitate swallowing during the thirsty condition by providing a source of cortical control (62) over nuclei in the medulla that control swallowing.
PPI Correlation with Ratings of Swallowing Effort During Oversated Condition.
During the oversated condition, those participants with the greatest increase in functional connectivity between the aMCC seed and the primary sensorimotor cortex experienced the least swallowing effort. As the primary sensorimotor cortex is the most consistently activated region in relation to voluntary swallowing (17, 18), this finding implies a facilitation of swallowing in these participants, despite all participants experiencing a general increase in swallowing effort during the oversated condition (28). Functional connectivity between the aMCC and primary sensorimotor cortex during the oversated condition could therefore represent a compensatory response to the increase in swallowing difficulty during this condition. Evidence for such a response has been presented in two other studies performed by our group. First, increased activation was reported in the mid and inferior primary motor cortex for the oversated condition compared with the thirsty condition during swallowing (27); second, when liquid was held in the mouth before swallowing, increased activation in the bilateral prefrontal cortex and primary motor cortex was reported during the oversated condition relative to the thirsty condition (28). Both findings were interpreted as the recruitment of these regions during the oversated condition to overcome the emergence of swallowing inhibition so that compliant drinking could continue. The findings of these two studies, along with those of the present study, are therefore consistent with the generation of a compensatory response, originating in the frontal cortex, which allows compliant swallowing to continue once excess fluid has been ingested.
Implications.
The results presented in this study provide converging evidence that activity in the aMCC is associated with drinking responses during a state of thirst and that this region is likely to contribute to a facilitation of drinking during this state. These findings have important implications for whether the experience of satiation, which is associated with a reduction in activity in the aMCC (11, 26), could help conclude a drinking response in the absence of relevant blood volume and osmolality signals from the circulatory system.
The origin of the signal representing satiation is currently unknown; however, it should be noted that oral and pharyngeal stimulation during swallowing has been associated with the satiation of thirst (36, 38). Studies in a variety of species have also found oral, pharyngeal, and esophageal factors are likely to contribute to the termination of drinking (34–36), along with gastric and postgastric factors (63–65). The presence of a satiation signal is also consistent with a feedback-mediated decision-making model for the dorsal aMCC (66), although the region implicated in the present study is located more ventrally. This discrepancy may be due to the cognitive role ascribed to the dorsal aMCC in the model, which involves integrating information to increase the efficiency of cognitive/motor decision-making and execution (66). This role contrasts with the relatively automated initiation and termination of drinking during the thirsty condition in the present study. The existing efficiency of this process therefore suggests the services of the dorsal aMCC are not required, a proposition supported by the finding that task difficulty distinguishes between the two regions (67).
In the present study, the evidence for the experience of satiation contributing to the conclusion of a drinking response is as follows. First, during a state of satiation, the aMCC and cortical regions associated with swallowing showed reduced activity relative to a state of thirst, suggesting that the cortical control of swallowing during drinking behavior is diminished once a sufficient volume of water has been ingested to correct the fluid deficit. Second, reduced functional connectivity between the aMCC and swallowing nuclei located in the medulla during the oversated condition is consistent with a reduction in the cortical control of swallowing, although care should be exercised in interpreting causal relationships on the basis of correlation results. Third, the PPI correlation involving effort ratings during the oversated condition showed an increase in effort was associated with a decrease in functional connectivity between the aMCC and the primary sensorimotor cortex, a region prominently involved in swallowing. These three findings, all related to the oversated condition, involve changes in brain activity and functional connectivity that are likely to occur before the absorption of fluid returns plasma osmolality to baseline, a process that can take as long as 50 min (36).
The aMCC may thus represent an allostasis mechanism (8) that anticipates the correction of fluid deficit in the body and adjusts drinking behavior accordingly. Because recent animal studies have identified neurons in the lamina terminalis that appear to perform this role (5–7, 9), the aMCC may function as a higher-order cortical supervisory region that supervenes over the lower-level operations performed by these neurons. It is interesting to speculate that the experience of satiation, which is associated with a reduction in aMCC activity in the absence of any relevant changes in blood chemistry (11), may represent a “predicted” correction of fluid deficit instead of the actual fluid deficit present within the body. Decreases in aMCC/ACC activity are also associated with decreases in the subjective state of pleasantness that accompanies progressive water intake during dehydration (26, 27); as these changes are also likely to occur in the absence of changes in blood chemistry, it is possible that this subjective state also represents a prediction. A reduction in reward may thus be predicted if drinking continues after predicted alleviation of the aversive thirst stimulus by current drinking behavior. Given the wide range of tasks and behaviors associated with aMCC activity, it would therefore be interesting to investigate whether other examples of aMCC activity could be found in which a predicted outcome, rather than an actual outcome, correlates with subjective experience.
Method
Dataset.
Twenty healthy participants (13 male; age range, 23–45 y; mean age, 30.0 ± 1.5 y) reported subjective ratings of thirst, pleasantness, and swallowing effort in a previous study that imaged preswallow and swallowing events when participants drank while thirsty and while oversated (28). We used the same dataset for the present study, performing a conjunction analysis on the fMRI data to combine the preswallow and swallow periods. The earlier study also used two liquids as stimuli: water and a sugar solution composed of 8% (wt/vol) sucrose added to water. Because no effect of stimulus was found in that study between the thirsty and oversated conditions, or in the individual conditions themselves, responses to both stimuli were amalgamated and treated as a single-liquid stimulus. The same approach was adopted for the present study.
MRI Scanning.
The fMRI data were collected at the Murdoch Children’s Research Institute (Melbourne, Australia) using a Siemens Magnetom Trio 3 Tesla scanner (Siemens AG) incorporating a 32-channel head coil. For details regarding acquisition parameters, refer to ref. 28. Four functional scans and one structural scan were acquired. The first two functional scans represented the thirsty condition; the third and fourth represented the oversated condition. After the second scan, participants were removed from the scanner and drank as much water as they could comfortably tolerate. While in the scanner, participants received 5-mL volumes of the liquid stimulus, which they briefly held in their mouth before swallowing. Participants subsequently rated the pleasantness of the liquid taste and the effort required to swallow it. For full details of the experimental protocol, see figure S1 of ref. 28.
Brain Activation Analysis.
Brain activity was analyzed during a period designated “drinking behavior,” a 10-s period incorporating both preswallow and swallow events. Preprocessing and analysis of functional images was performed using standard procedures with FEAT, v4.1.9 (68). Drinking behavior was modeled with four regressors, the first two representing brain responses to water and sugar solution during a 7-s “liquid in the mouth” period and the last two representing brain responses to water and sugar solution during a 3-s “swallowing” period. Separate regressors for the preswallow and swallow periods were used so that the analysis consisted of variance specifically associated with both events. Separate regressors for water and sugar solution were also included to model the two subsequent ratings events (swallowing effort and subjective pleasantness). Additional confound regressors were added related to head motion, physiological noise produced by respiratory maneuvers during swallowing (69, 70), and physical movement at the time of swallowing. Principle activation analyses were generated showing brain activity during either the thirsty condition or the oversated condition. Contrasts between the two conditions were produced, with each contrast masked with the relevant principle activation analysis (e.g., thirsty > oversated condition masked with thirsty activation). Significant activations were determined using a single-voxel inclusion threshold of z > 2.3 (P < 0.01) and a cluster level threshold of Pcorr < 0.05 corrected for multiple comparisons (71).
PPI Analysis.
The aMCC was chosen as the location for the PPI seed as this region is consistently implicated in the literature on thirst and drinking (see introductory paragraphs for details). The contrast between brain activity during drinking behavior in the thirsty condition compared with the oversated condition was used to define the seed. Voxel inclusion criteria for the seed were a z statistic > 3.0 and >50% gray matter. Selection was based on probabilities provided by the Harvard–Oxford Cortical Structural Atlas, one of the analytic tools provided by FSL to interrogate fMRI data.
For the PPI analysis, two additional regressors were added to the model used for the activation analysis: the PPI and the time series of the aMCC seed. The PPI was generated from the four regressors comprising drinking behavior and the time series of the seed. Principle functional connectivity analyses were produced for the thirsty condition and the oversated condition (Fig. S1C). Contrasts of functional connectivity between the two conditions were performed, with each contrast masked with the relevant principle activation analysis (Fig. S1E). Correlation analyses were also performed for each condition involving functional connectivity and participants’ mean effort ratings for that condition. Significant activations were determined using a single voxel inclusion threshold of z > 2.3 (P < 0.01) and a cluster level threshold of Pcorr < 0.05 corrected for multiple comparisons (71).
Supplementary Material
Acknowledgments
This work was supported by the G. Harold and Leila Y. Mathers Charitable Foundation, the Search Foundation, S. Baillieu Myer, Diana Gibson, Robert Albert, Dr. Mark Nelson, Tim Jones, Marcus Besen, and Jeanne Pratt.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1717646115/-/DCSupplemental.
References
- 1.Begg DP. Disturbances of thirst and fluid balance associated with aging. Physiol Behav. 2017;178:28–34. doi: 10.1016/j.physbeh.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Gardner JW. Death by water intoxication. Mil Med. 2002;167:432–434. [PubMed] [Google Scholar]
- 3.Hawken ER, et al. Mortality over a 20-year period in patients with primary polydipsia associated with schizophrenia: A retrospective study. Schizophr Res. 2009;107:128–133. doi: 10.1016/j.schres.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 4.Rosner MH, Kirven J. Exercise-associated hyponatremia. Clin J Am Soc Nephrol. 2007;2:151–161. doi: 10.2215/CJN.02730806. [DOI] [PubMed] [Google Scholar]
- 5.Oka Y, Ye M, Zuker CS. Thirst driving and suppressing signals encoded by distinct neural populations in the brain. Nature. 2015;520:349–352. doi: 10.1038/nature14108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zimmerman CA, et al. Thirst neurons anticipate the homeostatic consequences of eating and drinking. Nature. 2016;537:680–684. doi: 10.1038/nature18950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abbott SB, Machado NL, Geerling JC, Saper CB. Reciprocal control of drinking behavior by median preoptic neurons in mice. J Neurosci. 2016;36:8228–8237. doi: 10.1523/JNEUROSCI.1244-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sterling P. Allostasis: A model of predictive regulation. Physiol Behav. 2012;106:5–15. doi: 10.1016/j.physbeh.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Zocchi D, Wennemuth G, Oka Y. The cellular mechanism for water detection in the mammalian taste system. Nat Neurosci. 2017;20:927–933. doi: 10.1038/nn.4575. [DOI] [PubMed] [Google Scholar]
- 10.Vogt BA. Midcingulate cortex: Structure, connections, homologies, functions and diseases. J Chem Neuroanat. 2016;74:28–46. doi: 10.1016/j.jchemneu.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Denton D, et al. Neuroimaging of genesis and satiation of thirst and an interoceptor-driven theory of origins of primary consciousness. Proc Natl Acad Sci USA. 1999;96:5304–5309. doi: 10.1073/pnas.96.9.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egan G, et al. Neural correlates of the emergence of consciousness of thirst. Proc Natl Acad Sci USA. 2003;100:15241–15246. doi: 10.1073/pnas.2136650100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farrell MJ, et al. Unique, common, and interacting cortical correlates of thirst and pain. Proc Natl Acad Sci USA. 2006;103:2416–2421. doi: 10.1073/pnas.0511019103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denton D, et al. Correlation of regional cerebral blood flow and change of plasma sodium concentration during genesis and satiation of thirst. Proc Natl Acad Sci USA. 1999;96:2532–2537. doi: 10.1073/pnas.96.5.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farrell MJZF, et al. Effect of aging on regional cerebral blood flow responses associated with osmotic thirst and its satiation by water drinking: A PET study. Proc Natl Acad Sci USA. 2008;105:382–387. doi: 10.1073/pnas.0710572105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farrell MJ, et al. Cortical activation and lamina terminalis functional connectivity during thirst and drinking in humans. Am J Physiol Regul Integr Comp Physiol. 2011;301:R623–R631. doi: 10.1152/ajpregu.00817.2010. [DOI] [PubMed] [Google Scholar]
- 17.Hamdy S. Role of cerebral cortex in the control of swallowing. GI Motility Online. 2006 doi: 10.1038/gimo8. [DOI] [Google Scholar]
- 18.Humbert IA, Robbins J. Normal swallowing and functional magnetic resonance imaging: A systematic review. Dysphagia. 2007;22:266–275. doi: 10.1007/s00455-007-9080-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sörös P, Inamoto Y, Martin RE. Functional brain imaging of swallowing: An activation likelihood estimation meta-analysis. Hum Brain Mapp. 2009;30:2426–2439. doi: 10.1002/hbm.20680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kern MK, Jaradeh S, Arndorfer RC, Shaker R. Cerebral cortical representation of reflexive and volitional swallowing in humans. Am J Physiol Gastrointest Liver Physiol. 2001;280:G354–G360. doi: 10.1152/ajpgi.2001.280.3.G354. [DOI] [PubMed] [Google Scholar]
- 21.Malandraki GA, Sutton BP, Perlman AL, Karampinos DC, Conway C. Neural activation of swallowing and swallowing-related tasks in healthy young adults: An attempt to separate the components of deglutition. Hum Brain Mapp. 2009;30:3209–3226. doi: 10.1002/hbm.20743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin RE, et al. Cerebral areas processing swallowing and tongue movement are overlapping but distinct: A functional magnetic resonance imaging study. J Neurophysiol. 2004;92:2428–2443. doi: 10.1152/jn.01144.2003. [DOI] [PubMed] [Google Scholar]
- 23.Martin RE, Goodyear BG, Gati JS, Menon RS. Cerebral cortical representation of automatic and volitional swallowing in humans. J Neurophysiol. 2001;85:938–950. doi: 10.1152/jn.2001.85.2.938. [DOI] [PubMed] [Google Scholar]
- 24.Toogood JA, et al. Discrete functional contributions of cerebral cortical foci in voluntary swallowing: A functional magnetic resonance imaging (fMRI) “Go, No-Go” study. Exp Brain Res. 2005;161:81–90. doi: 10.1007/s00221-004-2048-1. [DOI] [PubMed] [Google Scholar]
- 25.Lowell SY, et al. Sensory stimulation activates both motor and sensory components of the swallowing system. Neuroimage. 2008;42:285–295. doi: 10.1016/j.neuroimage.2008.04.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Becker CA, Flaisch T, Renner B, Schupp HT. From thirst to satiety: The anterior mid-cingulate cortex and right posterior insula indicate dynamic changes in incentive value. Front Hum Neurosci. 2017;11:234. doi: 10.3389/fnhum.2017.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saker P, et al. Regional brain responses associated with drinking water during thirst and after its satiation. Proc Natl Acad Sci USA. 2014;111:5379–5384. doi: 10.1073/pnas.1403382111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saker P, Farrell MJ, Egan GF, McKinley MJ, Denton DA. Overdrinking, swallowing inhibition, and regional brain responses prior to swallowing. Proc Natl Acad Sci USA. 2016;113:12274–12279. doi: 10.1073/pnas.1613929113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bush G, et al. Dorsal anterior cingulate cortex: A role in reward-based decision making. Proc Natl Acad Sci USA. 2002;99:523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shima K, Tanji J. Role for cingulate motor area cells in voluntary movement selection based on reward. Science. 1998;282:1335–1338. doi: 10.1126/science.282.5392.1335. [DOI] [PubMed] [Google Scholar]
- 31.Williams ZM, Bush G, Rauch SL, Cosgrove GR, Eskandar EN. Human anterior cingulate neurons and the integration of monetary reward with motor responses. Nat Neurosci. 2004;7:1370–1375. doi: 10.1038/nn1354. [DOI] [PubMed] [Google Scholar]
- 32.Lang IM. Brain stem control of the phases of swallowing. Dysphagia. 2009;24:333–348. doi: 10.1007/s00455-009-9211-6. [DOI] [PubMed] [Google Scholar]
- 33.Miller AJ. The neurobiology of swallowing and dysphagia. Dev Disabil Res Rev. 2008;14:77–86. doi: 10.1002/ddrr.12. [DOI] [PubMed] [Google Scholar]
- 34.Bott E, Denton DA, Weller S. Water drinking in sheep with oesophageal fistulae. J Physiol. 1965;176:323–336. doi: 10.1113/jphysiol.1965.sp007553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brunstrom JM, Tribbeck PM, MacRae AW. The role of mouth state in the termination of drinking behavior in humans. Physiol Behav. 2000;68:579–583. doi: 10.1016/s0031-9384(99)00210-3. [DOI] [PubMed] [Google Scholar]
- 36.Figaro MK, Mack GW. Regulation of fluid intake in dehydrated humans: Role of oropharyngeal stimulation. Am J Physiol. 1997;272:R1740–R1746. doi: 10.1152/ajpregu.1997.272.6.R1740. [DOI] [PubMed] [Google Scholar]
- 37.Phillips PA, Rolls BJ, Ledingham JGG, Morton JJ. Body fluid changes, thirst and drinking in man during free access to water. Physiol Behav. 1984;33:357–363. doi: 10.1016/0031-9384(84)90154-9. [DOI] [PubMed] [Google Scholar]
- 38.Rolls BJ, et al. Thirst following water deprivation in humans. Am J Physiol. 1980;239:R476–R482. doi: 10.1152/ajpregu.1980.239.5.R476. [DOI] [PubMed] [Google Scholar]
- 39.Hamdy S, et al. Cortical activation during human volitional swallowing: An event-related fMRI study. Am J Physiol. 1999;277:G219–G225. doi: 10.1152/ajpgi.1999.277.1.G219. [DOI] [PubMed] [Google Scholar]
- 40.Zald DH, Pardo JV. The functional neuroanatomy of voluntary swallowing. Ann Neurol. 1999;46:281–286. [PubMed] [Google Scholar]
- 41.de Araujo IE, Kringelbach ML, Rolls ET, McGlone F. Human cortical responses to water in the mouth, and the effects of thirst. J Neurophysiol. 2003;90:1865–1876. doi: 10.1152/jn.00297.2003. [DOI] [PubMed] [Google Scholar]
- 42.Zald DH, Pardo JV. Cortical activation induced by intraoral stimulation with water in humans. Chem Senses. 2000;25:267–275. doi: 10.1093/chemse/25.3.267. [DOI] [PubMed] [Google Scholar]
- 43.Friston KJ, et al. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 44.O’Reilly JX, Woolrich MW, Behrens TE, Smith SM, Johansen-Berg H. Tools of the trade: Psychophysiological interactions and functional connectivity. Soc Cogn Affect Neurosci. 2012;7:604–609. doi: 10.1093/scan/nss055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allen WE, et al. Thirst-associated preoptic neurons encode an aversive motivational drive. Science. 2017;357:1149–1155. doi: 10.1126/science.aan6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Satow T, et al. Role of primary sensorimotor cortex and supplementary motor area in volitional swallowing: A movement-related cortical potential study. Am J Physiol Gastrointest Liver Physiol. 2004;287:G459–G470. doi: 10.1152/ajpgi.00323.2003. [DOI] [PubMed] [Google Scholar]
- 47.Mosier K, Bereznaya I. Parallel cortical networks for volitional control of swallowing in humans. Exp Brain Res. 2001;140:280–289. doi: 10.1007/s002210100813. [DOI] [PubMed] [Google Scholar]
- 48.Mao C-C, Coull BM, Golper LAC, Rau MT. Anterior operculum syndrome. Neurology. 1989;39:1169–1172. doi: 10.1212/wnl.39.9.1169. [DOI] [PubMed] [Google Scholar]
- 49.Schmahmann JD, et al. Three-dimensional MRI atlas of the human cerebellum in proportional stereotaxic space. Neuroimage. 1999;10:233–260. doi: 10.1006/nimg.1999.0459. [DOI] [PubMed] [Google Scholar]
- 50.Parsons LM, et al. Neuroimaging evidence implicating cerebellum in support of sensory/cognitive processes associated with thirst. Proc Natl Acad Sci USA. 2000;97:2332–2336. doi: 10.1073/pnas.040555497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suzuki M, et al. Activation of cerebellum and basal ganglia on volitional swallowing detected by functional magnetic resonance imaging. Dysphagia. 2003;18:71–77. doi: 10.1007/s00455-002-0088-x. [DOI] [PubMed] [Google Scholar]
- 52.Mottolese C, et al. Mapping motor representations in the human cerebellum. Brain. 2013;136:330–342. doi: 10.1093/brain/aws186. [DOI] [PubMed] [Google Scholar]
- 53.Mauk MD, Medina JF, Nores WL, Ohyama T. Cerebellar function: Coordination, learning or timing? Curr Biol. 2000;10:R522–R525. doi: 10.1016/s0960-9822(00)00584-4. [DOI] [PubMed] [Google Scholar]
- 54.Jean A. Brain stem control of swallowing: Neuronal network and cellular mechanisms. Physiol Rev. 2001;81:929–969. doi: 10.1152/physrev.2001.81.2.929. [DOI] [PubMed] [Google Scholar]
- 55.Contreras RJ, Beckstead RM, Norgren R. The central projections of the trigeminal, facial, glossopharyngeal and vagus nerves: An autoradiographic study in the rat. J Auton Nerv Syst. 1982;6:303–322. doi: 10.1016/0165-1838(82)90003-0. [DOI] [PubMed] [Google Scholar]
- 56.Torvik A. Afferent connections to the sensory trigeminal nuclei, the nucleus of the solitary tract and adjacent structures; an experimental study in the rat. J Comp Neurol. 1956;106:51–141. doi: 10.1002/cne.901060104. [DOI] [PubMed] [Google Scholar]
- 57.Kalia M, Mesulam MM. Brain stem projections of sensory and motor components of the vagus complex in the cat: II. Laryngeal, tracheobronchial, pulmonary, cardiac, and gastrointestinal branches. J Comp Neurol. 1980;193:467–508. doi: 10.1002/cne.901930211. [DOI] [PubMed] [Google Scholar]
- 58.Miller AJ. Characteristics of the swallowing reflex induced by peripheral nerve and brain stem stimulation. Exp Neurol. 1972;34:210–222. doi: 10.1016/0014-4886(72)90168-9. [DOI] [PubMed] [Google Scholar]
- 59.Doty RW. Influence of stimulus pattern on reflex deglutition. Am J Physiol. 1951;166:142–158. doi: 10.1152/ajplegacy.1951.166.1.142. [DOI] [PubMed] [Google Scholar]
- 60.Steele CM, Miller AJ. Sensory input pathways and mechanisms in swallowing: A review. Dysphagia. 2010;25:323–333. doi: 10.1007/s00455-010-9301-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kessler JP, Jean A. Identification of the medullary swallowing regions in the rat. Exp Brain Res. 1985;57:256–263. doi: 10.1007/BF00236530. [DOI] [PubMed] [Google Scholar]
- 62.Michou E, Hamdy S. Cortical input in control of swallowing. Curr Opin Otolaryngol Head Neck Surg. 2009;17:166–171. doi: 10.1097/MOO.0b013e32832b255e. [DOI] [PubMed] [Google Scholar]
- 63.Kurozumi C, Yamagata R, Himi N, Koga T. Emetic stimulation inhibits the swallowing reflex in decerebrate rats. Auton Neurosci. 2008;140:24–29. doi: 10.1016/j.autneu.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 64.Towbin EJ. Gastric distention as a factor in the satiation of thirst in esophagostomized dogs. Am J Physiol. 1949;159:533–541. doi: 10.1152/ajplegacy.1949.159.3.533. [DOI] [PubMed] [Google Scholar]
- 65.Wood RJ, Maddison S, Rolls ET, Rolls BJ, Gibbs J. Drinking in rhesus monkeys: Role of presystemic and systemic factors in control of drinking. J Comp Physiol Psychol. 1980;94:1135–1148. doi: 10.1037/h0077745. [DOI] [PubMed] [Google Scholar]
- 66.Bush G. Dorsal anterior midcingulate cortex: Roles in normal cognition and disruption in attention-deficit/hyperactivity disorder. In: Vogt BA, editor. Cingulate Neurobiology and Disease. Oxford Univ Press; New York: 2009. pp. 245–274. [Google Scholar]
- 67.Paus T, Koski L, Caramanos Z, Westbury C. Regional differences in the effects of task difficulty and motor output on blood flow response in the human anterior cingulate cortex: A review of 107 PET activation studies. Neuroreport. 1998;9:R37–R47. doi: 10.1097/00001756-199806220-00001. [DOI] [PubMed] [Google Scholar]
- 68.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 69.Mazzone SB, Cole LJ, Ando A, Egan GF, Farrell MJ. Investigation of the neural control of cough and cough suppression in humans using functional brain imaging. J Neurosci. 2011;31:2948–2958. doi: 10.1523/JNEUROSCI.4597-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Birn RM, Murphy K, Handwerker DA, Bandettini PA. fMRI in the presence of task-correlated breathing variations. Neuroimage. 2009;47:1092–1104. doi: 10.1016/j.neuroimage.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab. 1992;12:900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.