Significance
The LipB–LipA and lipoyl-relay pathways seem restricted to gram-negative and gram-positive bacteria, respectively. Braakman and Smith have persuasively argued that the lipoyl-relay pathway involving transfer of lipoic acid from lipoylated GcvH is the primordial pathway and that the LipB–LipA pathway arose later. The chemically challenging nature of lipoyl transfer strengthens this argument. Given the roughly 3 billion years since the two groups of bacteria diverged, it is unexpected and puzzling that the lipoyl transfer properties of many GcvHs—those from eukaryotes and bacteria, including Escherichia coli, and the deep-branching hyperthermophilic chemoautotrophic bacterium, Aquifex aeolicus—have lipoyl-relay activity in Bacillus subtilis. This provides a remarkable case in which a protein “moonlighting” function is found throughout evolution without selection for the function.
Keywords: lipoic acid, glycine cleavage, enzyme moonlighting, aerobic metabolism, C1 metabolism
Abstract
Lipoic acid is synthesized by a remarkably atypical pathway in which the cofactor is assembled on its cognate proteins. An octanoyl moiety diverted from fatty acid synthesis is covalently attached to the acceptor protein, and sulfur insertion at carbons 6 and 8 of the octanoyl moiety form the lipoyl cofactor. Covalent attachment of this cofactor is required for function of several central metabolism enzymes, including the glycine cleavage H protein (GcvH). In Bacillus subtilis, GcvH is the sole substrate for lipoate assembly. Hence lipoic acid-requiring 2-oxoacid dehydrogenase (OADH) proteins acquire the cofactor only by transfer from lipoylated GcvH. Lipoyl transfer has been argued to be the primordial pathway of OADH lipoylation. The Escherichia coli pathway where lipoate is directly assembled on both its GcvH and OADH proteins, is proposed to have arisen later. Because roughly 3 billion years separate the divergence of these bacteria, it is surprising that E. coli GcvH functionally substitutes for the B. subtilis protein in lipoyl transfer. Known and putative GcvHs from other bacteria and eukaryotes also substitute for B. subtilis GcvH in OADH modification. Because glycine cleavage is the primary GcvH role in ancestral bacteria that lack OADH enzymes, lipoyl transfer is a “moonlighting” function: that is, development of a new function while retaining the original function. This moonlighting has been conserved in the absence of selection by some, but not all, GcvH proteins. Moreover, Aquifex aeolicus encodes five putative GcvHs, two of which have the moonlighting function, whereas others function only in glycine cleavage.
Lipoic acid is an essential sulfur-containing coenzyme required by key enzymes of central metabolism in all three domains of life (1, 2). Unlike most coenzymes, lipoic acid functions only after covalent attachment to its cognate enzyme proteins. These are the E2 subunits of the 2-oxoacid dehydrogenase (OADH) complexes and the H proteins (called GcvH or GcsH, depending on the organism) of the glycine cleavage system (glycine decarboxylase in plants), all of which have been well studied (1). Although the OADH lipoyl domains (LDs) and the GcvH proteins both contain a strictly conserved lysine residue to which lipoic acid is attached and have remarkably similar structures, the proteins have only low sequence identities (1, 3). Lipoic acid attachment to these proteins is via an amide linkage formed between the lipoic acid carboxyl group and the conserved lysine ε-amino group (1, 3). Once attached, the lipoyl moiety becomes competent for enzyme catalysis. The terminal dithiolane ring becomes reduced and acylated with a reaction intermediate and the lipoyl-lysine arm serves to shuttle reaction intermediates between the active sites of the multienzyme complexes (3).
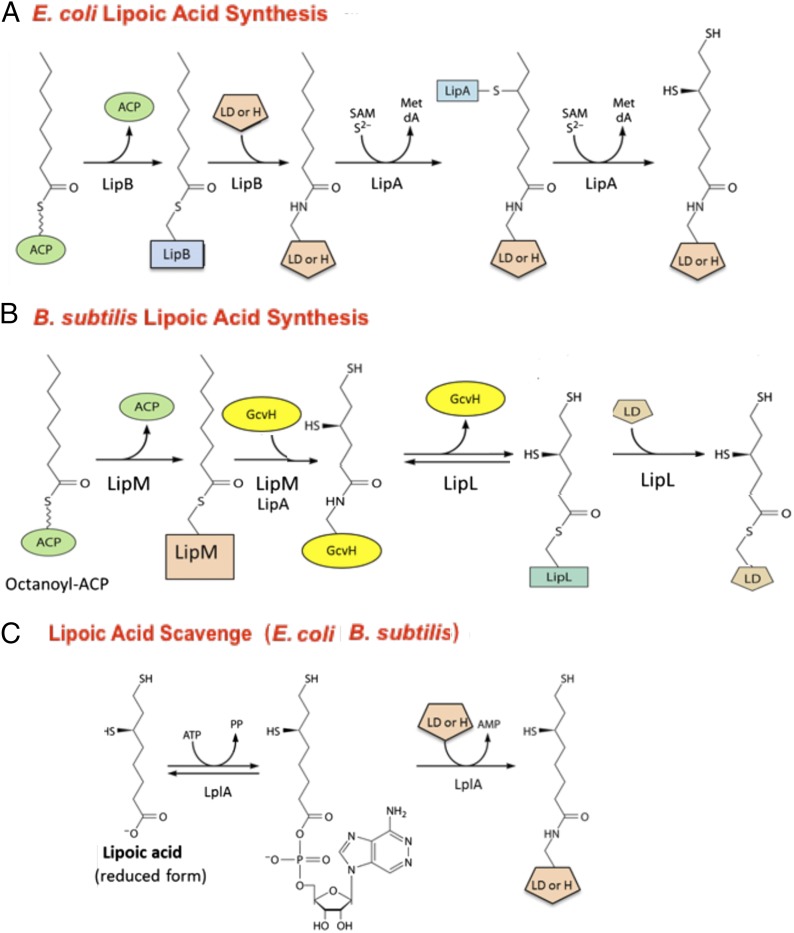
Lipoic acid assembly is best understood in Escherichia coli, where only two enzymes are required. LipB, an octanoyl transferase transfers the octanoyl moiety from fatty acid biosynthetic intermediate octanoyl-acyl carrier protein (ACP), to the specific lysine side chain of GcvH or an OADH LD (1, 4). The resulting octanoyl-protein is a substrate for the sulfur insertion step catalyzed by the radical iron-sulfur protein, LipA. LipA donates two sulfur atoms to carbons 6 and 8 of octanoate to give a dihydrolipoyl moiety which becomes oxidized to lipoyl-LD (Fig. 1A). Hence, lipoic acid is an atypical enzyme cofactor in that it is assembled on its cognate proteins rather than being wholly assembled before its covalent attachment, as is the case with biotin, another covalently attached coenzyme (5).
Fig. 1.
Lipoic acid synthesis and scavenging in E. coli and B. subtilis. (A) The E. coli lipoic acid synthetic pathway requires only two proteins, LipB and LipA. The LipB octanoyl-ACP transferase transfers the octanoyl moiety from the fatty acid biosynthetic intermediate, octanoyl-ACP, to both OADH LDs and GcvH, where LipA-catalyzed sulfur insertion converts the octanoyl moiety to lipoate. (B) The B. subtilis lipoic acid synthetic pathway requires four proteins (three enzymes plus the GcvH protein, on which lipoate is assembled and donated to the OADH proteins) rather than the two enzymes of the simpler E. coli pathway (A). Like E. coli, B. subtilis has the LipA sulfur insertion enzyme and an octanoyl transferase, LipM, although LipM is an isozyme rather than a homolog of the E. coli LipB octanoyl transferase. The octanoylated GcvH then becomes a substrate for LipA-catalyzed sulfur insertion at carbons 6 and 8, as in A. The lipoyl moiety is then relayed from GcvH to the OADH subunits by an amidotransferase called LipL. (C) Both bacteria scavenge lipoate from the medium by use of the reaction shown for the E. coli lipoate ligase, LplA. The B. subtilis ligase is LplJ (8). The ligases (which are 33% identical) are required for lipoate supplementation of strains blocked in lipoic acid synthesis. E. coli LplA efficiently lipoylates both its cognate GcvH and the OADH domains (43), whereas in B. subtilis GcvH is a much better substrate for LplJ than are the OADH proteins (8).
The straightforward two-enzyme, LipB plus LipA, E. coli lipoate synthesis pathway is found in many, but not all, bacterial species. A more complex pathway is found in Bacillus subtilis (6–9). The impetus to study this bacterium was the lack of a gene encoding a LipB homolog (6), although a validated LipA homolog was present (9). Moreover, the B. subtilis genome encoded what appeared to be three lipoate ligase homologs, the paradigm of which, E. coli LplA, scavenges lipoate from the environment (8). Screening of a plasmid library of B. subtilis genome fragments for complementation of an E. coli strain lacking LipB gave one of the putative lipoate ligase genes. However, the gene encoded an octanoyl transferase called LipM that had no ligase activity and virtually no sequence resemblance to E. coli LipB (6). Another putative lipoate ligase gene (LplJ) encoded a true lipoate ligase, whereas the third putative lipoate ligase gene (lipL) remained an enigma, although it was essential for lipoate synthesis (8). The enzymological characterization of LipM presented a quandary in that the purified enzyme was unable to transfer octanoate from octanoyl-ACP to its cognate pyruvate dehydrogenase (PDH) LDs, although the proteins were good substrates for the E. coli LipB octanoyl transferase (7). A concern was the possibility that LipM recognition of the B. subtilis PDH domains required assembly of the E2 subunit into the very large PDH complexes that contain many copies of E2, plus the E1 and E3 subunits. To avoid the possibility of a complex being the substrate, GcvH, a protein of only 127 residues (and thus only slightly larger than the OADH LDs of ∼80 residues), was tested as a LipM substrate. In contrast to the cognate PDH LDs, GcvH turned out to be an excellent LipM substrate (7), which raised the possibility that GcvH might somehow act as an intermediate between LipM and the OADH LDs. Indeed when LipL, the third putative B. subtilis lipoate ligase, was purified, the protein lacked ligase activity but was found to transfer an octanoyl moiety from its amide linkage on GcvH to the PDH LD using an acyl-enzyme (acyl-cysteine) intermediate (7). These biochemical experiments argued that GcvH was an obligate intermediate in lipoylation of the B. subtilis OADH proteins, and therefore B. subtilis ∆gcvH strains should be unable to make lipoate and be auxotrophic for lipoate. This was the case: the small GcvH protein is required for both lipoylation of the B. subtilis subunits (PDH for aerobic metabolism and the branched-chain OADH for fatty acid synthesis) and for glycine cleavage (7). Hence, a small protein of single-carbon metabolism has a second “moonlighting” (10–12) function that, for simplicity, we will refer to as the lipoyl-relay pathway. Therefore, although it is a protein of only 127 residues, the B. subtilis glycine cleavage H protein (GcvH) has three separate functions in central metabolism. Note that essentially the same pathway has recently been demonstrated in Staphylococcus aureus (13), and phenotypic analyses of Saccharomyces cerevisiae argue that this yeast also may use a similar pathway (14).
Brocklehurst and Perham (15) first deduced that despite low sequence identities (15–20%), the OADH LD structures could be used to accurately predict the pea GcvH structure. Although a similar low sequence identity is seen between the E. coli OADH LDs and its GcvH, the LipB octanoyl transferase efficiently modifies both proteins (16, 17). This is not the case with the B. subtilis LipM octanoyl transferase, which efficiently modifies its cognate GcvH but fails to modify its cognate OADH LDs (7), and thus the OADH domains must obtain bound lipoate from GcvH by LipL-catalyzed transfer (7) (Fig. 1B). This lipoyl transfer reaction is chemically challenging because the LipL active site thiol must attack a kinetically stable amide bond. Given this challenge, it seems likely that GcvH provides an environment that facilitates the LipL reaction and that the OADH domains lack this property. To test this hypothesis, we replaced the B. subtilis gcvH with genes encoding diverse GcvH and OADH LD proteins and tested for restoration of growth in the absence of lipoic acid. We also tested the proteins as acceptors of LipM-catalyzed octanoyl transfer in vitro. Substitution of E. coli GcvH for the B. subtilis protein would be particularly interesting because the E. coli does not use the lipoyl-relay pathway (Fig. 1A). Successful substitution would indicate that E. coli GcvH retains the ability to support lipoyl relay despite a lack of selection for this function. Moreover we also tested the seemingly redundant five copies of GcvH encoded in the small genome (1.5 Mb) of the deep-branching bacterium, Aquifex aeolicus. These proteins were analyzed because Braakman and Smith (18) developed their hypothesis that GcvH is the primordial lipoylated protein based on their analysis of A. aeolicus. To our surprise, two of these proteins were able to provide lipoyl-relay function to B. subtilis.
Results
Complementation of B. subtilis ∆gcvH Lipoate Auxotrophy by Genes Encoding Alternative GcvH and OADH LD Proteins.
To test the lipoyl-relay abilities of known and putative GcvH proteins from diverse organisms, we used a B. subtilis ∆gcvH strain (NM20) in which deletion of the gcvH gene resulted in a requirement for lipoic acid (7–9). To test GcvH and LD proteins from other organisms for the ability to substitute for B. subtilis GcvH, the single GcvHs of E. coli, Streptomyces coelicolor, Pisum sativum (pea), Homo sapiens, all five putative GcvHs from A. aeolicus (Fig. 2), together with LD proteins of E. coli, S. coelicolor, and H. sapiens, were tested. Each gene was cloned and integrated into the chromosomal amyE site of the B. subtilis ∆gcvH strain under the isopropyl-β-d-thiogalactopyranoside (IPTG)-dependent promoter, Pspac.
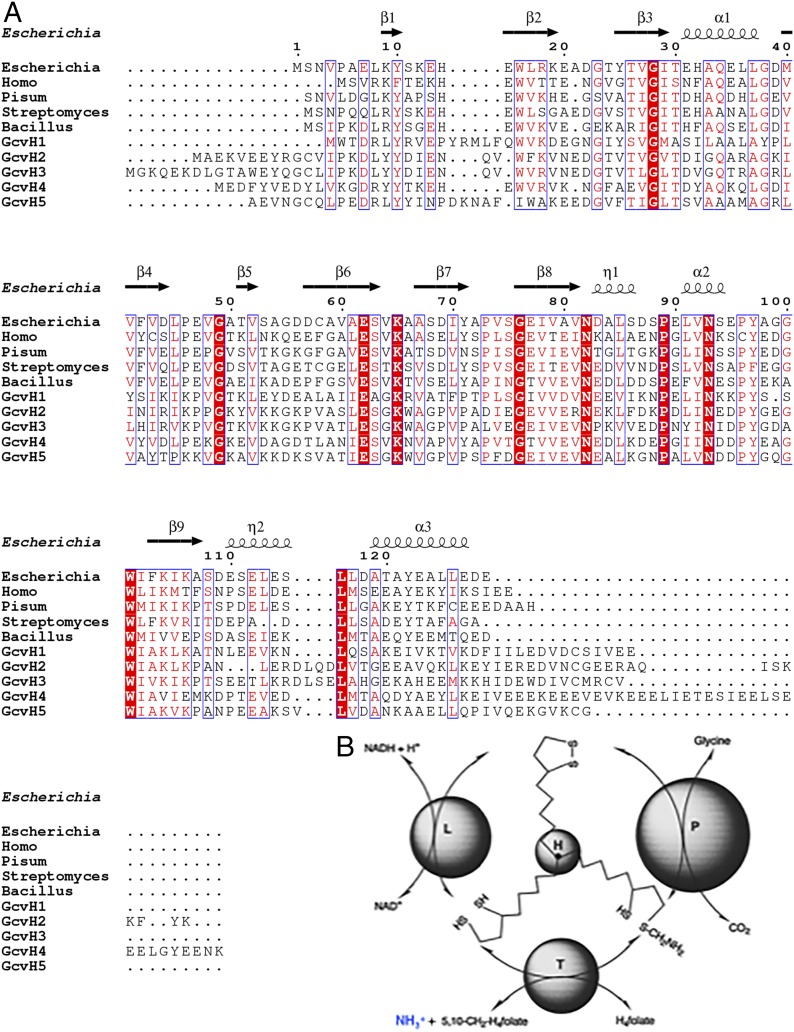
Fig. 2.
Amino acid sequence alignments of known and putative GcvH proteins and the central role of GcvH in the glycine cleavage mechanism. (A) Sequence alignment of GcvH proteins of E. coli, H. sapiens, P. sativum, S. coelicolor, B. subtilis, and five putative A. aeolicus GcvH proteins (GcvH1, GcvH2, GcvH3, GcvH4, GcvH5). Conserved residues are shown as white letters on a red background, and similar residues are shown as red letters in blue boxes. The E. coli GcvH secondary structure (PDB ID code 3A7L) is shown at the top of the panel. The vertical arrow denotes the lysine residue that becomes modified. (B) The glycine cleavge system and the central role of GcvH. The ammonia that provides an assay for GcvH activity is colored blue. Note the L protein that oxidizes the lipoyl cofactor is the same protein as the dihydrolipoyl dehydrogenase (Lpd) of the OADH complexes.
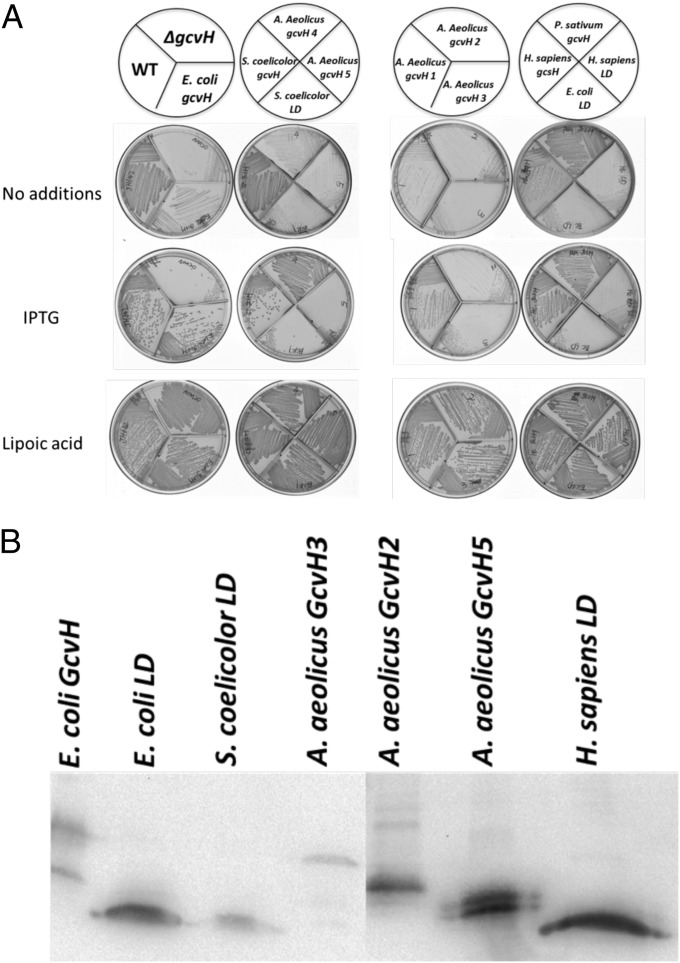
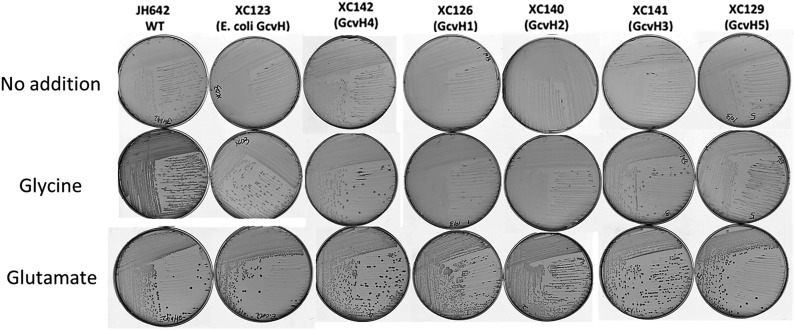
This set of B. subtilis ∆gcvH strains was tested for relief of lipoic acid auxotrophy by colony formation in the absence of lipoic acid on minimal medium plates containing glucose as carbon source (Fig. 3A). Slight background growth was observed on plates lacking IPTG, which is readily attributed to the known basal expression of the Pspac promoter and the low levels of lipoic acid required for growth. All of the strains grew well in the presence of lipoic acid, indicating that the expressed proteins were not toxic to growth (Fig. 3A). Interestingly, the GcvH proteins of E. coli and S. coelicolor supported robust growth of the B. subtilis ∆gcvH strain in the absence of lipoic acid, despite the fact that in their native hosts these GcvHs do not participate in lipoate synthesis. Expression of two eukaryotic GcvH proteins, the human (H. sapiens) and pea (P. sativum) proteins, both of which are known to function in glycine cleavage (19, 20), also supported robust growth in the absence of lipoic acid. Indeed, the pea GcvH gave strong growth even in the absence of IPTG induction.
Fig. 3.
(A) Growth of B. subtilis ∆gcvH strains expressing genes encoding various GcvH and LD proteins. The genes were integrated into the chromosomal amyE site of the B. subtilis ∆gcvH strain under control of Pspac promoter. The B. subtilis ∆gcvH NM20 strain is auxotrophic for lipoic acid and thus growth in the absence of lipoic acid-denoted complementation. Colony formation was scored after 36 h at 37 °C on minimal agar plates with glucose as the sole carbon source in the presence or absence of lipoic acid. The IPTG concentration was 0.5 mM. The wild-type (JH642) and B. subtilis ∆gcvH (NM20) strains served as positive and negative controls, respectively. (B) Western blot analysis of GcvH and LD expression. The strains expressing proteins that failed to complement growth of the B. subtilis ∆gcvH strain were grown for 22 h in minimal medium with lipoic acid and 0.5 mM IPTG. Crude cell extracts were separated on SDS/PAGE gels and immunoblotted with antihexahistidine antibody (Materials and Methods). Crude extracts of the B. subtilis ∆gcvH amyE:: Pspac-E. coli gcvH-His6 strain (XC173) were used as the positive control. Crude extracts of B. subtilis ∆gcvH derivatives expressing the various gcvH and OADH LD proteins are as indicated.
The five putative A. aeolicus GcvH proteins were problematic both in their number and lack of genome context. The lack of genome context is characteristic of the A. aeolicus genome (21). For example, although the genes encoding the glycine cleavage system GcvP and GcvT proteins are almost always contiguous genes in bacteria, this is not the case in A. aeolicus, where even the two GcvP subunits are encoded remotely. The only clue as to which of the five putative GcvHs might be the “real” GcvH is GcvH4, which is encoded next to the GcvP2 subunit gene, and indeed this protein gave robust complementation. The GcvH1 protein also had complementation activity but more weakly than GcvH4. The GcvH2, GcvH3, and GcvH5 proteins were completely inactive in complementation, as were all three of the OADH LDs tested (those from E. coli, S. coelicolor, and H. sapiens).
To exclude the possibility that the lack of complementation was due to poor protein expression, we constructed a set of B. subtilis ∆gcvH derivatives in which the proteins that had failed to complement (GcvH2, GcvH3, and GcvH5 from A. aeolicus) and the OADH LDs from E. coli, S. coelicolor, and H. sapiens were tagged with a C-terminal hexahistidine sequence to allow immunological detection. The B. subtilis ∆gcvH strain expressing a tagged E. coli GcvH provided a positive control. As shown in Fig. 3B, the three GcvH proteins (GcvH2, GcvH3, and GcvH5) from A. aeolicus and three LDs of E. coli, S. coelicolor, and H. sapiens that failed to complement the B. subtilis ∆gcvH strain were all detected by Western blotting and thus were expressed. Hence, the failure to complement could not be attributed to a lack of expression.
The Proteins That Failed to Complement Cannot be Modified by LipM.
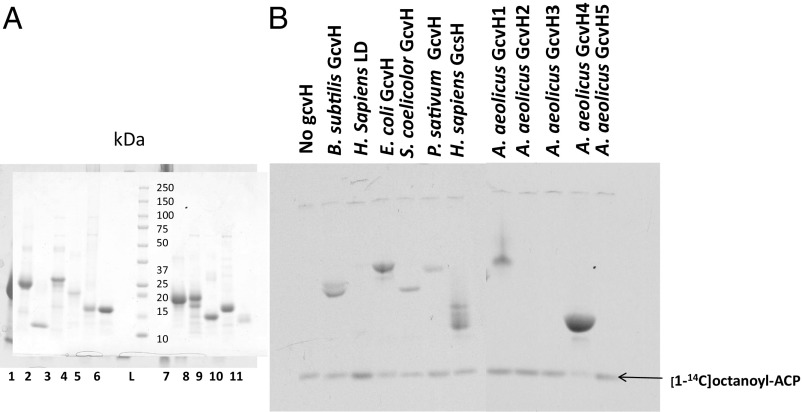
The inability of a given GcvH or OADH LD to complement the lipoate requirement of the B. subtilis ∆gcvH strain could be due to an inability to accept octanoate in the reaction catalyzed by LipM or to transfer lipoate from GcvH to the OADH subunits, the LipL reaction (Fig. 1). It is possible that a given protein could be inactive in both enzymatic reactions, but in the absence of LipM activity, LipL would have no acceptor substrate for transfer. Hence, the ability of a given protein to be modified by LipM but unable to complement OADH lipoylation could be attributed to defective LipL-catalyzed transfer. On the other hand, modification by LipM would be expected for the proteins that showed complementation. To test these predictions, we purified the GcvH proteins of E. coli, P. sativum, H. sapiens, S. coelicolor, the two complementing and three inactive putative GcvHs from A. aeolicus, plus the LD of H. sapiens PDH by nickel affinity chromatography. Some purified proteins migrated as doublets when analyzed on SDS/PAGE gel (Fig. 4A), which is attributed to deamidation during purification and did not affect modification (22, 23). Note that GcvH and LD proteins migrate on SDS/PAGE at rates expected for proteins of about twice their size. This is because both GcvH and LD are very acidic proteins (calculated pIs ∼3.9), and hence bind abnormally low amounts of SDS relative to the protein standards. We tested each protein for its ability to accept LipM-catalyzed [1-14C]octanoyl-transfer in vitro. The products were then analyzed by electrophoresis using conformationally sensitive urea PAGE (20%) rather than SDS/PAGE because some GcvH proteins comigrated with B. subtilis acyl-ACP in the SDS system. Upon autoradiographic analysis of acceptor protein modification the GcvH proteins of B. subtilis, E. coli, S. coelicolor, H. sapiens, P. sativum, as well as GcvH1 and GcvH4 from A. aeolicus, were readily octanoylated by LipM (Fig. 4B). In contrast, no octanoyl transfer to the A. aeolicus GcvH2, GcvH3, and GcvH5 proteins or to the LD of H. sapiens PDH was observed. These data indicate that the proteins that are unable to restore lipoic acid synthesis in vivo fail to complement due to the lack of modification by LipM. Note that the [1-14C]octanoyl-ACP was generated in situ using the AasS acyl-ACP synthetase, as described in Materials and Methods. B. subtilis GcvH, the natural substrate of LipM, served as positive control in these reactions (6).
Fig. 4.
Purification and octanoyl transferase assays of GcvH and LD proteins. (A) Purification of the GcvH and LD proteins. The proteins were purified as described in Materials and Methods and analyzed by SDS/PAGE on a 4–20% polyacrylamide gel. The molecular weights of the prestained broad-range protein standards (Bio-Rad) are indicated. 1: B. subtilis GcvH, 2: H. sapiens LD, 3: E. coli GcvH, 4: S. coelicolor GcvH, 5: P. sativum GcvH, 6: H. sapiens GcsH, 7–11: GcvH1, GcvH2, GcvH3, GcvH4, and GcvH5 from A. aeolicus, respectively. (B) Assay of [1-14C]octanoyl transfer from [1-14C]octanlyl-ACP to GcvH and LD proteins as indicated in the picture. The [1-14C]octanoyl-ACP was synthesized in situ from [1-14C]octanoate by AasS added to the reaction (ATP was added for the AasS reaction). Synthesis of [1-14C]octanoyl-ACP required [1-14C]octanoate, AasS, ATP and holo-ACP, whereas formation of [1-14C]octanoyl-GcvH/LD required apo-GcvH or LD, LipM and [1-14C]octanoyl-ACP.
The Putative GcvH2, GcvH3, and GcvH5 Proteins of A. aeolicus Cannot Replace B. subtilis GcvH in Glycine Cleavage.
Glycine cleavage produces 5,10-methylene tetrahydrofolate, CO2, and NH3 (Fig. 2B). Bacteria lacking function of one of the gcv genes have two phenotypes. These are an inability to use glycine as a nitrogen source and an inability to supplement serine auxotrophs with glycine in place of serine (the 5,10-methylene tetrahydrofolate produced by cleavage of a glycine molecule is used to convert another glycine molecule to serine). We have taken advantage of the first of these phenotypes to assess the abilities of the various GcvH and OADH LD proteins to function in B. subtilis glycine cleavage. The ammonium sulfate of Spizizen salts minimal medium was replaced with potassium sulfate and glycine was added as the major nitrogen source (the NM20 strain requires tryptophan and phenylalanine and grows best when the medium contains a trace amount of acid hydrolyzed casein). The presence of other nitrogen sources gave some background growth of the parental strain that ceased when the hydrolyzed casein nitrogen (mainly glutamate) was exhausted, whereas in the presence of glycine growth continued robustly. In contrast, the B. subtilis ∆gcvH strain NM20 showed only background growth. Strains expressing each of the GcvH proteins known to function in glycine cleavage (the human, pea, E. coli, and B. subtilis proteins) allowed growth of the B. subtilis ∆gcvH strain on glycine as nitrogen source (Table 1). The strain expressing A. aeolicus GcvH4 grew well with glycine as a nitrogen source (Fig. 5), consistent with the genomic location of the encoding gene. Therefore, each of these proteins had both glycine cleavage and lipoyl-relay activity. The other annotated A. aeolicus GcvH proteins gave a variety of glycine cleavage phenotypes (Fig. 5 and Table 1). The strain expressing the GcvH2 protein failed to grow on the glycine medium and the strain expressing GcvH5 gave only barely detectable growth, and thus these proteins have either no or extremely weak glycine cleavage activity and lack lipoyl-relay activity. The strain expressing the GcvH1 protein failed to grow on the glycine medium, although it has weak lipoyl-relay activity. In contrast, the strain expressing the GcvH3 protein, which lacks lipoyl-relay activity, grew on the glycine medium (Fig. 5). These data argue that the residues responsible for lipoyl relay and glycine cleavage are not identical. Although most GcvH proteins have both functions, some have only lipoyl-relay activity or glycine cleavage activity. To our knowledge this evidence that foreign GcvH proteins can function with noncognate GcvP and GcvT proteins is unique. As expected, the OADH LD proteins were inactive in glycine cleavage.
Table 1.
Functional activities of the putative A. aeolicus GcvH proteins
| Function | GcvH1 | GcvH2 | GcvH3 | GcvH4 | GcvH5 |
| Lipoyl relay | + | − | − | + | − |
| Glycine cleavage | − | − | (+) | + | ((+)) |
Symbols: The plus signs mean strong activity whereas the minus signs mean no detectable activity. The plus signs in parentheses indicate modest activity (+) or barely detectable activity ((+)).
Fig. 5.
Glycine cleavage activitites of the A. aeolicus GcvH homologs. The medium was a modified Spizizen salts that lacked ammonium sulfate and was supplemented with glucose, 0.01% each of l-phenylalanine and l-tryptophan and 0.015% acid hydrolyzed casein. Either glycine or l-glutamic acid (a preferred nitrogen source for this bacterium) was added at 0.1%. The plates were incubated at 37 °C for 72 h. No discrete colonies were formed by the strains that expressed GcvH1 and GcvH2 (the imperfections are scratches from the streaking), whereas barely visible colonies were formed within the scratches by the strain that expressed GcvH5. Note that this figure is a collage of scans.
Discussion
Despite the well-characterized role of lipoic acid as a protein-bound coenzyme, the detailed mechanisms of its synthesis and attachment to cognate proteins are still unclear in many organisms, including bacteria and eukaryotes (1). The most straightforward lipoic acid synthesis pathway is the LipB–LipA pathway of E. coli (Fig. 1) and numerous other bacteria, where lipoyl-GcvH functions only in the glycine cleavage cycle. In marked contrast, B. subtilis GcvH is not only a glycine cleavage system component, but also has a moonlighting function that is required for lipoylation of the OADH enzymes required for aerobic metabolism and branched-chain fatty acid synthesis.
In their modeling studies of early evolution of metabolism based on the deep-branching hyperthermophilic chemoautotrophic bacterium, A. aeolicus, Braakman and Smith (18) argued that the glycine cleavage system first arose as part of the ancestral glycine/serine pathway and the sole function of lipoic acid was in shuttling the intermediates of this pathway. These authors further argued that utilization of lipoate in glycine cleavage preceded its use by the OADH enzymes because the TCA cycle is not essential in many bacteria, whereas glycine cleavage is almost totally conserved. Their case was further buttressed by noting that those bacteria that lack the oxidative TCA cycle should lack LipL because there would be no OADH proteins to modify, and this is the case (18). Moreover, the few bacteria that lack the genes of the glycine cleavage system would be expected to also lack the genes of lipoic acid synthesis and scavenging, and again this is the case. Taken together, these considerations argue strongly that the B. subtilis pathway is derived from the ancestral pathway of lipoic acid attachment (18). It follows that transfer of lipoic acid via the lipoyl-relay pathway to the OADH proteins can be considered as an energetically unfavorable “make-do” arrangement that was superseded by the energetically favorable direct transfer of octanoate to the OADH (and GcvH) proteins by broad specificity octanoyl transferases, such as E. coli LipB. In B. subtilis, transfer of lipoic acid from GcvH to the OADH proteins requires LipL, which based on sequence and structural similarities, seems to have been derived from LipM, presumably by duplication of LipM with one of the copies subsequently acquiring amidotransferase activity (10, 11, 18, 24, 25). Indeed, the above lipoate metabolism enzymes plus the biotin protein ligases that constitute Pfam PF03099 are all constructed on the same scaffold, albeit from diverse sequences and extra substrate-activating domains in the ligases (1). As mentioned above, LipB and LipM share almost no sequence conservation, and moreover the enzymes diverge in that their active site cysteine residues are on different loops of the common scaffold (6). Hence, alteration of a lipM gene to encode LipL activity may have been easier than conversion of either LipM or LplJ into a protein having LipB activity.
At present LipB homologs are found only in the proteobacteria, whereas LipM and LipL homologs are found only in the Firmicutes. Based on sequence analysis of 32 proteins believed essential for growth (and hence resistant to horizontal gene transfer), Battistuzzi et al. (26) argue that these bacterial phyla diverged about 0.7 billion y before the rise in oxygen levels (2.3 billion y ago). Emergence of the organelles of bacterial origin, mitochondria, and chloroplasts, which contain the lipoate-modified proteins, is thought to have occurred at least 1.5 billion y ago (27). Hence, there was ample time available for genetic drift in the sequences of the bacterial and eukaryotic GcvHs we tested. Indeed, genetic drift is consistent with the low levels of strictly conserved residues (<10% or less, if the glycine residues required for the turns of antiparallel β-sheets are put aside). However, despite sequence drift the ability of GcvH to function in lipoate relay clearly arose early in bacterial evolution because two of the A. aeolicus proteins (GcvH4 and GcvH1) functionally replace B. subtilis GcvH, although this bacterium lacks any OADH substrates to accept relayed lipoyl moieties. Moreover, lipoate-relay ability has been retained in the GcvH proteins of bacteria (E. coli, S. coelicolor) that have no use for this proficiency because they utilize a direct and more efficient lipoylation pathway.
The most straightforward explanation of the curious state of affairs in which extremely diverse bacteria (A. aeolicus and E. coli) possess an activity that is metabolically extraneous would be that lipoate-relay ability is “hard-wired’ into GcvH proteins. In this scenario, sequences required for glycine cleavage would also play important roles in the lipoyl-relay pathway. However, the A. aeolicus proteins show this is not the case (Table 1). The two proteins that are active in lipoate relay, GcvH4 and GcvH1, differ in their glycine cleavage activities. GcvH4 has good activity, whereas GcvH1 lacks activity. Moreover GcvH3 and GcvH5 have modest and very weak glycine cleavage activities, respectively, but lack lipoate-relay activity. Finally GcvH2 lacks both activities.
It has been argued that during evolution, acquisition of specific new interactions is neither difficult nor rare (12). In this scenario proteins continually gain and lose new activities, only a few of which contribute to fitness and are retained. Indeed, examples are known in which one or two residue changes can alter enzyme specificity and protein–protein interactions (12). This view may provide an explanation for the diverse activities of the five annotated A. aeolicus GcvH proteins. It may be that the GcvH structural scaffold permits lipoate-relay ability to arise frequently and that we have fortuitously “caught” the GcvH1 and GcvH4 proteins before lipoate relay will be lost by the stochastic processes of mutation and drift. Note that the presence of other A. aeolicus proteins having glycine cleavage activity argues that the proteins capable of lipoate relay would not be required for glycine cleavage. Indeed the presence of five closely similar proteins in A. aeolicus can be viewed as license to allow evolutionary tinkering without loss of a fitness requirement. To extend these considerations to single copy GcvH proteins (e.g., that of E. coli) that have lipoyl-relay activity but perform OADH lipoylation by a direct mechanism raises the possibility that the residues required for lipoyl relay may aid glycine cleavage and provide a modest contribution to fitness. Sequence alignments give no obvious clues to explain the diversity among the lipoyl relay and glycine cleavage phenotypes. This puzzle will have to be approached by genetic analyses. We seek mutant GcvH proteins that have lost lipoyl-relay ability while retaining glycine cleavage activity and vice versa.
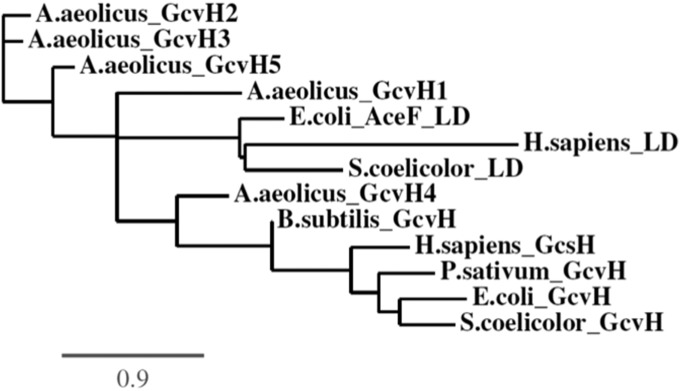
The putative GcvH protein, GcvH2, which failed to function in either lipoyl relay or glycine cleavage must be involved in another pathways or is a (temporary?) evolutionary remnant. This may also be true of GcvH5, which has very weak glycine cleavage activity and no lipoyl-relay activity. These two proteins seem closely related to one other and to GcvH3, which has only glycine cleavage activity (Fig. 6). The GcvH1 protein, which has only lipoyl-relay activity, seems off by itself, whereas the GcvH4 protein, which has both activities, clusters with known GcvH proteins. However, a caveat to these comparisons is that these are small proteins and thus only a few residue substitutions are needed to alter groupings. As expected from prior analyses (6), the OADH LDs and the GcvH proteins (perhaps excepting GcvH1) fall into separate clades.
Fig. 6.
The phylogenetic tree of the GcvH and OADH LD obtained using the PhyML program maximum-likelihood method (34, 35). The tree is drawn to scale. The branch lengths are in the same units as the evolutionary distances.
It should be noted that full transcription of bacterial glycine cleavage system genes proceeds only when high concentrations of glycine are present in the environment. For example, addition of glycine to a wild-type E. coli culture growing in minimal medium results in a 30- to 60-fold increase in glycine cleavage activity (28, 29). In E. coli the proteins required for glycine cleavage, including GcvH, are all encoded in a coordinately regulated operon except the dihydrolipoyl dehydrogenase subunit, which is shared with the OADH complexes (28). In B. subtilis, induction of the glycine cleavage pathway is mediated by a glycine riboswitch, which allows transcription in the presence of glycine (30). A problem would arise if GcvH were under control of the riboswitch because OADH lipoylation (hence aerobic growth and fatty acid synthesis) would occur only in the presence of high glycine concentrations. However, B. subtilis avoids this predicament because the riboswitch controls only the genes that encode the GcvP and GcvT enzymes (30). GcvH is encoded at a distant site in the genome and appears to be constitutively expressed (as required for OADH lipoylation). Indeed, a survey of genome sequences indicates that the genomic location of gcvH in relation to gcvP and gcvT genes may provide a diagnostic for the mode of OADH lipoylation. That is, when the gene encoding GcvH is remote from those encoding the P and T subunits, the bacterium uses the lipoyl-relay pathway, whereas in genomes where GcvH is encoded together with the P and T subunits, the direct (LipB) pathway is used.
Note that a natural lipoate auxotroph, Listeria monocytogenes, uses a modified lipoyl-relay pathway. This bacterium acquires lipoic acid from its mammalian host cells and uses a ligase to attach it to GcvH followed by LipL-catalyzed lipoyl relay to its OADH proteins (31). Finally, differential lipoylation of GcvH and the OADH proteins has recently been reported in Plasmodium falciparum, the causative agent of malaria. In this pathway, which seems descended from Chlamydia bacterial pathogens (32), a lipoate ligase called LipL1 proceeds through the usual lipoyl-AMP intermediate to attach host-derived lipoate to GcvH. However, LipL1 is unable to directly attach lipoate to the OADH proteins. Lipoylation of the OADH requires a second enzyme, called LipL2, to transfer lipoate from the lipoyl-AMP intermediate produced by LipL1 to the OADH proteins (but not to GcvH) (32). Curiously, the extant annotations indicate that neither P. falciparum nor Chlamydia species perform glycine cleavage. Chlamydia species lack recognizable genes that encode GcvP and GcvT subunits, whereas P. falciparum lacks GcvP encoding genes. Hence, the physiological advantage of GcvH lipoylation is unclear in these organisms and it seems that these GcvH proteins—like A. aeolicus Gcv2, Gcv3, and Gcv5—have other metabolic functions.
Materials and Methods
Growth Media and Conditions.
Bacterial strains used in the present study are listed in Table S1. E. coli and B. subtilis strains were routinely grown in Luria Bertani (LB) broth. For complementation assays, Spizizen salts (33), supplemented with 0.5% glucose, trace elements, and 0.01% each of tryptophan and phenylalanine were used as the minimal medium for B. subtilis. Antibiotics were added to medium at the following concentrations: sodium ampicillin (Amp) 100 μg mL−1; chloramphenicol (Cm) 5 μg mL−1; kanamycin sulfate (Km) 5 μg mL−1; and spectinomycin sulfate (Spec) 50 μg mL−1. Lipoic acid (racemic) was added at 40 μM. IPTG was added at 0.5 mM. Note that the lipoic acid requirement of ∆gcvH strains can be satisfied by a mixture of acetate and branched chain fatty acid precursors that bypass the lack of OADH activities.
Phylogenetic Analysis.
The amino acid sequences of different species were retrieved from the National Center for Biotechnology Information. Both the number of best-scoring sequences and the number of best alignments were set to 1,000. The e-values of the sequences selected were between 10−40 and 10−50. The phylogenetic tree was constructed using the maximum-likelihood method with the PhyML program (34, 35). PHYLIP Interleaved was used for alignment. The Bootstrap analysis was set to 1,000 replicates.
Plasmid and Strain Constructions.
The primers used for PCR amplification are given in Table S2. Strain NM20 was complemented with ectopic gcvHs or OADH domains under control of an IPTG-dependent promoter (Pspac). Plasmids pXC.045-pXC.056 was constructed by inserting PCR-amplified gcvH or OADH domain gene fragments into pDR111, a gift of D. Rudner, Harvard Medical School, Boston, MA (36). The five putative gcvH genes from A. aeolicus gcvH1 [Kyoto Encyclopedia of Genes and Genomes (KEGG) entry aq_1052; gcvH2, KEGG entry aq_1657; gcvH3, KEGG entry aq_944, gcvH4: KEGG entry aq_1108; gcvH5, KEGG entry aq_402], the LD of S. coelicolor branched-chain 2-oxoacid dehydrogenase E2 (KEGG entry SCO3815), E. coli gcvH (KEGG entry b2904), and S. coelicolor gcvH (KEGG entry SCO5471) were amplified from the corresponding genomic DNAs using a Pfu DNA polymerase with primers oXC241/242, oXC247/248, oXC249/250, oXC251/252, oXC239/240, oXC259/oXC260, oXC233/0XC234, and oXC271/272, respectively, which added a ribosome binding site (5′-AAAACCATTATATTAGGAGGAAATAAC-3′) plus SphI and HindIII restriction sites. The E. coli PDH E2 (KEGG entry EC 1.2.4.1) lipoyl domain gene was amplified from plasmid pGS331 (37, 38) using primers oXC235/236, which added the above ribosome binding site plus SalI and HindIII restriction sites. The H. sapiens sequence of the lipoyl domain of the PDH E2 subunit (KEGG entry 8050), the H. sapiens gcsH (KEGG entry K02437), and P. sativum gcvH (GenBank accession no. CAJ13840) were synthesized by IDT (Integrated DNA Technologies) as gBlocks Gene Fragments and were amplified with primers oXC257 and 258, oXC231 and 232, and oXC237 and 238, respectively, adding ribosome binding site plus SphI and HindIII restriction sites as given above. Plasmids pXC.045-pXC.056 encoding GcvHs or OADH lipoyl domains were linearized and used to transform The B. subtilis ∆gcvH strain NM20, yielding strains XC.123-XC.129, XC.140, XC.141, XC.142, XC.147, XC.148, and XC.161, respectively. Transformants were selected on plates containing kanamycin and spectinomycin and screened for the amyE phenotype. The amyE phenotype was assayed with colonies grown for 24 h in LB starch plates by adding 10 mL of 1% I2-KI solution (39). Under these conditions, amy+ colonies give a clear halo, whereas ∆amyE colonies produce no halo.
To detect the expression levels of the proteins that failed to complement growth of strain NM20, insertion of a sequence encoding the C-terminal His6 tag into the NM20 genomic gcvH/LD was performed. The genes that failed to complement were PCR-amplified with a forward primer containing the above ribosome binding site and a reverse primer encoding a His6 tag and a stop codon. E. coli gcvH, E. coli LD, H. sapiens LD, S. coelicolor LD, A. aeolicus gcvH2, gcvH3, and gcvH5 genes were amplified with primers oXC233/277, oXC235/278, oXC257/282, oXC259/283, oXC247/280, oXC249/281, and oXC239/279, respectively, and ligated into pDR111 to give the pXC.057 to pXC.063 plasmids. These plasmids were then linearized and transformed into NM20 generating strains XC.173-XC.179. Transformants were selected and screened for amyE phenotype as above.
For purification of the protein products of the genes, the five A. aeolicus putative gcvH genes (gcvH1, gcvH2, gcvH3, gcvH4, and gcvH5) were amplified from corresponding genomic DNA using Pfu DNA polymerase with primers oHYQ188/189, oHYQ190/191, oHYQ192/193, oHYQ194/195, and oHYQ165/166, respectively, which added NdeI and HindIII restriction sites. The products were digested with NdeI and HindIII and ligated into vector pET28b to express the N-terminally hexahistidine-tagged proteins. Plasmid pGS331, which expresses E. coli AceF LD under the control of the tac promoter, was the source of the E. coli AceF LD protein. The LD of H. sapiens PDH E2 and H. sapiens gcsH were PCR-amplified with NdeI and BamHI restriction sites added. The NdeI- and BamHI-digested PCR fragments were ligated to pET28b generating pXC.043 and pXC.044, respectively. The expression vectors containing B. subtilis gcvH (KEGG entry BSU32800), LD of S. coelicolor branched-chain OADH E2, and S. coelicolor gcvH were from laboratory stocks (Table S1).
Protein Purification.
GcvH and LD proteins were expressed and purified from strain BL21(DE3) (Table S1). Briefly, cultures were grown to OD600 0.5–0.8, in which 1 mM IPTG was added to induce protein expression. The cells were harvested after 3–5 h and suspended in lysis buffer [50 mM Tris⋅HCl (pH 8.0), 300 mM NaCl, 1 mM Tris(2-carboxyethyl)phosphine (TCEP), 10 mM imidazole]. The cells were lysed by three passages through a French Press apparatus after which the insoluble matter was removed by high-speed centrifugation and then filtration. Clear lysates were then incubated with Ni-NTA agarose for 3–12 h at 5 °C, and washed/eluted using buffer with 10–250 mM imidazole. The concentrated proteins were dialyzed against 25 mM sodium phosphate buffer (pH 7.2) containing 1 mM TCEP and further purified by ion-exchange chromatography using a Q column on an AFTA Purifier with increasing concentrations of NaCl. The purified proteins were then concentrated and dialyzed against 50 mM sodium phosphate (pH 7.2), 50 mM NaCl, and 1 mM TCEP. The B. subtilis Sfp, apo-ACP, and LipM proteins, the Vibrio harveyi AasS, and the E. coli AceF LD were purified as described previously (40, 41).
Conversion of apo-ACP to holo-ACP.
The reaction mixture (100 μL) contained 100 mM 2-(N-morpholino)ethanesulfonic acid (pH 6.0), 10 mM MgCl2, 5 mM DTT, 500 μM lithium CoA, 200 μM apo-ACP, and 10 μM of the B. subtilis Sfp 4′-phosphopantetheinyl transferase. The reaction was performed at 37 °C for 3 h followed by incubation at 65 °C for 2 h and the precipitates were removed by centrifugation. The remaining fraction containing holo-ACP was analyzed by conformationally sensitive 2 M urea PAGE (15% arylamide), and then dialyzed against 25 mM sodium phosphate (pH 7.0), 300 mM NaCl, 1 mM TCEP, and 10% glycerol.
AasS/LipM Coupled Octanoyl-Transfer Reaction.
The coupled reaction mixture (25 μL) contained 100 mM sodium phosphate (pH 7.2), 50 mM NaCl, 5 mM disodium ATP, 5 mM TCEP, 2 mM MgCl2, 0.25 mM [1-14C] sodium octanoate, 50 µM holo-ACP, 2.5 μM of the V. harveyi AasS acyl-ACP synthetase, 10 μM LipM, and ∼20 μM of one of the GcvH proteins. The reaction was performed at 37 °C for 5 h. The products were electrophoresed on 2 M urea PAGE (20% arylamide), then dried under vacuum at 65 °C for 2 h and exposed to preflashed Biomax XAR film (Kodak) at −80 °C for 24 h.
Western Blot Analysis.
Anti-His6 primary antibody was utilized to probe protein expression, as described previously (42). Strains XC173 to XC179, which encode genomic C-terminal His6-tagged versions of either a gcvH or a LD, were grown to midlog phase in Spizizen salts minimum medium plus trace elements, 0.5% (wt/vol) glucose, 0.01% tryptophan, 0.01% phenylalanine, 40 μM lipoic acid, and 0.5 mM IPTG. A 2-mL aliquot of each cell culture was harvested after 22 h of growth. Soluble fractions of whole-cell extracts were analyzed by SDS/PAGE. Equal amounts of protein were loaded and separated on a 12% SDS-polyacrylamide gel and transferred by electrophoresis to Immobilon-P membranes (Millipore) for 30 min at 60 V. The membranes were preblocked with TBS buffer (100 mM Tris base and 0.9% NaCl, pH 7.5) containing 0.1% Tween 20 and 5% nonfat milk powder. The membranes were probed for 1 h with an anti-His6 protein primary antibody (ThermoFisher Scientific) diluted 1:2,000 in the above buffer. Following incubation with a peroxidase labeled anti-mouse secondary antibody (diluted 1:5,000; GE Healthcare Life Sciences), the labeled proteins (His6-tagged GcvH or LD) were detected using Quantity One software.
Supplementary Material
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1718653115/-/DCSupplemental.
References
- 1.Cronan JE. Assembly of lipoic acid on its cognate enzymes: An extraordinary and essential biosynthetic pathway. Microbiol Mol Biol Rev. 2016;80:429–450. doi: 10.1128/MMBR.00073-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spalding MD, Prigge ST. Lipoic acid metabolism in microbial pathogens. Microbiol Mol Biol Rev. 2010;74:200–228. doi: 10.1128/MMBR.00008-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perham RN. Swinging arms and swinging domains in multifunctional enzymes: Catalytic machines for multistep reactions. Annu Rev Biochem. 2000;69:961–1004. doi: 10.1146/annurev.biochem.69.1.961. [DOI] [PubMed] [Google Scholar]
- 4.Zhao X, Miller JR, Jiang Y, Marletta MA, Cronan JE. Assembly of the covalent linkage between lipoic acid and its cognate enzymes. Chem Biol. 2003;10:1293–1302. doi: 10.1016/j.chembiol.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 5.Cronan JE. Biotin and lipoic acid: Synthesis, attachment, and regulation. In: Böck RCI, et al., editors. EcoSal Plus. ASM Press; Washington, DC: 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christensen QH, Cronan JE. Lipoic acid synthesis: A new family of octanoyltransferases generally annotated as lipoate protein ligases. Biochemistry. 2010;49:10024–10036. doi: 10.1021/bi101215f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christensen QH, Martin N, Mansilla MC, de Mendoza D, Cronan JE. A novel amidotransferase required for lipoic acid cofactor assembly in Bacillus subtilis. Mol Microbiol. 2011;80:350–363. doi: 10.1111/j.1365-2958.2011.07598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin N, Christensen QH, Mansilla MC, Cronan JE, de Mendoza D. A novel two-gene requirement for the octanoyltransfer reaction of Bacillus subtilis lipoic acid biosynthesis. Mol Microbiol. 2011;80:335–349. doi: 10.1111/j.1365-2958.2011.07597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin N, Lombardía E, Altabe SG, de Mendoza D, Mansilla MC. A lipA (yutB) mutant, encoding lipoic acid synthase, provides insight into the interplay between branched-chain and unsaturated fatty acid biosynthesis in Bacillus subtilis. J Bacteriol. 2009;191:7447–7455. doi: 10.1128/JB.01160-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Copley SD. Moonlighting is mainstream: Paradigm adjustment required. BioEssays. 2012;34:578–588. doi: 10.1002/bies.201100191. [DOI] [PubMed] [Google Scholar]
- 11.Copley SD. An evolutionary perspective on protein moonlighting. Biochem Soc Trans. 2014;42:1684–1691. doi: 10.1042/BST20140245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siddiq MA, Hochberg GK, Thornton JW. Evolution of protein specificity: Insights from ancestral protein reconstruction. Curr Opin Struct Biol. 2017;47:113–122. doi: 10.1016/j.sbi.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zorzoli A, Grayczyk JP, Alonzo F., 3rd Staphylococcus aureus tissue infection during sepsis is supported by differential use of bacterial or host-derived lipoic acid. PLoS Pathog. 2016;12:e1005933. doi: 10.1371/journal.ppat.1005933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schonauer MS, Kastaniotis AJ, Kursu VA, Hiltunen JK, Dieckmann CL. Lipoic acid synthesis and attachment in yeast mitochondria. J Biol Chem. 2009;284:23234–23242. doi: 10.1074/jbc.M109.015594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brocklehurst SM, Perham RN. Prediction of the three-dimensional structures of the biotinylated domain from yeast pyruvate carboxylase and of the lipoylated H-protein from the pea leaf glycine cleavage system: A new automated method for the prediction of protein tertiary structure. Protein Sci. 1993;2:626–639. doi: 10.1002/pro.5560020413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris TW, Reed KE, Cronan JE., Jr Lipoic acid metabolism in Escherichia coli: The lplA and lipB genes define redundant pathways for ligation of lipoyl groups to apoprotein. J Bacteriol. 1995;177:1–10. doi: 10.1128/jb.177.1.1-10.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reed KE, Cronan JE., Jr Lipoic acid metabolism in Escherichia coli: Sequencing and functional characterization of the lipA and lipB genes. J Bacteriol. 1993;175:1325–1336. doi: 10.1128/jb.175.5.1325-1336.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braakman R, Smith E. Metabolic evolution of a deep-branching hyperthermophilic chemoautotrophic bacterium. PLoS One. 2014;9:e87950. doi: 10.1371/journal.pone.0087950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Douce R, Bourguignon J, Neuburger M, Rébeillé F. The glycine decarboxylase system: A fascinating complex. Trends Plant Sci. 2001;6:167–176. doi: 10.1016/s1360-1385(01)01892-1. [DOI] [PubMed] [Google Scholar]
- 20.Kikuchi G, Motokawa Y, Yoshida T, Hiraga K. Glycine cleavage system: Reaction mechanism, physiological significance, and hyperglycinemia. Proc Jpn Acad, Ser B, Phys Biol Sci. 2008;84:246–263. doi: 10.2183/pjab/84.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deckert G, et al. The complete genome of the hyperthermophilic bacterium Aquifex aeolicus. Nature. 1998;392:353–358. doi: 10.1038/32831. [DOI] [PubMed] [Google Scholar]
- 22.Jordan SW, Cronan JE., Jr Biosynthesis of lipoic acid and posttranslational modification with lipoic acid in Escherichia coli. Methods Enzymol. 1997;279:176–183. doi: 10.1016/s0076-6879(97)79021-9. [DOI] [PubMed] [Google Scholar]
- 23.Robinson NE, Robinson AB. Prediction of protein deamidation rates from primary and three-dimensional structure. Proc Natl Acad Sci USA. 2001;98:4367–4372. doi: 10.1073/pnas.071066498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khersonsky O, Tawfik DS. Enzyme promiscuity: A mechanistic and evolutionary perspective. Annu Rev Biochem. 2010;79:471–505. doi: 10.1146/annurev-biochem-030409-143718. [DOI] [PubMed] [Google Scholar]
- 25.Tawfik DS. Messy biology and the origins of evolutionary innovations. Nat Chem Biol. 2010;6:692–696. doi: 10.1038/nchembio.441. [DOI] [PubMed] [Google Scholar]
- 26.Battistuzzi FU, Feijao A, Hedges SB. A genomic timescale of prokaryote evolution: Insights into the origin of methanogenesis, phototrophy, and the colonization of land. BMC Evol Biol. 2004;4:44. doi: 10.1186/1471-2148-4-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Archibald JM. Endosymbiosis and eukaryotic cell evolution. Curr Biol. 2015;25:R911–R921. doi: 10.1016/j.cub.2015.07.055. [DOI] [PubMed] [Google Scholar]
- 28.Steiert PS, Stauffer LT, Stauffer GV. The lpd gene product functions as the L protein in the Escherichia coli glycine cleavage enzyme system. J Bacteriol. 1990;172:6142–6144. doi: 10.1128/jb.172.10.6142-6144.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson RL, Steiert PS, Stauffer GV. Positive regulation of the Escherichia coli glycine cleavage enzyme system. J Bacteriol. 1993;175:902–904. doi: 10.1128/jb.175.3.902-904.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mandal M, et al. A glycine-dependent riboswitch that uses cooperative binding to control gene expression. Science. 2004;306:275–279. doi: 10.1126/science.1100829. [DOI] [PubMed] [Google Scholar]
- 31.Christensen QH, Hagar JA, O’Riordan MX, Cronan JE. A complex lipoate utilization pathway in Listeria monocytogenes. J Biol Chem. 2011;286:31447–31456. doi: 10.1074/jbc.M111.273607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Afanador GA, et al. A novel lipoate attachment enzyme is shared by Plasmodium and Chlamydia species. Mol Microbiol. 2017;106:439–451. doi: 10.1111/mmi.13776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spizizen J. Transformation of biochemically deficient strains of Bacillus subtilis by deoxyribonucleate. Proc Natl Acad Sci USA. 1958;44:1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chevenet F, Brun C, Bañuls AL, Jacq B, Christen R. TreeDyn: Towards dynamic graphics and annotations for analyses of trees. BMC Bioinformatics. 2006;7:439. doi: 10.1186/1471-2105-7-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- 36.Wagner JK, Marquis KA, Rudner DZ. SirA enforces diploidy by inhibiting the replication initiator DnaA during spore formation in Bacillus subtilis. Mol Microbiol. 2009;73:963–974. doi: 10.1111/j.1365-2958.2009.06825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ali ST, Guest JR. Isolation and characterization of lipoylated and unlipoylated domains of the E2p subunit of the pyruvate dehydrogenase complex of Escherichia coli. Biochem J. 1990;271:139–145. doi: 10.1042/bj2710139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ali ST, Moir AJ, Ashton PR, Engel PC, Guest JR. Octanoylation of the lipoyl domains of the pyruvate dehydrogenase complex in a lipoyl-deficient strain of Escherichia coli. Mol Microbiol. 1990;4:943–950. doi: 10.1111/j.1365-2958.1990.tb00667.x. [DOI] [PubMed] [Google Scholar]
- 39.Sekiguchi J, Takada N, Okada H. Genes affecting the productivity of alpha-amylase in Bacillus subtilis Marburg. J Bacteriol. 1975;121:688–694. doi: 10.1128/jb.121.2.688-694.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang Y, Chan CH, Cronan JE. The soluble acyl-acyl carrier protein synthetase of Vibrio harveyi B392 is a member of the medium chain acyl-CoA synthetase family. Biochemistry. 2006;45:10008–10019. doi: 10.1021/bi060842w. [DOI] [PubMed] [Google Scholar]
- 41.De Lay NR, Cronan JE. In vivo functional analyses of the type II acyl carrier proteins of fatty acid biosynthesis. J Biol Chem. 2007;282:20319–20328. doi: 10.1074/jbc.M703789200. [DOI] [PubMed] [Google Scholar]
- 42.Cao X, Zhu L, Hu Z, Cronan JE. Expression and activity of the BioH esterase of biotin synthesis is independent of genome context. Sci Rep. 2017;7:2141. doi: 10.1038/s41598-017-01490-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morris TW, Reed KE, Cronan JE., Jr Identification of the gene encoding lipoate-protein ligase A of Escherichia coli. Molecular cloning and characterization of the lplA gene and gene product. J Biol Chem. 1994;269:16091–16100. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.