Abstract
Surface carbohydrate moieties are essential for bacterial communication, phage-bacteria and host-pathogen interaction. Most Staphylococcus aureus produce polyribitolphosphate type Wall teichoic acids (WTAs) substituted with α- and/or β-O-linked N-acetyl-glucosamine (α-/β-O-GlcNAc) residues. GlcNAc modifications have attracted particular interest, as they were shown to govern staphylococcal adhesion to host cells, to promote phage susceptibility conferring beta-lactam resistance and are an important target for antimicrobial agents and vaccines. However, there is a lack of rapid, reliable, and convenient methods to detect and quantify these sugar residues. Whole cell Fourier transform infrared (FTIR) spectroscopy could meet these demands and was employed to analyse WTAs and WTA glycosylation in S. aureus. Using S. aureus mutants, we found that a complete loss of WTA expression resulted in strong FTIR spectral perturbations mainly related to carbohydrates and phosphorus-containing molecules. We could demonstrate that α- or β-O-GlcNAc WTA substituents can be clearly differentiated by chemometrically assisted FTIR spectroscopy. Our results suggest that whole cell FTIR spectroscopy represents a powerful and reliable method for large scale analysis of WTA glycosylation, thus opening up a complete new range of options for deciphering the staphylococcal pathogenesis related glycocode.
Introduction
The Staphylococcus aureus cell envelope comprises a variety of secondary surface glycopolymers including capsule polysaccharide (CP), poly-β(1–6)-N-acetylglucosamine (PNAG), and wall teichoic acid (WTA)1. These cell surface glycostructures are known to affect bacterial interactions with hosts in multiple ways and are important targets for antimicrobial agents and vaccines2,3. In particular, WTAs are highly abundant surface polymers that play important roles in S. aureus physiology and pathogenesis such as cell division, phage susceptibility, biofilm formation, interaction with host cells and complement activation4–6. One of the main sources of structural diversity in polysaccharides is the type of glycosidic linkage. Recently, the WTA glycosylation pathways in S. aureus have been elucidated, involving two glycosyltransferase TarM and TarS that attach N-Acetylglucosamine (GlcNAc) to the WTA main chain via O-linkage in α- and β-anomeric configuration, respectively7,8. α-O-GlcNAc modified WTA plays an important role as phage receptor and promotes the transduction of staphylococcal DNA via horizontal gene transfer (HGT), whereas, a lack in β-O-GlcNAc modification sensitize methicillin resistant S. aureus (MRSA) strains to β-lactams6,7,9. This variable structure of glycosylated WTA constitutes a specific glycocode and it remains elusive why the presence or absence of both anomeric linkage types differ among clinical isolates.
Although, the presence of the tarM or tarS genes can be easily detected by PCR, to date, detection and quantification of WTA GlcNAc residues remains challenging. Often, purified WTA samples and nuclear magnetic resonance (NMR) spectroscopy are required, rendering detection and quantification of WTA GlcNAc residues both laborious and time consuming. Therefore a rapid, cost-effective, and a reliable method is urgently needed to analyse WTA α- and β-O-GlcNAc modifications in S. aureus.
Whole cell molecular fingerprinting by Fourier transform infrared (FTIR) spectroscopy represents a particularly promising approach. This user-friendly, high-throughput tool has not only been successfully implemented in bacterial identification and subtyping, but also allows users to follow phenotypic responses10–12. In S. aureus, FTIR spectroscopy was applied to analyse the charge of extracted WTA in relation to its alanine substitutions and the phosphate group in its main chain13. Recently, we were able to introduce chemometric-assisted FTIR spectroscopy as a highly discriminatory and reliable tool in the differentiation of S. aureus capsular serotypes CP5, CP8, and non-typeable (NT)14. We further demonstrated that highly discriminatory subtyping of S. aureus by FTIR spectroscopy primarily relies on the differential expression of capsular serotypes and/or additional surface glycopolymers such as WTA, peptidoglycan, and lipoteichoic acid15.
In this study, we aim to evaluate the suitability of FTIR spectroscopy as a tool for discrimination of WTA glycoepitopes, particularly between α-O-GlcNAc and β-O-GlcNAc modified WTA.
Results
Complete loss of wall teichoic acids causes strong perturbations in whole cell FTIR spectra of S. aureus
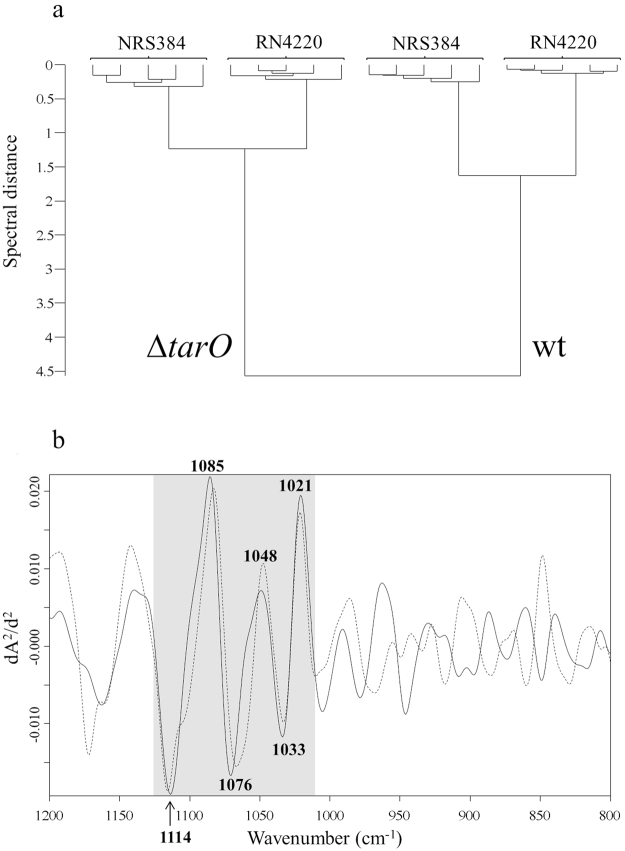
Whole cell FTIR spectra of wild type S. aureus strains RN4220 and NRS384 (also known as USA300) were compared to those of isogenic ΔtarO mutants. As shown in in Fig. 1, the mutation of the ΔtarO gene results in the complete loss of S. aureus WTA. Growth experiments and FTIR measurements were carried out five times independently. The original, average absorbance spectra over the whole spectral range (4000–500 cm−1) of strain RN4220 and their mutants can be found as Supplementary Fig. S1. 2nd-derivatives were calculated from the original absorbance spectra to increase spectral resolution and to minimize contributions of baseline shifts followed by vector normalization to adjust for variations in bacterial biomass among different sample preparation. An exploratory, hierarchical cluster analysis (HCA) was carried out to detect inherent spectral differences between wild type strains and strains lacking WTA using the spectral range of 1200–800 cm−1, previously described to be highly discriminatory for S. aureus surface glycostructures. Both ΔtarO mutants clustered separately from their isogenic wild type strains (Fig. 2a). Subtractive spectral analysis was performed to obtain relevant information of spectral differences by subtracting 2nd derivative/vector normalized spectra of ∆tarO from those of the corresponding wild types (RN4220, NRS384). The complete loss of WTA synthesis caused highly discriminatory spectral changes at wavenumbers between 1125 cm−1 and 1010 cm−1, as shown by subtractive spectral analysis in Fig. 2b. Within this spectral range, a series of prominent differences at 1114 cm−1, 1085 cm−1, 1076 cm−1, 1048 cm−1, 1033 cm−1 and 1021 cm−1 could be detected.
Figure 1.
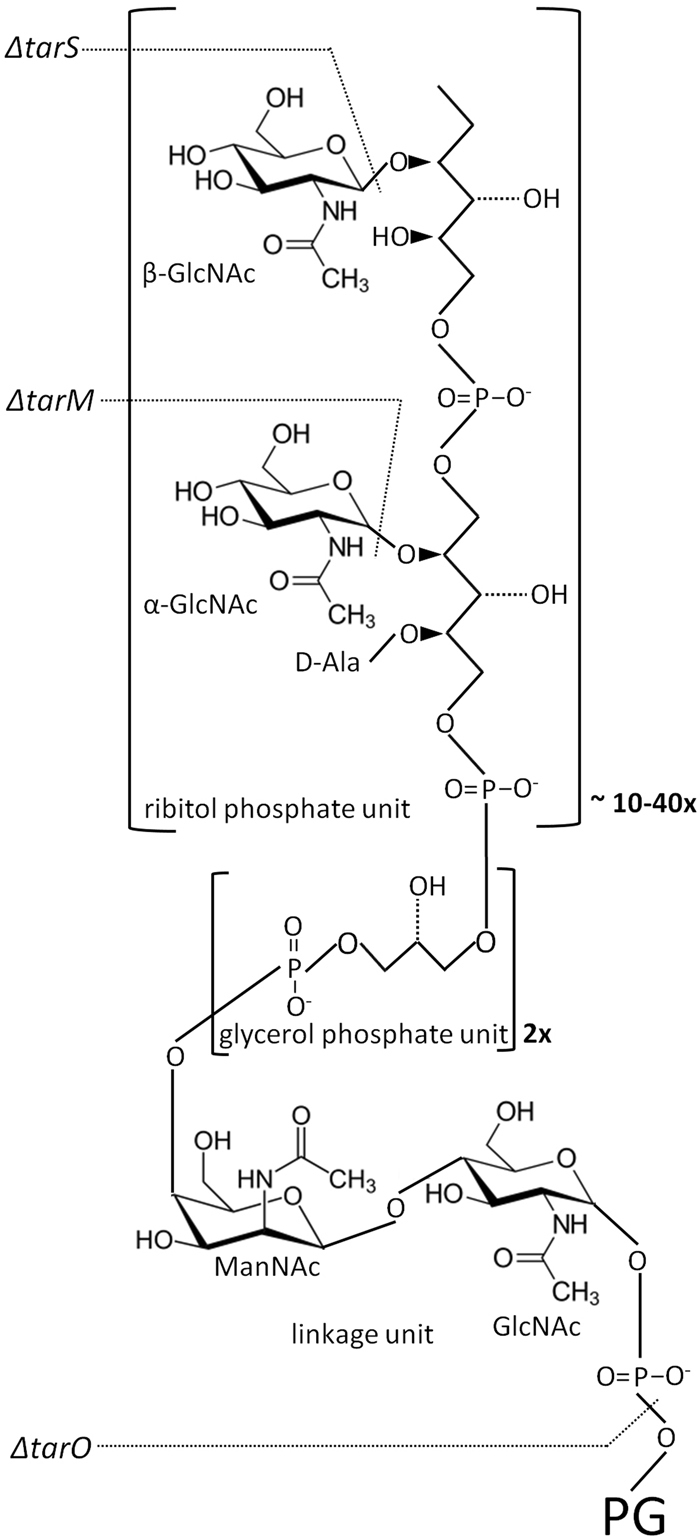
Schematic S. aureus WTA structure. S. aureus WTA consists of an ManNAc-GlcNAc disaccharide linkage unit connected to the peptidoglycan layer in the bacterial cell wall, two glycerol phosphate units and a long chain of ribitol phosphate repeating units substituted with α- or β-GlcNAc and D-alanine (adapted from Kurokawa et al.23). Structural changes by mutations of ΔtarS, ΔtarM, or ΔtarO are indicated. GlcNAc, N-acetylglucosamine; ManNAc, N-acetylmannoseamine; D-Ala, D-alanine; PG, peptidoglycan.
Figure 2.
Spectral impact of lack of S. aureus WTA. (a) FTIR spectroscopy-based dendrogram (HCA) of intact wild type (RN4220, NRS384) and isogenic ∆tarO mutant strains, each comprising measurements at five different days. (b) Subtraction spectra were generated from second derivative, vector-normalized, average FTIR spectra by subtracting spectra of ∆tarO from those of the wild type (RN4220, solid; NRS384, dashed). Most pronounced differences for both strains (RN4220, NRS384) were observed in the spectral range of 1120–1010 cm−1, which can be assigned to carbohydrate-associated vibrations and phosphodiester functional groups (see also Table 1).
Spectral differentiation of S. aureus α- and β-O-GlcNAc WTA substituents
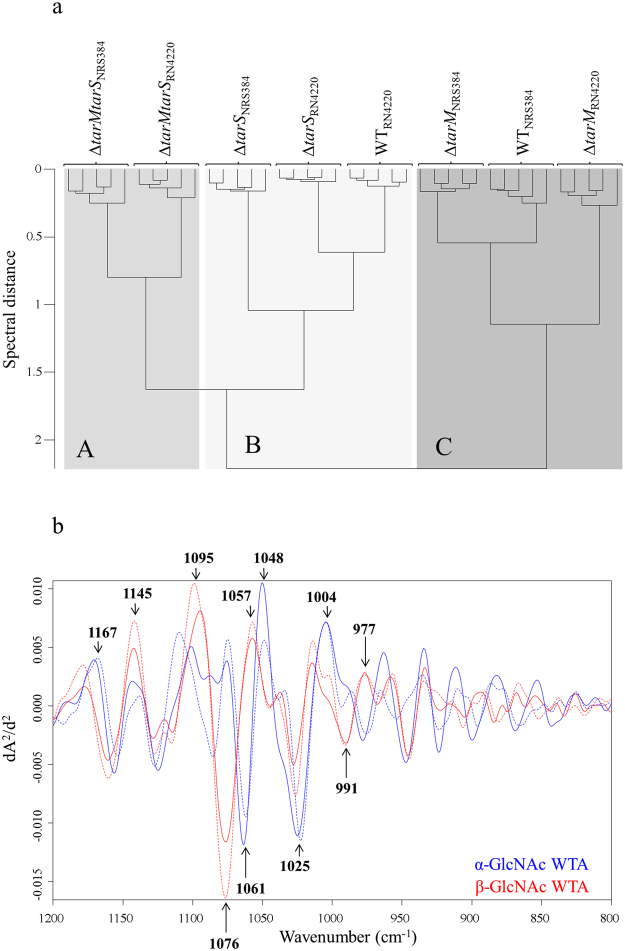
Various mutant strains derived from wild type S. aureus strain RN4220 and NRS384 were subjected to whole cell FTIR spectroscopic analysis. These include ΔtarM mutants deficient in WTA α-O-GlcNAc, ΔtarS mutants lacking WTA β-O-GlcNAc residues and double mutants (ΔtarMΔtarS) without any GlcNAc residues on WTA (Fig. 1). FTIR-HCA (Fig. 3a) revealed distinct clustering (A-C) for WTA glycosyltransferase mutants ΔtarM, ΔtarS, and ΔtarMΔtarS, respectively. Interestingly, the wild type strain RN4220 was grouped into cluster B, which includes all ΔtarS mutants deficient in WTA β-O-GlcNAc. This is in good agreement with previous observations that 90% GlcNAc residues on WTA have α-linkage in RN4220. Of note, wild type NRS384 was grouped into cluster C, which includes all ΔtarM mutants lacking WTA α-O-GlcNAc suggesting that WTA GlcNAc residues in NRS384 most probably exhibit β-configuration. Data available at transcriptional level point to a constituently higher expression of tarS (WTA β-O-GlcNAc) compared to tarM, which largely depends on growth conditions16. Subtraction spectral analysis supports this assumption showing differences in intensities of several vibrational frequencies for the α- and β-glycosidic substitution of GlcNAc residues (Fig. 3b): 1167 cm−1, 1145 cm−1, 1095 cm−1, 1076 cm−1, 1061 cm−1, 1057 cm−1, 1048 cm−1, 1025 cm−1, 1004 cm−1, 991 cm−1 and 977 cm−1. However, this cannot be finally validated, since the exact amount and relation of α- and β-O-GlcNAc for strain NRS384 are currently unknown.
Figure 3.
Spectral differentiation of S. aureus α- and β-O-GlcNAc WTA substituents. (a) FTIR spectroscopy-based dendrogram (HCA) of intact wild type (RN4220, NRS384) and isogenic ∆tarM, ∆tarS and ∆tarMS mutants, each comprising duplicate measurements at five different days. (b) Spectral assignments of α-GlcNAc and β-GlcNAc modified RboP WTA. Subtraction spectra were generated using second derivative, vector-normalized FTIR average spectra of ∆tarM and ∆tarS strains and the respective isogenic double mutant ∆tarMS of RN4220 (solid) and NRS384 (dashed). Spectra from ∆tarM∆tarS mutant strains were substracted from either (1) ∆tarM exhibiting the glycosyltransferase TarS expression corresponding to the β-GlcNAc WTA (red) or (2) ∆tarS exhibiting the glycosyltransferase TarM expression corresponding to the α-GlcNAc WTA (blue). Main spectral differences in the modification of RboP WTA with α-GlcNAc and β-GlcNAc substituents are in the spectral range of 1175–970 cm−1, which can be assigned to vibrations of carbohydrates and their glyosidic linkages.
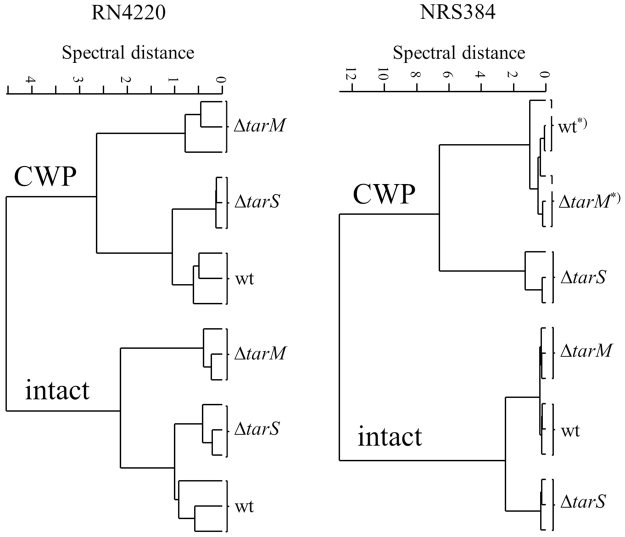
In order to evaluate if these differences obtained by FTIR spectroscopy can be particularly assigned to changes at the cellular surface and not to possible changes in the entire cellular composition of the bacteria, spectra of cell wall preparations were compared to spectra obtained from intact bacterial cells using HCA (Fig. 4). Since spectra derived from cell wall preparations of wild type, ΔtarM and ΔtarS mutants from each strain background (RN4220, NRS384) clustered consistent with spectra from intact bacterial cells, it can be assumed that the major discriminatory components are related to the bacterial surface. Thus, spectral changes due to α/β-O-GlcNAc modifications of RboP WTA can be characterized directly from intact S. aureus cells in a significantly faster and less-laborious manner.
Figure 4.
Spectral comparison between intact bacterial cells and their cell wall preparation. FTIR spectroscopy-based dendrogram of RN4220 and NRS384 WT and their corresponding mutant strains ∆tarM and ∆tarS recorded from intact bacterial cells and after cell wall preparation (CWP). Spectra derived from independent growth experiments and subsequent cell wall preparations at three different days. Since a consistent clustering of wild type and mutant strains derived from cell wall preparations and intact bacteria cells was achieved it can be assumed that major discriminatory components are related to the bacterial surface. *Several cell wall sample preparations steps result in a higher technical variation, which interferes differential clustering of minor spectral differences.
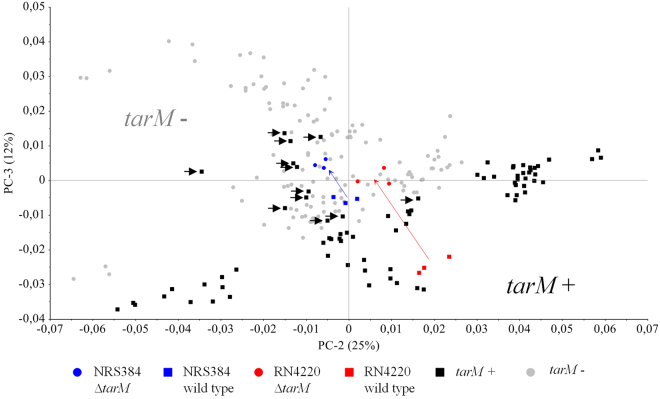
Reliable prediction of the expression ratio of α-O-GlcNAc and β-O-GlcNAc glycoepitopes by FTIR spectroscopy based on a single measurement of intact bacterial cells requires a sufficient amount of characterized strains. This would allow for the establishment of a supervised chemometric algorithm as shown previously for artificial neural network -assisted discrimination of S. aureus CP expression14. Due to the fact that expression data of the WTA α/β-O-GlcNAc –ratio of individual strains are not available and the tarS gene exits in every sequenced S. aureus genome, we focused on the correlation between the presence of the tarM gene and the signal signature of WTA α-O-GlcNAc glycoepitopes in the FTIR spectra. A well-characterized set of 70 S. aureus strains from human, animal, and food sources, which includes clonal complexes (CC) commonly isolated from asymptomatic and infected humans (CC5, CC8, CC30, and CC45) and animals (CC705,CC398) was used to determine the presence of the tarM gene. A total of 26 out of 70 strains (37.1%) were tested positive for tarM. Of note, while all tested CC705 strains are tarM positive, all tested CC398, CC5 and CC45 strains are tarM negative. CC30 and CC8 strains were found to be predominantly tarM positive, 70% and 60%, respectively (Suppl. 2). Our data also suggest that the presence of α-O-GlcNAc WTA may additionally contribute to the discrimination of S. aureus strains by FTIR spectroscopy, which is primarily based on CP expression (Suppl. 2). In particular, the tarM negative CC45 and tarM positive CC705 strains formed individual subclusters (A1 and A2) within the main cluster A of CP8 expressing strains. In addition, subcluster B2 only comprised tarM positive strains (n = 6) derived from different CC (CC15, CC8, CC22, CC30). Moreover, a principal component analysis (PCA) of FTIR spectra from this data set was carried out to correlate the inherent spectral data structures to the presence or absence of the tarM gene in a specific strain. PCA, an unsupervised multivariate data analysis technique, reduces the large spectral data space to several principal components (PCs) in an unbiased manner. As shown in Fig. 5, tarM negative and tarM positive strains can be mainly discriminated from each other by using the PC 2 (25%) and 3 (12%), while PC 1 discriminates mainly according to the CP expression of each strain. Each PC explains a certain percentage of the total variation in the original dataset and each sample has a score on it, which reflects the location of the sample along the PC. Thus, proximate samples at the scores plot are more similar to each other by forming a cluster. As expected, both tarM mutant strains (derived from either NRS384 or RN4220) were located in the tarM negative cluster, whereas the RN4220 wild type strain known to express 90% GlcNAc residues on WTA with the α-glycosidic linkage type was found in the tarM-positive strain cluster. However, triplicate measurements of four strains harbouring the tarM gene (all belong to CC8; highlighted with an arrow) and the NRS384 wild type (CC8) strain were located in the tarM-negative cluster. It can be assumed that these strains either do not or only marginally produce amounts of β-GlcNAc WTA. Further investigations with additional characterized WTA α/β-O-GlcNAc strains are needed to set up a functional, reliable supervised algorithm which can quantify the S. aureus α/β-O-GlcNAc WTA content or ratio.
Figure 5.
Spectral discrimination of α-O-GlcNAc glycoepitopes based on the presence of the tarM gene. PCA was performed from triplicate spectra of tarM positive and tarM negative strains from a diverse strain collection (n = 70) including various CCs. PC2 was plotted against PC3. PCA shows a distinct separation of the tarM positive and tarM negative strains, except the triplicate measurements of four strains (incl. NRS384 wild type) highlighted with an arrow. The tarM mutant strains of NRS384 and RN4220 were located in the tarM negative cluster, whereas the RN4220 wild type strain known for its high α-GlcNAc WTA content is located in the tarM positive cluster.
Discussion
In this study, we demonstrate that whole cell FTIR spectroscopy can be used to detect changes in S. aureus WTA glycoepitope composition. We focused on spectral bands in the wavenumber range of 1200–800 cm−1, known to be highly discriminatory for S. aureus capsule polysaccharide typing and subspecies differentiation14,15. A complete loss of S. aureus WTA expression (ΔtarO mutant) resulted in highly discriminatory changes within this spectral region, which had been previously assigned to various functional groups: (i) C-OH str, C-O-C str, Ring str vib of carbohydrates and (ii) P = O symmetric stretching of -PO2− (Table 1). These assignments can be structurally correlated to the loss of carbohydrates and phosphodiester groups in the WTA of the ΔtarO mutant strain (Fig. 1).
Table 1.
Assignment of bands of FTIR spectra mentioned in this report.
| Frequency (cm-1) | Assignment | References |
|---|---|---|
| 1200–900 | C-OH stretching modes and C-O-C, C-O ring vibrations of carbohydrates in the bacterial cell wall | 24–32 |
| 1175–1140 | Differences in glycosidic linkage configuration | 19 |
| 1160–1130 1120–1020 | C-O str of C-O-C glycosidic linkage C-O-C str of carbohydrates | 17,31 |
| 1085 | P=O sym str of >PO2− | 25,28,30,31 |
| 1080–1060 | Ring str vib of carbohydrates | 31 |
| 1080–1000 | C-OH str of carbohydrates | 31 |
| 1000–970 | Differences in glycosidic linkage configuration | 19 |
| 999–965 | C-O str in C-O-C glycosidic linkages | 18 |
Str, stretching; sym, symmetrical; vib, vibration.
The clustering of the glycosyltransferase ΔtarM and ΔtarS mutant as well as double ΔtarMΔtarS mutant strains showed the high sensitivity of FTIR spectroscopy to specifically detect α- and β-GlcNAc substitutes of WTA in intact S. aureus cells. We could demonstrate that these spectral signatures are originated from the bacterial surface comparing spectra of cell wall preparations to spectra obtained from intact bacterial cells. Vibrational spectroscopy can detect not only carbohydrate molecule-specific bands but also bands interfering with the glycosidic linkage17–19. We could previously link a highly discriminatory spectral band at 834 cm−1 to a different α- and β-glycosidic linkage for N-acetyl-D-fucosamine (D-FucNAc) of S. aureus CP8 and CP5 capsular polysaccharides, respectively14. For differences of α- and β-O-GlcNAc WTA, the spectral range of 1175–970 cm−1 showed several different bands, which predominantly arise from (i) C-OH str, C-O-C str, Ring str vib of carbohydrates, and (ii) differences in the glycosidic linkage type, and are suggested to be affected by the removal of α- and β-GlcNAc WTA (Fig. 1, Table 1). Thus, they might be helpful to predict the presence or even the ratio of α-O-GlcNAc and β-O-GlcNAc WTA in the infrared spectrum of S. aureus. We found first indications for this, since the RN4220 and NRS384 wild type strains cluster either next to the ΔtarS or ΔtarM mutant strains. Moreover, PCA analysis could roughly correlate FTIR spectral patterns to the presence or absence of the tarM gene in S. aureus.
Recently, it was shown that in contrast to tarS which is present in almost all S. aureus genomes, tarM is strain specific and missing in certain CCs (CC398, CC5, CC45)20. We could confirm the lack of tarM for these CCs using a larger number of strains. We were also able to show that all tested CC705 (former CC151) isolates, which is very common among bovine mastitis, does harbour the tarM gene. Interestingly, some CC8 and CC30 strains turned out to be lacking the tarM gene. This supports the previously raised hypothesis that tarM-dependent WTA (α-glycosidic substitution) could play a role in host tropism3. We found 36.1% of all tested strains were tarM positive, which is in good agreement with the previously published observation for nasal isolates (35.7%)20.
Our data suggest that whole cell FTIR spectroscopy is a promising approach to detect WTA GlcNAc epitopes. However, current limitations in the availability of strains, which are well characterized for the presence of WTA α- and β-GlcNAc residues, impair accurate detection by FTIR spectroscopy. Thus, to further explore the potential of FTIR spectroscopy and to analyse the abundance of α- and β-GlcNAc modified WTA, more strains serving as reference have to be analysed for their specific glycosidic linkage type by specific immunobased detection and/or NMR spectroscopy. This would enable the establishment of FTIR spectroscopy as a rapid phenotyping method to decipher the role of surface carbohydrate moieties important for bio-communication to further decipher the S. aureus glycocode. These may include, but are not limited to, the carbohydrate structures contributing to S. aureus host specificity/tropism, the glycostructural dynamics due to time-dependent monitoring across various phases of growth and the dynamic arrangement due to exposure to host cells. In particular, as WTA β-GlcNAc residues are specifically required for the beta-lactam resistance in S. aureus, a typing scheme including whole cell FTIR spectroscopy would be more advantageous to predict the resistance phenotype when compared to conventional PCR based genotyping or more recent typing by Matrix Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry (MALDI-TOF-MS), as these approaches could neither provide the configuration nor the abundance of the WTA glycoepitopes. As it is appreciated that bacterial cell surface structure such as lipopolysaccharides (LPS), CP, PNAG, glycoproteins and WTAs play crucial roles in bacterial pathogenesis, antibiotic resistance, host-pathogen interaction and antibiotic resistance, clearly, further development of whole cell FTIR spectroscopy will enable efficient and specific detection and quantification of bacterial cell surface glycostructures of important diagnostic value, help developing novel specific, fast, convenient, phenotyping and diagnostics tools for detecting bacterial infections.
Conclusion
Our study revealed that FTIR spectroscopy can detect changes in S. aureus WTA glycoepitope composition. A complete loss of S. aureus WTA expression resulted in strong perturbations in the spectral region assigned to carbohydrates and phosphorus-containing molecules. We successfully showed that FTIR spectroscopy is able to discriminate between α-/β-O-GlcNAc substitutions of S. aureus WTA. This novel approach complements the recently developed ANN-assisted FTIR spectroscopy capsular polysaccharide typing system and fosters the implementation of FTIR spectroscopy of whole bacterial cells for deciphering the S. aureus glycocode during colonization and the establishment and progression of staphylococcal infection.
Methods
Bacterial strains
RN4220 and NRS384 (US300) wild type and their isogenic mutant strains represent different glycosylation on WTA, which are listed in Table 2. A collection of well-characterized S. aureus strains (n = 70) from human, animal, and food sources was included in the study. Detailed information on all strains, including origin, clonal complex assignment, as well as comprehensive virulence and resistance gene profile determined by DNA microarray have been previously published15. All strains were analysed by PCR for the presence of the intact tarM locus according to Winstel et al.20.
Table 2.
S. aureus strains used.
| Strains | Glycosylation phenotype on WTA backbones | Source or reference |
|---|---|---|
| RN4220 | WTA with predominant α-O-GlcNAc and β-O-GlcNAc glycoepitopes | Kreiswirth et al.33 |
| RN4220 ΔtarO | Cell wall with no WTA | Xia et al.8 |
| RN4220 ΔtarM | WTA with β-O-GlcNAc glycoepitopes | Brown et al.7 |
| RN4220 ΔtarS | WTA with α-O-GlcNAc glycoepitopes | Brown et al.7 |
| RN4220 ΔtarM ΔtarS | WTA with neither α-O-GlcNAc nor β-O-GlcNAc glycoepitopes | Brown et al.7 |
| NRS384 (USA300) | Glycosylated WTA with both α-O-GlcNAc and β-O-GlcNAc glycoepitopes | NARSA collection |
| NRS384 ΔtarO | Cell wall with no WTA | Kurokawa et al.23 |
| NRS384 ΔtarM | WTA with β-O-GlcNAc glycoepitopes | Kurokawa et al.23 |
| NRS384 ΔtarS | WTA with α-O-GlcNAc glycoepitopes | Kurokawa et al.23 |
| NRS384 ΔtarM ΔtarS | WTA with neither α-O-GlcNAc nor β-O-GlcNAc glycoepitopes | Kurokawa et al.23 |
Whole cell FTIR spectroscopic measurements
FTIR spectroscopic measurements and spectral quality determination were performed as previously reported15,21. Duplicate measurements were performed on five different days. All strains were grown as a bacterial lawn on tryptone soy agar plates (Oxoid) at 30 °C. After 24 hours one loop-full of intact bacterial cells was suspended in 100 µl sterile deionized water. A 30 µL aliquot of the suspension was transferred to a zinc selenite (ZnSe) optical plate and dried at 40 °C for 40 min. Subsequently, FTIR spectra were recorded in transmission mode with an HTS-XT microplate adapter coupled to a Tensor 27 FTIR spectrometer (Bruker Optics GmbH, Ettlingen, Germany) using the following parameters: 4000–500 cm−1 spectral range, 6 cm−1 spectral resolution, zero-filling factor 4, Blackmann-Harris 3-term apodization, and 32 interferograms were averaged with background subtraction for each spectrum. Spectral quality assessment was performed for each spectrum with thresholds for minimum (0.300) and maximum (1.300) absorbance, signal to-noise (S/N) ratio, and water vapor content.
Spectral processing and chemometrics
FTIR spectra were preprocessed as follows: (1) second derivatives were calculated over the whole spectral range using a second-order 9-point Savitzky–Golay algorithm, (2) spectra were vector normalized. Chemometric analysis was performed on preprocessed data employing unsupervised hierarchical cluster analysis (HCA) and principal component analysis (PCA). The spectral region between1200–800 cm−1 was selected for further analyses, as this region is highly discriminatory for S. aureus subtyping15 and is associated to polysaccharides and their specific types of glycosidic linkages (see also Table 1). For HCA, a dendrogram was generated using the average linkage algorithm at repro-level 30 using the OPUS software (Version 7.2; Bruker Optics GmbH). PCA computation was based on the NIPALS algorithm and the second and third components were projected for PCA using the software Unscrambler X (CAMO Software, Oslo, Norway).
Cell wall extraction from S. aureus strains for FTIR spectroscopy
The S. aureus cell wall preparation was conducted according to Weidenmaier et al.22 and adapted for FTIR spectroscopic measurements. S. aureus cultures were centrifuged at 5000 × g for 10 min at RT and subsequently washed with 20 mM NH4Ac buffer (pH 4.8). After disruption in an Oneshot Frenchpress (Constant systems, Daventry, UK) using the same buffer, the cell lysates were centrifuged at 5000 × g for 10 min to remove potential intact cells. Next, SDS (final concentration of 2%) was added to the collected supernatant followed by ultra-sonication for 15 min to remove any cell membrane contaminations. After heating at 65 °C for one hour, the cell wall preparations were washed six times with 20 mM NH4Ac buffer by centrifugation at RT, 12000 × g for 10 minutes. Finally, the cell wall preparations were re-suspended in distilled water and measured by FTIR spectroscopy.
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on request.
Electronic supplementary material
Acknowledgements
We thank the Rob van Dalen and Nina van Sorge (UMC Utrecht, The Netherlands) for helpful discussions.
Author Contributions
T.G. and G.X. conceived and designed this study. G.X. and S.J. provided the strains. T.G., D.J. and W.S. performed the experiments. T.G., D.J., W.S., S.J., C.W., M.E.-S. and G.X. analyzed and interpreted the data. T.G. and G.X. wrote the manuscript. T.G. has supervised and acted as overall study director. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-20222-6.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Weidenmaier, C. & Lee, J. C. Structure and Function of Surface Polysaccharides of Staphylococcus aureus. Current topics in microbiology and immunology, 10.1007/82_2015_5018 (2016). [DOI] [PubMed]
- 2.Tuchscherr L, Loffler B, Buzzola FR, Sordelli DO. Staphylococcus aureus adaptation to the host and persistence: role of loss of capsular polysaccharide expression. Future microbiol. 2010;5:1823–1832. doi: 10.2217/fmb.10.147. [DOI] [PubMed] [Google Scholar]
- 3.Winstel V, Xia G, Peschel A. Pathways and roles of wall teichoic acid glycosylation in Staphylococcus aureus. IJMM. 2014;304:215–221. doi: 10.1016/j.ijmm.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Baur S, et al. A nasal epithelial receptor for Staphylococcus aureus WTA governs adhesion to epithelial cells and modulates nasal colonization. PLoS Pathog. 2014;10:e1004089. doi: 10.1371/journal.ppat.1004089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wanner S, et al. Wall teichoic acids mediate increased virulence in Staphylococcus aureus. Nat. Microbiol. 2017;2:16257. doi: 10.1038/nmicrobiol.2016.257. [DOI] [PubMed] [Google Scholar]
- 6.Winstel V, et al. Wall teichoic acid structure governs horizontal gene transfer between major bacterial pathogens. Nat. Commun. 2013;4:2345. doi: 10.1038/ncomms3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown S, et al. Methicillin resistance in Staphylococcus aureus requires glycosylated wall teichoic acids. Proc Natl Acad Sci USA. 2012;109:18909–18914. doi: 10.1073/pnas.1209126109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xia G, et al. Glycosylation of wall teichoic acid in Staphylococcus aureus by TarM. J Biol Chem. 2010;285:13405–13415. doi: 10.1074/jbc.M109.096172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xia G, et al. Wall teichoic Acid-dependent adsorption of staphylococcal siphovirus and myovirus. J. Bacteriol. 2011;193:4006–4009. doi: 10.1128/JB.01412-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehling-Schulz M, et al. Emetic toxin formation of Bacillus cereus is restricted to a single evolutionary lineage of closely related strains. Microbiology. 2005;151:183–197. doi: 10.1099/mic.0.27607-0. [DOI] [PubMed] [Google Scholar]
- 11.Mester P, et al. FTIR metabolomic fingerprint reveals different modes of action exerted by active pharmaceutical ingredient based ionic liquids (API-ILs) on Salmonella typhimurium. RSC Advances. 2016;6:32220–32227. doi: 10.1039/C5RA24970H. [DOI] [Google Scholar]
- 12.Naumann D, Helm D, Labischinski H. Microbiological characterizations by FT-IR spectroscopy. Nature. 1991;351:81–82. doi: 10.1038/351081a0. [DOI] [PubMed] [Google Scholar]
- 13.Latteyer F, Peisert H, Gohring N, Peschel A, Chasse T. Vibrational and electronic characterisation of Staphylococcus aureus wall teichoic acids and relevant components in thin films. Anal Bioanal Chem. 2010;397:2429–2437. doi: 10.1007/s00216-010-3832-3. [DOI] [PubMed] [Google Scholar]
- 14.Grunert T, et al. Rapid and reliable identification of Staphylococcus aureus capsular serotypes by means of artificial neural network-assisted Fourier transform infrared spectroscopy. J Clin Microbiol. 2013;51:2261–2266. doi: 10.1128/JCM.00581-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johler, S., Stephan, R., Althaus, D., Ehling-Schulz, M. & Grunert, T. High-resolution subtyping of Staphylococcus aureus strains by means of Fourier-transform infrared spectroscopy. Syst. Appl. Microbiol., 10.1016/j.syapm.2016.03.003 (2016). [DOI] [PubMed]
- 16.Mader U, et al. Staphylococcus aureus Transcriptome Architecture: From Laboratory to Infection-Mimicking Conditions. PLoS genetics. 2016;12:e1005962. doi: 10.1371/journal.pgen.1005962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kacurakova M, Capek P, Sasinkova V, Wellner N, Ebringerova A. FT-IR study of plant cell wall model compounds: pectic polysaccharides and hemicelluloses. Carbohydr Polym. 2000;43:195–203. doi: 10.1016/S0144-8617(00)00151-X. [DOI] [Google Scholar]
- 18.Kacurakova M, Mathlouthi M. FTIR and laser-Raman spectra of oligosaccharides in water: characterization of the glycosidic bond. Carbohydr Res. 1996;284:145–157. doi: 10.1016/0008-6215(95)00412-2. [DOI] [PubMed] [Google Scholar]
- 19.Nikonenko NA, Buslov DK, Sushko NI, Zhbankov RG. Investigation of stretching vibrations of glycosidic linkages in disaccharides and polysaccharides with use of IR spectra deconvolution. Biopolymers. 2000;57:257–262. doi: 10.1002/1097-0282(2000)57:4<257::AID-BIP7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Winstel V, et al. Wall Teichoic Acid Glycosylation Governs Staphylococcus aureus Nasal Colonization. mBio. 2015;6:e00632. doi: 10.1128/mBio.00632-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kummerle M, Scherer S, Seiler H. Rapid and reliable identification of food-borne yeasts by Fourier-transform infrared spectroscopy. Appl Environ Microbiol. 1998;64:2207–2214. doi: 10.1128/aem.64.6.2207-2214.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weidenmaier C, et al. Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nature medicine. 2004;10:243–245. doi: 10.1038/nm991. [DOI] [PubMed] [Google Scholar]
- 23.Kurokawa K, et al. Glycoepitopes of staphylococcal wall teichoic acid govern complement-mediated opsonophagocytosis via human serum antibody and mannose-binding lectin. J Biol Chem. 2013;288:30956–30968. doi: 10.1074/jbc.M113.509893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Helm D, Labischinski H, Schallehn G, Naumann D. Classification and identification of bacteria by Fourier-transform infrared spectroscopy. J Gen Microbiol. 1991;137:69–79. doi: 10.1099/00221287-137-1-69. [DOI] [PubMed] [Google Scholar]
- 25.Naumann, D. Infrared Spectroscopy in Microbiology in Encyclopedia of Analytical Chemistry (ed. Meyers, RA) 102-131 (John Wiley & Sons, Ltd, 2006).
- 26.Naumann D, Barnickel G, Bradaczek H, Labischinski H, Giesbrecht P. Infrared spectroscopy, a tool for probing bacterial peptidoglycan. Potentialities of infrared spectroscopy for cell wall analytical studies and rejection of models based on crystalline chitin. Eur J Biochem. 1982;125:505–515. doi: 10.1111/j.1432-1033.1982.tb06711.x. [DOI] [PubMed] [Google Scholar]
- 27.Amiali NM, et al. Rapid identification of coagulase-negative staphylococci by Fourier transform infrared spectroscopy. J Microbiol Methods. 2007;68:236–242. doi: 10.1016/j.mimet.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 28.Beekes M, Lasch P, Naumann D. Analytical applications of Fourier transform-infrared (FT-IR) spectroscopy in microbiology and prion research. Vet Microbiol. 2007;123:305–319. doi: 10.1016/j.vetmic.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 29.Bosch A, et al. Characterization of Bordetella pertussis growing as biofilm by chemical analysis and FT-IR spectroscopy. Appl Microbiol Biotechnol. 2006;71:736–747. doi: 10.1007/s00253-005-0202-8. [DOI] [PubMed] [Google Scholar]
- 30.Maquelin, K. et al. Vibrational Spectroscopic Studies of Microorganisms in Handbook of Vibrational Spectroscopy (ed. Griffiths, PR) 3308-3334 (John Wiley & Sons, Ltd., 2006).
- 31.Socrates, G. Infrared and Raman Characteristic Group Frequencies, Tables and Charts. Vol. 3 339 (John Wiley & Sons, Ltd., 2001).
- 32.Yu C, Irudayaraj J. Spectroscopic characterization of microorganisms by Fourier transform infrared microspectroscopy. Biopolymers. 2005;77:368–377. doi: 10.1002/bip.20247. [DOI] [PubMed] [Google Scholar]
- 33.Kreiswirth BN, et al. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature. 1983;305:709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the corresponding author on request.