Abstract
Lnc2Catlas (http://lnc2catlas.bioinfotech.org/) is an atlas of long noncoding RNAs (lncRNAs) associated with cancer risk. LncRNAs are a class of functional noncoding RNAs with lengths over 200 nt and play a vital role in diverse biological processes. Increasing evidence shows that lncRNA dysfunction is associated with many human cancers/diseases. It is therefore important to understand the underlying relationship between lncRNAs and cancers. To this end, we developed Lnc2Catlas to compile quantitative associations between lncRNAs and cancers using three computational methods, assessing secondary structure disruption, lncRNA-protein interactions, and co-expression networks. Lnc2Catlas was constructed based on 27,670 well-annotated lncRNAs, 31,749,216 SNPs, 1,473 cancer-associated proteins, and 10,539 expression profiles of 33 cancers from The Cancer Genome Atlas (TCGA). Lnc2Catlas contains 247,124 lncRNA-SNP pairs, over two millions lncRNA-protein interactions, and 6,902 co-expression clusters. We deposited Lnc2Catlas on Alibaba Cloud and developed interactive, mobile device-compatible, user-friendly interfaces to help users search and browse Lnc2Catlas with ultra-low latency. Lnc2Catlas can aid in the investigation of associations between lncRNAs and cancers and can provide candidate lncRNAs for further experimental validation. Lnc2Catlas will facilitate an understanding of the associations between lncRNAs and cancer and will help reveal the critical role of lncRNAs in cancer.
Introduction
Long noncoding RNAs (lncRNAs), a class of noncoding RNAs with lengths exceeding 200 nucleotides, play a vital role in dysfunctional networks in cancer1. Increasing evidence suggests that single nucleotide polymorphisms (SNPs) in lncRNAs may alter their functions and induce cancer2–4. In addition, lncRNAs can silence other gene loci by interacting with diverse genes or acting as sponges in binding processes5–7. Moreover, overexpressed lncRNAs in tumour cells correlate with genes that impact cell cycle regulation, survival, pluripotency, immune responses or other functions8. Understanding the underlying relationship between lncRNAs and cancer is an important task9.
Many databases compiling lncRNAs have been developed and provide abundant resources for exploring the function of lncRNAs. LncRNAdb provides comprehensive annotations of eukaryotic long non-coding RNAs10. LNCipedia is an integrated database that contains annotated human lncRNA transcripts obtained from different sources11. NONCODE provides non-coding RNAs across 17 species12. LncATLAS is a comprehensive resource for information on lncRNA localization in cells based on human RNA-sequencing data13. In a recent study, Hon CC et al. used FANTOM5 cap analysis of gene expression data to generate a reliable long non-coding RNA (lncRNA) dataset across 1,829 human samples14. Several resources that indicate associations between lncRNAs and diseases/cancers have been constructed and have contributed to studies on the interactions of lncRNAs and cancer. LincSNP links disease-associated SNPs to human lincRNAs and identifies disease-associated SNPs in lncRNA transcription factor binding sites (TFBSs)15. LncRNASNP identifies SNPs in lncRNAs and analyses their potential impacts on lncRNA structure and function16. LncRNADisease curates and annotates 478 entries of experimentally supported lncRNA-disease associations, including 128 human lncRNAs and 166 diseases17. The LncRNADisease database also curates lncRNA interaction partners at various molecular levels. Lnc2Cancer provides more than 1,000 manually curated associations between 531 lncRNAs and 86 human cancers18. TANRIC represents a resource for exploring the function and clinical relevance of lncRNAs in 20 cancer types19. lnCaNet analyses the interactions between 9,641 lncRNAs and 2,544 cancer genes and provides 8,494,907 significant co-expression pairs20. However, a database compiling quantitative associations between lncRNAs and cancer from distinct aspects is still needed.
In this study, we developed Lnc2Catlas to compile the underlying quantitative associations between lncRNAs and cancer using multiple methods and data sources. Lnc2Catlas assesses lncRNA-cancer associations with three quantified ranking methods, assessing secondary structure disruptions, lncRNA-protein interactions, and co-expression networks. Lnc2Catlas was constructed based on 27,670 well-annotated lncRNAs, 31,749,216 SNPs, 1,473 cancer-associated proteins, and 10,539 expression profiles of 33 cancers from The Cancer Genome Atlas (TCGA, https://portal.gdc.cancer.gov/). It contains 247,124 lncRNA-SNP pairs, over two millions lncRNA-protein interactions, and 6,902 co-expression clusters. We deposited Lnc2Catlas on Alibaba Cloud and developed interactive, mobile device-compatible, user-friendly interfaces to assist users in searching and browsing with ultra-low latency. Lnc2Catlas is a valuable resource for investigating the associations between lncRNAs and cancer from distinct aspects. Lnc2Catlas is now freely available at http://lnc2catlas.bioinfotech.org.
Results
Database interface
Lnc2Catlas provides browse and search functions for users to query the database, and these functions are accessed from the top navigation bar (Fig. 1A). All data in the database can be obtained on the ‘Download’ page. User-defined content on the detail pages for each lncRNA is also provided for download. Detailed help materials are available on the ‘Help’ page to ensure easy use for first-time visitors.
Figure 1.
Screenshots of the Lnc2Catlas web interface. (A) Navigation bar at the top of the pages; (B) pie chart on the ‘Home’ page and the list of cancers collected in Lnc2Catlas on the ‘Browse’ page; (C) browse page for a certain cancer; (D) search box provided on the ‘Home’ and ‘Search’ pages; and (E) detail page of a queried lncRNA.
We have provided direct entry to all 33 cancers collected by Lnc2Catlas. Users can select a cancer type on the left of the ‘Browse’ page or can tap on a region of the pie chart on the ‘Home’ page (Fig. 1B). For each cancer, the ‘Browse’ page displays a brief description of the cancer and cancer-associated SNPs, proteins, and lncRNAs obtained using the three methods indicated above. In addition, each SNP and protein are linked to their corresponding pages in dbSNP21 and GeneCards22, respectively. Clicking the corresponding detail button in the lncRNA list will redirect the user to the lncRNA detail page. In addition, thumbnail blocks of the weighted co-expression network generated through weighted gene co-expression network analysis (WGCNA)23 are shown. Users can click the detail button to zoom in their view of the network and can select a node of interest to see its connected lncRNAs and proteins. Additionally, users can click the lncRNA ID in the dialog box to be redirected to the lncRNA detail page. Only the 85,000 most-weighted connections are presented in each network due to memory and time costs, but the full-scale network can still be accessed on the ‘Download’ page for further research (Fig. 1C).
The search box accepts case-insensitive inputs to query Lnc2Catlas using different identifiers on the ‘Search’ and ‘Home’ pages (Fig. 1D). Three types of input are supported: SNP (rs ID), protein (Gene Symbol) and lncRNA transcript ID (ENST ID). For each SNP input, Lnc2Catlas returns lncRNAs ranked by p-values based on secondary structure disruption analysis. The SNP data source and associated cancer are also presented in a tabular manner. For each protein input, the returned results are presented similarly to those of a SNP query; the protein’s associated lncRNAs ranked by Global Score and its related cancer are listed. For lncRNA inputs, the case is more complicated. If the query returns multiple results, two tables are displayed, listing lncRNA-SNP interactions and lncRNA-protein interactions. In contrast, if the query returns a definite lncRNA, the page will directly redirect the user to the corresponding lncRNA detail page.
On the lncRNA detail page, Lnc2Catlas displays basic information on the lncRNA and the computational results obtained through secondary structure disruption analysis, lncRNA-protein interaction analysis, expression analysis, co-expression network analysis, and gene ontology (GO) and pathway analysis of co-expression clusters. A local base pair probability matrix and secondary structures are shown when users select a SNP in the SNP-structure disruption table. The expression value of the corresponding cancer is shown when the mouse is moved over the bar, providing an interactive view of the results. Moreover, the graph of the co-expression network can be scaled and dragged, and any node of the network and its adjacent nodes can be highlighted by hovering over it (Fig. 1E). In the GO and pathway analysis results, users can select a cancer type in the drop-down box to obtain term types, enrichment p-values and matched gene lists. The publications experimentally supporting the above lncRNA-cancer associations are presented in the bottom of the detail page.
Secondary structure disruption
Distinctive secondary structures are a primary prerequisite for the function of noncoding RNAs; therefore, alterations caused by disease-associated SNPs confer a predisposition to dysfunction and the possibility of severe disease. We used RNAsnp24 to quantitatively evaluate the impacts of the disruptions caused by SNPs to lncRNA secondary structure. In total, 19,745 lncRNA transcripts were found to be significantly altered by 247,124 dbSNPs. Among these lncRNA transcripts, 298 were defined as significantly altered by 914 cancer-associated SNPs. In this analysis, the secondary structures of all lncRNA transcripts and the local base pair probability matrix and secondary structures of the wild-type and mutant type of each lncRNA are presented in the detail page.
LncRNA-protein interactions
The identification of interactions between lncRNAs and proteins through experimental methods is challenging. Thus, a database quantifying the strength of interaction between lncRNAs and cancer-associated proteins is extremely beneficial. To prioritize candidate lncRNA-protein pairs in cancer, we used Global Score25 to calculate binding scores between 27,670 lncRNA transcripts and 1,040 cancer-associated proteins. For each cancer type, only the expressed lncRNAs and proteins with fragments per kilobase million (FPKM) values > 0 were selected to calculate the binding interaction scores using Global Score. A higher score indicates a greater binding interaction strength between a lncRNA and a cancer-associated protein. Due to the large number of computations, Lnc2Catlas currently compiles over two million lncRNA-protein interactions, covering 27,670 lncRNA transcripts, with at least the top three genes for each cancer. All of these interactions are available in Lnc2Catlas.
Co-expression network
LncRNAs are expressed in a tissue-specific and heterogeneously regulated manner in tumour/normal cells. The underlying regulatory expression patterns correlated with genes with key roles in cancer were explored by constructing weighted co-expression networks based on 10,539 expression profiles from TCGA through WGCNA. For each cancer, the co-expression network calculated via WGCNA was divided into small blocks with weighted edges between the lncRNAs and protein-coding genes. Twelve blocks on average were created for each cancer. In total, 395 blocks, ranging from 2,831 lncRNAs x 1,549 protein-coding genes in kidney chromophobe cancer to 3 lncRNAs x 2 protein-coding genes in breast invasive carcinoma, are presented in Lnc2Catlas. The results of co-expression network analysis were presented as clusters (modules) of highly correlated lncRNAs and genes for each cancer. In total, 6,902 clusters were defined in 33 cancer types, ranging from 59 clusters for breast invasive carcinoma to 348 clusters for brain lower-grade glioma. Further GO analysis of the 6,902 clusters in 33 cancer types revealed that 2,415 clusters were enriched with various GO and pathway terms.
Case studies
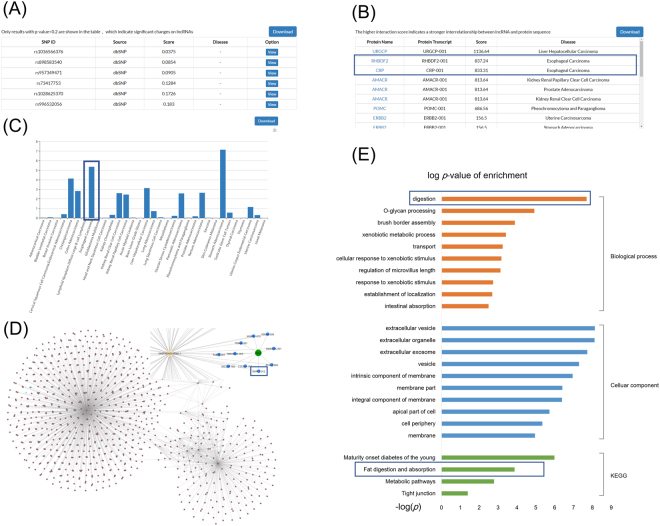
Lnc2Catlas compiles quantitative associations between lncRNAs and cancer. Lnc2Catlas can aid in the investigation of the potential role of lncRNAs in cancer by exploring the associations between lncRNAs and cancer. Two lncRNA transcripts are taken as examples to demonstrate how to explore lncRNA-cancer associations using Lnc2Catlas. ENST00000535301.1 is a lncRNA transcript located on chromosome 12 whose knockdown greatly impacts the proliferation of oesophageal adenocarcinoma cells26. This lncRNA transcript can be accessed in Lnc2Catlas by searching for its ID (Fig. 2). Secondary structure disruption analysis showed that 6 SNPs greatly altered the secondary structure of this transcript (Fig. 2A). LncRNA-protein interaction analysis demonstrated that this lncRNA transcript showed high interaction scores with RHBDF2 and CRP, of 837.24 and 833.31, respectively. RHBDF2, also known as IRHOM2, has been reported to play a critical role in activating or downregulating the EGFR signalling pathway connected to oesophageal adenocarcinoma27,28 (Fig. 2B). The postoperative levels of CRP were used to predict prognosis after treatment of oesophageal adenocarcinoma29. In addition, this transcript was differentially expressed in 33 cancer types and exhibited the second-highest ranked expression in oesophageal cancer samples (Fig. 2C). Furthermore, weighted co-expression analysis showed that this transcript displays a similar expression pattern to DAPK1, which mediates programmed cell death and is associated with diverse cancers30,31 (Fig. 2D). GO and pathway analysis of the highly correlated clusters involving this transcript and its associated genes in oesophageal adenocarcinoma revealed enrichment for genes involved in the functions of digestion and pathways of fat digestion and absorption (Fig. 2E). Together, the results provided by Lnc2Catlas suggest that the ENST00000535301.1 transcript may play an important role in oesophageal cancer.
Figure 2.
An example illustrating how Lnc2Catlas helps reveal the potential function of a lncRNA in oesophageal cancer. (A) A SNP located in the ENST00000535301.1 transcript significantly impacts secondary structure; (B) genes (RHBDF2 and CRP) with key roles in oesophageal cancer were highly associated with the transcript by Global Score; (C) ENST00000535301.1 was expressed at a relatively higher level in oesophageal cancer; and (D) ENST00000535301.1 s was found to correlate with the death-related gene DAPK1 (indicated with a red box) in oesophageal cancer based on co-expression networks. (E) GO and pathway analysis of genes that were highly correlated with ENST00000535301.1 involved in the cluster derived from co-expression analysis of oesophageal adenocarcinoma.
ENST00000422494.1, a lncRNA transcript located on chromosome 20, has been reported to exhibit a function related to papillary thyroid carcinoma32. We searched for this transcript in Lnc2Catlas to examine its association with thyroid cancer (Figure S3). We found 11 SNPs located in ENST00000422494.1 that significantly disrupted its secondary structure (Figure S3A). In addition, this transcript presented the highest expression associated with thyroid cancer, exhibiting 6-times higher expression than the second-ranked transcript (Figure S3B). Co-expression network analysis showed that in thyroid cancer cells, ENST00000422494.1 was expressed in the same pattern as MPPED2, which has been widely reported to be overexpressed and to affect the malignancy of lesions in thyroid tissues33,34 (Figure S3C). Further GO and pathway enrichment analysis revealed that genes in cluster from thyroid cancer are involved in the thyroid hormone signalling pathway. These results suggest that ENST00000422494.1 may function in thyroid carcinoma (Figure S3D). These two examples demonstrate that Lnc2Catlas can truly aid in the exploration of associations between lncRNAs and cancer and provide clues to the potential roles of lncRNAs in cancer.
Discussion
Evidence has suggested that lncRNAs play a crucial role in cancer; however, the screening of candidate lncRNAs and definition of their function in specific cancers is still challenging. To this end, we present Lnc2Catlas, which compiles multiple quantitative assessments of the associations between lncRNAs and cancer to help researchers prioritize candidate lncRNAs. A study based on TCGA project demonstrated that 60% of dysregulated lncRNAs were specific to only one tumour35. These tumour-specific dysregulated lncRNAs participate in diverse regulatory process as regulators or regulatees. To help researchers infer the underlying functional lncRNAs in specific tumours, we link lncRNAs with disease-associated SNPs and cancer-associated proteins and genes. Lnc2Catlas integrates multiple resources and constructs lncRNA-centric networks that associate cancer-specific lncRNAs with SNPs, proteins and genes related to each cancer. The two presented case studies demonstrate that our Lnc2Catlas is helpful to complement the experimental design and validation processes in studies of lncRNA functions in cancer by prioritizing candidate lncRNAs based on three quantitative assessments.
Other groups have also constructed lncRNA-cancer association databases, such as lncRNASNP, LncRNADisease, Lnc2Cancer, TANRIC, and lnCaNet. A comparison between Lnc2Catlas and these similar databases is presented in Table 1, which demonstrates some strong advantages of Lnc2Catlas in comparison with these other databases. One of the advanced characteristics distinguishing Lnc2Catlas is the ability to explore the quantitative associations between lncRNAs and cancer from distinct aspects. In studies of lncRNA functions, the prioritization of candidate lncRNAs in the experimental design and validation processes is challenging. Lnc2Catlas adopts three scoring methods to quantify the associations between lncRNAs and cancer. Thus, Lnc2Catlas can help researchers to rank candidate lncRNAs and explore their potential roles in cancer, on the basis of secondary structure disruption, lncRNA-protein interactions, and co-expression networks. To enhance the experimentally supported ability of our Lnc2Catlas, we curated 1,038 publications related to lncRNA-cancer associations in Lnc2Catlas.
Table 1.
Comparison between Lnc2Catlas and other five cancer-associated databases.
| Database | LncRNADisease | Lnc2Cancer | lncRNASNP | TANRIC | lnCaNet | Lnc2Catlas | |
|---|---|---|---|---|---|---|---|
| Data | Number of lncRNAs | 914 | 531 | 17,436 | 12,727 | 9,641 | 27,670 |
| Number of diseases | 329 | 86 | 28 | 20 | 11 | 33 | |
| SNPs | GWAS | Not available | GWAS and LD analysis | Not available | Not available | GWAS, Clinvar and LD analysis | |
| Genes | Not available | Not available | Not available | Collected from publications | Collected from publications | MalaCard, DisGeNet, Human protein Atlas | |
| Gene expression | Not available | Not available | Not available | TCGA | TCGA | TCGA | |
| Publication support | Available | Available | Not Available | Not available | Not Available | Available | |
| Analysis | Analysis based on SNPs | Based on genomic neighbourhood | Not available | Based on miRNA-lncRNA target | Not available | Not available | Based on secondary structure |
| Analysis based on physical interaction with genes | Not available | Not available | Not available | Not available | Not available for lncRNA-protein interactions | Available for 27,670 lncRNAs and 1,759 cancer-related genes | |
| Analysis based on gene expression | Not available | Not available | Not available | Differential expression and correlation with clinical data | Co-expression network analysis | Weighted gene co-expression network analysis | |
| Computational result coverage | 1,564 lncRNAs | Not available | 17,436 lncRNAs | 12,727 lncRNAs | 9,641 lncRNAs | 27,670 lncRNAs | |
| Interface | Mobile device compatibility | No | No | Yes | No | No | Yes |
| Graphic visualization | No | No | Yes | No | No | Yes | |
We will continually update the database by integrating up-to-date resources and methods that quantify the associations between lncRNAs and cancer. In summary, Lnc2Catlas will facilitate our understanding of the associations between lncRNAs and cancer and will aid in the investigation of the potential role of lncRNAs in cancer.
Materials and Methods
LncRNAs, SNPs, and proteins
The relationship between lncRNAs and cancers was explored by selecting 10,539 expression files from 10,237 samples of 33 cancer types from TCGA; this dataset included profiles of 15,899 lncRNA genes and 20,241 coding genes. Annotations of the corresponding 27,670 lncRNA transcripts were collected from GENCODE V22 (hg38) for consistency with the data protocol for TCGA. A total of 31,749,216 SNPs located in lncRNA transcripts were collected from dbSNP (build 147). Among these SNPs, 92,604 were defined as cancer-associated SNPs, including 941 SNPs from genome-wide association studies (GWAS)36, 39,558 ClinVar SNPs37 marked as “Pathogenic” or “Likely Pathogenic”, and 52,105 SNPs in strong linkage disequilibrium (LD) with GWAS or ClinVar SNPs (r2 > 0.8). We also collected 1,473 curated cancer-associated proteins from MalaCards38, DisGeNET39, and the Human Protein Atlas in a recent study40, with an average 45 proteins for each cancer, ranging from 4 proteins in uterine corpus endometrial carcinoma to 321 proteins in breast invasive carcinoma.
Quantifying associations between lncRNAs and cancer
Three different scoring methods were used to quantify associations between lncRNAs and cancer in Lnc2Catlas (Table 2). To quantify the impacts of the SNPs on lncRNA secondary structure, we used RNAsnp to calculate the disruption of secondary structure between the wild type and the mutant type and to predict the significance of disruptive impacts using default parameters. For each lncRNA-SNP pair, a p-value was calculated to indicate the significance of the disruptive impact of the SNP on the lncRNA. Disruptions with p-values < 0.2 (default threshold of RNAsnp) are considered significant, indicating that the corresponding lncRNA is more likely to undergo major structural changes. The secondary structures of all lncRNA transcripts were calculated using RNAfold from the ViennRNA packages41. The local base pair probability matrix and secondary structures of the wild-type and mutant type of each lncRNA were generated using ViennRNA packages and VARNA software42, respectively. For the analysis of lncRNA-protein interactions, we calculated the potential binding strength between 27,670 lncRNA transcripts and 1,473 cancer-associated proteins from MalaCards and other databases using Global Score with the default parameters. For the analysis of co-expression networks, the expression profiles of tumour and normal samples were merged into one matrix to produce the topological overlap matrix for each cancer type, which had an average size of approximately 55,000 × 55,000. The topological overlap matrix was then divided into small blocks with weighted edges between the lncRNAs and protein-coding genes, composed of the clusters (modules) of highly correlated lncRNAs and genes. Furthermore, we used g:Profiler43 to investigate GO and pathway enrichment for each cluster, employing a threshold p-value of 0.05. The GO of biological processes, cellular components, and molecular functions and the pathways of KEGG and Reactome were used in the enrichment analysis.
Table 2.
Data source and methods adopted in Lnc2Catlas.
| Secondary structure disruption | lncRNA-protein binding interaction | Co-expression network analysis | |
|---|---|---|---|
| Method | RNAsnp | GlobalScore | WGCNA |
| Input | SNP and sequence of lncRNA | Sequences of lncRNAs and cancer-related proteins | Expression profiles of lncRNAs and genes in different cancer |
| Data Sources | 31,749,216 SNPs from dbSNP; 941 cancer-related SNPs from GWAS; and 39,558 cancer-related SNPs from Clinvar | 1,040 cancer-related proteins from Malacard; 664 cancer-related proteins from DisGeNet; and 55 genes from Human Protein Atlas. In total, 1,473 genes. | 10,539 expression files from 10,237 samples of 33 cancer types |
| Results | p-value indicating the significance of the impact of secondary structure disruption | Score indicating the binding strength | Clusters (modules) of highly correlated lncRNAs and genes |
| Results statistic | 19,745 lncRNAs significantly altered by 247,124 SNPs. 298 lncRNAs altered by 914 cancer-associated SNPs. | Over two million lncRNA-protein interactions | 6,902 clusters in 33 co-expression networks of 33 cancer types. 2,415 clusters with enriched GO and pathway terms |
| Publication supports | 113 | 144 | 905 |
Experimentally validated support
To enhance the experimentally supported ability of Lnc2Catlas, publications addressing lncRNAs and cancer were curated from PubMed. First, we used “(lncRNA or long noncoding RNA) AND (cancer OR tumour OR neoplasms)” as keywords to search the PubMed database. Second, we manually curated these publications to identify lncRNA-cancer associations. We classified these publications into different classes to support the associations in Lnc2Catlas. Publications describing the study of variants were used to support the existence of SNPs in lncRNAs in tumour/cancer samples. Publications addressing lncRNA-protein binding interactions were used to support the prediction of lncRNA-protein interactions. Publications reporting investigations of the expression pattern of lncRNAs and genes in tumours and cancers were used to support co-expression network analysis. Finally, we checked the aliases of lncRNAs and replaced them with unified GENCODE annotations for each lncRNA processed in our study. Furthermore, to compile more comprehensive publication support, we also integrated publications from several other databases, including lncRNAdb, LNCipedia, NONCODE, LncRNADisease, and Lnc2Cancer. In total, we compiled 1,038 publications related to lncRNA-cancer associations in Lnc2Catlas, among which 113, 144, and 905 supported the predictions of secondary structure disruptions, lncRNA-protein interactions, and co-expression networks, respectively, covering 562 lncRNAs.
Implementation
Lnc2Catlas is deployed on an Apache HTTP Server 2.4.7 with a Linux (Ubuntu14.04) operating system. The database was built using a Django web framework (version 1.11.1) and the MySQL database (version 5.5.55). We deposited Lnc2Catlas on Alibaba Cloud, which provides fast memory and the latest Intel CPUs to help users achieve ultra-low latency. The web interface was based on Bootstrap 3, a framework integrating HTML, CSS, and JavaScript, which can easily and efficiently scale websites on both handheld devices and older browsers (Figure S1). Graphs and charts are generated for users to interactively visualize and access the data using echarts3 (http://echarts.baidu.com/) and cytoscape.js44. Five MySQL tables were created to efficiently organize and store the huge amount of data in Lnc2Catlas. The first four tables store the results obtained with RNAsnp, Global Score, WGCNA, and g:Profiler. The fifth table contains basic information on the lncRNAs, including sequences, gene expression, and publication support. LncRNA transcript IDs act as a primary key in all tables (Figure S2).
Electronic supplementary material
Acknowledgements
We would like to thank TCGA Project Consortium for making their data publicly available. We thank Dr. Alexandros Armaos of Tartaglia Lab (Centre for Genomic Regulation, The Barcelona Institute of Science and Technology, Barcelona, Spain) for sharing Global Score with us. This work was supported by grants from the Major Research Plan of the National Key R&D Program of China (No. 2016YFC0901600), the National Natural Science Foundation of China (No. U1435222), and the National High Technology R&D Program of China (No. 2015AA020108).
Author Contributions
W.S. conceived the study. W.S. and X.B. designed all of the experiments. W.S., C.R. and G.A. drafted the manuscript. C.R. and G.A. wrote the programs and analysed the results. C.Z. and Z.O. assisted in the analysis and discussion and provided useful comments. All authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Chao Ren and Gaole An contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-20232-4.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiaochen Bo, Email: boxc@bmi.ac.cn.
Wenjie Shu, Email: shuwj@bmi.ac.cn.
References
- 1.Huarte M. The emerging role of lncRNAs in cancer. Nat Med. 2015;21:1253–1261. doi: 10.1038/nm.3981. [DOI] [PubMed] [Google Scholar]
- 2.Yang C, et al. Tag SNPs in long non-coding RNA H19 contribute to susceptibility to gastric cancer in the Chinese Han population. Oncotarget. 2015;6:15311–15320. doi: 10.18632/oncotarget.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma X, et al. Tag SNPs of long non-coding RNA TINCR affect the genetic susceptibility to gastric cancer in a Chinese population. Oncotarget. 2016;7:87114–87123. doi: 10.18632/oncotarget.13513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin Y, et al. The association of rs710886 in lncRNA PCAT1 with bladder cancer risk in a Chinese population. Gene. 2017;627:226–232. doi: 10.1016/j.gene.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 5.Marin-Bejar O, et al. Pint lincRNA connects the p53 pathway with epigenetic silencing by the Polycomb repressive complex 2. Genome Biol. 2013;14:R104. doi: 10.1186/gb-2013-14-9-r104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhai N, Xia Y, Yin R, Liu J, Gao F. A negative regulation loop of long noncoding RNA HOTAIR and p53 in non-small-cell lung cancer. Onco Targets Ther. 2016;9:5713–5720. doi: 10.2147/OTT.S110219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang GW, Zhang YL, Liao LD, Li EM, Xu LY. Natural antisense transcript TPM1-AS regulates the alternative splicing of tropomyosin I through an interaction with RNA-binding motif protein 4. Int J Biochem Cell Biol. 2017;90:59–67. doi: 10.1016/j.biocel.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 8.Shih JW, et al. Long noncoding RNA LncHIFCAR/MIR31HG is a HIF-1alpha co-activator driving oral cancer progression. Nat Commun. 2017;8:15874. doi: 10.1038/ncomms15874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guttman M, et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458:223–227. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quek XC, et al. lncRNAdbv2.0: expanding the reference database for functional long noncoding RNAs. Nucleic Acids Res. 2015;43:D168–D173. doi: 10.1093/nar/gku988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volders PJ, et al. LNCipedia: a database for annotated human lncRNA transcript sequences and structures. Nucleic Acids Res. 2013;41:D246–D251. doi: 10.1093/nar/gks915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao Y, et al. NONCODE 2016: an informative and valuable data source of long non-coding RNAs. Nucleic Acids Res. 2016;44:D203–208. doi: 10.1093/nar/gkv1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mas-Ponte D, et al. LncATLAS database for subcellular localization of long noncoding RNAs. RNA. 2017;23:1080–1087. doi: 10.1261/rna.060814.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hon CC, et al. An atlas of human long non-coding RNAs with accurate 5’ ends. Nature. 2017;543:199–204. doi: 10.1038/nature21374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ning S, et al. LincSNP 2.0: an updated database for linking disease-associated SNPs to human long non-coding RNAs and their TFBSs. Nucleic Acids Res. 2017;45:D74–D78. doi: 10.1093/nar/gkw945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong J, Liu W, Zhang J, Miao X, Guo A. Y. lncRNASNP: a database of SNPs in lncRNAs and their potential functions in human and mouse. Nucleic Acids Res. 2015;43:D181–186. doi: 10.1093/nar/gku1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen G, et al. LncRNADisease: a database for long-non-coding RNA-associated diseases. Nucleic Acids Res. 2013;41:D983–986. doi: 10.1093/nar/gks1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ning S, et al. Lnc2Cancer: a manually curated database of experimentally supported lncRNAs associated with various human cancers. Nucleic Acids Res. 2016;44:D980–985. doi: 10.1093/nar/gkv1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, et al. TANRIC: An Interactive Open Platform to Explore the Function of lncRNAs in Cancer. Cancer Res. 2015;75:3728–3737. doi: 10.1158/0008-5472.CAN-15-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu YN, Zhao M. lnCaNet: pan-cancer co-expression network for human lncRNA and cancer genes. Bioinformatics. 2016;32:1595–1597. doi: 10.1093/bioinformatics/btw017. [DOI] [PubMed] [Google Scholar]
- 21.Sherry ST, et al. dbSNP: the NCBI database of genetic variation. Nucleic Acids Res. 2001;29:308–311. doi: 10.1093/nar/29.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stelzer, G. et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr Protoc Bioinformatics54, 1 30 31-31 30 33, 10.1002/cpbi.5 (2016). [DOI] [PubMed]
- 23.Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9:559. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sabarinathan R, et al. RNAsnp: efficient detection of local RNA secondary structure changes induced by SNPs. Hum Mutat. 2013;34:546–556. doi: 10.1002/humu.22323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cirillo D, et al. Quantitative predictions of protein interactions with long noncoding RNAs. Nat Methods. 2016;14:5–6. doi: 10.1038/nmeth.4100. [DOI] [PubMed] [Google Scholar]
- 26.Yang X, et al. Long non-coding RNA HNF1A-AS1 regulates proliferation and migration in oesophageal adenocarcinoma cells. Gut. 2014;63:881–890. doi: 10.1136/gutjnl-2013-305266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siggs OM, et al. Genetic interaction implicates iRhom2 in the regulation of EGF receptor signalling in mice. Biol Open. 2014;3:1151–1157. doi: 10.1242/bio.201410116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee MY, Nam KH, Choi KC. iRhoms; Its Functions and Essential Roles. Biomol Ther (Seoul) 2016;24:109–114. doi: 10.4062/biomolther.2015.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ibuki Y, et al. Role of Postoperative C-Reactive Protein Levels in Predicting Prognosis After Surgical Treatment of Esophageal Cancer. World J Surg. 2017;41:1558–1565. doi: 10.1007/s00268-017-3900-3. [DOI] [PubMed] [Google Scholar]
- 30.Dai L, et al. DAPK Promoter Methylation and Bladder Cancer Risk: A Systematic Review and Meta-Analysis. PLoS One. 2016;11:e0167228. doi: 10.1371/journal.pone.0167228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao J, et al. Death-associated protein kinase 1 promotes growth of p53-mutant cancers. J Clin Invest. 2015;125:2707–2720. doi: 10.1172/JCI70805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lan X, et al. Genome-wide analysis of long noncoding RNA expression profile in papillary thyroid carcinoma. Gene. 2015;569:109–117. doi: 10.1016/j.gene.2015.05.046. [DOI] [PubMed] [Google Scholar]
- 33.Finn SP, et al. Expression microarray analysis of papillary thyroid carcinoma and benign thyroid tissue: emphasis on the follicular variant and potential markers of malignancy. Virchows Arch. 2007;450:249–260. doi: 10.1007/s00428-006-0348-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazzanti C, et al. Using gene expression profiling to differentiate benign versus malignant thyroid tumors. Cancer Res. 2004;64:2898–2903. doi: 10.1158/0008-5472.CAN-03-3811. [DOI] [PubMed] [Google Scholar]
- 35.Yan X, et al. Comprehensive Genomic Characterization of Long Non-coding RNAs across Human Cancers. Cancer Cell. 2015;28:529–540. doi: 10.1016/j.ccell.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacArthur J, et al. The new NHGRI-EBI Catalog of published genome-wide association studies (GWAS Catalog) Nucleic Acids Res. 2017;45:D896–D901. doi: 10.1093/nar/gkw1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Landrum MJ, et al. ClinVar: public archive of interpretations of clinically relevant variants. Nucleic Acids Res. 2016;44:D862–868. doi: 10.1093/nar/gkv1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rappaport N, et al. MalaCards: an amalgamated human disease compendium with diverse clinical and genetic annotation and structured search. Nucleic Acids Res. 2017;45:D877–D887. doi: 10.1093/nar/gkw1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinero J, et al. DisGeNET: a comprehensive platform integrating information on human disease-associated genes and variants. Nucleic Acids Res. 2017;45:D833–D839. doi: 10.1093/nar/gkw943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uhlen M, et al. A pathology atlas of the human cancer transcriptome. Science. 2017;357:660−+. doi: 10.1126/science.aan2507. [DOI] [PubMed] [Google Scholar]
- 41.Lorenz R, et al. ViennaRNA Package 2.0. Algorithms Mol Biol. 2011;6:26. doi: 10.1186/1748-7188-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Darty K, Denise A, Ponty Y. VARNA: Interactive drawing and editing of the RNA secondary structure. Bioinformatics. 2009;25:1974–1975. doi: 10.1093/bioinformatics/btp250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reimand J, et al. g:Profiler-a web server for functional interpretation of gene lists (2016 update) Nucleic Acids Res. 2016;44:W83–W89. doi: 10.1093/nar/gkw199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Franz M, et al. Cytoscape.js: a graph theory library for visualisation and analysis. Bioinformatics. 2016;32:309–311. doi: 10.1093/bioinformatics/btv557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.