Abstract
Background
Patients with cerebral small vessel disease may suffer from varying levels of cognitive deficit and may progress on to vascular dementia. The extent of involvement, as seen on conventional magnetic resonance (MR) measures, correlates poorly with the level of cognitive decline. The purpose of this study was to investigate the utility of diffusion tensor imaging (DTI) as a marker for white matter damage in small vessel disease and to assess its correlation with cognitive function.
Methods
Thirty consecutive patients with cerebral small vessel disease underwent conventional MR imaging, DTI, and neuropsychological assessment.
Results
On tractographic analysis, fractional anisotropy was significantly reduced while mean diffusivity significantly increased in several white matter tracts. The alteration in DTI indices correlated well with cognitive function. No significant correlation was identified between T2 lesion load and cognitive performance.
Conclusions
Tractographic analysis of white matter integrity is a useful measure of disease severity and correlates well with cognitive function. It may have a significant potential in monitoring disease progression and may serve as a surrogate marker for treatment trials.
Keywords: Cognitive function, small vessel disease, tractography, diffusion tensor imaging
Introduction
Cerebral small vessel disease refers to ischemia in the territory of small perforating cerebral end arterioles.1 Pathological examination in these patients reveals the presence of small regions of lacunar infarction along with more diffuse areas of neuronal loss, demyelination, and gliosis.2 The neuroimaging correlates—which are best seen on T2-weighted magnetic resonance imaging (MRI)—are discrete focal lesions representing lacunar infarcts, and more diffuse white matter hyperintensity referred to as leukoaraiosis. Leukoaraiosis is a common white matter change in the elderly and appears as hypoattenuated areas in computed tomography (CT) scans and as hyperintense areas on T2-weighted MR images. The correlation of leukoaraiosis with arterial hypertension and history of stroke and some neuropathological studies suggest a small vessel alteration as the underlying cause.3–5
Patients with small vessel disease may progress on to vascular dementia with impairment of executive functions, attention, and mental processing speed.1 Cerebral small vessel disease can also occur independent of dementia. It is likely that a much larger number of individuals have more subtle cognitive disturbance because of cerebral small vessel disease and leukoaraiosis. This group of patients probably has a high risk of developing dementia, and may be a particularly suitable target for preventive treatment.6
Previous studies on patients with small vessel disease have found only a weak correlation between lesion load on T2-weighted imaging and the degree of cognitive impairment, which therefore limits the usefulness of T2-weighted MRI in the assessment and monitoring of disease severity. This may be owing to the fact that the high signal intensity reflects a spectrum of pathological changes, ranging from complete axonal degeneration to relatively benign pathology with preserved fiber architecture.1 Efficient functioning of different neural networks is central to various cognitive processes. A disruption in the white matter tracts connecting these networks could adversely affect cognitive performance.
Diffusion tensor imaging (DTI) is a promising method for characterizing microstructural integrity of white matter tracts.7 Many developmental, aging, and pathologic processes of the central nervous system influence the microstructural composition and architecture of the affected tissues. The diffusion of water within the tissues will be altered by changes in the tissue microstructure and organization; consequently, diffusion-weighted (DW) MRI methods including DTI are potentially powerful probes for characterizing the effects of disease and aging on microstructure. The most robust indices for quantitative evaluation of white matter tract integrity are fractional anisotropy (FA), which depicts the degree of alignment of the white matter tracts, and mean diffusivity (MD), which represents the degree of overall restrictions to water diffusion.8 The purpose of this study was to investigate the utility of DTI as a marker for white matter damage in small vessel disease and to assess its correlation with cognitive function.
Methods
Thirty consecutive patients with small vessel disease underwent DTI and conventional MRI assessment, following detailed clinical assessment. Small vessel disease was defined as a clinical lacunar stroke syndrome, which was accompanied by characteristic changes on MRI including a lacunar infarct, in addition to confluent leukoaraiosis, viz. Fazekas grade II or higher.9 Patients with a history of previous neurological or psychiatric disease, those with cortical or nonlacunar subcortical infarcts (size more than 1.5 cm), or with stroke mechanisms other than small vessel disease were excluded from the study.10 Additionally, 30 age- and sex-matched healthy volunteers also underwent the same MR study protocol. All patients underwent a detailed clinical and neuropsychological assessment. The study was undertaken after obtaining approval from the institutional ethics committee. Written informed consent was obtained from all subjects.
MRI data was collected on a Siemens Skyra 3.0 T scanner using a 20 channel phased array head coil. The MR protocol was as follows: (a) three-plane localizer imaging (repetition time (TR, in ms)/echo time (TE, in ms): 8.6/4.0); (b) axial and coronal fluid-attenuated inversion recovery (FLAIR) imaging (TR/TE/inversion time (in ms): 9000/81/2500); (c) axial fast spin-echo T2-weighted imaging (5600/100); (d) sagittal T2-weighted spin-echo imaging (5401/87); and (e) axial T1-weighted inversion recovery (2000/12/898). This was followed by the DTI sequence. For DTI, a single shot echo planar (EPI) sequence was used, with 30 isotropic gradient directions with b = 1000 s/mm2 and one b = 0 acquisition with a total scan time of 9 min. Axial images were acquired (matrix size 128 × 128, field of view 230 × 230 mm, slice thickness 3 mm, with no inter-slice gap). To enhance the signal-to-noise ratio and reduce the phase fluctuations, magnitude-constructed images were repeated (average = 2) and temporally averaged. Diffusion tensor tractography (DTT) was performed to assess the diffusion tensor properties of the white matter tracts. Major white-matter fiber tracts, including the corpus callosum, superior longitudinal fasciculus, inferior longitudinal fasciculus, corticospinal tracts, cingulum, inferior fronto-occipital fasciculus, superior cerebellar peduncle, middle cerebellar peduncle, inferior cerebellar peduncle, anterior thalamic radiations, superior thalamic radiations, posterior thalamic radiations, and uncinate fasciculus and fornix were generated and quantified using Java-based software based on the principal eigenvector field segmentation methodology.11
In-house DICOM-based Visual C# based software was used, which performed the segmentation of hyperintense lesions visualized on T2-weighted images. The software allowed the user to manually draw the region of interest on T2-weighted images and obtain the volumetric information of the same. Neuropsychological assessment was done within one week of MRI. A comprehensive battery of neuropsychological tests was incorporated using the AIIMS Adult Comprehensive Neuropsychological Battery. This battery is formed keeping the age, education, and handedness of patients in mind. The method followed in this battery neutralizes the effect of above-mentioned variables in its final scoring. It is administered in the local vernacular, for the convenience of the patient. Tests were grouped into cognitive domains encompassing motor function, memory function and global intellectual function. The motor scale is one of the longest (35 items) and most complex. Tests included those that elicited simple to complex motor movements of hands, feet, and fingers, paper–pencil tests, and oral movements. The memory scale had 12 items, for assessment of remote and recent memory. The intellectual functions scale comprised of 14 items to test comprehension, logical reasoning, ability to form analogies and concepts, determine similarities and differences, etc. Administration of the battery took between one and a half to two hours.
The differences in participant demographics between patients and controls were tested for statistical significance using the Student’s t-test for age and chi-square test for gender. DTI parameters of cases and controls were statistically compared using the unpaired t-test. A p value < 0.05 was considered as statistically significant. The Pearson correlation test was used to assess the correlation between the DTI indices (FA and MD) in various white matter tracts and the cognitive scores obtained in the domains of memory, intellectual, and motor function. The correlation of T2 lesion load with neuropsychological tests in the above cognitive domains was also analyzed using the Pearson correlation test. Here too, a p value < 0.05 was considered as statistically significant. All statistical analysis was performed using SPSS 23 version (SPSS Inc, Chicago, IL, USA) software.
Results
All 30 patients included in the study had leukoaraiotic lesions (Fazekas grade II or III) as well as lacunar infarcts (Figure 1). Mean age of cases was 66.93 ± 8.40 (18 males and 12 females), while that of controls was 65.56 ± 3.90 (14 males and 16 females), with no significant difference between cases and controls.
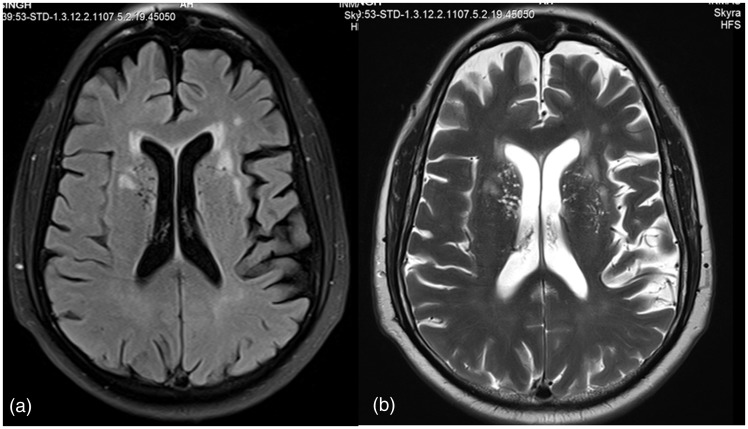
Figure 1.
Axial FLAIR (a) and T2-weighted (b) image in 75-year-old male with small vessel disease showing leukoaraiosis (Fazekas grade II).
On tractographic analysis (Figure 2), it was observed that the FA was significantly less (p < 0.05) in cases as compared to controls (Table 1), in several white matter tracts, namely the corpus callosum (CC), bilateral superior and inferior longitudinal fasciculus (SLF and ILF), corticospinal tracts (CST), cingulum (CNG), inferior fronto-occipital fasciculus (IFO), uncinate fasciculus, fornix, posterior and superior thalamic radiations (PTR and STR), and right anterior thalamic radiation (ATR).
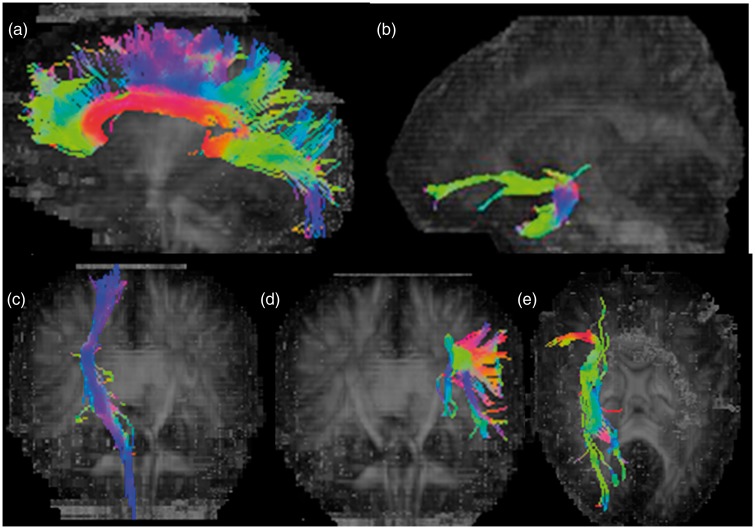
Figure 2.
Diffusion tensor tractography in 75-year-old male depicting corpus callosum (a), left uncinate fasciculus (b), right corticospinal tract (c), left superior longitudinal fasciculus (d), and right inferior fronto-occipital fasciculus (e).
Table 1.
Tractography differences in fractional anisotropy (FA) between small vessel disease cases and controls.
| No. | Fibers | Cases (mean ± SD) | Controls (Mean ± SD) | p value |
|---|---|---|---|---|
| 1 | CC | 0.42 ± 0.03 | 0.47 ± 0.01 | <0.05 |
| 2 | FX | 0.26 ± 0.02 | 0.29 ± 0.01 | <0.05 |
| 3 | R-CNG | 0.33 ± 0.02 | 0.35 ± 0.00 | <0.05 |
| 4 | L-CNG | 0.33 ± 0.02 | 0.36 ± 0.01 | <0.05 |
| 5 | R-UNC | 0.32 ± 0.02 | 0.33 ± 0.01 | <0.05 |
| 6 | L-UNC | 0.32 ± 0.02 | 0.35 ± 0.01 | <0.05 |
| 7 | R-AF | 0.38 ± 0.02 | 0.43 ± 0.01 | <0.05 |
| 8 | L-AF | 0.40 ± 0.02 | 0.43 ± 0.01 | <0.05 |
| 9 | R-CST | 0.46 ± 0.02 | 0.47 ± 0.01 | <0.05 |
| 10 | L-CST | 0.44 ± 0.02 | 0.48 ± 0.02 | <0.05 |
| 11 | R-IFO | 0.40 ± 0.02 | 0.43 ± 0.02 | <0.05 |
| 12 | L-IFO | 0.39 ± 0.02 | 0.44 ± 0.01 | <0.05 |
| 13 | R-SLF | 0.38 ± 0.02 | 0.41 ± 0.01 | <0.05 |
| 14 | L-SLF | 0.38 ± 0.02 | 0.42 ± 0.01 | <0.05 |
| 15 | R-ILF | 0.40 ± 0.02 | 0.42 ± 0.02 | <0.05 |
| 16 | L-ILF | 0.39 ± 0.02 | 0.42 ± 0.01 | <0.05 |
| 17 | R-STR | 0.40 ± 0.01 | 0.42 ± 0.02 | <0.05 |
| 18 | L-STR | 0.38 ± 0.02 | 0.43 ± 0.02 | <0.05 |
| 19 | R-ATR | 0.34 ± 0.02 | 0.35 ± 0.01 | <0.05 |
| 20 | L-ATR | 0.33 ± 0.02 | 0.34 ± 0.01 | >0.05 |
| 21 | R-PTR | 0.40 ± 0.02 | 0.41 ± 0.02 | <0.05 |
| 22 | L-PTR | 0.39 ± 0.01 | 0.41 ± 0.02 | <0.05 |
| 23 | R-SCP | 0.35 ± 0.04 | 0.36 ± 0.02 | >0.05 |
| 24 | L-SCP | 0.36 ± 0.02 | 0.35 ± 0.01 | >0.05 |
| 25 | R-MCP | 0.37 ± 0.03 | 0.38 ± 0.03 | >0.05 |
| 26 | L-MCP | 0.38 ± 0.02 | 0.39 ± 0.04 | >0.05 |
| 27 | R-ICP | 0.36 ± 0.02 | 0.35 ± 0.02 | >0.05 |
| 28 | L-ICP | 0.35 ± 0.02 | 0.36 ± 0.02 | >0.05 |
The MD was significantly raised (p < 0.05) in cases as compared to controls (Table 2) in nearly all white matter tracts, namely the corpus callosum (CC), bilateral superior longitudinal fasciculus (SLF), corticospinal tracts (CST), cingulum (CNG), inferior fronto-occipital fasciculus (IFO), superior, middle, and inferior cerebellar peduncle (SCP, MCP, and ICP), anterior, superior, and posterior thalamic radiations (ATR, STR, PTR), uncinate fasciculus, fornix, and left inferior longitudinal fasciculus (ILF).
Table 2.
Tractography differences in mean diffusivity (MD) between small vessel disease cases and controls.
| No. | Tracts | Cases (Mean ± SD) | Controls – ADC | p value |
|---|---|---|---|---|
| 1 | CC | 1.02 ± 0.06 | 0.47 ± 0.02 | <0.05 |
| 2 | R-PTR | 0.90 ± 0.06 | 0.85 ± 0.03 | <0.05 |
| 3 | L-PTR | 0.90 ± 0.07 | 0.82 ± 0.01 | <0.05 |
| 4 | R-CST | 0.92 ± 0.06 | 0.81 ± 0.02 | <0.05 |
| 5 | L-CST | 0.93 ± 0.07 | 0.81 ± 0.01 | <0.05 |
| 6 | R-AF | 0.86 ± 0.04 | 0.80 ± 0.02 | <0.05 |
| 7 | L-AF | 0.85 ± 0.05 | 0.78 ± 0.02 | <0.05 |
| 8 | R-UNC | 0.92 ± 0.04 | 0.87 ± 0.02 | <0.05 |
| 9 | L-UNC | 0.91 ± 0.04 | 0.86 ± 0.02 | <0.05 |
| 10 | R-CNG | 0.88 ± 0.04 | 0.82 ± 0.02 | <0.05 |
| 11 | L-CNG | 0.87 ± 0.45 | 0.83 ± 0.02 | <0.05 |
| 12 | R-STR | 0.84 ± 0.06 | 0.77 ± 0.02 | <0.05 |
| 13 | L-STR | 0.84 ± 0.06 | 0.78 ± 0.02 | <0.05 |
| 14 | FX | 1.69 ± 0.18 | 1.43 ± 0.07 | <0.05 |
| 15 | R-SLF | 0.84 ± 0.05 | 0.77 ± 0.01 | <0.05 |
| 16 | L-SLF | 0.83 ± 0.05 | 0.77 ± 0.01 | <0.05 |
| 17 | R-IFO | 0.91 ± 0.07 | 0.84 ± 0.01 | <0.05 |
| 18 | L-IFO | 0.88 ± 0.04 | 0.82 ± 0.01 | <0.05 |
| 19 | R-ATR | 0.92 ± 0.05 | 0.81 ± 0.02 | <0.05 |
| 20 | L-ATR | 0.92 ± 0.06 | 0.85 ± 0.02 | <0.05 |
| 21 | R-MCP | 1.02 ± 0.09 | 0.94 ± 0.10 | <0.05 |
| 22 | L-MCP | 1.09 ± 0.13 | 0.96 ± 0.07 | <0.05 |
| 23 | R-SCP | 1.12 ± 0.11 | 1.00 ± 0.09 | <0.05 |
| 24 | L-SCP | 1.12 ± 0.11 | 1.02 ± 0.09 | <0.05 |
| 25 | R-ICP | 1.05 ± 0.13 | 0.90 ± 0.06 | <0.05 |
| 26 | L-ICP | 1.03 ± 0.14 | 0.86 ± 0.06 | <0.05 |
| 27 | R-ILF | 0.89 ± 0.04 | 0.88 ± 0.02 | >0.05 |
| 28 | L-ILF | 0.92 ± 0.05 | 0.85 ± 0.01 | <0.05 |
Neuropsychological testing was performed using the AIIMS Adult Neuropsychology Test Battery in the domains of memory, motor, and intellectual function. A higher score indicated a deterioration of function. There was a significant correlation between the tractography scores and the neuropsychological test scores in these domains (Tables 3 and 4). Significant positive correlation (p < 0.05) was observed between the motor performance and the MD in the corpus callosum, right corticospinal tract, right uncinate fasciculus, left superior longitudinal fasciculus, right superior thalamic radiation, and right inferior cerebellar peduncle. Significant negative correlation (p < 0.05) was observed between motor performance and the FA in the right posterior thalamic radiation. FA in the right uncinate fasciculus showed significant negative correlation with memory score, while MD in corpus callosum, fornix, left uncinate fasciculus, and right cortico-spinal tract showed significant positive correlation with the memory score. Further, there was significant positive correlation of scores on intellectual function with MD in the fornix, bilateral uncinate fasciculus, right corticospinal tract, and right posterior thalamic radiation. Also, significant negative correlation was observed between scores on intellectual function and FA in right cingulum, bilateral uncinate fasciculus, right inferior fronto-occipital fasciculus, and left superior thalamic radiation. There was no significant correlation between the neuropsychological test performance and the T2 lesion load.
Table 3.
Relation between neuropsychological scores and fractional anisotropy (FA).
| Motor | Memory | Intellectual | |
|---|---|---|---|
| Corpus callosum | −0.108 | −0.138 | −0.333 |
| Fornix | −0.100 | −0.273 | −0.205 |
| Rt. cingulum | −0.040 | −0.244 | −0.371 |
| Lt. cingulum | −0.125 | −0.018 | −0.240 |
| Rt. uncinate fasciculus | −0.261 | −0.405* | −0.461* |
| Lt. uncinate fasciculus | −0.372 | −0.340 | −0.456* |
| Rt. corticospinal tract | −0.196 | −0.207 | −0.255 |
| Lt. corticospinal tract | −0.079 | −0.149 | −0.233 |
| Rt. inferior fronto-occipital fasciculus | −0.166 | −0.229 | 0.367* |
| Lt. inferior fronto-occipital fasciculus | −0.030 | −0289 | −0.266 |
| Rt. superior longitudinal fasciculus | −0.040 | −0.296 | −0.321 |
| Lt. superior longitudinal fasciculus | −0.392* | −0.042 | −0.250 |
| Rt. inferior longitudinal fasciculus | −0.001 | −0.121 | −0.223 |
| Lt. inferior longitudinal fasciculus | −0.047 | −0.322 | −0.233 |
| Rt. superior thalamic radiation | −0.168 | −0.017 | −0.075 |
| Lt. superior thalamic radiation | −0.272 | −0.158 | −0.362* |
| Rt. anterior thalamic radiation | −0.148 | −0.170 | −0.106 |
| Lt. anterior thalamic radiation | −0.156 | −0.200 | −0.255 |
| Rt. posterior thalamic radiation | −0.441* | −0.193 | −0.084 |
| Lt. posterior thalamic radiation | −0.234 | −0.122 | −0.203 |
| Rt. superior cerebellar peduncle | −0.254 | 0.038 | −0.020 |
| Lt. superior cerebellar peduncle | −0.330 | −0.064 | −0.174 |
| Rt. middle cerebellar peduncle | −0.124 | −0.283 | −0.341 |
| Lt. middle cerebellar peduncle | −0.129 | −0.124 | 0.051 |
| Rt. inferior cerebellar peduncle | −0.198 | −0.234 | −0.350 |
| Lt. inferior cerebellar peduncle | −0.042 | 0.077 | −0.131 |
p < 0.05
Table 4.
Relation between neuropsychological scores and mean diffusivity (MD).
| Motor | Memory | Intellectual | |
|---|---|---|---|
| Corpus callosum | 0.416* | 0.540* | 0.048 |
| Fornix | 0.014 | 0.372 | 0.380 |
| Rt. cingulum | 0.324 | 0.194 | 0.266 |
| Lt. cingulum | 0.095 | 0.027 | 0.184 |
| Rt. uncinate fasciculus | 0.452* | 0.282 | 0.381* |
| Lt. uncinate fasciculus | 0.098 | 0.392* | 0.341* |
| Rt. corticospinal tract | 0.388* | 0.368* | 0.402* |
| Lt. corticospinal tract | 0.091 | 0.337 | 0.323 |
| Rt. inferior fronto-occipital fasciculus | 0.018 | 0.134 | 0.236 |
| Lt. inferior fronto-occipital fasciculus | 0.167 | 0.245 | 0.302 |
| Rt. superior longitudinal fasciculus | 0.313 | 0.211 | 0.336 |
| Lt. superior longitudinal fasciculus | 0.379* | 0.162 | 0.207 |
| Rt. inferior longitudinal fasciculus | 0.057 | 0.327 | 0.338 |
| Lt. inferior longitudinal fasciculus | 0.143 | 0.068 | 0.120 |
| Rt. superior thalamic radiation | 0.377* | 0.201 | 0.327 |
| Lt. superior thalamic radiation | 0.306 | 0.018 | 0.140 |
| Rt. anterior thalamic radiation | 0.149 | 0.134 | 0.329 |
| Lt. anterior thalamic radiation | 0.068 | 0.104 | 0.207 |
| Rt. posterior thalamic radiation | 0.230 | 0.249 | 0.402* |
| Lt. posterior thalamic radiation | 0.354 | 0.037 | 0.421 |
| Rt. superior cerebellar peduncle | 0.212 | 0.071 | 0.204 |
| Lt. superior cerebellar peduncle | 0.104 | 0.146 | 0.086 |
| Rt. middle cerebellar peduncle | 0.156 | 0.014 | 0.017 |
| Lt. middle cerebellar peduncle | 0.219 | 0.286 | 0.343 |
| Rt. inferior cerebellar peduncle | 0.403* | 0.059 | 0.139 |
| Lt. Inferior cerebellar peduncle | 0.063 | 0.144 | 0.259 |
p < 0.05
Discussion
Cerebral small vessel disease can be associated with cognitive decline, which has been hypothesized to occur secondary to white matter damage.12 Cognitive functions depend on efficient functioning of distributed brain networks connected by white matter tracts. Small vessel disease pathologies could disrupt these connections, impairing network functioning and cognition via a disconnection “syndrome.”1 DTI is a noninvasive imaging technique that provides information about tissue microstructure and architecture, based on the directionality of diffusion of water molecules. White matter tractography based on DTI has become a well-accepted noninvasive tool for exploring the white matter architecture of the human brain in vivo.13–15
DTI is likely to outperform conventional MRI in delineating the extent of tissue damage for two reasons. Firstly, pathological studies have shown that the severity of damage is highly variable in the region of T2 hyperintensities, thus reducing the effectiveness of T2 lesion load assessment as a marker of disease severity.16 Secondly, changes in the normal appearing white matter may also contribute to the cognitive decline, thereby weakening correlations with lesion burden. Overall, no or weak correlation of T2 lesion load with quantitative measurements of cognitive deficit was observed in cross-sectional studies evaluating a small sample of patients.1,17 Our study, which incorporates T2 lesion load assessment of 30 patients, is in conformity with these observations, with no significant correlation between T2 lesion load and cognitive performance. However, certain studies which use greater sample sizes, typically of hundreds of patients, have shown correlation between the extent of leukoaraiosis and global cognitive impairment.18
Abnormalities in white matter that appear normal on conventional MRI sequences have been observed using DTI in both CADASIL,19,20 and in patients with lacunar stroke and leukoaraiosis.1 Various studies using different DTI approaches have revealed a reduction in FA along with an increase in MD in the normal appearing white matter of patients with small vessel disease. Based on these studies, a relationship between DTI abnormalities and cognitive deficit is emerging. A DTI study on patients with leukoaraiosis revealed that histogram and voxel-based analyses of the whole-brain MD and FA maps showed a good correlation with the degree of motor and cognitive impairment.21 The present study which incorporated a tractography approach could identify white matter tract abnormalities with reduction in FA and increase in MD in nearly all tracts. Further, a good correlation was obtained between the tractography indices in several white matter tracts, and the degree of motor and cognitive deficit.
A region of interest analysis by O’Sullivan et al. on 36 patients with ischemic leukoaraiosis revealed abnormalities in the anterior periventricular white matter and the centrum semiovale.1 In their series, highest correlation was found between MD of normal appearing white matter and full scale IQ (intelligence quotient) and tests of executive function. Another study performed on 40 patients with small vessel disease having mild cognitive impairment revealed that MD in several white matter regions, (not limited to the frontal lobes) correlated well with executive functions.12 A three-year follow-up of a cohort of patients with cerebral small vessel disease, revealed a decline in FA and increase in diffusivity in white matter tissue with time. In this study too, MD was found to be the most sensitive marker to assess disease progression.22 In our study, both FA and MD in several white matter tracts correlated well with cognitive function; however, a greater number of tracts showed significant correlation of MD with cognitive performance. A recent study performed using tract-based spatial statistics, showed that the fall in FA and rise in MD correlated well with the cognitive performance. They observed that the loss of microstructural integrity of the white matter at specific locations in the normal-appearing white matter was related to specific cognitive disturbances.23 Another cross-sectional study which applied graph-based efficiency analysis to DTT data to estimate structural connectivity between various cortical and subcortical brain regions revealed that network connectivity was significantly reduced in small vessel disease. The degree of brain network disruption correlated well with cognitive function.24
DTI is presently the sole in vivo method for assessing white matter connectivity but it has certain limitations, especially in regions of white matter fiber crossing, kissing, or twisting.25 Newer techniques using high angular resolution diffusion-weighted MRI aimed to address these issues show improved accuracy of tract delineation.
Conclusion
Tractographic analysis of white matter integrity is a useful measure of disease severity and correlates well with cognitive function. DTI thus shows promise as a useful tool for exploring the mechanisms of cognitive dysfunction in small vessel disease and has the potential to be used as a surrogate marker in therapeutic trials.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.O’Sullivan M, Morris RG, Huckstep B, et al. Diffusion tensor MRI correlates with executive dysfunction in patients with ischaemic leukoaraiosis. J Neurol Neurosurg Psychiatry 2004; 75: 441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babikian V, Ropper AH. Binswanger’s disease: a review. Stroke 1987; 18: 2–12. [DOI] [PubMed] [Google Scholar]
- 3.Basile AM, Pantoni L, Pracucci G, et al. Age, hypertension, and lacunar stroke are the major determinants of the severity of age-related white matter changes. The LADIS (Leukoaraiosis and Disability in the Elderly) Study. Cerebrovasc Dis 2006; 21: 315–322. [DOI] [PubMed] [Google Scholar]
- 4.Brown WR, Moody DM, Thore CR, et al. Apoptosis in leukoaraiosis. Am J Neuroradiol 2000; 21: 79–82. [PMC free article] [PubMed] [Google Scholar]
- 5.Moody DM, Thore CR, Anstrom JA, et al. Quantification of afferent vessels shows reduced brain vascular density in subjects with leukoaraiosis. Radiology 2004; 233: 883–890. [DOI] [PubMed] [Google Scholar]
- 6.Erkinjuntti T, Inzitari D, Pantoni L, et al. Limitations of clinical criteria for the diagnosis of vascular dementia in clinical trials. Is a focus on subcortical vascular dementia a solution? Ann NY Acad Sci 2000; 903: 262–272. [DOI] [PubMed] [Google Scholar]
- 7.Mayer AR, Ling J, Mannell MV, et al. A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology 2010; 74: 643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B 1996; 111: 209–219. [DOI] [PubMed] [Google Scholar]
- 9.Fazekas F, Chawluk JB, Alavi A, et al. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. Am J Roentgenol 1987; 149: 351–356. [DOI] [PubMed] [Google Scholar]
- 10.Lawrence AJ, Patel B, Morris RG, et al. Mechanisms of cognitive impairment in cerebral small vessel disease: multimodal MRI results from the St George’s Cognition and Neuroimaging in Stroke (SCANS) Study. PLoS One 2013; 8: e61014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rathore RK, Gupta RK, Agarwal S, et al. Principal eigenvector field segmentation for reproducible diffusion tensor tractography of white matter structures. Magn Reson Imaging 2011; 29: 1088–1100. [DOI] [PubMed] [Google Scholar]
- 12.Ciulli S, Citi L, Salvadori E, et al. Prediction of impaired performance in trail making test in MCI patients with small vessel disease using DTI data. IEEE J Biomed Health Inf 2016; 20: 1026–1033. [DOI] [PubMed] [Google Scholar]
- 13.Holodny A, Gor D, Watts R, et al. Diffusion-tensor MR tractography of somatotopic organization of corticospinal tracts in the internal capsule: initial anatomic results in contradistinction to prior reports. Radiology 2005; 234: 649–653. [DOI] [PubMed] [Google Scholar]
- 14.Kleiser R, Staempfli P, Valavanis A, et al. Impact of fMRI-guided advanced DTI fiber tracking techniques on their clinical applications in patients with brain tumors. Neuroradiology 2010; 52: 37–46. [DOI] [PubMed] [Google Scholar]
- 15.Yamada K, Kizu O, Kubota T, et al. The pyramidal tract has a predictable course through the centrum semiovale: a diffusion-tensor based tractography study. J Magn Reson Imaging 2007; 26: 519–524. [DOI] [PubMed] [Google Scholar]
- 16.Awad IA, Johnson PC, Spetzler RF, et al. Incidental subcortical lesions identified on magnetic resonance imaging in the elderly. II. Postmortem pathological correlations. Stroke 1986; 17: 1090–1097. [DOI] [PubMed] [Google Scholar]
- 17.Van der Flier WM, van Straaten ECW, Barkhof F, et al. Medial temporal lobe atrophy and white matter hyperintensities are associated with mild cognitive deficits in non-disabled elderly people: the LADIS study. J Neurol Neurosurg Psychiatry 2005; 76: 1497–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pantoni L, Poggesi A, Basile AM, et al. Leukoaraiosis predicts hidden global functioning impairment in nondisabled older people: the LADIS (leukoaraiosis and disability in the elderly) study. J Am Geriatr Soc 2006; 54: 1095–1110. [DOI] [PubMed] [Google Scholar]
- 19.Chabriat H, Pappata S, Poupon C, et al. Clinical severity in CADASIL related to ultrastructural damage in white matter: in vivo study with diffusion tensor MRI. Stroke 1999; 30: 2637–2643. [DOI] [PubMed] [Google Scholar]
- 20.Mascalchi M, Pantoni L, Giannelli M, et al. Diffusion tensor imaging to map brain microstructural changes in CADASIL. J Neuroimaging. Epub ahead of print 30 June 2016. DOI: 10.1111/jon.12374. [DOI] [PubMed]
- 21.Della Nave R, Foresti S, Pratesi A, et al. Whole-brain histogram and voxel based analyses of diffusion tensor imaging in patients with leukoaraiosis: correlation with motor and cognitive impairment. Am J Neuroradiol 2007; 28: 1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeestraten EA, Benjamin P, Lambert C, et al. Application of diffusion tensor imaging parameters to detect change in longitudinal studies in cerebral small vessel disease. PLoS One 2016; 11: e0147836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tuladhar AM, van Norden AG, de Laat KF, et al. White matter integrity in small vessel disease is related to cognition. Neuroimage Clin 2015; 7: 518–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lawrence AJ, Chung AW, Morris RG, et al. Structural network efficiency is associated with cognitive impairment in small-vessel disease. Neurology 2014; 83: 304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johansen-Berg H, Behrens TEJ. Just pretty pictures? What diffusion tractography can add in clinical neuroscience. Curr Opin Neurol 2006; 19: 379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]