Abstract
Primary involvement of leptomeninges with melanocytic tumours is rarely seen and its diagnosis is challenging. Here we summarise two cases of primary leptomeningeal melanomatosis presenting as subacute meningitis. Both cases have pleocytosis and high protein on cerebrospinal fluid analysis, and demonstrated atypical cells on cytology. On magnetic resonance imaging, there is diffuse leptomeningal thickening and avid enhancement of intracranial and intraspinal leptomeninges. One of them demonstrates T1 shortening due to magnetic effects of melanin, the other case is amelanotic and shows hypointensity on precontrast T1-weighted images. Both cases can be diagnosed with biopsy. In conclusion, these cases highlight the importance of the correct interpretation of cytological and magnetic resonance imaging findings in patients with atypical findings.
Keywords: Magnetic resonance imaging, leptomeningeal melanocytosis, meningeal neoplasm
Introduction
Malignant infiltration of the leptomeninges by melanocytic tumours is highly uncommon and poses a significant diagnostic and therapeutic challenge for the treating physician. Herein, we report two cases of primary diffuse leptomeningeal melanomatosis (melanocytosis) that were initially considered to have subacute meningitis, and received the correct diagnosis only after histopathological examinations.
Patient 1
A 21-year-old previously healthy man was admitted to a local hospital with complaints of headache, fever, and vomiting. Based on the symptoms, cerebrospinal fluid (CSF) examination which has a protein level of 85 mg/dL and leptomeningeal contrast enhancement in magnetic resonance imaging (MRI), the diagnosis of bacterial meningitis was considered, and empirical antibiotic treatment with ceftriaxone was started. Apart from the CSF cultures remaining sterile, the clinical picture did not improve. A new lumbar puncture revealed CSF pleocytosis (100/mm3, mainly mononuclear cells) and hypoglycorrhachia, with persistently elevated protein levels. Gram and Ziehl–Nielsen stains were not remarkable, and CSF cultures remained sterile. Polymerase chain reaction (PCR) evaluations were negative for Mycobacterium tuberculosis, herpes simplex virus and Enterovirus. Nonetheless, because of the high endemic rate of tuberculosis, anti-tuberculosis treatment including methylprednizolon was initiated at the local hospital; although a definitive diagnosis was not present. The patient was then transferred to our institution for further work-up.
At the time of admission, the neurological examination was normal except for a slight decrease of attention. There was no neck stiffness. Blood and urine cultures, together with cultures from a repeat lumbar puncture examination, were negative. An MRI study showed bright diffuse lesions along the spinal and intracranial leptomeninges on pre-contrast T1-weighted images with leptomeningeal thickening and avid contrast enhancement on post-contrast T1-weighted images (Figure 1). CSF cytology showed atypical cells suggesting an epithelial neoplasia. A meningeal biopsy performed by open craniotomy revealed a thick brown-coloured coat of tissue along the cerebral sulci in the subarachnoid space. Microscopic analysis of the biopsy specimen confirmed the diagnosis of leptomeningeal melanocytosis with malignant transformation. The neoplastic cells filled and expanded the subarachnoid space and extended through the Virchow–Robin spaces. Immunohistochemically the neoplastic cells were positive for S-100, HMB-45 (an antigen present in melanocytic tumours such as melanomas, and stands for homatropine methylbromide 45) and MART-1, with a high Ki-67 labelling index (Figure 2). A single-point mutation in exon-15 of the BRAF gene (a human gene that encodes a protein called B-Raf) was negative. A thorough physical examination did not show any abnormal skin lesions or intraocular lesions. Furthermore, no other hypermetabolic foci were identified elsewhere in the body on the whole-body positron emission tomography–computed tomography (PET-CT) examination. The patient had no family history of malignant melanoma. The patient meets three of the Haywards criteria, which are especially used to diagnose primary central nervous system (CNS) melanoma.1 Haywards criteria are defined as: (a) no malignant melanoma tumour outside the CNS; (b) involvement of the leptomeninges (spinal or cranial); (c) intramedullary spinal lesions; (d) hydrocephalus; (e) tumour in the pituitary or pineal gland; (f) a single intracerebral lesion. The patient received chemotherapy with cisplatin and dacarbazine for five cycles and whole-brain radiotherapy (30 Gy in 10 fractions) and died one year after the diagnosis.
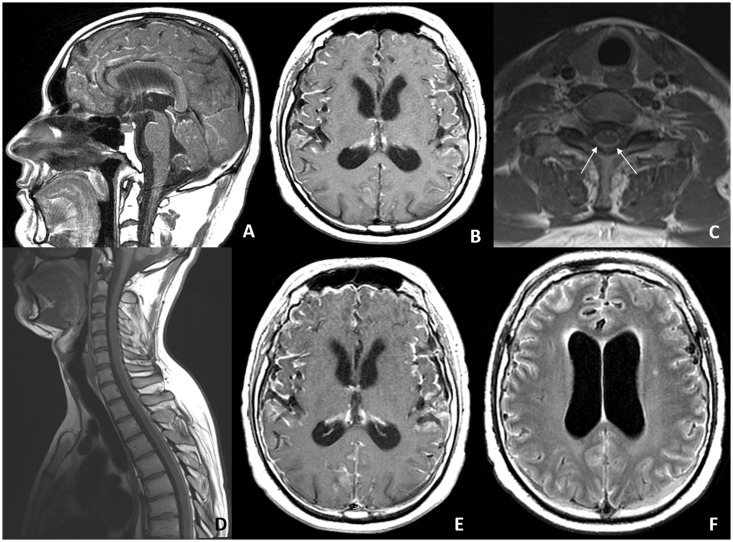
Figure 1.
Pre-contrast sagittal and axial T1-weighted images of the brain (a, b) and spine (c, d) show hyperintensity from a T1 shortening of the paramagnetic properties of melanin. Axial post-gadolinium T1-weighted image (e) shows diffuse leptomeningeal enhancement. Axial fluid-attenuated inversion recovery image (f) reveals hyperintensity in the cerebral sulci.
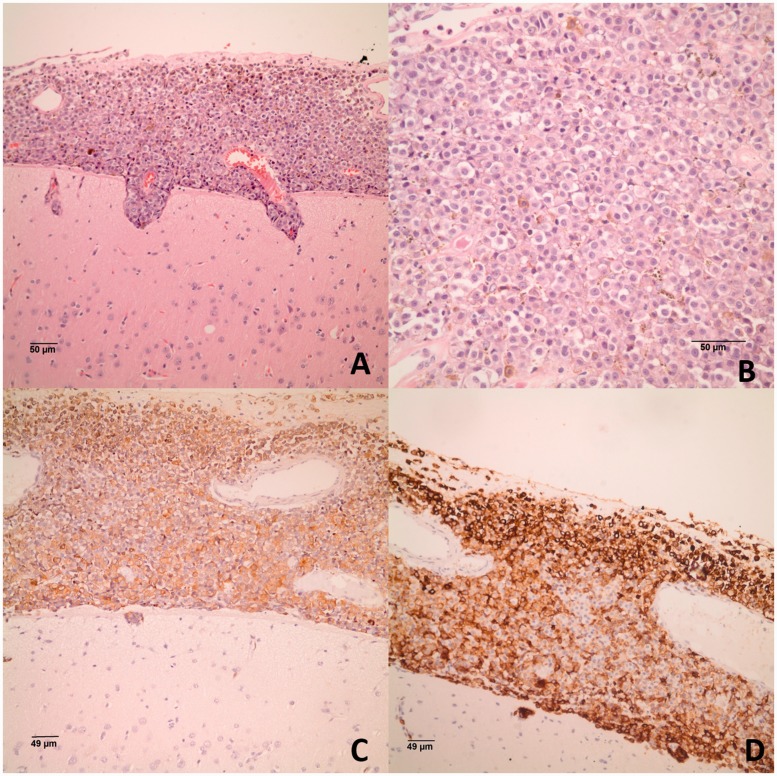
Figure 2.
(a) Histological section of meninges and underlying brain, showing cellular infiltration of the meninges penetrating perivascular Virchow–Robin spaces into the cerebral cortex (haematoxylin-eosin); and (b) monotonous polygonal cells with prominent nuclei. The neoplastic cells are positive for melanocytic markers (c) HMB-45; and (d) MART-1 (immunohistochemistry).
Patient 2
A 44-year-old woman without significant past medical history presented to a local hospital with complaints of nausea, vomiting, and weight loss. She also complained of slight forgetfulness over the course of the past few weeks. She was initially diagnosed with acute cholecystitis and underwent cholecystectomy. However, following the operation, there was deterioration in her cognitive performance. Her family noticed mental slowness, poor concentration, and impairments in short-term memory. A short-stepped gait was also evident in her neurological examination. A cranial computed tomography examination revealed enlargement in the lateral ventricles suggestive of acute hydrocephalus. The patient was thereafter transferred to the neurosurgery unit in our hospital.
At the time of admission, she was uncooperative and mute. She was spontaneously moving all her extremities, and no lateralising deficit was evident. Repeat lumbar punctures disclosed elevated CSF pressure, protein levels ranging from 728 to 2453 mg/dl CSF analysis with normal glucose levels and lymphocytic pleocytosis. Due to a presumptive diagnosis of tuberculous meningitis, anti-tuberculosis therapy was started; however, the treatment was stopped following negative PCR findings on repeat CSF examinations. A craniospinal MRI showed thick contrast enhancement along intracranial and spinal leptomeninges (Figure 3). Malignant cells were noticed in one of the cytological examinations. However, no evidence of malignancy was apparent on dural biopsy. Also immunohistochemical staining with EMA, LCA, pankeratin and CD68 was performed; there was only arachnoid cell proliferation on EMA staining. A slight improvement in the clinical condition of the patient was attained following a ventriculoperitoneal shunt procedure. However, the patient developed paraparesis and urinary incontinence over the following days. A whole-body PET-CT examination showed no abnormal findings. A repeat craniospinal MRI showed persistent and diffuse enhancement in the leptomeninges, showing no difference from the first MRI. Due to the progressive clinical course and absence of a diagnosis, a right frontal biopsy was performed. Microscopic analysis of the biopsy specimen revealed leptomeningeal melanocytosis with malignant transformation. Immunohistochemically the neoplastic cells were positive for S-100, HMB-45 and Melan-A (Figure 4). Ki-67 labelling index was not so high. A single-point mutation in exon-15 and exon-11 of the BRAF gene was negative. Physical examination showed no abnormal skin lesions or intraocular lesions. The patient had no family history of malignant melanoma. The patient met three of the Haywards criteria.1 A chemotherapy regimen with temozolomide was initiated. The patient died four months later after diagnosis.
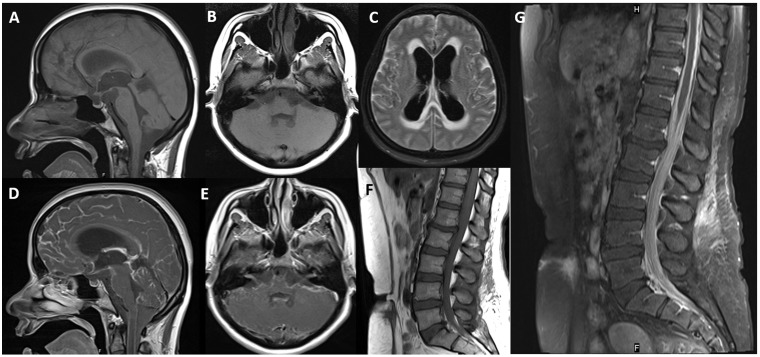
Figure 3.
Pre-contrast sagittal (a) and axial (b) T1-weighted images of the brain and spine (f) do not show any hyperintensity or T1 shortening. Post-gadolinium T1-weighted images (d–g) show diffuse leptomeningeal enhancement. Note the 7–8th cranial nerve enhancement (e). Axial fluid-attenuated inversion recovery image (c) reveals hyperintensity in the cerebral sulci and hydrocephalus.
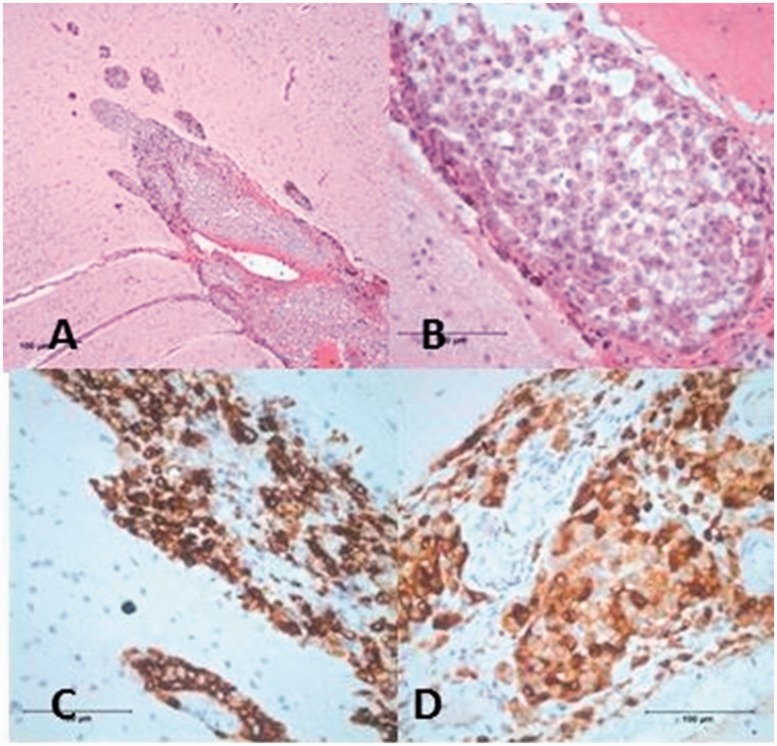
Figure 4.
Histological section of meninges shows cellular infiltration of the meninges (a) and monotonous polygonal cells with prominent nuclei (b) (haematoxylin-eosin). Immunohistochemically the neoplastic cells are positive for HMB-45 (c) and Melan-A (d).
Discussion
Primary diffuse leptomeningeal melanomatosis (melanocytosis) is an extremely rare tumour, with the related literature primarily consisting of individual case reports.2–5 Melanocytic lesions involving the CNS include both benign (leptomeningeal melanocytosis, melanocytoma) and malignant (leptomeningeal melanomatosis, melanoma) pathologies.6 The signs and symptoms of these pathologies depend on the site of anatomical involvement (cerebral hemispheres, cranial nerves, spinal cord and nerve roots).7 In addition to focal neurological deficits, subarachnoid haemorrhage, seizures, signs of increased intracranial pressure can be observed. A major clinical challenge occurs, as leptomeningeal melanomatosis can mimic infectious meningitis both clinically and radiologically. In the setting of suggestive clinical (encephalopathy, headache, vomiting), laboratory (low CSF glucose, elevated CSF protein) and imaging (leptomeningeal enhancement) findings, a presumptive diagnosis such as bacterial or tuberculous meningitis can easily be considered in leptomeningeal melanomatosis cases. A critical finding that is helpful for differential diagnosis is the presence of hyperintense signals on pre-contrast T1-weighted images in the setting of leptomeningeal melanocytosis as a reflection of the paramagnetic properties of melanin. Unfortunately, T1 shortening might not always be evident in all cases despite melanosis (as in our case no. 2), and especially amelanotic melanoma lesions remain hypointense on T1-weighted images.8 A biopsy specimen showing the tumour cells or melanin-loaded macrophages is generally necessary for establishing the diagnosis.9 But there is a recent case report in which the diagnosis could be proved with CSF cytological analysis with immunohistochemical staining and molecular analysis.10 As meninges are diffusely involved in leptomeningeal melanomatosis, the site of biopsy may not be a concern, but the biopsy specimen should include leptomeninges and also cortex in order to demonstrate dissemination along Virchow–Robin spaces. Our second patient’s first biopsy was normal, probably as it was obtained from the dura through burr-hole defects and did not include leptomeningeal specimens. Overall, all of these challenges delay the diagnosis of these cases for weeks or months. The survival for melanoma diagnosis with leptomeningeal involvement is poor so this delay is remarkable. It is known that there are no specific guidelines for the management of both the primary CNS melanomas and leptomeningeal melanomatosis as they are rarely seen. However, radiotherapy, especially after surgical resection of a primary tumour, systemic chemotherapy with agents such as dacarbazine, cisplatin, thalidomide or temozalamid or intrathecal therapy with methothraxate, cytarabine and sometimes immunmodulatory agents such as peginterferon alpha-2b have all been used as treatments.11 The prognosis and life expectancy for patients with primary leptomeningeal malign melanomatosis is worse despite treatment.12 Most authors quote a median survival of less than one year.3,4,11 Vemurafenib is a BRAF inhibitor that has demonstrated dramatic activity in BRAF V600E-mutated melanoma. There is a report of a patient who had melanoma with leptomeningeal melanomatosis, describing that the patient had remarkable clinical and radiological improvement after the initiation of vemurafenib.13
In conclusion, these cases highlight the importance of the correct interpretation of cytological and MRI findings in patients with a suspicion of meningitis but harbouring atypical clinical, laboratory and radiological features together with symptoms unresponsive to antimicrobial regimens.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Haywards RD. Malignant melanoma of the central nervous system. A guide for classification based on the clinical findings. J Neurol Neurosurg Psychiatry 1976; 39: 525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pirini MG, Mascalchi M, Salvi F, et al. Primary diffuse meningeal melanomatosis: radiologic–pathologic correlation. AJNR Am J Neuroradiol 2003; 24: 115–118. [PMC free article] [PubMed] [Google Scholar]
- 3.Szathmari A, Perbet R, Hermier M, et al. Primary amelonotic leptomeningeal melanomatosis in a child: a rare but severe disease. World Neurosurg 2016; 92: 581.e15–581.e20. [DOI] [PubMed] [Google Scholar]
- 4.Kim DH, Choi CY, Lee CH, et al. Primary intracranial leptomeningeal melanomatosis. J Korean Neurosurg Soc 2015; 58: 554–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trinh V, Medina-Flores R, Taylor CL, et al. Primary melonocytic tumors of the central nervous system: report of two cases and review of literature. Surg Neurol Int 2014; 5: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liubinas SV, Maartens N, Drummond KJ. Primary melanocytic neoplasms of the central nervous system. J Clin Neurosci: Official journal of the Neurosurgical Society of Australasia 2010; 17: 1227–1232. [DOI] [PubMed] [Google Scholar]
- 7.Harstad L, Hess KR, Groves MD. Prognostic factors and outcomes in patients with leptomeningeal melanomatosis. Neuro-oncology 2008; 10: 1010–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Escott EJ. A variety of appearances of malignant melanoma in the head: a review. Radiographics: A review publication of the Radiological Society of North America, Inc 2001; 21: 625–639. [DOI] [PubMed] [Google Scholar]
- 9.Miro J, Velasco R, Majos C, et al. Meningeal melanocytosis: a possibly useful treatment for a rare primary brain neoplasm. J Neurol 2011; 258: 1169–1171. [DOI] [PubMed] [Google Scholar]
- 10.Kolin DL, Geddie WR, Ko HM. CSF cytology diagnosis of NRAS-mutated primary leptomeningeal melanomatosis with neurocutaneous melanosis. Cytopathology 2016. Epub ahead of print 2 October 2016. DOI: 10.1111/cyt.12366. [DOI] [PubMed] [Google Scholar]
- 11.Angelino G, De Pasquale, De Sio L, et al. NRASQ61K mutated primary leptomeningeal melanoma in a child: case presentation and discussion on clinical and diagnostic implications. BMC Cancer 2016; 16: 512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balakrishnan R, Porag R, Asif DS, et al. Primary intracranial melanoma with early leptomeningeal spread: a case report and treatment options available. Case Rep Oncol Med 2015; 2015: 293802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schäfer N, Scheffler B, Stuplich M, et al. Vemurafenib for leptomeningeal melanomatosis. J Clin Oncol 2013; 31: e173–e174. [DOI] [PubMed] [Google Scholar]