Abstract
Migraine has been hypothesized to be a syndrome of chronic low serotonin (5-HT) levels, but investigations of brain 5-HT levels have given equivocal results. Here, we used positron emission tomography (PET) imaging of the 5-HT4 receptor as a proxy for brain 5-HT levels. Given that the 5-HT4 receptor is inversely related to brain 5-HT levels, we hypothesized that between attacks migraine patients would have higher 5-HT4 receptor binding compared to controls. Eighteen migraine patients without aura (migraine free >48 h), and 16 age- and sex-matched controls underwent PET scans after injection of [11C]SB207145, a specific 5-HT4 receptor radioligand. An investigator blinded to group calculated a neocortical mean [11C]SB207145 binding potential (BPND). Three migraine patients reported a migraine attack within 48 h after the scan and were excluded from the primary analysis. Comparing 15 migraine patients and 16 controls, we found that migraine patients have significantly lower neocortical 5-HT4 receptor binding than controls (0.60 ± 0.09 vs. 0.67 ± 0.05, p = .024), corrected for 5-HTTLPR genotype, sex and age. We found no association between 5-HT4 receptor binding and attack frequency, years with migraine or time since last migraine attack. Our finding of lower 5-HT4 receptor binding in migraine patients is suggestive of higher brain 5-HT levels. This is in contrast with the current belief that migraine is associated with low brain 5-HT levels. High brain 5-HT levels may represent a trait of the migraine brain or it could be a consequence of migraine attacks.
Keywords: Headache, Pain, Neuroimaging, Brain, Serotonergic mechanisms
Highlights
-
•
For the first time PET imaging of the 5-HT4 receptor was used to examine if migraine is associated with low brain 5-HT levels
-
•
Compared to controls, migraine patients had lower neocortical 5-HT4 receptor binding, indicating higher brain 5-HT levels
-
•
We found no association between years of migraine, attack frequency or days since last attack and 5-HT4 receptor binding.
-
•
Migraine pathophysiology involves serotonergic mechanisms, but migraine patients have high rather than low brain 5-HT levels
1. Introduction
Migraine is a highly debilitating and socioeconomically costly neurological disorder, affecting 16% of the population worldwide (Olesen et al., 2012; Steiner et al., 2013). Despite intensive research during the past several decades, the neurobiological basis and pathophysiology of migraine remains largely unknown. Serotonin (5-hydroxytryptamine, 5-HT) has been directly implicated in the pathophysiology of migraine (Hamel, 2007) and studies on plasma and urinary levels of 5-HT and its main metabolite, 5-hydoxyindoleacetic acid (5-HIAA) suggest that between their migraine attacks, patients have decreased levels of plasma 5-HT (Ferrari et al., 1989; Sicuteri et al., 1961). Accordingly, although plasma levels of 5-HT do not necessarily reflect brain 5-HT levels, migraine has been considered a syndrome of chronically low brain 5-HT levels. Several studies have attempted to assess brain 5-HT levels in migraine patients, but results have been equivocal, showing both higher and lower levels compared to controls (Deen et al., 2017a). We here use a novel neuroimaging method to investigate if migraine is a syndrome associated with low 5-HT brain levels.
The 5-HT4 receptor, one of 14 5-HT receptors, is inversely related to central serotonergic tonus and can thus be used as an indirect biomarker of brain 5-HT levels. In rats, brain 5-HT4 receptor binding decreased after 14 days of selective 5-HT reuptake inhibitor (SSRI) administration (Licht et al., 2009). In humans, carriers of the short allele of the 5-HT transporter (5-HTT) gene, which is associated with relatively increased synaptic 5-HT levels, had lower neocortical 5-HT4 receptor binding compared to carriers of the long allele (Fisher et al., 2012). Furthermore, the final support for the 5-HT4 receptor being inversely related to brain 5-HT levels, came from a study showing that three weeks of SSRI intervention led to a significant decrease in brain 5-HT4 receptor binding in humans (Haahr et al., 2014).
Here we investigated differences in brain 5-HT levels between migraine patients without aura and controls using PET imaging of the 5-HT4 receptor as an in vivo biomarker of brain 5-HT levels. According to existing beliefs, we hypothesized that migraine patients had higher 5-HT4 receptor binding compared to controls.
2. Materials and methods
2.1. Subjects
Participants were recruited through a Danish website for recruitment of volunteers to health research and from a local database. All patients fulfilled the following inclusion criteria: 1) 18–65 years old, 2) a verified diagnosis of migraine without aura according to the International Headache Society Criteria (HCC IHS, 2013). 3) at least one migraine attack every other month but less than five migraine days per month, 4) self-reported previous effect of treatment of migraine attacks with sumatriptan (a 5-HT1B/1D receptor agonist drug). The last criterion was applied, since the subjects were also included in a study investigating the 5-HT1B receptor (Deen et al., 2017b). A standardized interview of all patients was conducted at screening including the following items: duration of disease (years), duration of attack when untreated (hours), migraine days pr. month, frequency of attack (number per month), maximum pain intensity of untreated headache as measured with the Numerical Rating Scale (NRS) (number 0–10), intake of acute pain medication including triptans (days per month), and date of their last migraine attack. Inclusion criteria for age- and sex-matched controls included: 1) no history of migraine including probable migraine and no first-degree relatives with migraine. For all participants, the following exclusion criteria were applied: 1) a history of any other primary headache (except tension-type headache <5 days per month), 2) psychiatric, cerebro- or cardiovascular disease, 3) contraindications for magnetic resonance imaging (MRI), 4) pregnancy or nursing, 5) daily intake of medication including migraine prophylaxis.
All subjects reported to be headache free on the day of their PET scan, and no medication intake was allowed for the last 24 h prior to the scan. All migraine patients were migraine free for at least 48 h prior to the PET scan. In addition, to ensure that all included subjects were truly between two migraine attacks, headache diaries were obtained from all patients for 48 h after the scan. All included participants had a normal physical and neurological examination and unremarkable brain MRI. All participants filled out the major depression inventory (MDI) on the day of the PET scan.
The study was approved by the Ethics Committee of The Region of Copenhagen (H-6-2014-057). In accordance with the Declaration of Helsinki of 1964, with later revisions, all participants gave written consent after detailed oral and written information about the study.
2.2. PET and MR imaging
Synthesis of the radioligand, [11C]SB207145, was performed using an automated radiosynthesis system as previously described (Marner et al., 2009). An intravenous bolus injection of the radioligand was given over 20 s, followed by 120-minute dynamic data acquisition with a high-resolution research tomography (HRRT) PET scanner (CTI/Siemens, Knoxville, TN, USA). To minimize head movement, all subjects had their head stabilized in a specialized head holder. The scans were reconstructed into 38 frames (6 × 5, 10 × 15, 4 × 30, 5 × 120, 5 × 300, and 8 × 600 s) using a 3D-OSEM-PSF algorithm (16 subsets, 10 iterations) with TXTV based attenuation correction (image matrix, 256 × 256 × 207; voxel size, 1.22 × 1.22 × 1.22 mm), as previously described (Hong et al., 2007; Keller et al., 2013; Sureau et al., 2008). T1 and T2 weighted MRI scans used for co-registration were acquired for each subject using a Siemens Prisma 3T scanner (Siemens, Erlangen, Germany) with a 64-channel head coil.
2.3. Quantification of 5-HT4 receptor binding
Single-subject PET images were corrected for intra-scan movement by aligning the frames 10–38 to a reference frame (frame 26) using a scaled least-squares cost function in AIR 5.2.5. Co-registration and alignment of PET images to the corresponding T1-weighted MRI image was done using SPM8. Regions of interest (ROIs) were automatically delineated on each subject's MRI using PVElab software (www.nru.dk) as previously described (Svarer et al., 2005). Accurate co-registration and ROI placement were confirmed by visual inspection for each subject, across all planes. Time activity curves (TAC) and grey matter volumes for each ROI were then extracted.
The Simplified Reference Tissue Model (SRTM) was applied to calculate the non-displaceable binding potential (BPND) of [11C]SB207145. This model has previously been validated for quantification of [11C]SB207145 in the human brain (Marner et al., 2009). Cerebellum (excluding vermis) was used as a reference region since it has a negligible density of 5-HT4 receptors (Ganz et al., 2017; Marner et al., 2009). Kinetic modeling was performed in MATLAB R2013a (8.1.0.604) 64 bit (Mathworks Inc., MA) using an in-house script and the person performing the kinetic modeling was blinded to group status (migraine patient or control). Parametric 5-HT4 receptor binding images for voxel based analysis were generated using PETSurfer (http://surfer.nmr.mgh.harvard.edu, version 6.0), as previously described (Greve et al., 2014). In summary, a combined volumetric and surface registration algorithm was used to normalize each single-subject structural T1 to Montreal Neurological Institute (MNI) space (Postelnicu et al., 2009). After application to the co-registered PET-images, these were then volume-smoothed with a 6-mm full-width half-maximum 3D Gaussian kernel. The Multilinear Reference Tissue Model 2 (MRTM2), using cerebellum as reference region and high-binding regions (putamen, pallidum and caudate) for estimation of k2′, was applied to estimate voxel-level BPNDs.
2.4. Genotyping
Participants were genotyped for the tri-allelic 5-HT transporter-linked polymorphic region (5-HTTLPR) polymorphism. Briefly, genotyping was performed by PCR amplification from forward primer 5′-TAATGTCCCTACTGCAGCCC-3′ and reverse primer 5′-GGGACTGAGCTGGACAACC-3′. The fragments were then digested by the restriction enzyme MspI and separated by gel electrophoresis. Participants were categorized into two groups: 1. Carriers of the short allele (S-carriers) or the long LG allele (LG-carriers). 2. Homozygotes of the long LA allele (LA-homozygotes). This dichotomization was based on a previous study showing that carriers of the low-expressing alleles, S and LG have lower neocortical 5-HT4 receptor binding compared to homozygotes of the high-expressing allele, LA (Fisher et al., 2012).
2.5. Experimental design and statistical analysis
This study was an observational, cross-sectional study comparing interictal migraine without aura patients (>48 hour migraine free before and after the scan) with sex- and age matched controls. Sample size was based on a previous study showing that n = 15 is sufficient to detect a 15% difference between groups with a power of 0.80 in very large brain regions (>50 cm3), such as e.g. neocortex (Marner et al., 2010). Differences between groups in demographics, genotypes and PET variables were evaluated using two-sample t-tests.
To evaluate differences in brain 5-HT levels between migraine patients and controls we used the BPND of a large neocortical region as a proxy of central serotonergic tonus. The neocortical BPND was chosen, since previous studies investigating brain 5-HT levels in migraine mostly focused on cortical regions (for review see (Deen et al., 2017a)). The cortical brain regions receive numerous serotonergic projections from the raphe nuclei and 5-HT plays an important role in the modulation of cortical activity (Celada et al., 2013). Additionally, the relationship between the 5-HTTLPR genotype and 5-HT4 receptor binding is most pronounced in neocortex (Fisher et al., 2012).
The mean neocortical [11C]SB207145 BPND was calculated based on 11 neocortical brain regions (occipital cortex, orbitofrontal cortex, superior, medial and inferior frontal gyri, insula, superior, medial and inferior temporal gyri, sensory motor cortex and parietal cortex) by volume weighting grey matter segmented brain region BPND's:
As our primary investigation, a general linear model including neocortical BPND as the primary dependent variable and group status (patients vs. controls) as the predictive variable was then used to model effects of group on neocortical 5-HT4 receptor binding. To this model, 5-HTTLPR-status (S or LG carriers vs. LA homozygotes), sex and age were added as covariates, since all are known to affect neocortical 5-HT4 receptor binding (Fisher et al., 2012; Madsen et al., 2015, Madsen et al., 2011a). All subjects received tracer doses (injected mass of SB207145 < 0.024 μg/kg) obviating the inclusion of injected mass a covariate (Madsen et al., 2011b). Effects of interaction between group and genotype were evaluated and excluded unless statistically significant. To detect any regional (including subcortical) specific group differences in 5-HT4 receptor binding, whole brain voxel-wise multiple regression was performed using the same linear model as in the primary analysis. Only voxels (sized 1 × 1 × 1 mm) with an average BPND > 0.3 were evaluated within a whole brain mask. In addition, associations between measures of clinical severity (attack frequency, years with migraine and time since last migraine attack) and neocortical 5-HT4 receptor binding were evaluated in the patient group only using a general linear model including 5-HTTLPR-status, age and sex as co-variates.
Statistical tests were carried out using R Studio 3.2.3 and SPM8. We ensured that model assumptions were met by examination of quantile-quantile plots, distribution of the residuals, and predicted values plotted against residuals. In the ROI analyses, the significance threshold was set at p < .05 (two-tailed). In the voxel based analysis, a p-value threshold of p < .001 at voxel level was used. To correct for multiple comparisons only clusters at p < .05 corrected using the family wise error rate (FWE) were assumed significant. All other p-values are reported without correction for multiple comparison.
3. Results
3.1. Demographics and migraine characteristics
Out of the 18 migraine patients who completed the study, three reported to have a migraine attack within 48 h after the PET scan. These were excluded from the primary analysis. Sixteen controls completed the study. One migraine patient (subject 15) was scanned for 90 instead of 120 min, because she felt anxious in the scanner. To ensure that this patient did not affect our results, we conducted the primary analysis both with and without this patient. Thus, data from 15 migraine patients and 16 controls were included in the primary analysis.
A summary of demographics and PET variables are presented in Table 1. Clinical data of the migraine group is shown in Table 2. The regional distribution of the tracer was in concordance with previous studies with lowest binding in neocortex and highest binding in striatum. We found no differences in grey matter volume or MDI score between the two groups and no interaction between group and genotype.
Table 1.
Demographics and PET variables.
| Patients | Controls | p-Valuea | |
|---|---|---|---|
| Number of subjects (men/women) | 15 (2/13) | 16 (3/13) | |
| Genotype (LA homozygote/S or LG carrier) | 6/9 | 6/10 | |
| Age (years) | 29.6 ± 10.2 | 28.9 ± 10.2 | .85 |
| BMI (kg/m2) | 22.6 ± 1.7 | 23.9 ± 4.8 | .33 |
| Major depression inventory | 7.87 ± 7.6 | 7.13 ± 4.8 | .75 |
| Injected radioactivity (MBq) | 584 ± 16 | 591 ± 19 | .34 |
| Specific radioactivity (GBq/μmol) | 567 ± 282 | 486 ± 217 | .38 |
| [11C]SB injected mass per kg (μg/kg) | 0.008 ± 0.006 | 0.008 ± 0.005 | .90 |
| [11C]SB cerebellum AUC/specific radioactivity (GBq/μmol) | 31.1 ± 23 | 32.6 ± 21 | .85 |
Continuous variables are presented as mean ± SD.
Two-sample t-test.
Table 2.
Migraine history.
| Subject | Migraine history (years) |
Attack frequency (n/month) |
Time since last migraine attack (days) |
|---|---|---|---|
| 1 | 8 | 2 | 17 |
| 2 | 7 | 2 | 12 |
| 3 | 21 | 3 | 11 |
| 4 | 25 | 1 | 22 |
| 5 | 15 | 2 | 15 |
| 6 | 20 | 1 | 31 |
| 7 | 19 | 1 | 19 |
| 8 | 8 | 0.5 | 50 |
| 9 | 17 | 2 | 15 |
| 10 | 7 | 1 | 29 |
| 11 | 6 | 3 | 10 |
| 12 | 2 | 2 | 7 |
| 13 | 36 | 4 | 4 |
| 14 | 16 | 3 | 5 |
| 15 | 10 | 2 | 13 |
| Median (range) | 15 (2–36) | 2 (0.5–4) | 15 (4–50) |
3.2. Differences in neocortical binding
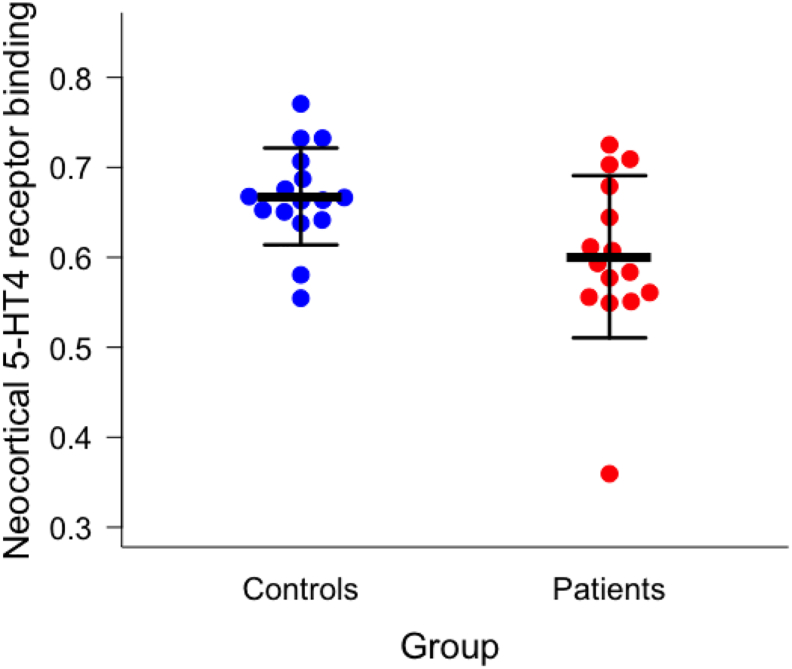
We found that migraine patients had significant lower neocortical [11C]SB207145 binding compared to controls (0.60 ± 0.09 (mean ± SD) vs. 0.67 ± 0.05 (mean ± SD), p = .024) (Fig. 1) after adjustment for covariates. This difference remained after excluding subject 15 (0.63 ± 0.06 vs. 0.68 ± 0.05, p = .038). Post hoc explorative analysis of the ROIs included in the neocortical region showed that the low binding was most pronounced within the orbitofrontal cortex (p = .009), insula (p = .018), superior temporal gyrus (p = .019), parietal cortex (p = .026), medial and inferior temporal gyrus (p = .032), and superior frontal gyrus (p = .040). The voxel-based analysis revealed no significant clusters after rigorous FWE correction.
Fig. 1.
Migraine patients have lower neocortical 5-HT4 receptor binding than controls (0.60 ± 0.09 vs. 0.67 ± 0.05, p = .024) after adjusting for covariates (5-HTTLPR status, age, and sex). Black bars indicate mean ± SD. The difference remained after exclusion of subject 15 (the patient with the lowest BPND) (0.63 ± 0.06 vs. 0.68 ± 0.05, p = .038).
3.3. Associations with migraine characteristics
We found no associations between neocortical 5-HT4 receptor binding and attack frequency (slope estimate −0.017, CI: [−0.081;0.047], p = .56), years with migraine (slope estimate 0.003, CI: [−0.007;0.012], p = .53), or days since last attack (slope estimate 0.002, CI: [−0.003;0.007], p = .40).
4. Discussion
The key finding of this study is that between attacks, migraine patients have lower 5-HT4 receptor binding within neocortex compared to controls. Our post hoc ROI analysis showed that this difference was most pronounced within the orbitofrontal, parietal, temporal and insular cortices, but no significant clusters were found when using a whole brain voxel-based analysis. This discrepancy is probably due to the conservative statistical method applied to the voxel-based analysis (p-value threshold of p < .001 at voxel level, and correction for multiple comparisons using FWE) and due to the large whole-brain search volume. The latter was applied in order to detect any possible subcortical differences. Further, the detected difference between migraine patients and healthy controls was rather small, around 10%. This is, however, in line with previous studies finding significant differences in 5-HT4 receptor binding between groups differing with regards to intervention, genes or disease. Thus, a 5.2% decrease was found after 3 weeks of fluoxetine intervention (Haahr et al., 2014), BDNF met-carriers were shown to have 7% higher neocortical binding relative to val/val-carriers, whereas 5-HTTLPR S-carriers had 7% lower binding compared to LL homozygotes (Fisher et al., 2015, Fisher et al., 2012), and lastly, in Alzheimer's patients, PIB-positive patients had 13% higher binding compared to PIB-negative patients (Madsen et al., 2011c).
Although an acute increase in brain 5-HT levels does not affect [11C]SB207145 BPND (Marner et al., 2010), we took great care to ensure that all included patients were scanned during an attack-free interval (migraine free 48 h before and after scan) to exclude possible effects of attack related changes in brain 5-HT levels on 5-HT4 receptor binding. However, inclusion of the three subjects who reported to have a migraine attack within 48 after the scan did not change our results (p = .024). Furthermore, to reduce the possibility of variability in [11C]SB207145 binding due to diurnal 5-HT variations, we scanned all patients at the same time of the day (±1 h). Since we found no difference in grey matter volume between patients and controls within neocortex this is likewise not thought to affect the magnitude of 5-HT4 receptor binding. Lastly, the low 5-HT4 receptor binding could reflect changes in affinity of the 5-HT4 receptor in migraine patients. However, in colliculi neurons desensitization of the 5-HT4 receptor is caused by a loss of binding sites after continued 5-HT exposure (Ansanay et al., 1996). Thus, we interpret our findings of low [11C]SB207145 binding to reflect low neocortical density of the 5-HT4 receptor in migraine patients.
The low neocortical 5-HT4 receptor density in patients could be explained by repeated surges of 5-HT during migraine attacks – causing an on average higher brain 5-HT level with subsequent downregulation of the cerebral 5-HT4 receptor. In support of this, our post hoc analysis identified lower 5-HT4 receptor binding in several regions implicated in migraine attacks; e.g. functional neuroimaging studies reported increased activation of insula, the prefrontal cortex and the temporal lobe during attacks compared to the attack free interval (Afridi et al., 2005; Weiller et al., 1995). Moreover, both insula and the orbitofrontal cortex are involved in pain modulation (Tracey, 2008). However, we found no direct association between 5-HT4 receptor binding and migraine history (years), attack frequency (number per month) or days since the last migraine attack. It would be interesting to investigate whether duration of attack combined with frequency – as a measure of hours with pain pr. month – is associated with 5-HT4 receptor binding, but this analysis would have required a detailed, prospective headache diary. In addition, we cannot rule out a possible relationship between migraine severity and brain 5-HT levels in high frequency or chronic migraine. Future studies should include this patient group to investigate further whether the low 5-HT4 receptor density is a consequence of repeating migraine attacks.
Alternatively, the low neocortical 5-HT4 receptor density may reflect high brain 5-HT levels in migraine patients in the attack free interval. This interpretation challenges the longstanding belief that migraine patients have low brain 5-HT levels between attacks. To date, electrophysiological studies have provided the most substantial evidence for the 5-HT deficiency hypothesis (Coppola et al., 2009). Migraine patients between attacks showed a lack of habituation of visual evoked potentials (VEP) (Afra et al., 2000) and an increased intensity dependence of auditory evoked potentials (AEP) (Wang et al., 1996), both thought to reflect low brain 5-HT levels (Schoenen, 1996; Wutzler et al., 2008). However, most of these studies were unblinded and did not report time to the next migraine attack. In addition, the findings were not reproduced in other studies (Omland et al., 2013; Sand, 2013). In the current study, all included migraine patients had been migraine free for 48 h before and after the scan and all data analyses were conducted by an investigator who was blinded to diagnoses and clinical data.
In addition to electrophysiological studies, some PET studies have suggested that brain 5-HT levels are low in migraine patients, but the results have been inconsistent. In a 5-HT1A-receptor PET neuroimaging study, higher cortical BPND in migraine patients compared to controls was interpreted as reflecting low brain 5-HT levels (Lothe et al., 2008). However, the 5-HT1A receptor radioligand, [18F]MPPF, is not convincingly sensitive to endogenous 5-HT levels in humans (Paterson et al., 2010). One study reported a higher brain 5-HT synthesis capacity in migraine patients without aura when using α-[11 C]methyl-l-tryptophan as a surrogate marker of brain 5-HT synthesis capacity (Chugani et al., 1999). This was interpreted as being consistent with a high 5-HT turnover and thus, low brain 5-HT levels. Interestingly, in another study using the same method, findings of a low cortical 5-HT synthesis capacity in migraine patients was likewise interpreted as reflecting low cortical 5-HT levels (Sakai et al., 2008). Taking the limitations of these studies (Chugani et al., 1999; Sakai et al., 2008) into account (the reliability of α-[11 C]methyl-l-tryptophan as a surrogate marker of brain 5-HT synthesis capacity has been questioned (Shoaf et al., 2000)), one might speculate whether a low 5-HT synthesis capacity could indeed reflect high 5-HT levels – as found in the current study – due to a negative feedback regulation on 5-HT synthesis. On the other hand, a high synthesis capacity may potentially lead to high 5-HT levels. In further support of our findings, high brain 5-HT levels in migraine patients between attacks may, at least partly, explain, why selective 5-HT reuptake inhibitors are not efficient as migraine prophylaxis (Banzi et al., 2015).
4.1. Possible migraine-inducing mechanisms of serotonin
Serotonergic agents such as m-chlorophenylpiperazine (m-CPP) (Leone et al., 2000), reserpine (Curzon et al., 1969), and fenfluramine (Sicuteri et al., 1976) induce migraine attacks more frequently in migraine patients than in controls, and most likely via increasing brain 5-HT levels (Panconesi and Sicuteri, 1997). Since our data suggest the presence of increased brain 5-HT levels in migraine patients between attacks, we propose that migraine patients could be more susceptible to additional acute increases in 5-HT, which lead to migraine induction.
5-HT has generally been considered an inhibiting agent of pain and is one of the major neurotransmitters of the descending pain-inhibition pathway (Stamford, 1995). In vitro, 5-HT exerts an antinociceptive effect in the trigeminal system (Kilinc et al., 2016) and in healthy animals 5-HT inhibits pain (Viguier et al., 2013). However, recent preclinical studies have shown that 5-HT may be involved in pain facilitation as well (Bardin, 2011). In 5-HT depleted rats, the acute pain reaction was intact but pain behavior in the second phase after formalin injection was attenuated. In addition, in persistent pain models 5-HT depleted rats exhibited a decrease in thermal hyperalgesia and mechanical allodynia (Wei et al., 2010). These pathophysiological-dependent properties of 5-HT may be due to the complex role of the different 5-HT receptor subtypes: The 5-HT1 and 5-HT3 receptors are generally considered antinociceptive, whereas the 5-HT2A and the 5-HT7 receptors are considered pronociceptive (Viguier et al., 2013). Thus, 5-HT may be both pro- and antinociceptive depending on receptor type, affinity and concentration (Sommer, 2006). Interestingly, low density of the antinociceptive 5-HT1B receptor was recently found in pain modulating regions in migraine patients between attacks (Deen et al., 2017b). In light of our present findings we therefore speculate that the pathophysiology of migraine includes an imbalance in the pain modulating system caused by high interictal brain 5-HT levels and changes in expression of different 5-HT receptor subtypes, resulting in loss of inhibition and enhancement of pain facilitation.
4.2. Limitations
Even though several studies have corroborated the inverse relationship between 5-HT4 receptor binding and brain 5-HT levels (Haahr et al., 2014; Licht et al., 2009), the method used in the present study is still an indirect measure of brain 5-HT levels. In addition, we cannot rule out that the effect of migraine status on the 5-HT4 receptor is specific for this receptor, and not due to changes in brain 5-HT levels.
5. Conclusions
Migraine patients have low neocortical 5-HT4 receptor binding. Since pharmacological studies of 5-HT4 receptor binding suggest an inverse relationship with brain 5-HT levels, this most likely indicates higher brain 5-HT levels in migraine patients compared to controls. Our results, therefore, support the involvement of the serotonergic system in migraine pathophysiology, but are in contrast with the current hypothesis that migraine is a syndrome of low brain 5-HT levels. Future studies must explore whether our observation is due to high brain 5-HT levels between attacks, predisposing to migraine, or is the result of recurring migraine attacks with surges of 5-HT.
Acknowledgements
We thank all participants for volunteering to this study. Patrick Fisher is gratefully acknowledged for statistical counseling and Claus Svarer, Bente Dall, Lone Ibsgaard Freyr, Martin Korsbak Madsen, Erik Perfalk and Gerda Thomsen are gratefully acknowledged for their excellent technical assistance.
Funding
This work was supported by Innovation Fund Denmark (NeuroPharm), the Lundbeck Foundation (grant no R180-2014-3398), the Migraine Research Foundation, the A.P. Møller Foundation for the Advancement of Medical Science, and the Cool Sorption Foundation. The John and Birthe Meyer Foundation is gratefully acknowledged for sponsoring the HRRT scanner. The funding sources were not involved in the study design or in the collection, analysis, writing or publication of data.
Conflicts of interest
Dr. Knudsen has received honoraria as a board member of Brain Prize and the Elsass Foundation. She is also on the advisory board for the Kristian G. Jebsen Foundation and a field editor for Int J Neuropsychopharm. Messoud Ashina is a consultant and/or scientific adviser/speaker for the ATI, Allergan, Amgen, Alder and Eli Lilly. All other authors declare no competing financial interests.
References
- Afra J., Proietti Cecchini A., Sándor P.S., Schoenen J. Comparison of visual and auditory evoked cortical potentials in migraine patients between attacks. Clin. Neurophysiol. 2000;111:1124–1129. doi: 10.1016/s1388-2457(00)00271-6. [DOI] [PubMed] [Google Scholar]
- Afridi S.K., Giffin N.J., Kaube H., Friston K.J., Ward N.S., Frackowiak R.S.J., Goadsby P.J. A positron emission tomographic study in spontaneous migraine. Arch. Neurol. 2005;62:1270–1275. doi: 10.1001/archneur.62.8.1270. [DOI] [PubMed] [Google Scholar]
- Ansanay H., Sebben M., Bockaert J., Dumuis A. Pharmacological comparison between [3H]GR 113808 binding sites and functional 5-HT4 receptors in neurons. Eur. J. Pharmacol. 1996;298:165–174. doi: 10.1016/0014-2999(95)00786-5. [DOI] [PubMed] [Google Scholar]
- Banzi R., Cusi C., Randazzo C., Sterzi R., Tedesco D., Moja L. Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) for the prevention of migraine in adults (Review) Cochrane Database Syst. Rev. 2015;5 doi: 10.1002/14651858.CD002919.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardin L. The complex role of serotonin and 5-HT receptors in chronic pain. Behav. Pharmacol. 2011;22:390–404. doi: 10.1097/FBP.0b013e328349aae4. [DOI] [PubMed] [Google Scholar]
- Celada P., Puig M.V., Artigas F. Serotonin modulation of cortical neurons and networks. Front. Integr. Neurosci. 2013;7 doi: 10.3389/fnint.2013.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani D.C., Niimura K., Chaturvedi S., Muzik O., Fakhouri M., Lee M.L., Chugani H.T. Increased brain serotonin synthesis in migraine. Neurology. 1999;53:1473–1479. doi: 10.1212/wnl.53.7.1473. [DOI] [PubMed] [Google Scholar]
- Coppola G., Pierelli F., Schoenen J. Habituation and migraine. Neurobiol. Learn. Mem. 2009;92:249–259. doi: 10.1016/j.nlm.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Curzon G., Barrie M., Wilkinson M.I. Relationships between headache and amine changes after administration of reserpine to migrainous patients. J. Neurol. Neurosurg. Psychiatry. 1969;32:555–561. doi: 10.1136/jnnp.32.6.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen M., Christensen C.E., Hougaard A., Hansen H.D., Knudsen G.M., Ashina M. Serotonergic mechanisms in the migraine brain – a systematic review. Cephalalgia. 2017;37:251–264. doi: 10.1177/0333102416640501. [DOI] [PubMed] [Google Scholar]
- Deen M., Hansen H.D., Hougaard A., da Cunha-Bang S., Nørgaard M., Svarer C., Keller S.H., Thomsen C., Ashina M., Knudsen G.M. Low 5-HT 1B receptor binding in the migraine brain: a PET study. Cephalalgia. 2017;33310241769870 doi: 10.1177/0333102417698708. [DOI] [PubMed] [Google Scholar]
- Ferrari M., Odink J., Tapparelli C., Van Kempen G.M.J., Pennings E.J., Bruyn G. Serotonin metabolism in migraine. Neurology. 1989;39:1239–1242. doi: 10.1212/wnl.39.9.1239. [DOI] [PubMed] [Google Scholar]
- Fisher P.M., Holst K.K., Mc Mahon B., Haahr M.E., Madsen K., Gillings N., Baaré W.F., Jensen P.S., Knudsen G.M. 5-HTTLPR status predictive of neocortical 5-HT 4 binding assessed with [11C]SB207145 PET in humans. NeuroImage. 2012;62:130–136. doi: 10.1016/j.neuroimage.2012.05.013. [DOI] [PubMed] [Google Scholar]
- Fisher P.M., Holst K.K., Adamsen D., Klein A.B., Frokjaer V.G., Jensen P.S., Svarer C., Gillings N., Baare W.F.C., Mikkelsen J.D., Knudsen G.M. BDNF Val66met and 5-HTTLPR polymorphisms predict a human in vivo marker for brain serotonin levels. Hum. Brain Mapp. 2015;36:313–323. doi: 10.1002/hbm.22630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz M., Feng L., Hansen H.D., Beliveau V., Svarer C., Knudsen G.M., Greve D.N. Cerebellar heterogeneity and its impact on PET data quantification of 5-HT receptor radioligands. J. Cereb. Blood Flow Metab. 2017 doi: 10.1177/0271678X16686092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve D.N., Svarer C., Fisher P.M., Feng L., Hansen A.E., Baare W., Rosen B., Fischl B., Knudsen G.M. Cortical surface-based analysis reduces bias and variance in kinetic modeling of brain PET data. NeuroImage. 2014;92:225–236. doi: 10.1016/j.neuroimage.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haahr M.E., Fisher P.M., Jensen C.G., Frokjaer V.G., Mahon B.M., Madsen K., Baare W.F.C., Lehel S., Norremolle A., Rabiner E.A., Knudsen G.M. Central 5-HT4 receptor binding as biomarker of serotonergic tonus in humans: a [lsqb]11C[rsqb]SB207145 PET study. Mol. Psychiatry. 2014;19:427–432. doi: 10.1038/mp.2013.147. [DOI] [PubMed] [Google Scholar]
- Hamel E. Serotonin and migraine: biology and clinical implications. Headache Curr. 2007;27:1293–1300. doi: 10.1111/j.1468-2982.2007.01476.x. [DOI] [PubMed] [Google Scholar]
- Hong I.K., Chung S.T., Kim H.K., Kim Y.B., Son Y.D., Cho Z.H. Ultra fast symmetry and SIMD-based projection-backprojection (SSP) algorithm for 3-D PET image reconstruction. IEEE Trans. Med. Imaging. 2007;26:789–803. doi: 10.1109/tmi.2007.892644. [DOI] [PubMed] [Google Scholar]
- Keller S.H., Svarer C., Sibomana M. Attenuation correction for the HRRT PET-scanner using transmission scatter correction and total variation regularization. IEEE Trans. Med. Imaging. 2013;32:1611–1621. doi: 10.1109/TMI.2013.2261313. [DOI] [PubMed] [Google Scholar]
- Kilinc E., Guerrero-Toro C., Zakharov A., Vitale C., Gubert-Olive M., Koroleva K., Timonina A., Luz L.L., Shelukhina I., Giniatullina R., Tore F., Safronov B.V., Giniatullin R. Serotonergic mechanisms of trigeminal meningeal nociception: implications for migraine pain. Neuropharmacology. 2016;116:160–173. doi: 10.1016/j.neuropharm.2016.12.024. [DOI] [PubMed] [Google Scholar]
- Leone M., Attanasio A., Croci D., Filippini G., D'Amico D., Grazzi L., Nespolo A., Bussone G. The serotonergic agent m-chlorophenylpiperazine induces migraine attacks: a controlled study. Neurology. 2000;55:136–139. doi: 10.1212/wnl.55.1.136. [DOI] [PubMed] [Google Scholar]
- Licht C.L., Marcussen A.B., Wegener G., Overstreet D.H., Aznar S., Knudsen G.M. The brain 5-HT4 receptor binding is down-regulated in the flinders sensitive line depression model and in response to paroxetine administration. J. Neurochem. 2009;109:1363–1374. doi: 10.1111/j.1471-4159.2009.06050.x. [DOI] [PubMed] [Google Scholar]
- Lothe A., Merlet I., Demarquay G., Costes N., Ryvlin P., Mauguière F. Interictal brain 5-HT1A receptors binding in migraine without aura: a 18F-MPPF-PET study. Cephalalgia. 2008;28:1282–1291. doi: 10.1111/j.1468-2982.2008.01677.x. [DOI] [PubMed] [Google Scholar]
- Madsen K., Haahr M.T., Marner L., Keller S.H., Baaré W.F., Svarer C., Hasselbalch S.G., Knudsen G.M. Age and sex effects on 5-HT(4) receptors in the human brain: a [(11)C]SB207145 PET study. J. Cereb. Blood Flow Metab. 2011;31:1475–1481. doi: 10.1038/jcbfm.2011.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen K., Marner L., Haahr M., Gillings N., Knudsen G.M. Mass dose effects and in vivo affinity in brain PET receptor studies - a study of cerebral 5-HT 4 receptor binding with [11C]SB207145. Nucl. Med. Biol. 2011;38:1085–1091. doi: 10.1016/j.nucmedbio.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Madsen K., Neumann W.J., Holst K., Marner L., Haahr M.T., Lehel S., Knudsen G.M., Hasselbalch S.G. Cerebral serotonin 4 receptors and amyloid-β in early Alzheimer's disease. J. Alzheimers Dis. 2011;26:457–466. doi: 10.3233/JAD-2011-110056. [DOI] [PubMed] [Google Scholar]
- Madsen K., Torstensen E., Holst K.K., Haahr M.E., Knorr U., Frokjaer V.G., Brandt-Larsen M., Iversen P., Fisher P.M., Knudsen G.M. Familial risk for major depression is associated with lower striatal 5-HT(4) receptor binding. Int. J. Neuropsychopharmacol. 2015;18 doi: 10.1093/ijnp/pyu034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marner L., Gillings N., Comley R.A., Baaré W.F.C., Rabiner E.A., Wilson A.A., Houle S., Hasselbalch S.G., Svarer C., Gunn R.N., Laruelle M., Knudsen G.M. Kinetic modeling of 11C-SB207145 binding to 5-HT4 receptors in the human brain in vivo. J. Nucl. Med. 2009;50:900–908. doi: 10.2967/jnumed.108.058552. [DOI] [PubMed] [Google Scholar]
- Marner L., Gillings N., Madsen K., Erritzoe D., Baaré W.F.C., Svarer C., Hasselbalch S.G., Knudsen G.M. Brain imaging of serotonin 4 receptors in humans with [11C]SB207145-PET. NeuroImage. 2010;50:855–861. doi: 10.1016/j.neuroimage.2010.01.054. [DOI] [PubMed] [Google Scholar]
- Olesen J., Gustavsson A., Svensson M., Wittchen H.-U., Jönsson B. The economic cost of brain disorders in Europe. Eur. J. Neurol. 2012;19:155–162. doi: 10.1111/j.1468-1331.2011.03590.x. [DOI] [PubMed] [Google Scholar]
- Omland P.M., Nilsen K.B., Uglem M., Gravdahl G., Linde M., Hagen K., Sand T. Visual evoked potentials in interictal migraine: no confirmation of abnormal habituation. Headache J. Head Face Pain. 2013;53:1071–1086. doi: 10.1111/head.12006. [DOI] [PubMed] [Google Scholar]
- Panconesi A., Sicuteri R. Headache induced by serotonergic agonists – a key to the interpretation of migraine pathogenesis? Cephalalgia. 1997;17:3–14. doi: 10.1046/j.1468-2982.1997.1701003.x. [DOI] [PubMed] [Google Scholar]
- Paterson L.M., Tyacke R.J., Nutt D.J., Knudsen G.M. Measuring endogenous 5-HT release by emission tomography: promises and pitfalls. J. Cereb. Blood Flow Metab. 2010;30:1682–1706. doi: 10.1038/jcbfm.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postelnicu G., Zöllei L., Fischl B. Combined volumetric and surface registration. IEEE Trans. Med. Imaging. 2009;28:508–522. doi: 10.1109/TMI.2008.2004426. (doi:19273000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai Y., Dobson C., Diksic M., Aubé M., Hamel E. Sumatriptan normalizes the migraine attack-related increase in brain serotonin synthesis. Neurology. 2008;70:431–439. doi: 10.1212/01.wnl.0000299095.65331.6f. [DOI] [PubMed] [Google Scholar]
- Sand T. We were blind, so now we can see: the EP/ERP story in migraine. Clin. Neurophysiol. 2013;125:433–434. doi: 10.1016/j.clinph.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Schoenen J. Deficient habituation of evoked cortical potentials in migraine: a link between brain biology, behavior and trigeminovascular activation? Biomed Pharmacother. 1996;50:71–78. doi: 10.1016/0753-3322(96)84716-0. [DOI] [PubMed] [Google Scholar]
- Shoaf S.E., Carson R.E., Hommer D., Williams W.A., Higley J.D. The suitability of [11C]-a-methyl-l-tryptophan as a tracer for serotonin synthesis: studies with dual administration of [11C] and [14C] labeled tracer. J. Cereb. Blood Flow Metab. 2000;20:244–252. doi: 10.1097/00004647-200002000-00004. [DOI] [PubMed] [Google Scholar]
- Sicuteri F., Testi A., Anselmi B. Biochemical investigations in headache: increase in the hydroxyindoleacetic acid excretion during migraine attacks. Int. Arch. Allergy Immunol. 1961;19:55–58. [Google Scholar]
- Sicuteri F., Del Bene E., Anselmi B. Fenfluramine headache. Headache. 1976;16:185–188. doi: 10.1111/j.1526-4610.1976.hed1604185.x. [DOI] [PubMed] [Google Scholar]
- Society, H.C.C. of the I.H.S. The international classification of headache disorders, 3rd edition (beta version) Cephalalgia. 2013;33:629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- Sommer C. Is serotonin hyperalgesic or analgesic? Curr. Pain Headache Rep. 2006;10:101–106. doi: 10.1007/s11916-006-0020-4. [DOI] [PubMed] [Google Scholar]
- Stamford J.A. Descending control of pain. Prog. Neurobiol. 1995;75:217–227. doi: 10.1093/bja/75.2.217. [DOI] [PubMed] [Google Scholar]
- Steiner T.J., Stovner L.J., Birbeck G.L. Migraine: the seventh disabler. Cephalalgia. 2013;33:289–290. doi: 10.1177/0333102412473843. [DOI] [PubMed] [Google Scholar]
- Sureau F.C., Reader A.J., Comtat C., Leroy C., Ribeiro M.J., Buvat I., Trebossen R. Impact of image-space resolution modeling for studies with the high-resolution research tomograph. J. Nucl. Med. 2008;49:1000–1008. doi: 10.2967/jnumed.107.045351. [DOI] [PubMed] [Google Scholar]
- Svarer C., Madsen K., Hasselbalch S.G., Pinborg L.H., Haugbøl S., Frøkjær V.G., Holm S., Paulson O.B., Knudsen G.M. MR-based automatic delineation of volumes of interest in human brain PET images using probability maps. NeuroImage. 2005;24:969–979. doi: 10.1016/j.neuroimage.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Tracey I. Imaging pain. Br. J. Anaesth. 2008;101:32–39. doi: 10.1093/bja/aen102. [DOI] [PubMed] [Google Scholar]
- Viguier F., Michot B., Hamon M., Bourgoin S. Multiple roles of serotonin in pain control mechanisms – implications of 5-HT₇ and other 5-HT receptor types. Eur. J. Pharmacol. 2013;716:8–16. doi: 10.1016/j.ejphar.2013.01.074. [DOI] [PubMed] [Google Scholar]
- Wang W., Timsit-berthier M., Schoenen J. Intensity dependence of auditory evoked potentials is pronounced in migraine: an indication of cortical potentiation and low serotonergic neurotransmission? Neurology. 1996;46:1404–1409. doi: 10.1212/wnl.46.5.1404. [DOI] [PubMed] [Google Scholar]
- Wei F., Dubner R., Zou S., Ren K., Bai G., Wei D., Guo W. Molecular depletion of descending serotonin unmasks its novel facilitatory role in the development of persistent pain. J. Neurosci. 2010;30:8624–8636. doi: 10.1523/JNEUROSCI.5389-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiller C., May A., Limmroth V., Jüptner M., Kaube H., Schayck R.V., Coenen H.H., Diener H.C. Brain stem activation in spontaneous human migraine attacks. Nat. Med. 1995;1:658–660. doi: 10.1038/nm0795-658. [DOI] [PubMed] [Google Scholar]
- Wutzler A., Winter C., Kitzrow W., Uhl I., Wolf R.J., Heinz A., Juckel G. Loudness dependence of auditory evoked potentials as indicator of central serotonergic neurotransmission: simultaneous electrophysiological recordings and in vivo microdialysis in the rat primary auditory cortex. Neuropsychopharmacology. 2008;33:3176–3181. doi: 10.1038/npp.2008.42. [DOI] [PubMed] [Google Scholar]