Abstract
Background: Spinal cord injury (SCI) is associated with both a state of chronic inflammation and an increased prevalence of cardiovascular disease (CVD). These disorders are closely linked and have been shown to negatively influence one another. Participation in regular exercise has been shown to be an effective intervention strategy in the treatment of each of these disorders. For individuals with SCI who may lack the lower limb motor capabilities to perform certain traditional exercise modalities, functional electrical stimulation (FES) cycling may provide an effective alternative. Objective: The purpose of this study was to examine the effects of 12 weeks of FES training performed 3 times per week on physiological indices of cardiovascular function as well as molecular indices of inflammation and cardiovascular health. Methods: Ten individuals with chronic SCI were included. Measures of central and peripheral cardiovascular function as well as hematological and immunological markers were assessed before and after the 12-week exercise program. Results: Enhancements in exercise performance as well as a corresponding increase in peripheral cardiovascular function were achieved, as shown by a significant 34% increase in pulse volume (P = .04) and trends toward increases in cross-sectional area (P = .09) and arterial inflow volume (P = .11) of the common femoral artery. Despite this, no change in any hematological or immunological markers was evident. Conclusion: Although the efficacy of FES exercise in enhancing exercise performance (time and distance to fatigue) and peripheral cardiovascular function has been reaffirmed, no alterations in any molecular indices of cardiovascular risk were achieved.
Keywords: cardiovascular disease, chronic inflammation, functional electrical stimulation, spinal cord injury
Cardiovascular disease (CVD) is one of the most prevalent health complications after spinal cord injury (SCI), with morbidity and mortality rates exceeding those from renal and pulmonary complications.1,2 The loss of physical function and lack of accessible options for participation in physical activity often result in the adoption of a more sedentary lifestyle, making individuals with SCI more prone to a variety of metabolic conditions such as obesity, type 2 diabetes, and hyperlipidemia.3,4 In addition to directly contributing to the risk of CVD, each of these conditions is independently associated with an inflammatory state1,5,6 and may contribute to the chronic inflammatory status typically observed after SCI.7,8
There is a well-established bidirectional link between inflammation and CVD whereby atherosclerotic plaques associated with CVD are influenced by and also further induce elevations in proinflammatory mediators.9 A number of proinflammatory mediators have the ability to induce vasoconstriction, thereby increasing shear force on vessels and the likelihood of endothelial damage.10 Further, as proinflammatory mediators increase chemotaxis and the production of adhesion molecules, they may act to increase leukocyte infiltration to the damaged areas, which increases the risk of plaque formation and/or worsens preexisting plaques.10 These plaques, in turn, contribute to a greater state of inflammation. Such an influence demonstrates the role that chronic inflammation after SCI plays in the development of CVD and provides a rationale for the use of inflammatory markers as estimates of cardiovascular risk.11
Considerable evidence exists to suggest that a suitably designed exercise program may help attenuate or reverse many aspects of CVD after SCI, including abnormalities in glucose homeostasis, lipid lipoprotein profiles, and cardiovascular fitness.12 Regular exercise has also been consistently demonstrated to produce anti-inflammatory effects in able-bodied individuals as a result of a unique exercise-induced inflammatory response and improvements in metabolic health.13 Therefore, if exercise can be performed at a high enough intensity, it may produce similar improvements in individuals with SCI. The primary caveat concerning exercise as a treatment or preventative measure for CVD is that many individuals with SCI lack the motor capabilities required to perform traditional exercises such as arm ergometry. The use of functional electrical stimulation (FES) cycling provides an opportunity for individuals to participate in exercise that may not otherwise be possible. It also provides a means of enhancing blood flow to the lower limbs, providing peripheral cardiovascular benefits not afforded by arm ergometry.14 Multiple physiological systems have been shown to benefit from FES cycling, including cardiovascular,15,16 respiratory,16,17 muscular,18,19 and skeletal systems.18 In previous studies, investigators have evaluated the cardiovascular benefit of FES training after SCI on aspects such as vascular dimension,20 femoral artery blood flow,20,21 and myocardial atrophy.22 Each of these studies has utilized FES cycling performed 3 times weekly for durations ranging from 4 to 8 weeks. Despite the various cardiovascular benefits achieved in these studies, none have assessed changes in molecular indices of immunological and cardiovascular health.
In this study, we examined the effects of 12 weeks of FES cycling on physiological measures of cardiovascular function and on molecular indices of cardiovascular and immunological health in individuals with chronic SCI. We hypothesized that this training intervention would result in improvements in both physiological measures of cardiovascular function and molecular indices of risk.
Methods
Participants
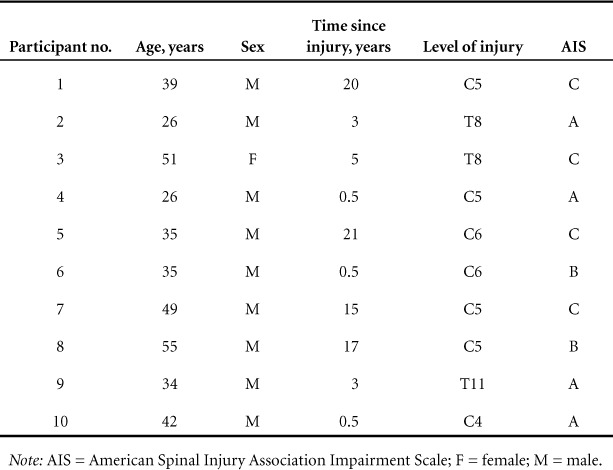
Participant characteristics are described in Table 1. Ten community-dwelling individuals with chronic (>6 months) SCI, aged 26 to 55 years, were recruited for participation in the study. All participants had neurologically stable injuries with severities ranging from American Spinal Injury Association Impairment Scale (AIS) A to C and levels ranging from C5-T11. Participants were excluded from the study if there was evidence of a lower motor neuron injury, active infectious disease, symptoms of autonomic dysreflexia caused by stimulation, or excessive spasticity that precluded the use of the FES cycle.
Table 1.
Participant characteristics
Exercise protocol
The exercise protocol consisted of FES cycling ergometer training performed on the RT-300 rehabilitation system (Restorative Therapies, Baltimore, MD). Training was performed 3 times per week for 12 weeks. Electrical stimulation was provided to the hamstrings, gluteal muscles, and quadriceps via 2 × 4 self-adhesive surface electrodes. Electrical stimulation was provided as biphasic square wave pulses, 500 μs in duration, at 50 Hz, in a coordinated sequence, allowing for cyclic patterns of muscle contractions resulting in a cycle motion. Each session began with a 1-minute passive warm-up period during which no electrical stimulation was provided to the muscles. After the warm-up period, progressive stimulation of the leg muscles began, allowing active FES movement. Participants with some degree of lower limb motor control were permitted to contribute to the cycling motion along with the assistance of the electrical stimulation. The FES cycling exercise then continued at a predetermined stimulation amplitude (ranging from 50 to 140 mA), pulse width (500 μs), frequency (50 Hz), and target speed depending on individual pain tolerance, until muscle fatigue was eventually sensed by the ergometer. The ergometer sensed muscle fatigue after the predetermined stimulation (measured in milliamps) reached 100% and the participant was unable to maintain the target speed (as assessed by a reduction in revolutions per minute). Specifically, when the participant's speed dropped 5 or more rpm below the target speed for 2 consecutive seconds, the ergometer sensed fatigue and stopped stimulation. After this point, the participant underwent the final 2-minute passive cool-down period whereby the cycling motion was produced by motor support only. This was followed by a 5-minute rest period before the next training bout was attempted. Throughout the 12-week intervention, the stimulation amplitude and target cycling cadence (rpm) were increased based on individual pain tolerance and ability, ultimately achieving the maximum stimulation of 140 mA and a speed of 35 to 49 rpm. Increasing the stimulation amplitude induced a stronger muscle contraction, allowing participants to cycle against greater resistance and at greater speeds. The duration of any given session was also gradually increased, based on the amount of time stimulation could be tolerated and the participant's ability to withstand fatigue (1 to 45 minutes). Once the participant achieved 45 minutes of FES cycling without fatigue for 3 consecutive days, the resistance provided by the FES ergometer was increased by 1 unit (1.2 Nm increments). The percentage of resistance during any given session was altered to maintain the stimulation in the 90% to 95% range. This allowed for a level that would challenge the participant while avoiding early fatigue, because an inappropriately high resistance and corresponding increase in stimulation (to 100%) may lead to an earlier onset of muscle fatigue and drop in speed.
Doppler ultrasound measures of cardiovascular function
Central measures of cardiovascular function were assessed before and after the exercise intervention and included cross-sectional area (CSA) of the aorta, measured at the left ventricular output track, stroke volume (SV), and cardiac output (CO). Peripheral measures of cardiovascular function included CSA of the common femoral artery (CFA), pulse volume (PV) of the CFA, and arterial inflow volume of the CFA. Peripheral vascular ultrasonography was performed by a single examiner as described by Nash et al.22 Images were acquired using a 2.5-MHz transducer on a Sonos 5500 ultrasound platform (Hewlett-Packard Sonos 5500 system, Cheshire, MA). The femoral artery was imaged in short axis at the level of the saphenofemoral venous junction, and the maximal internal diameter was measured. CSA of the femoral artery was calculated using the formula A = πr2. Pulsed Doppler images of femoral artery flow were then obtained by positioning a 1- to 2-mm sample volume within the femoral artery. Care was taken to ensure that the angle of the insonating beam was parallel to flow as demonstrated by color Doppler imaging. The Doppler envelope was traced to allow determination of the velocity time integral (VTI) and peak systolic velocity of femoral arterial flow. Femoral artery volume per beat was calculated as: Volume = CSA × VTI.
Measurement of hematological and immunological markers
Blood samples were drawn from participants, under fasting conditions, by means of standard venipuncture. Each whole blood sample was collected aseptically by a trained phlebotomist or nurse into anticoagulant-free tubes (for serum preparation). The samples were allowed to clot for 30 minutes then centrifuged at 3,400 rpm for 10 to 15 minutes. The serum was then separated into plastic vials and frozen at −20°C until just before immunoassay via enzyme-linked immunosorbent assay (Quantibody Human Array kit; Raybiotech Inc., Narcross, GA). All assays were sent to a certified commercial laboratory for quantification (Raybiotech Inc.).
Immunological markers of interest included the acute-phase reactant and C-reactive protein, as well as a panel of cytokines including interleukin-1α (IL-1α), IL-1β, IL-4, IL-6, IL-8, IL-10, monocyte chemoattractant protein-1 (MCP-1), interferon gamma (IFN-γ), and tumor necrosis factor alpha (TNF-α). These inflammatory proteins have been shown to be elevated in the sera of individuals with SCI and may be indicative of cytokine dysregulation or a protective autoimmunity.7 Proinflammatory cytokines such as TNF-α, IL-6, and CRP have also been shown to be associated with many forms of cardiac disease9 and, as such, were also examined as an indication of risk of cardiac disease. Additionally, levels of cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglycerides were each quantified as indicators of cardiovascular risk.
Statistical analysis
Differences between preintervention and postintervention values were assessed by means of a paired-sample t test. Statistical significance was set at P < .05 for all tests, which were conducted using IBM SPSS version 20 (IBM Corp., Armonk, NY).
Results
Exercise performance
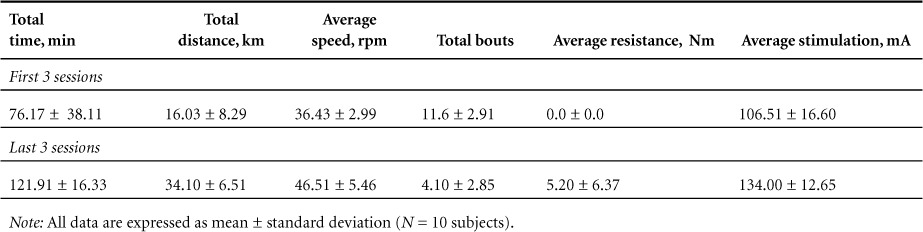
The results of the performance measures are described in Table 2. All participants experienced improvements in performance over time with no adverse events. Subjects were required to meet an adherence rate of at least 85% to be included in the study. Summary statistics show that across all subjects, total distance achieved increased from 16.03 ± 8.29 km within the initial 3 bouts to 34.10 ± 6.51 km during the final 3 bouts [t(9) = 7.39, P = .00]. This was also accomplished with progressively fewer bouts (initial mean = 11.60 to final mean = 4.10), indicating that participants were able to travel longer distances before experiencing fatigue and required fewer breaks [t(9) = 7.23, P = .00]. Average stimulation was also increased from an initial level of 106.51 mA to a final level of 134.00 mA, and average resistance was increased from an initial level of 0.0 Nm to a final level of 5.20 Nm.
Table 2.
Exercise performance
Doppler ultrasound measures of cardiovascular function
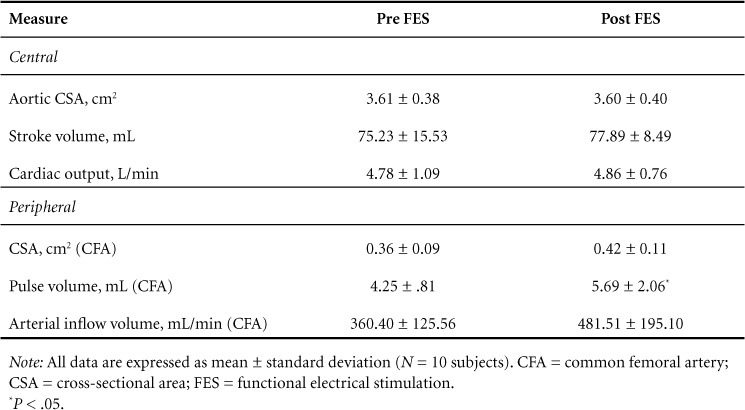
The results for both central and peripheral cardiovascular changes are described in Table 3. There was a significant increase in femoral artery PV after the 12-week exercise intervention as values increased by 34% from an average preintervention value of 4.25 ± 0.81 mL to 5.69 ± 2.06 mL (P = .04). Trends toward increases in CSA and arterial inflow volume of the CFA were also observed with respective changes of 0.36 ± 0.09 cm2 to 0.42 ± 0.11 cm2 (P = .09) and 360.40 ± 125.56 mL/min to 481.51 ± 195.10 mL/min (P = .11). No alterations were observed for any measures of central cardiovascular function after the intervention (Table 3).
Table 3.
Doppler ultrasound measures of cardiovascular function
Hematological and immunological measures
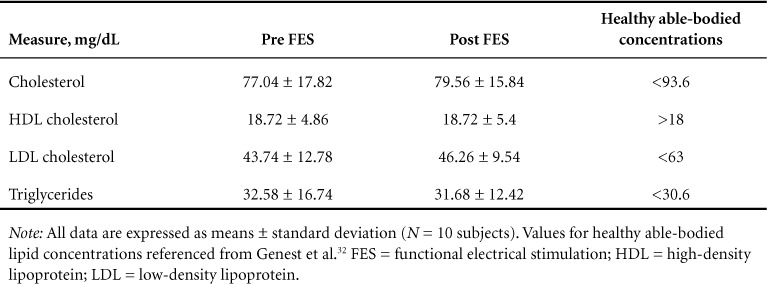
The results for changes in hematological and immunological measures are described in Tables 4 and 5. No significant changes were observed for any hematological or immunological measures after the 12-week intervention.
Table 4.
Immunologic measures
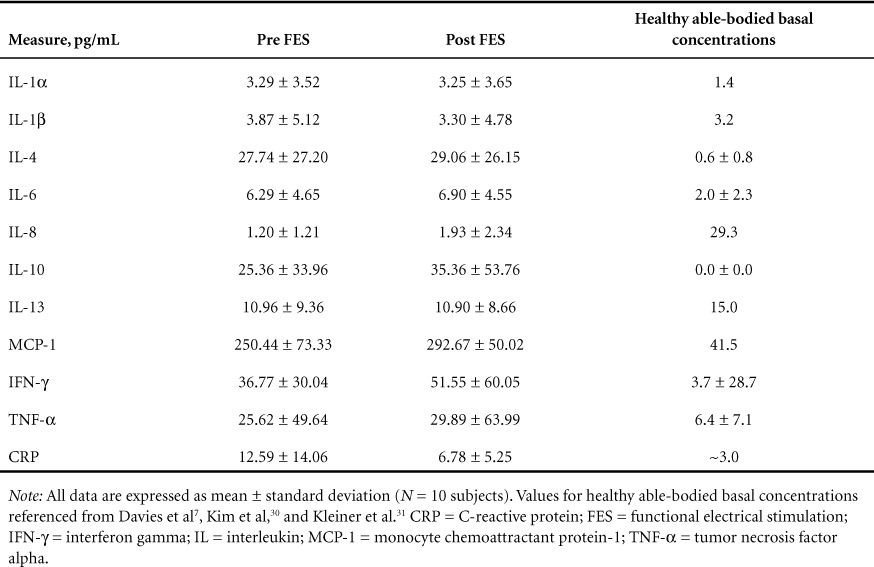
Table 5.
Hematological measures
Discussion
Considerable evidence suggests that a suitably designed exercise program can help improve the impaired immunological and cardiovascular status of persons with SCI.23–25 A number of studies have also established physiological benefits after FES exercise training related to aspects such as increased lower limb muscle mass,26 increased vascular dimension,20 and improved femoral artery blood flow.20,21 Despite these positive findings, it may be ill advised to assume that such physiological changes translate into actual reductions in the risk of corresponding CVD. Such a link between exercise-induced improvements in physiological outcomes and actual reductions in disease risk is often assumed but is not well established in the literature. The findings of this study highlight this concept.
It is important to note that this study successfully demonstrated exercise-induced improvements in exercise capacity and lower limb blood flow. Improvements in exercise capacity were demonstrated by significant increases in total exercise duration, distance, and speed, while the number of bouts needed to complete each session was significantly reduced. Likewise, exercise-induced improvements in lower limb blood flow were demonstrated with a 17% increase in resting femoral artery diameter (CSA), a 34% increase in PV within the femoral artery, and a 34% increase in resting femoral artery blood flow (arterial inflow volume) (see Table 3). Such findings correspond to previous findings whereby improvements in femoral artery blood flow of 30% to 40% were demonstrated after long-term FES cycling.20,21 Thus, the exercise protocol employed was sufficient to induce a training effect, and any lack of benefit in molecular indices cannot be attributed to an insufficient intensity or volume of the FES cycling protocol used in this study. It should be noted that an FES cycling program may simply not be of high enough intensity to induce such molecular changes or a program of longer duration may be required. It is also possible that other clinical risk factors not assessed in this study may have been reduced. However, the fact that the molecular indices showed no change after the intervention highlights the important fact that physiological improvements, such as enhanced peripheral blood flow, do not necessarily translate to a reduction in clinical risk.
There is a well-established link between immune markers and cardiac risk,9 and individuals with SCI have elevations in both of these domains as compared with their able-bodied counterparts.8,27 The American Heart Association has set clinical guidelines for CRP with values above 3.0 mg/L corresponding to high CVD risk.11 Although CRP levels were reduced by 46% across all subjects after the intervention, the change was not significant and postintervention values remained in the category of high CVD risk. Additionally, other inflammatory markers related to CVD,28 such as IL-6 and TNF-α levels, were elevated across all participants compared with previously reported values from healthy able-bodied control subjects (2.0 ± 2.3 and 6.4 ± 7.1)7 and were not reduced after the intervention (see Table 4). Lastly, blood lipid levels were assessed as indicators of cardiovascular risk. Increases in HDL cholesterol and decreases in LDL cholesterol levels have been shown to be associated with a reduced risk of CVD29; however, no alterations in any blood lipids were evident after the intervention (see Table 5).
As such, the results of this study indicate that despite a substantial improvement in exercise performance and corresponding improvements in peripheral blood flow, 12 weeks of thrice weekly FES cycling was not sufficient to cause a corresponding reduction in clinical risk in terms of any of hematological or immunological markers of cardiovascular disease.
Conclusion
Overall, the results of this study confirm that 12 weeks of FES exercise training is associated with a strong exercise training response (time and distance to fatigue) as well as positive physiological adaptations to lower limb vascular dimension and resting blood flow. However, despite the efficacy of FES exercise in this regard, there were no alterations in any molecular indices of cardiovascular risk.
Acknowledgments
The authors declare no conflicts of interest.
REFERENCES
- 1. Myers J, Lee M, Kiratli J.. Cardiovascular disease in spinal cord injury. Am J Phys Med Rehabil. 2007; 86 2: 142– 152. [DOI] [PubMed] [Google Scholar]
- 2. Garshick E, Kelley A, Cohen SA, . et al. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord. 2005; 43 7: 408– 416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gorgey AS, Gater DR.. Prevalence of obesity after spinal cord injury. Top Spinal Cord Inj Rehabil. 2007; 12 4: 1– 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cragg JJ, Noonan VK, Dvorak M, Krassioukov A, Mancini GBJ, Borisoff JF.. Spinal cord injury and type 2 diabetes: Results from a population health survey. Neurology. 2013; 81 21: 1864– 1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gregor MF, Hotamisligil GS.. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011; 29: 415– 445. [DOI] [PubMed] [Google Scholar]
- 6. Lee MY, Myers J, Hayes A, . et al. C-reactive protein, metabolic syndrome, and insulin resistance in individuals with spinal cord injury. J Spinal Cord Med. 2005; 28 1: 20– 25. [DOI] [PubMed] [Google Scholar]
- 7. Davies AL, Hayes KC, Dekaban GA.. Clinical correlates of elevated serum concentrations of cytokines and autoantibodies in patients with spinal cord injury. Arch Phys Med Rehabil. 2007; 88 11: 1384– 1393. [DOI] [PubMed] [Google Scholar]
- 8. Hayes KC, Hull TCL, Delaney GA, . et al. Elevated serum titers of proinflammatory cytokines and CNS autoantibodies in patients with chronic spinal cord injury. J Neurotrauma. 2002; 19 6: 753– 761. [DOI] [PubMed] [Google Scholar]
- 9. Knuefermann P, Vallejo J, Mann DL.. The role of innate immune responses in the heart in health and disease. Trends Cardiovasc Med. 2004; 14 1: 1– 7. [DOI] [PubMed] [Google Scholar]
- 10. Hansson GK. Immune mechanisms in atherosclerosis. Arterioscler Thromb Vasc Biol. 2001; 21 12: 1876– 1890. [DOI] [PubMed] [Google Scholar]
- 11. Pearson TA, Mensah GA, Alexander RW, . et al. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003; 107 3: 499– 511. [DOI] [PubMed] [Google Scholar]
- 12. Warburton D, Eng J, Krassioukov A, Sproule S.. Cardiovascular health and exercise rehabilitation in spinal cord injury. Top Spinal Cord Inj Rehabil. 2007; 13 1: 98– 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Petersen AMW, Pedersen BK.. The anti-inflammatory effect of exercise. J Appl Physiol. 2005; 98: 1154– 1162. [DOI] [PubMed] [Google Scholar]
- 14. Gerrits HL, de Haan A, Sargeant AJ, van Langen H, Hopman MT.. Peripheral vascular changes after electrically stimulated cycle training in people with spinal cord injury. Arch Phys Med Rehabil. 2001; 82 June: 832– 839. [DOI] [PubMed] [Google Scholar]
- 15. Hooker SP, Figoni SF, Glaser RM, Rodgers MM, Ezenwa BN, Faghri PD.. Physiologic responses to prolonged electrically stimulated leg-cycle exercise in the spinal cord injured. Arch Phys Med Rehabil. 1990; 71 11: 863– 869. [PubMed] [Google Scholar]
- 16. Mutton DL, Scremin AM, Barstow TJ, Scott MD, Kunkel CF, Cagle TG.. Physiologic responses during functional electrical stimulation leg cycling and hybrid exercise in spinal cord injured subjects. Arch Phys Med Rehabil. 1997; 78 7: 712– 718. [DOI] [PubMed] [Google Scholar]
- 17. Hooker SP, Figoni SF, Rodgers MM, . et al. Physiologic effects of electrical stimulation leg cycle exercise training in spinal cord injured persons. Arch Phys Med Rehabil. 1992; 73 5: 470– 476. [PubMed] [Google Scholar]
- 18. Mohr T, Andersen JL, Biering-Sørensen F, . et al. Long-term adaptation to electrically induced cycle training in severe spinal cord injured individuals. Spinal Cord. 1997; 35 1: 1– 16. [DOI] [PubMed] [Google Scholar]
- 19. Baldi JC, Jackson RD, Moraille R, Mysiw WJ.. Muscle atrophy is prevented in patients with acute spinal cord injury using functional electrical stimulation. Spinal Cord. 1998; 36 7: 463– 469. [DOI] [PubMed] [Google Scholar]
- 20. Gerrits HL, de Haan A, Sargeant AJ, van Langen H, Hopman MT.. Peripheral vascular changes after electrically stimulated cycle training in people with spinal cord injury. Arch Phys Med Rehabil. 2001; 82 6: 832– 839. [DOI] [PubMed] [Google Scholar]
- 21. Hopman MTE, Groothuis JT, Flendrie M, Gerrits KHL, Houtman S.. Increased vascular resistance in paralyzed legs after spinal cord injury is reversible by training. J Appl Physiol. 2002; 93 6: 1966– 1972. [DOI] [PubMed] [Google Scholar]
- 22. Nash MS, Bilsker S, Marcillo AE, . et al. Reversal of adaptive left ventricular atrophy following electrically-stimulated exercise training in human tetraplegics. Paraplegia. 1991; 29: 590– 599. [DOI] [PubMed] [Google Scholar]
- 23. Phillips WT, Kiratli BJ, Sarkarati M, . et al. Effect of spinal cord injury on the heart and cardiovascular fitness. Curr Probl Cardiol. 1998; 23 11: 641– 716. [DOI] [PubMed] [Google Scholar]
- 24. Jacobs PL, Nash MS.. Exercise recommendations for individuals with spinal cord injury. Sports Med. 2004; 34 11: 727– 751. [DOI] [PubMed] [Google Scholar]
- 25. Hooker SP, Wells CL.. Effects of low- and moderate-intensity training in spinal cord-injured persons. Med Sci Sports Exerc. 1989; 21 1: 18– 22. [DOI] [PubMed] [Google Scholar]
- 26. Chilibeck PD, Jeon J, Weiss C, Bell G, Burnham R.. Histochemical changes in muscle of individuals with spinal cord injury following functional electrical stimulated exercise training. Spinal Cord. 1999; 37 4: 264– 268. [DOI] [PubMed] [Google Scholar]
- 27. DeVivo MJ, Black KJ, Stover SL.. Causes of death during the first 12 years after spinal cord injury. Arch Phys Med Rehabil. 1993; 74 3: 248– 254. [PubMed] [Google Scholar]
- 28. Van Gaal LF, Mertens IL, De Block CE.. Mechanisms linking obesity with cardiovascular disease. Nature. 2006; 444 7121: 875– 880. [DOI] [PubMed] [Google Scholar]
- 29. Manninen V, Tenkanen L, Koskinen P, . et al. Joint effects of serum triglyceride and LDL cholesterol and HDL cholesterol concentrations on coronary heart disease risk in the Helsinki Heart Study. Implications for treatment. Circulation. 1992; 85 1: 37– 45. [DOI] [PubMed] [Google Scholar]
- 30. Kim YK, Myint AM, Lee BH, . et al. Th1, Th2 and Th3 cytokine alteration in schizophrenia. Prog Neuro-Psychopharmacology Biol Psychiatry. 2004; 28: 1129– 1134. [DOI] [PubMed] [Google Scholar]
- 31. Kleiner G, Marcuzzi A, Zanin V, Monasta L, Zauli G.. Cytokine levels in the serum of healthy subjects. Mediat Inflamm. 2013; 2013: 434010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Genest J, McPherson R, Frohlich J, . et al. 2009 Canadian Cardiovascular Society/Canadian guidelines for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease in the adult - 2009 recommendations. Can J Cardiol. 2009; 25 10: 567– 579. [DOI] [PMC free article] [PubMed] [Google Scholar]


