Abstract
Objectives
To describe a series of miniature schnauzers diagnosed with histiocytic sarcoma and assess for possible breed predisposition.
Materials and Methods
Medical records of miniature schnauzers with a diagnosis of histiocytic sarcoma between January 2008 and April 2015 were reviewed. Data collected included signalment, body weight, presenting complaint, date of diagnosis, clinicopathologic and diagnostic imaging findings, treatment, therapeutic response, date of death or last follow-up and necropsy findings. Breed predisposition was assessed with odds ratios, using breed-matched dogs without histiocytic sarcoma admitted during the study period as controls. Pedigree analysis was performed for dogs with available registration information.
Results
Fourteen miniature schnauzers were diagnosed with histiocytic sarcoma during the study period, making them over-represented among the hospital population (odds ratio=4.8, P=0.0009). Disease was considered localised in ten dogs and disseminated in four. Of the dogs with localised disease, nine were diagnosed with primary pulmonary histiocytic sarcoma based on the presence of a large pulmonary mass with (n=7) or without (n=2) evidence of intra-thoracic metastasis, and one had gastric histiocytic sarcoma with nodal metastasis. Treatments varied, but an aggressive clinical course was found in most patients. Pedigree analysis revealed a recent common ancestor for a subset of the dogs assessed.
Clinical Significance
Miniature schnauzers were over-represented among dogs with histiocytic sarcoma in this patient population. Pedigree analysis supports an inherited risk factor, which has not previously been suggested in the breed. Primary pulmonary involvement with or without intra-thoracic metastasis was common in this cohort.
Introduction
Canine histiocytic sarcoma (HS) is a malignancy of macrophages and dendritic cells that can manifest as either localised or disseminated disease. This distinction has historically been made based upon the number of organ systems affected at the time of diagnosis because the morphology of HS is variable and cannot be used to reliably differentiate between the two anatomic forms (Affolter & Moore 2002). Disseminated disease is characterised by multiple tumours within several organ systems, while localised HS originates from a single organ. Common sites of localised disease include periarticular locations, bone, skin, subcutaneous tissues and lung; however, metastasis is common, occurring in 70 to 91% of cases (Craig et al. 2002, Fidel et al. 2006, Schultz et al. 2007). Dogs with nonresectable or disseminated disease have a median survival time of four months when treated with chemotherapy (Skorupski et al. 2007, Clifford et al. 2012). Localised disease may have an improved prognosis when treated with a combination of local and systemic therapy, with median survival times ranging from 6 to 19 months (Craig et al. 2002, Fidel et al. 2006, Skorupski et al. 2009, Klahn et al. 2011).
Breeds over-represented among dogs diagnosed with HS include Bernese mountain dogs, flat-coated retrievers, golden retrievers, Labrador retrievers and rottweilers (Affolter & Moore 2002, Craig et al. 2002, Fidel et al. 2006, Skorupski et al. 2007, Constantino-Casas et al. 2011, Clifford et al. 2012, Erich et al. 2013, Dervisis et al. 2016). Pembroke Welsh corgis have also been found to be commonly affected in Japan (Takahashi et al. 2014, Kagawa et al. 2015, Mariani et al. 2015). These breed predispositions suggest a genetic role in the pathogenesis of HS, and an oligogenic mode of inheritance has been proposed in Bernese mountain dogs, with a trait heritability of 0.3 (Moore & Rosin 1986, Rosin et al. 1986, Padgett et al. 1995, Abadie et al. 2009).
Although HS can occur in any breed of dog, cases in miniature schnauzers have rarely been reported (Friedrichs et al. 2010, Cannon et al. 2015, Wouda et al. 2015). The purpose of this retrospective study is to describe a series of miniature schnauzers diagnosed with HS, assess for over-representation of the breed compared to the overall hospital population, and to evaluate pedigrees for common ancestors.
Materials And Methods
Study population
Medical records of miniature schnauzers with a possible diagnosis of HS between January 2008 and April 2015 at the University of Tennessee Center of Veterinary Medicine (UT-CVM) were reviewed. Potential cases were initially identified by searching the UT-CVM anatomic and clinical pathology electronic databases for the key word “sarcoma” in the pathology report from January 2008 through April 2014. Pathology reports were then reviewed by the authors. A definitive cytologic or histopathologic (±CD18 immunohistochemistry) diagnosis of HS by a veterinary pathologist was required to be included in the study. For cases in which the histopathologic diagnosis was not confirmed by immunohistochemistry (IHC) at the time of initial presentation, samples were reviewed by a single, board-certified anatomic pathologist (LEC) and CD18 IHC was retrospectively performed. Cases for which HS was listed as possible, probable or included as a differential diagnosis were not included unless the diagnosis could be confirmed with IHC. Additional miniature schnauzer cases were prospectively identified by the authors on presentation to the clinic between April 2014 and April 2015.
For breeds other than miniature schnauzer, cases diagnosed with HS were identified using the same search described above. However, in addition to definitive cytologic or histopathologic diagnoses, cases with a suspected diagnosis of HS were included in the study. All cases identified via the pathology databases were required to have been referred and evaluated at UT-CVM during the specified time period.
The control population for breed-specific odds ratio calculations consisted of all canine hospital admissions to the UT-CVM between January 2008 and April 2014 for each breed, with the number of dogs from that breed with a diagnosis of HS subtracted from the total.
Miniature schnauzers were included only if the medical record was available, including date and cause of death. When necessary, additional follow-up information were obtained by contacting the referring veterinarian. Breeder information, club registration numbers and pedigree certificates were collected via telephone and email communication with the owners. Although specific ethical approval was not sought for this study, owner consent was obtained for all cases. At UT-CVM, institutional approval for retrospective studies is not required.
Medical records review
Data collected included signalment, body weight, presenting complaint, clinicopathologic and diagnostic imaging findings, treatment, therapeutic response, survival time, and necropsy information (when available). Dogs were considered to have primary pulmonary HS if a single, large pulmonary mass was identified and all metastatic disease was confined to the thoracic cavity. Intra-thoracic metastasis consisted of smaller intrapulmonary nodules, extension into the mediastinum, and/or enlarged intrathoracic lymph nodes.
Statistical analysis
Our hypothesis that the miniature schnauzer breed was over-represented among dogs diagnosed with HS in this patient population was tested using odds ratios. Odds ratios were calculated for any breed with one or more dog diagnosed with HS between January 2008 and April 2014 using the following formula: OR=[(Number of cases for breed)/(Number of controls for breed)]/[(Number of cases for all other breeds)/(Number of controls for all other breeds)]. Cases and controls were defined as described under Study Population. Two-sided Fisher's exact tests were used to determine the 95% confidence intervals for each breed odds ratio and to calculate P-values to assess statistical significance of breed predispositions. Raw P-values were corrected for multiple comparisons using the Bonferroni correction, and the adjusted P-values are reported. A Bonferonni adjusted P-value <0.05 was considered significant. Analyses were performed with R software for statistical computing (R Core Team 2014). Two miniature schnauzers identified after April 2014 were excluded from statistical analysis.
Pedigree analysis
All owners were contacted for breeder information and registration. Pedigrees were available for seven dogs. Six dogs were registered by the American Kennel Club and one was registered by the American Canine Association. The pedigree figure was created using Genial Pedigree Draw (Genial Genetic Solutions (2014)).
Results
Clinicopathologic findings
Seventeen cases of miniature schnauzers diagnosed with HS were identified on initial review. Fifteen were identified from pathology database review and two on clinical presentation between April 2014 and April 2015. These two cases were included in the study, but excluded from statistical analysis since they were identified following the time period that the hospital pathology databases were reviewed. Eleven had a definitive diagnosis, and six had HS considered as a top differential. Of the cases without a definitive diagnosis, HS was confirmed via pathologic review and positive CD18 IHC in three dogs. The remaining three cases were excluded due to non-supportive CD18 IHC (n=2) or lack of histopathologic confirmation of a suspected cytologic diagnosis (n=1). Thus, a total of 14 dogs were included in the study. Six dogs were diagnosed by cytology without histopathology, three by cytology with histopathologic confirmation post-mortem, and five by histopathology (Table 1). CD18 IHC was performed for all cases with a histopathologic diagnosis (n=8), either at the time of diagnosis (n=3) or retrospectively (n=5), and seven cases were positive. One case was negative for CD18; however, the internal control (alveolar macrophages) was also negative in this sample. Therefore, we concluded that this was likely a false negative resulting from prolonged formalin fixation or paraffin block storage (Ramos-Vara et al. 2014).
Table 1. Clinical features, treatment and survival of fourteen miniature schnauzers with histiocytic sarcoma.
| Dog | Age (years) | Sex | Disseminated versus localised | Primary tumour location | Metastasis at diagnosis | Method of diagnosis | Treatment | OS (days) |
|---|---|---|---|---|---|---|---|---|
| 1 | 11 | FS | D | – | Lung (multifocal), liver, adrenal glands, diaphragm, LN (multicentric) | C, N (CD18+) | Paclitaxel | 19 |
| 2 | 8 | MN | D | – | Lung (multifocal), liver, vertebral body * | N (CD18+) | None | 0 |
| 3 | 8 | FS | D | – | Lung (mutltifocal) *, liver, mediastinal LN, right adrenal gland * | C | None | 0 |
| 4 | 13 | FS | D | – | Lung (solitary/LCD), liver, hepatic LN, mediastinum | C, N | None | 0 |
| 5 | 11 | FS | L | Stomach | Gastric LN | H (CD18+) | Sx | 3 |
| 6 | 12 | FS | L | Lung (RM) | Lung (RCR) | H (CD18+) | Sx | 1620 ** |
| 7 | 11 | FS | L | Lung (RM) | Tracheobronchial LN *, Lung (RA) * | H (CD18+) | Doxorubicin, rapamycin | 18 |
| 8 | 7 | FS | L | Lung (RCR) | Mediastinum | C, N (CD18+) | None | 0 |
| 9 | 10 | FS | L | Lung (RCR) | Mediastinum | C | Doxorubicin, prednisone | 19 |
| 10 | 7 | FS | L | Lung (RCD) | Lung (RCR) * | C | CCNU | 145 |
| 11 | 9 | FS | L | Lung (RCD) | – | C | None | 57 |
| 12 | 9 | FS | L | Lung (LCR) | Lung (RM, RCR) * | C | Masitinib, prednisone | 10 |
| 13 | 10 | FS | L | Lung (LCD) | – | C | None | 94 |
| 14 | 10 | FS | L | Lung (LCD) | Lung (RCD) * | H (CD18+) | None | 88 |
FS female spayed, MN male castrated, D disseminated, L localised, CT computed tomography, LN lymph node, C Cytology, H Histopathology, N Necropsy, OS overall survival, Sx surgery, RCR right cranial, RM right middle, RA right accessory, RCD right caudal, LCR left cranial, LCD left caudal
Metastasis suspicious from imaging findings, but not confirmed
Alive at the time of data collection
There were 13 spayed females and one castrated male, with a median age of 10 years (range 7 to 13 years) and a median weight of 7.8 kg (range 6.1 to 9.4 kg) at the time of diagnosis. Presenting clinical signs included: cough (n=8), respiratory distress (n=3), dysphagia (n=1), forelimb lameness (n=1), melena (n=1), vomiting (n=1), epistaxis (n=1), and non-ambulatory paraparesis (n=1). One case was incidentally diagnosed during routine staging for a mast cell tumour. Primary tumour locations included the lung (n=9) and stomach (n=1). The remaining four dogs had disseminated disease without an obvious primary tumour. Of the dogs with primary lung tumours, two had no evidence of metastasis and seven had evidence of local metastasis to intrathoracic lymph nodes (n=1), mediastinum (n=2), and/or other pulmonary sites (n=5). None of the dogs considered to have primary pulmonary HS had evidence of abdominal organ involvement (Table 1).
Complete blood count and serum biochemistry evaluation was performed in all dogs. Abnormalities are shown in Table 2. Thrombocytopenia was not noted in any dog in this series, though it is reportedly common in dogs with HS (Skorupski et al. 2007, Rassnick et al. 2010). Anaemia in the affected dogs was considered mild (Table 2) and there was no indication of haemophagocytic HS on medical record review. Urinalysis was performed in five dogs, and was unremarkable in all. Bone marrow evaluation was not performed in any dog. Four dogs underwent full necropsy examination.
Table 2. Laboratory abnormalities at the time of diagnosis in miniature schnauzers with histiocytic sarcoma.
| Abnormality | Number of dogs | Median | Range | Reference | Units |
|---|---|---|---|---|---|
| Anaemia (HCT) | 3 | 39.4 | 31.7 to 40.3 | 41 to 60 | % |
| Neutrophilia | 3 | 18.5 | 11.9 to 33 | 2.65 to 9.8 | K/uL |
| Left shift | 3 | 1.0 | 0.5 to 1.16 | 0 to 0.3 | K/uL |
| Thrombocytosis | 4 | 489 | 429 to 646 | 147 to 423 | K/uL |
| Lymphopaenia | 7 | 0.6 | 0.3 to 0.9 | 1.1 to 4.6 | K/uL |
| Monocytosis | 3 | 1.4 | 1.0 to 1.8 | 0.165 to 0.85 | K/uL |
| Hypoalbuminaemia | 8 | 2.7 | 2.3 to 3.1 | 3.2 to 4.1 | g/dL |
| Hyperglobulinaemia | 6 | 3.8 | 3.5 to 5.7 | 2.0 to 3.2 | g/dL |
| Hypoglycaemia | 2 | 74 | 68 to 80 | 84 to 120 | mg/dL |
| Hyperglycaemia | 2 | 120 | 116 to 123 | 84 to 120 | mg/dL |
| Hypocalcaemia | 1 | 9.2 | 9.2 | 10.0 to 11.9 | mg/dL |
| Hypercalcaemia | 1 | 12.2 | 12.2 | 10.0 to 11.9 | mg/dL |
| Elevated ALP | 7 | 299 | 200 to 1042 | 15 to 164 | U/L |
| Elevated ALT | 1 | 103 | 103 | 21 to 97 | U/L |
| Elevated AST | 3 | 72 | 61 to 105 | 15 to 51 | U/L |
| Elevated CK | 2 | 1004 | 383 to 1624 | 49 to 324 | U/L |
HCT haematocrit, ALP alkaline phosphatase, ALT alanine aminotransferase, AST aspartate aminotransferase, CK creatine kinase
Diagnostic imaging findings
Thoracic radiographs were performed in all dogs and thoracic CT was performed in three dogs. Pulmonary involvement of HS was diagnosed in 13/14 dogs (92.9%). A suspected primary lung tumour was identified in nine dogs. Location of primary pulmonary HS varied and affected lung lobes were equally represented. Pleural effusion was noted in five dogs. Fluid analysis in these cases revealed modified transudate (n=2), inflammatory exudate (n=1) and malignant effusion (n=2).
Abdominal imaging was performed in 11 dogs, 10 via ultrasonography and one via CT. Intra-abdominal disease was detected in three dogs, of which two had disseminated disease and one had apparent primary gastric HS with local lymph node metastasis. Abdominal imaging was not performed for three dogs; however, two of these dogs were euthanased due to multifocal lung metastasis on the date of diagnosis and were confirmed to have disseminated HS with abdominal organ involvement at necropsy. The remaining dog underwent lung lobectomy and was still alive at the time of writing, over four years from diagnosis. Due to the long-term survival of this patient, abdominal involvement is considered unlikely.
Treatment and outcome
Two dogs had surgery. One dog underwent gastric mass resection and regional lymph node removal, but died due to post-operative septic peritonitis. The other dog had lung lobectomy (complete right middle and partial right cranial) to remove two pulmonary tumours, both confirmed as HS. No adjunctive chemotherapy was pursued. This patient was still alive at the time of writing (1620 days).
Five dogs were treated with chemotherapy, including doxorubicin (Adriamycin; Pfizer, n=2), lomustine (CCNU, Corden Pharma, n=1), paclitaxel (Paccal Vet, Oasmia Pharmaceutical AB, n=1), and masitinib (Kinavet; AB Science, n=1). One dog received rapamycin (Sirolimus, Pfizer) and two dogs received prednisone (Hikma Pharmaceuticals) in combination with their chemotherapy protocols. The dog receiving masitinib started treatment one month prior to HS diagnosis for a concurrent mast cell tumour with local lymph node metastasis. All dogs undergoing chemotherapy were euthanased because of radiographically progressive disease (n=2) or re-accumulation of pleural effusion (n=3) causing significant dyspnoea. Survival times for dogs treated with chemotherapy were 10, 18, 19, 19 and 145 days (median 19 days, Table 1). Response rates were difficult to assess due to short survival times and low case number; however, the dog living for 145 days maintained stable disease throughout the duration of the protocol.
Four dogs were euthanased at the time of diagnosis and the remaining three dogs received palliative treatment with antitussive and pain medications. Dogs treated with palliative medications had survival times of 57, 88 and 94 days.
Breed analyses
During the period from January 2008 to April 2014 there were 24.896 new hospital admissions for dogs at the UT-CVM. HS was diagnosed in 103 dogs. Miniature schnauzers comprised 12/103 (11.7%) of all HS cases, while accounting for 2.7% of the total patient population (679/24.896). Thirty-six different breeds were diagnosed with HS, but only five were found to be over-represented relative to the control population: miniature schnauzer (OR=4.8, CI=8.8, P<0.001), golden retriever (OR=3.9, CI=7.0, P=0.003), flat-coated retriever (OR=34, CI=115, P=0.005), shar pei (OR=16, CI=44, P=0.006) and Bernese mountain dog (OR=15, CI=48, P=0.042) (Table 3).
Table 3. Breed odds ratios of dogs diagnosed with HS at University of Tennessee Center of Veterinary Medicine between January 2008 and April 2014.
| Breed | Dogs with HS | Dogs without HS | Odds ratio | Lower 95% CI | Upper 95% CI | Adjusted P value |
|---|---|---|---|---|---|---|
| Miniature schnauzer | 12 | 667 | 4.8 | 2.4 | 8.8 | <0.001 |
| Golden retriever | 13 | 896 | 3.9 | 2.0 | 7.0 | 0.003 |
| Flat-coated retriever | 3 | 22 | 34 | 6.4 | 115 | 0.005 |
| Shar pei | 4 | 63 | 16 | 4.1 | 44 | 0.006 |
| Bernese mountain dog | 3 | 49 | 15 | 3.0 | 48 | 0.042 |
| Rottweiler | 4 | 192 | 5.2 | 1.4 | 14 | 0.280 |
| Mixed breed | 14 | 5740 | 0.5 | 0.3 | 0.9 | 0.570 |
| Standard poodle | 2 | 137 | 3.6 | 0.4 | 13 | 1 |
| Beagle | 3 | 350 | 2.1 | 0.4 | 6.4 | 1 |
| Bichon frise | 2 | 238 | 2.0 | 0.2 | 7.7 | 1 |
| Labrador | 11 | 1488 | 1.9 | 0.9 | 3.5 | 1 |
| English bulldog | 2 | 305 | 1.6 | 0.2 | 5.9 | 1 |
| German shepherd | 4 | 623 | 1.6 | 0.4 | 4.2 | 1 |
| Pomeranian | 2 | 333 | 1.5 | 0.2 | 5.4 | 1 |
| Cocker spaniel | 2 | 410 | 1.2 | 0.1 | 4.4 | 1 |
| Boxer | 3 | 684 | 1.1 | 0.2 | 3.2 | 1 |
CI confidence interval, HS histiocytic sarcoma
Bonferroni-adjusted P values corrected for multiple comparisons, P<0.05 is considered significant
Bold values highlight over-represented breeds. The following breeds were diagnosed with one case each and had P values equal to 1: Norwich terrier, Keeshond, Bouvier des flandres, Alaskan malamute, Staffordshire terrier, English setter, German short-haired pointer, Australian shepherd, Greyhound, Australian cattle dog, Siberian husky, Walker hound, Boston terrier, springer spaniel, basset hound, Chihuahua, Maltese, Weimeraner, Doberman, American eskimo
Pedigree analysis
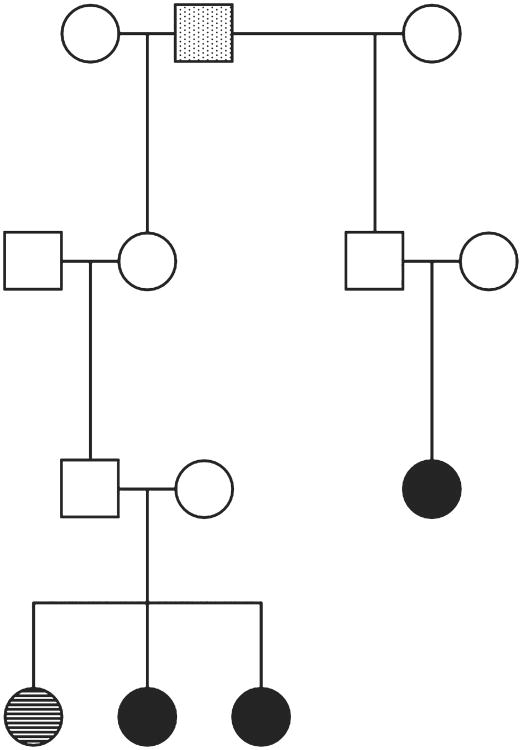
Pedigree information was available for seven dogs, three of which shared a close common ancestor within three generations (Fig 1). Two of these dogs were discovered to be full siblings from successive litters, and the third was related to the siblings through a recent common ancestor. An additional dog from the same breeding pair was also evaluated at UT-CVM for a pulmonary mass and pleural effusion. Cytology of this mass revealed histiocytic proliferation. However, as a definitive diagnosis was not reached and additional diagnostics were not pursued, this dog was excluded from the study. All three of these siblings had the same owner and home environment, and neither parent of the siblings died due to cancer-related disease. Two additional dogs with HS included in the study were more distantly related to this lineage, tracing back greater than five generations.
Fig 1.
Pedigree demonstrating familial aggregation of HS. Three cases, 2 dogs with confirmed HS included in the study and 1 additional dog with presumptive HS (lung mass with histiocytic proliferation), were discovered to be full siblings from separate litters. A fourth confirmed HS case was related to these dogs through a recent common ancestor. Solid, histiocytic sarcoma; Lines, histiocytic proliferation; Dots, common ancestor
Discussion
Miniature schnauzers were over-represented among dogs with HS in this patient population. Pedigree analysis revealed that two of the miniature schnauzers with confirmed HS and one with suspected HS were full siblings. Another dog with HS was related within a few generations (Fig 1). In a popular breed, such as the miniature schnauzer, this is not expected to occur by chance alone, particularly for an uncommon disease such as HS. Thus, the common ancestry within the breed further suggests an inherited component to HS risk in this cohort of miniature schnauzers. As miniature schnauzers have not previously been reported to be predisposed to HS, it is possible that the predisposition noted in this study is limited to a lineage in the southeast United States. Less likely, a common environmental exposure or gene-environment interaction could have also contributed to risk as the three full siblings shared a home environment.
Golden retrievers, flat-coated retrievers and Bernese mountain dogs were also found to be over-represented among dogs with HS, consistent with previous reports (Padgett et al. 1995, Affolter & Moore 2002, Fidel et al. 2006, Nielsen et al. 2010, Constantino-Casas et al. 2011, Clifford et al. 2012, Erich et al. 2013, van Kuijk et al. 2013). Retrievers and mountain breeds (including Bernese mountain dogs and rottweilers) cluster together based on genetic analyses, suggesting that these breeds may share a common genetic risk factor for HS (Parker 2012). Schnauzers are not part of this cluster, but rather belong to a cluster with Doberman pinschers (Parker 2012, Streitberger et al. 2012). While Dober-mans were not over-represented in this study population, they have been previously reported to be at increased risk for HS (Kerlin & Hendrick 1996). Thus, the predispositions towards HS in miniature schnauzer and Doberman pinscher breeds could again be an indication of a genetic risk factor shared across breeds. Shar pei dogs were also found to be over-represented in this study, but belong to an ancient cluster that branched off before all non-ancient breeds and, thus, are not closely related to any of the other predisposed breeds (Parker 2012). Nevertheless, this does not preclude the existence of an ancient mutation that was inherited by diverse breeds.
In this series of miniature schnauzers, 9/14 dogs had suspected primary pulmonary tumours. Interestingly, two miniature schnauzers previously identified in the literature were also discovered to have primary pulmonary lesions via personal communication with the corresponding authors (Cannon et al. 2015, Wouda et al. 2015). Although canine primary pulmonary HS has not been well documented, a site predilection for the right middle and left cranial lung lobes has been suggested (Tsai et al. 2012, Barrett et al. 2014). Dog breeds that have been previously reported to be over-represented with pulmonary HS are consistent with those reported for other forms of the disease, and include Bernese mountain dogs, golden retrievers, rottweilers and Pembroke Welsh corgis (Tsai et al. 2012, Barrett et al. 2014, Kagawa et al. 2015).
One study evaluating the various forms of localised HS found the lung to be the most common primary tumour location, accounting for 31% of all cases (Skorupski et al. 2009). Interestingly, however, there appears to be some breed differences between the commonly reported locations of localised HS (Skorupski et al. 2009). While rottweilers and flat-coated retrievers have been found to be predisposed to the development of periarticular HS, Pembroke Welsh corgis have been found to be at risk for primary HS of the central nervous system (Craig et al. 2002, Fidel et al. 2006, Ide et al. 2011, Mariani et al. 2015). In addition to our series of miniature schnauzers, primary pulmonary involvement has also been reported in Pembroke Welsh corgis (Kagawa et al. 2015). Primary gastric HS is rare and, to the authors' knowledge, has only been documented in three previous cases (Fant et al. 2004, Skorupski et al. 2009, Elliott 2016). Additional studies are needed to investigate these breed differences and to identify possible genetic factors influencing which organ systems are affected.
The number of dogs that received definitive treatment is too low to make any meaningful judgments or comparisons regarding therapy. Survival times were short for most dogs, consistent with the previous literature. Outcomes of dogs with primary pulmonary HS tend to be similar to dogs with disseminated disease (Kagawa et al. 2015). It is interesting to note that one dog undergoing lung lobectomy with multifocal pulmonary lesions had long-term survival after treatment with surgery alone.
There are limitations of this study, including its retrospective nature and low number of cases. Additionally, this study included dogs with both cytologic and histopathologic diagnoses. All histopathologic diagnoses were reviewed by a single pathologist and CD18 IHC was supportive in all but one, which is suspected to be a false negative. However, additional IHC to rule out other round cell tumours was not performed.
While six cases were diagnosed via cytology alone, the cytological features of HS have been well described and shown to be specific (Brown et al. 1994, Wiley et al. 2009). This is supported by the fact that, in three cases in our study, an initial cytologic diagnosis was confirmed by histopathology at the time of necropsy. It should be noted that if all cases with a cytologic diagnosis (n=6) or a histopathologic diagnosis without confirmatory IHC staining (n=1) were removed from statistical analysis, the miniature schnauzer breed remains over-represented (n=7, OR=2.8, CI=1.1 to 6.0, raw P=0.017).
While the miniature schnauzers included in our study were limited to those with a definitive cytologic or histopathologic diagnosis of HS, the control group included cases with suspected as well as definitive diagnoses of HS. Thus, the inclusion criteria for miniature schnauzers with a diagnosis of HS was more stringent than the inclusion criteria for other breeds and the overall number of dogs diagnosed with HS among the control group may be over-estimated due to false positives in breeds other than miniature schnauzer. Although this may affect the accuracy of the odds ratio calculations, the expected effect for miniature schnauzers would be an underestimation of the breed predisposition. While this makes it more challenging to draw conclusions for the other breeds over-represented in our study, it strengthens our finding for the miniature schnauzer breed.
Here we present a case series of miniature schnauzers with HS and document over-representation of this breed among dogs with HS in our hospital population. A shared recent ancestor was identified in a subset of these cases which raises the possibility for one or more genetic risk factors, though a shared environmental risk factor cannot be ruled out. Another noteworthy finding was the predominance of primary pulmonary HS in the breed. Future studies are needed to better characterise this disease in miniature schnauzers and to assess response to therapy and outcome of pulmonary HS.
Acknowledgments
The authors thank Michele McConnell, Amanda Rainey, Chase Constant, and Nicole Tate for technical assistance. Partial funding for Dr. Furrow is provided by an NIH ORIP K01 Mentored Research Scientist Development Award (1K01OD019912-01). IHC for CD18 was paid for by the Small Animal Clinical Sciences Department at the University of Tennessee. No other funding or support to disclose.
Footnotes
Conflict of interest: None of the authors of this article has a financial or personal relationship with other people or organisations that could inappropriately influence or bias the content of the paper.
References
- Abadie J, Hédan B, Cadieu E, et al. Epidemiology, pathology, and genetics of histiocytic sarcoma in the Bernese mountain dog breed. Journal of Heredity. 2009;100(Supplement 1):S19–S27. doi: 10.1093/jhered/esp039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Affolter VK, Moore PF. Localized and disseminated histiocytic sarcoma of dendritic cell origin in dogs. Veterinary Pathology. 2002;39:74–83. doi: 10.1354/vp.39-1-74. [DOI] [PubMed] [Google Scholar]
- Barrett LE, Pollard RE, Zwingenberger A, et al. Radiographic characterization of primary lung tumors in 74 dogs. Veterinary Radiology & Ultrasound. 2014;55:480–487. doi: 10.1111/vru.12154. [DOI] [PubMed] [Google Scholar]
- Brown DE, Thrall MA, Getzy DM, et al. Cytology of canine malignant histiocytosis. Veterinary Clinical Pathology. 1994;23:118–122. doi: 10.1111/j.1939-165x.1994.tb00921.x. [DOI] [PubMed] [Google Scholar]
- Cannon C, Borgatti A, Henson M, et al. Evaluation of a combination chemotherapy protocol including lomustine and doxorubicin in canine histiocytic sarcoma. Journal of Small Animal Practice. 2015;56:425–429. doi: 10.1111/jsap.12354. [DOI] [PubMed] [Google Scholar]
- Clifford CA, Skorupski KA, Moore PF. Histiocytic diseases. In: Withrow SJ, Vail DM, Page R, editors. Withrow & MacEwen ' s Small Animal Clinical Oncology. 5th. W.B. Saunders; Philadelphia, PA, USA: 2012. pp. 706–714. [Google Scholar]
- Constantino-Casas F, Mayhew D, Hoather TM, et al. The clinical presentation and histopathologic-immunohistochemical classification of histiocytic sarcomas in the flat-coated retriever. Veterinary Pathology. 2011;48:764–771. doi: 10.1177/0300985810385153. [DOI] [PubMed] [Google Scholar]
- Craig LE, Julian ME, Ferracone JD. The diagnosis and prognosis of synovial tumors in dogs: 35 cases. Veterinary Pathology. 2002;39:66–73. doi: 10.1354/vp.39-1-66. [DOI] [PubMed] [Google Scholar]
- Dervisis NG, Kiupel M, Qin Q, et al. Clinical prognostic factors in canine histiocytic sarcoma. Veterinary and Comparative Oncology. 2016 doi: 10.1111/vco.12252. DOI: https://doi.org/10.1111/vco.12252. [DOI] [PubMed]
- Elliott J. Gastric histiocytic sarcoma in a dog. Journal of Small Animal Practice. 2016;57:719. doi: 10.1111/jsap.12602. [DOI] [PubMed] [Google Scholar]
- Erich SA, Rutteman GR, Teske E. Causes of death and the impact of histiocytic sarcoma on the life expectancy of the Dutch population of Bernese mountain dogs and flat-coated retrievers. The Veterinary Journal. 2013;198:678–683. doi: 10.1016/j.tvjl.2013.09.062. [DOI] [PubMed] [Google Scholar]
- Fant P, Caldin M, Furlanello T, et al. Primary gastric histiocytic sarcoma in a dog – a case report. Journal of Veterinary Medicine Series A. 2004;51:358–362. doi: 10.1111/j.1439-0442.2004.00645.x. [DOI] [PubMed] [Google Scholar]
- Fidel J, Schiller I, Hauser B, et al. Histiocytic sarcomas in flat-coated retrievers: a summary of 37 cases (November 1998 – March 2005) Veterinary and Comparative Oncology. 2006;4:63–74. doi: 10.1111/j.1476-5810.2006.00090.x. [DOI] [PubMed] [Google Scholar]
- Friedrichs KR, Thomas C, Plier M, et al. Evaluation of serum ferritin as a tumor marker for canine histiocytic sarcoma. Journal of Veterinary Internal Medicine. 2010;24:904–911. doi: 10.1111/j.1939-1676.2010.0543.x. [DOI] [PubMed] [Google Scholar]
- Genial Genetic Solutions. Chester, UK: 2014. [Accessed June 15, 2016]. http://www.genialgenetics.com. [Google Scholar]
- Ide T, Uchida K, Kagawa Y, et al. Pathological and immunohistochemical features of subdural histiocytic sarcomas in 15 dogs. Journal of Veterinary Diagnostic Investigation. 2011;23:127–132. doi: 10.1177/104063871102300123. [DOI] [PubMed] [Google Scholar]
- Kagawa Y, Nakano Y, Kobayashi T, et al. Localized pulmonary histiocytic sarcomas in Pembroke Welsh Corgi. Journal of Veterinary Medical Science. 2015;77:1659–1661. doi: 10.1292/jvms.15-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerlin RL, Hendrick ML. Malignant fibrous histiocytoma and malignant histiocytosis in the dog – convergent or divergent phenotypic differentiation? Veterinary Pathology. 1996;33:713–716. doi: 10.1177/030098589603300614. [DOI] [PubMed] [Google Scholar]
- Klahn SL, Kitchell BE, Dervisis NG. Evaluation and comparison of outcomes in dogs with periarticular and nonperiarticular histiocytic sarcoma. Journal of American Veterinary Medical Association. 2011;239:90–96. doi: 10.2460/javma.239.1.90. [DOI] [PubMed] [Google Scholar]
- van Kuijk L, van Ginkel K, de Vos JP, et al. Peri-articular histiocytic sarcoma and previous joint disease in Bernese mountain dogs. Journal of Veterinary Internal Medicine. 2013;27:293–299. doi: 10.1111/jvim.12059. [DOI] [PubMed] [Google Scholar]
- Mariani CL, Jennings MK, Olby NJ, et al. Histiocytic sarcoma with central nervous system involvement in dogs: 19 cases (2006-2012) Journal of Veterinary Internal Medicine. 2015;29:607–613. doi: 10.1111/jvim.12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore PF, Rosin A. Malignant histiocytosis of Bernese mountain dogs. Veterinary Pathology. 1986;23:1–10. doi: 10.1177/030098588602300101. [DOI] [PubMed] [Google Scholar]
- Nielsen L, Andreasen SN, Andersen SD, et al. Malignant histiocytosis and other causes of death in Bernese mountain dogs in Denmark. Veterinary Record. 2010;166:199–202. doi: 10.1136/vr.b4756. [DOI] [PubMed] [Google Scholar]
- Padgett GA, Madewell BR, Keller ET, et al. Inheritance of histiocytosis in Bernese mountain dogs. Journal of Small Animal Practice. 1995;36:93–98. doi: 10.1111/j.1748-5827.1995.tb02838.x. [DOI] [PubMed] [Google Scholar]
- Parker HG. Genomic analyses of modern dog breeds. Mammalian Genome. 2012;23:19–27. doi: 10.1007/s00335-011-9387-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R Foundation for Statistical Computing; Vienna, Austria: 2014. R: A Language and Environment for Statistical Computing. http://www.R-project.org/ [Google Scholar]
- Ramos-Vara JA, Webster JD, DuSold D, et al. Immunohistochemical evaluation of the effects of paraffin section storage on biomarker stability. Veterinary Pathology. 2014;51:102–109. doi: 10.1177/0300985813476067. [DOI] [PubMed] [Google Scholar]
- Rassnick KM, Moore AS, Russell DS, et al. Phase II, open-label trial of single-agent CCNU in dogs with previously untreated histiocytic sarcoma. Journal of Veterinary Internal Medicine. 2010;24:1528–1531. doi: 10.1111/j.1939-1676.2010.0588.x. [DOI] [PubMed] [Google Scholar]
- Rosin A, Moore P, Dubielzig R. Malignant histiocytosis in Bernese mountain dogs. Journal of American Veterinary Medical Association. 1986;188:1041–1045. [PubMed] [Google Scholar]
- Schultz RM, Puchalski SM, Kent M, et al. Skeletal lesions of histiocytic sarcoma in nineteen dogs. Veterinary Radiology & Ultrasound. 2007;48:539–543. doi: 10.1111/j.1740-8261.2007.00292.x. [DOI] [PubMed] [Google Scholar]
- Skorupski KA, Clifford CA, Paoloni MC, et al. CCNU for the treatment of dogs with histiocytic sarcoma. Journal of Veterinary Internal Medicine. 2007;21:121–126. doi: 10.1892/0891-6640(2007)21[121:cfttod]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Skorupski KA, Rodriguez CO, Krick EL, et al. Long-term survival in dogs with localized histiocytic sarcoma treated with CCNU as an adjuvant to local therapy. Veterinary and Comparative Oncology. 2009;7:139–144. doi: 10.1111/j.1476-5829.2009.00186.x. [DOI] [PubMed] [Google Scholar]
- Streitberger K, Schweizer M, Kropatsch R, et al. Rapid genetic diversification within dog breeds as evidenced by a case study on schnauzers. Animal Genetics. 2012;43:577–586. doi: 10.1111/j.1365-2052.2011.02300.x. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Tomiyasu H, Hotta E, et al. Clinical characteristics and prognostic factors in dogs with histiocytic sarcomas in Japan. Journal of Veterinary Medical Science. 2014;76:661–666. doi: 10.1292/jvms.13-0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S, Sutherland-Smith J, Burgess K, et al. Imaging characteristics of intrathoracic histiocytic sarcoma in dogs. Veterinary Radiology & Ultrasound. 2012;53:21–27. doi: 10.1111/j.1740-8261.2011.01863.x. [DOI] [PubMed] [Google Scholar]
- Wiley J, Walton R, Kennedy D, et al. Proceedings of the Veterinary Cancer Society. Austin, TX, USA: 2009. Oct 15 to 17, Comparison of cytology, flow cytometry using CD18 and histopathology with immunohistochemistry (CD18) for the diagnosis of canine histiocytic sarcoma; p. 28. [Google Scholar]
- Wouda RM, Miller ME, Chon E, et al. Clinical effects of vinorelbine administration in the management of various malignant tumor types in dogs: 58 cases (1997-2012) Journal of the American Veterinary Medical Association. 2015;246:1230–1237. doi: 10.2460/javma.246.11.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]