Abstract
BACKGROUND
The intraoperative evaluation of axillary sentinel lymph nodes (SLNs) allows the surgeon to complete axillary dissection in 1 setting at the time of the primary breast surgery. However, to the authors’ knowledge, there is no consensus regarding the optimal method for intraoperative evaluation of SLNs in breast cancer. The authors of this report prospectively compared touch imprint (TI) cytology with frozen section (FS) analysis and rapid cytokeratin immunostaining (RCI) of SLNs for the intraoperative evaluation of disease and compared the results with final pathologic examination (FP).
METHODS
Patients with invasive breast carcinoma who were diagnosed with lymph node-negative disease (based on preoperative clinical and sonographic evaluation with or without fine-needle aspiration of the indeterminate lymph nodes) and who subsequently were scheduled for lymphatic mapping were eligible to participate in this prospective protocol. TI and FS analysis were performed on all SLNs, and the lymph nodes were stained by the hematoxylin and eosin (H&E) method. RCI was performed using the enhanced polymer 1-step cytokeratin method. The results of TI, FS, RCI, TI plus FS, and FS plus RCI were compared with the results from FP, including 1 H&E stain and cytokeratin immunostain of the third level.
RESULTS
One hundred patients with invasive mammary carcinoma were accrued to the study. Eighty-five tumors were the ductal type, 8 tumors were lobular, 5 tumors were mixed ductal and lobular, 1 was an adenoid cystic tumor, and 1 tumor was metaplastic carcinoma. Seventy-two tumors were staged clinically as T1N0M0, 25 tumors were staged as T2N0M0, and 3 tumors were staged as T3N0M0. Metastatic carcinoma was detected in the SLNs by 1 or more methods, including TI, FS, RCI, and FP, in 20 tumors, which included 12 macrometastases and 8 micrometastases. TI detected 8 of 12 macrometastases (67%), FS detected 12 of 12 macrometastases (100%), RCI detected 12 of 12 macrometastases (100%), and FP detected 12 of 12 macrometastases (100%). TI detected 1 of 8 micrometastases (13%), FS detected 3 of 8 micrometastases (38%), RCI detected 4 of 8 micrometastases (50%), and FP detected 6 of 8 micrometastases (75%). The sensitivities of TI, FS, RCI, TI plus FS, and FS plus RCI (with FP as the gold standard) were 50%, 72%, 78%, and 83%, respectively, and the sensitivities of the same intraoperative methods were 45%, 75%, 80%, and 85%, respectively, with detection of metastatic disease by any method as the gold standard. The specificities of the different methods (with FP as the gold standard) were 100% for TI and 97.5% for FS, RCI, TI plus FS, and FS plus RCI. The specificity of each method was 100% when the detection of metastatic disease by any method was regarded as the gold standard. Although the difference in sensitivity between FS and TI was not statistically significant (P = .08), the difference between RCI and TI bordered on significance (P = .046); however, FS analysis plus RCI was significantly superior to TI (P = .03) and produced results comparable to those of FP.
CONCLUSIONS
The sensitivities of FS, RCI, TI plus FS, and FS plus RCI were better than the sensitivity of TI cytology of axillary SLNs. However, only the combination of FS and RCI was statistically superior to TI and generated results comparable to those of FP in SLNs. RCI can be completed within the time constraints for intraoperative use and, in conjunction with FS, can be useful for generating results closer to those generated by FP. FS analysis plus RCI have a role in the intraoperative evaluation of SLNs.
Keywords: breast cancer, final pathologic evaluation, intraoperative evaluation, sentinel lymph nodes
Sentinel lymph node (SLN) mapping is a routinely performed procedure for axillary staging in patients with early-stage breast cancer. SLNs are sensitive and specific predictors of the status of non-SLNs in breast cancer.1-3 Therefore, a thorough and focused examination of those lymph nodes with the highest probability for metastatic involvement is mandatory. Intraoperative evaluation of SLNs allows the surgeon to complete axillary dissection in 1 setting at the time of primary breast surgery, when they are positive for metastatic disease. Intraoperative evaluation can be done using imprint cytology, frozen section (FS) analysis, scrape cytology, or a combination of these methods; and each technique has advantages and disadvantages. However, the sensitivities of these conventionally used, intraoperative methods for detecting metastatic disease in SLNs is not fully equivalent to permanent histopathologic examination. Therefore, these tests can result in false-negative reporting of metastases to SLNs. Other currently available techniques, including rapid cytokeratin immunostaining (RCI) on FS and touch imprint (TI) and molecular methods, reportedly improve the sensitivity of intraoperative evaluation of SLNs in breast cancer.
We conducted a prospective study to evaluate the feasibility and utility of using RCI of FS analysis of SLNs for the intraoperative detection of metastatic tumors. We also compared the sensitivities and specificities of TI cytology with those of FS analysis alone, RCI alone, TI cytology combined with FS analysis, and FS analysis combined with RCI for the intraoperative evaluation of SLNs in breast cancer.
MATERIALS AND METHODS
This study was conducted at the M. D. Anderson Cancer Center after obtaining approval by the Institutional Review Board (LAB04-967). Informed consent was obtained from all patients. Patients who had a diagnosis of T1 through T3 invasive breast cancer with lymph node-negative disease and who may or may not have received neoadjuvant chemotherapy were considered for this study. Preoperative evaluation of axillary lymph node status was obtained by clinical examination and sonographic evaluation. Fine-needle aspiration (FNA) biopsy was performed on sonographically indeterminate lymph nodes to derive at a definite diagnosis. Patients who were regarded as negative for metastatic disease in the axilla by clinical examination or sonographic evaluation with or without FNA biopsy and who subsequently were scheduled to undergo lymphatic mapping and SLN biopsy were eligible for enrollment into this protocol. Patients with stage IV disease and those who otherwise were not eligible for lymphatic mapping were not eligible for accrual.
The SLNs were identified by using radiocolloid and/or isosulfan blue according to the surgeon’s preference. All identified SLNs were sent to pathology for a detailed gross and microscopic evaluation. SLNs received fresh in the FS suite were bisected if they measured <0.5 cm in greatest dimension and were sectioned at 2-mm intervals along the short axis if they measured >0.5 cm in greatest dimension. TIs were made of both surfaces of the lymph node sections, fixed in 95% alcohol, and stained by the hematoxylin and eosin (H&E) method. The results of the TIs alone were used in considering whether to complete axillary dissection at the time of primary breast surgery. In addition to the TIs, 2 frozen sections were prepared from the lymph sections, 1 for H&E staining and 1 for RCI. The results of FS and RCI were not used in the intraoperative decision-making process of whether to complete axillary dissection. TI cytology and FS of the SLNs were stained by H&E method.
The FS for RCI was fixed in 100% acetone for 20 seconds at room temperature and then air dried for 5 minutes. Then, the slides were rinsed with phosphate-buffered saline (PBS), treated with hydrogen peroxide for 5 minutes for blocking peroxidase in the tissue, and subsequently rinsed well with PBS. The slides then were incubated with 6 μL of enhanced polymer 1 step (EPOS) cytokeratin (DAKO, Carpinteria, Calif), incubated at 37°C for 7 minutes, and rinsed with distilled water. Finally, for evaluation of the targeted reaction and identification of the immunopositive cells in the tissue sections, the slides were incubated with diaminobenzidine tetrahydrochloride as the chromogen for 5 minutes at 37°C and rinsed with water. The slides were then counterstained with hematoxylin and coverslipped with Aquamount. Distinct cytoplasmic and/or membranous staining of the cells was regarded as a positive result. Frozen sections of breast tissue with invasive carcinoma were used as a positive control with each batch of immunostaining. The entire RCI procedure was completed in 25 minutes.
Patients with negative TI cytology waited until the final pathology result was received for further intervention. Final pathologic evaluation was performed on the formalin-fixed, paraffin-embedded tissue sections of the lymph node by H&E staining of the first section and pancytokeratin immunostaining of the third level of the tissue block.
Statistical Analysis
The sensitivities and specificities of TI, FS, RCI, TI plus FS, FS plus RCI, and permanent histopathologic examination with the corresponding 95% confidence intervals were determined for the detection of metastatic tumor in the SLNs. The sensitivities of TI, FS, TI plus FS, and FS plus RCI for the intraoperative detection of metastatic tumor, using the results of permanent section evaluation by H&E and cytokeratin immunostain as the gold standard, and taking all tests into consideration, were determined along with the corresponding 95% confidence intervals. TI was compared with FS, RCI, and FS plus RCI by using the McNemar test.
RESULTS
We accrued 100 patients with 297 SLNs in this prospective study for comparing TI, FS, and FS plus RCI in the intraoperative evaluation of SLNs from March 2005 to September 2006. The invasive carcinoma was diagnosed as ductal in 85 patients, lobular in 8 patients, ductal and lobular mixed in 5 patients, adenoid cystic carcinoma in 1 patient, and metaplastic carcinoma in 1 patient. The nuclear grade of the invasive tumor was modified Black nuclear grade 1 in 18 patients, grade 2 in 41 patients, and grade 3 in 41 patients. The tumors were staged preoperatively as T1N0M0 in 72 patients, T2N0M0 in 25 patients, and T3N0M0 in 3 patients.
Nineteen patients received preoperative neoadjuvant chemotherapy before they underwent the SLN mapping procedure. All patients underwent ultrasonography screening of the axillary basin in addition to routine clinical examination. Suspicious and indeterminate lymph nodes were selected for ultrasound-guided FNA biopsy at the discretion of the respective sonographer. Twenty-eight patients underwent ultrasound-guided FNA before the SLN surgery. All 28 patients were diagnosed as negative for metastatic carcinoma on conventional cytologic examination of the aspirated material.
The FS quality was satisfactory in all patients for histologic interpretation, although there were artifacts of freezing and cutting in some sections. RCI revealed weak staining of the dendritic cells, including the presence of dendritic processes emanating from the cells, in contrast to the strong cytoplasmic and membranous staining of the metastatic tumor cells. The morphologic features of these cells, in conjunction with the different staining patterns, were useful for the accurate interpretation of metastatic tumor. Figure 1 illustrates the difference in the staining patterns between the metastatic tumor cells (Fig. 1A) and the dendritic cells (Fig. 1B) in the SLN.
FIGURE 1.
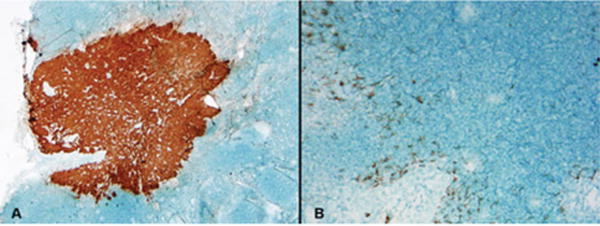
Rapid cytokeratin immunostaining of sentinel lymph nodes revealed strong cytoplasmic staining of the metastatic tumor cells (A) compared with the weak staining of dendritic reticulum cells (B). Note also the presence of dendritic processes emanating from the latter cells.
Metastases were noted in 20 patients by 1 of the 3 methods (intraoperative TI, FS, or RCI and permanent histopathologic examination). The metastases were classified according to the revised American Joint Committee on Cancer classification (sixth edition) as macrometastases when they measured >2 mm in greatest dimension and as micrometastases when they measured >0.2 mm but <2 mm in greatest dimension. Two metastases with isolated tumor cells that measured <0.2 mm were excluded in comparisons of the effectiveness of the different intraoperative methods. The metastatic tumors in the SLNs were classified as macrometastases in 12 of 20 patients and as micrometastases 8 of 20 patients. TI detected 8 macrometastases and only 1 micrometastasis. FS detected all 12 macrometastases but only 3 of 8 micrometastases. RCI detected all 12 of the macrometastases and 4 of the 8 micrometastases. Permanent histopathologic examination, including H&E and cytokeratin immunostaining of the third level, revealed macrometastases in 12 patients and micrometastases in 6 of the total 8 patients who had micrometastasis in the study. The results of the 3 intraoperative methods and permanent histopathologic examination with respect to the detection of macrometastases and micrometastases are illustrated in Table 1. Figure 2 is an illustration of a lymph node that was characterized as negative according to TI, RCI, and permanent histopathologic examination but positive according to FS analysis; and Figure 2B illustrates a lymph node that was characterized as positive according to RCI alone.
Table 1.
Detection of Metastatic Tumor in the Axillary Sentinel Lymph Nodes by Different Methods
| Method | Macrometastases, n = 12 (%) | Micrometastases, n = 8 (%) | Total (%) |
|---|---|---|---|
| TI | 8 (67) | 1 (13) | 9 (45) |
| FS | 12 (100) | 3 (38) | 15 (75) |
| RCI | 12 (100) | 4 (50) | 16 (80) |
| FP | 12 (100) | 6 (75) | 18 (90) |
TI indicates touch imprint; FS, frozen section; RCI, rapid cytokeratin immunostain, FP, final pathology examination.
FIGURE 2.
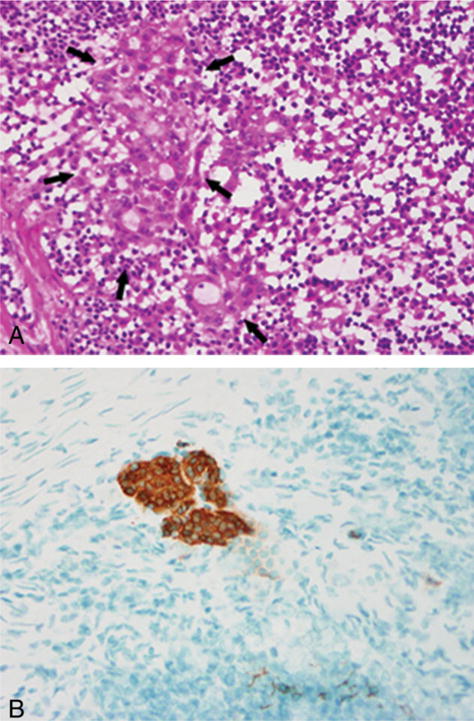
(A) This frozen section (FS) of a sentinel lymph node demonstrated the presence of a micrometastasis that was not noted in touch imprint, rapid cytokeratin immunostain of FS, or permanent histopathologic examination of the same lymph node. (B) Rapid cytokeratin immunostaining of a sentinel lymph node revealed a micrometastasis. This tumor deposit was not noted on touch imprint, frozen section, or permanent histopathologic examination of the same lymph node.
The sensitivity of TI with permanent histopathologic examination as the gold standard was 50%, and, taking all tests into consideration, it was 45%. Similarly, the sensitivities of FS, RCI, TI plus FS, and combined FS and RCI were 72%, 78%, 78%, and 83%, respectively, when considering permanent section evaluation as the gold standard, and 75%, 80%, 80%, and 85%, respectively, taking all tests into consideration. Although the specificity of TI was 100% in both calculations, FS, RCI, TI plus FS, and FS plus RCI produced a specificity of 97.5% when FP was used as the gold standard, because metastatic disease was detected in 2 tumors that were negative on permanent histopathologic examination. TI was compared with FS, RCI, and FS plus RCI by using the McNemar test. TI was not significantly different from FS alone (P = .08), and the significance of the difference between TI and RCI was borderline (P = .046); however, there was a statistically significant difference between TI and FS plus RCI (P = .03). Tables 2 and 3 present the sensitivities and specificities of each test alone, of TI plus FS, and of FS in combination with RCI and the corresponding 95% confidence intervals using permanent section evaluation and the combination of all tests as the gold standard.
Table 2.
Comparison of the Results of Different Intraoperative Methods With Final Pathologic Examination of the Sentinel Lymph Node
| Sensitivity | Specificity | |||
|---|---|---|---|---|
| Method | % | 95% CI, % | % | 95% CI, % |
| TI | 50 | 26–74 | 100 | 95.5–100 |
| FS | 72.2 | 46.5–90.3 | 97.5 | 91.3–99.7 |
| RCI | 77.8 | 52.4–93.6 | 97.5 | 91.3–99.7 |
| TI+FS | 77.8 | 52.4–93.6 | 97.5 | 91.3–99.7 |
| FS+RCI | 83.3 | 58.6–96.4 | 97.5 | 91.3–99.7 |
95% CI indicates 95% confidence interval; TI, touch imprint; FS, frozen section; RCI, rapid cytokeratin immunostain, FP, final pathology examination.
Table 3.
Effectiveness of Different Methods for Detecting Metastasis in Axillary Sentinel Lymph Nodes
| Sensitivity | Specificity | |||
|---|---|---|---|---|
| Method | % | 95% CI, % | % | 95% CI, % |
| TI | 45 | 23.1–68.5 | 100 | 95.4–100 |
| FS | 75 | 50.9–91.3 | 100 | 95.4–100 |
| RCI | 80 | 56.3–94.3 | 100 | 95.4–100 |
| FP | 90 | 68.3–98.8 | 100 | 95.4–100 |
| TI+FS | 80 | 56.3–94.3 | 100 | 95.4–100 |
| FS+RCI | 85 | 62.1–96.8 | 100 | 95.4–100 |
95% CI indicates 95% confidence interval; TI, touch imprint; FS, frozen section; RCI, rapid cytokeratin immunostain, FP, final pathology examination.
DISCUSSION
Our results with TI and FS for the intraoperative evaluation of SLNs in breast cancer in this prospective study are similar to the results from some previously reported studies. The 45% sensitivity of TI and the 75% sensitivity of FS with permanent histopathologic examination as the gold standard falls within the range of reported sensitivities: 33% to 96% for TI and 44% to 100% for FS.4-19 It is well recognized that variations in patient selection criteria and gross and permanent histopathologic examination largely account for the wide range of results from different studies. Our results, however, are comparable to the results of some studies in which the SLNs were sliced at 2-mm intervals and examined by H&E and cytokeratin immunostaining on permanent sections.
TI detected 67% of macrometastases and only 13% of micrometastasis, whereas FS detected 100% of macrometastases and 75% of micrometastases in our study. The lower sensitivity of TI in detecting micrometastasis and the higher false-negative rate compared with FS also have been addressed in the literature.4,6,9,11 The lowered sensitivity of TI usually is caused not by interpretive error but, rather, by sampling, in that the metastasis is uncovered after cutting through the tissue block. On direct comparison of TI and FS, although we observed that FS demonstrated higher sensitivity for detecting metastasis in general and micrometastasis in particular, the difference was not statistically significant. The majority of the previous studies that directly compared TI with FS analysis in SLNs arrived at similar conclusions.20-23 To our knowledge, to date, only Motomura et al have reported TI cytology to be better than FS analysis.23 Brogie et al reported that TI cytology and FS were comparable for detecting macrometastases and micrometastases in SLNs.5 Tew et al calculated the pooled sensitivity of 4 studies that directly compared TI with FS and observed an overall sensitivity of 62% for TI versus 76% for FS analysis.4
Few other studies have used RCI for the intraoperative evaluation of SLNs. Acceleration of the immunostaining procedure can be performed by different methods, such as using preformed antibody biotinperoxidase complexes, EPOS staining, or microwave heat treatment of the tissue after applying the antibody. The former 2 methods alone can be suitable for use on FS analysis. In the EPOS system, as in our study, primary antibodies and horseradish peroxidase were linked to a chemically inert polymer complex (dextran). This system offers a 1- step immunostaining procedure that can be accomplished easily with fast and reproducible results that are suitable for intraoperative use on either FS or TI analysis. Ever since the protocol for rapid immunostaining of FS first was published by Richter et al,24 few investigators have reported results from rapid immunostaining protocols on both TI and FS for the intraoperative evaluation of SLNs.25-30 The time taken for completion of the staining has varied from 8 minutes to 45 minutes in the reported studies. We standardized an RCI protocol on FS that can be completed in 20 to 25 minutes and, thus, is feasible for intraoperative use. We observed that RCI detected 100% of macrometastases and 50% of micrometastases with an overall sensitivity of 80% for detecting metastatic disease in SLNs. Therefore, although RCI was similar to FS analysis for detecting macrometastasis, it was slightly better than FS for detecting micrometastasis, because RCI detected 1 case with micrometastasis that was not detected on FS. All but 1 of the previous studies that used RCI indicated that RCI improved the sensitivity of TI and FS analysis to a variable extent and that they were particularly useful for detecting small-sized metastasis and lobular carcinomas. Although Aihara et al28 observed only a slight improvement in sensitivity with the combination of TI cytology and RCI (from 83% to 85%), other investigators, such as Johnston et al29 and Nahrig et al30 observed more than a marginal benefit of using RCI with TI or FS. In our study, RCI alone yielded a sensitivity of 80% compared with 75% for FS and 45% for TI alone.
When SLNs are sliced very thin, at 2-mm intervals, any of the currently used intraoperative methods, including TI or FS, can detect the majority of macrometastases. However, using FS and RCI can improve the detection of micrometastasis and can produce results comparable to the results produced by permanent section evaluation. In addition, compared with TI, both FS and RCI can allow measurement of the size of the metastasis and can distinguish isolated tumor cells from micrometastasis, which can be extremely useful in the intraoperative decision-making process regarding whether to complete axillary dissection. Completion of axillary dissection generally is recommended for micrometastasis alone and not for isolated tumor cells. RCI can be performed within 20 to 25 minutes, which may be acceptable to many surgeons in select (if not all) patients. The benefits of using RCI include a slight improvement in the detection of micrometastasis and, thus, the generation of results closer to those produced by permanent pathologic evaluation, as demonstrated in our study. This method certainly has a role to play in the confirmation of the presence or absence of metastatic carcinoma in select (if not all) patients who undergo SLN biopsy and particularly in patients who undergo plastic reconstructive surgery. The false-negative results of all intraoperative methodologies that we studied were associated with the ductal phenotype of invasive carcinoma; however, RCI can be particularly useful in avoiding false-negative results in patients with metastatic lobular carcinoma, as demonstrated by Weinberg et al,31 who tested the utility of RCI in the evaluation of TI cytology in patients with invasive lobular carcinoma and observed that the sensitivity increased from 41.9% to 54.8%, with an overall improvement of 12.9% in the detection of metastases.
Currently, molecular tests on automated platforms, such as the Gene Search BLN assay (Veridex, LLC, Warren, NJ) and the 1-step nucleic acid amplification assay (Sysmex, Japan), are available for intraoperative use and can provide sensitivity and specificity comparable to those provided by permanent histopathologic examination.32,33 The current study demonstrates that RCI in combination with FS also can produce results comparable to those produced by permanent section evaluation. Both of these methods, however, have advantages and disadvantages. In contrast to molecular tests, FS plus RCI can provide achievable proof of metastasis and allow measurement of the size of the metastatic tumor, which is an important determinant for the occurrence of metastasis in non-SLNs. However, molecular tests allow a thorough examination of the entire lymph node, enabling detection of all of the randomly distributed metastatic tumor that may be missed by limited histologic evaluation, including FS and RCI. Therefore future studies that integrate both methodologies with intraoperative FS and RCI followed by molecular testing for the intraoperative evaluation of SLNs in breast cancer should be considered seriously to achieve the benefits of both systems.
Although several studies have directly compared TI with FS, TI with RCI, and FS with RCI, to the best of our knowledge, no studies in the literature have systematically and simultaneously compared all techniques prospectively for the intraoperative detection of metastatic disease in axillary SLNs. In our study, the combination of FS and RCI was significantly better than TI for the intraoperative evaluation of SLNs, with results comparable to those produced by permanent histopathologic examination, including H&E and pancytokeratin immunostaining.
Acknowledgments
FUNDING STATEMENT:
This article was supported by NIH. The grant number is P30 CA016672.
We thank Kristine Broglio of the Biostatistics Department for her statistical input and Laura M. Pantoja, RN for consenting patients in the study.
Footnotes
Conflict of Interest Disclosures
The University of Texas M. D. Anderson Cancer Center Office of Clinical Research funded this study.
References
- 1.Guiliano AE, Dale PS, Turner RR, et al. Improved axillary staging of breast cancer with sentinel lymphadenectomy. Ann Surg. 1995;222:394–399. doi: 10.1097/00000658-199509000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Veronesi U, Paganelli G, Galimberti V, et al. Sentinelnode biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet. 1997;349:1864–1867. doi: 10.1016/S0140-6736(97)01004-0. [DOI] [PubMed] [Google Scholar]
- 3.Veronesi U, Paganelli G, Viale G, et al. Sentinel lymph node biopsy and axillary dissection in breast cancer: results in a large series. J Natl Cancer Inst. 1999;91:368–373. doi: 10.1093/jnci/91.4.368. [DOI] [PubMed] [Google Scholar]
- 4.Tew K, Irwig L, Matthews A, et al. Meta-analysis of sentinel lymph imprint cytology in breast cancer. Br J Surg. 2005;92:1068–1080. doi: 10.1002/bjs.5139. [DOI] [PubMed] [Google Scholar]
- 5.Brogi E, Torres-Matundan E, Tan LK, et al. The results of frozen section, touch preparation, and cytological smear are comparable for intraoperative examination of sentinel lymph nodes: a study in 133 breast cancer patients. Ann Surg Oncol. 2005;12:173–180. doi: 10.1245/ASO.2005.03.067. [DOI] [PubMed] [Google Scholar]
- 6.Pugliese MS, Tickman R, Wang NP, et al. The utility of intraoperative evaluation of sentinel lymph nodes in breast cancer. Ann Surg Oncol. 2007;14:1024–1030. doi: 10.1245/s10434-006-9270-y. [DOI] [PubMed] [Google Scholar]
- 7.Pugliese MS, Kohr JR, Allison KH, et al. Accuracy of intraoperative imprint cytology of sentinel lymph nodes in breast cancer. Am J Surg. 2006;192:516–519. doi: 10.1016/j.amjsurg.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 8.Tamiolakis D, Papadopoulos N, Venizelos J, et al. Intraoperative touch imprint cytological analysis of sentinel lymph nodes for the presence of metastases in breast cancer. Onkologie. 2007;29:372–375. doi: 10.1159/000094409. [DOI] [PubMed] [Google Scholar]
- 9.Cox C, Centeno B, Dickson D, Clark J, et al. Accuracy of intraoperative imprint cytology for sentinel lymph node evaluation in the treatment of breast carcinoma, a 6 year study. Cancer. 2005;105:13–20. doi: 10.1002/cncr.20738. [DOI] [PubMed] [Google Scholar]
- 10.Litz CE, Beitsch PD, Roberts CA, et al. Intraoperative cytologic diagnosis of 10. breast sentinel lymph nodes in the routine nonacademic setting: a highly specific test with limited sensitivity. Breast J. 2004;10:383–387. doi: 10.1111/j.1075-122X.2004.21381.x. [DOI] [PubMed] [Google Scholar]
- 11.Dabbs DJ, Fung M, Johnson R. Intraoperative cytologic examination of breast sentinel lymph nodes: test utility and patient impact. Breast J. 2004;10:190–194. doi: 10.1111/j.1075-122X.2004.21313.x. [DOI] [PubMed] [Google Scholar]
- 12.Pogacnik A, Klopcic V, Grazio-Frkovics, et al. The reliability and accuracy of intraoperative imprint cytology of sentinel lymph nodes in breast cancer. Cytopathology. 2005;16:71–76. doi: 10.1111/j.1365-2303.2004.00212.x. [DOI] [PubMed] [Google Scholar]
- 13.Chao C, Wong SL, Ackermann D, et al. Utility of intraoperative frozen section analysis of sentinel lymph nodes in breast cancer. Am J Surg. 2001;182:609–615. doi: 10.1016/s0002-9610(01)00794-2. [DOI] [PubMed] [Google Scholar]
- 14.Tanis PJ, Boom RP, Koops HS, et al. Frozen section investigation of the sentinel node in malignant melanoma and breast cancer. Ann Surg Oncol. 2001;8:222–226. doi: 10.1007/s10434-001-0222-2. [DOI] [PubMed] [Google Scholar]
- 15.Weiser MR, Montgomery LL, Susnik B, Tan LK, Borgen PI, Cody HS. Is routine intraoperative frozen-section examination of sentinel nodes in breast cancer worthwhile? Ann Surg Oncol. 2000;7:651–655. doi: 10.1007/s10434-000-0651-3. [DOI] [PubMed] [Google Scholar]
- 16.Rahusen FD, Pijpers R, Van Diest PJ, et al. The implementation of the sentinel node biopsy as a routine procedure for patients with breast cancer. Surgery. 2000;128:6–12. doi: 10.1067/msy.2000.107229. [DOI] [PubMed] [Google Scholar]
- 17.Noguchi M, Bando E, Tsugawa K, et al. Staging efficiency of breast cancer with sentinel lymphadenectomy. Breast Caner Res Treat. 1999;57:221–229. doi: 10.1023/a:1006268426526. [DOI] [PubMed] [Google Scholar]
- 18.Wada N, Imoto S, Hasebe T, et al. Evaluation of intraoperative frozen section diagnosis of sentinel lymph nodes in breast cancer. Jpn J Clin Oncol. 2004;34:113–117. doi: 10.1093/jjco/hyh023. [DOI] [PubMed] [Google Scholar]
- 19.Leidenius MH, Krogerus LA, Toivonen TS, et al. The feasibility of intraoperative diagnosis of sentinel lymph node metastases in breast cancer. J Surg Oncol. 2003;84:68–73. doi: 10.1002/jso.10296. [DOI] [PubMed] [Google Scholar]
- 20.Beach RA, Lawson D, Waldrop SM, et al. Rapid immunohistochemistry for cytokeratin in the intraoperative evaluation of sentinel lymph nodes for metastatic breast carcinoma. Appl Immunohistochem Mol Morphol. 2003;11:45–50. doi: 10.1097/00129039-200303000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Menes TS, Tartter PI, Mizrachi H, et al. Touch preparation or frozen section of sentinel lymph node metastases from breast cancer. Ann Surg Oncol. 2003;10:1166–1170. doi: 10.1245/aso.2003.04.023. [DOI] [PubMed] [Google Scholar]
- 22.Van Diest PJ, Torrenga H, Borgstein PJ, et al. Reliability of intraoperative frozen section and imprint cytological investigation of sentinel lymph nodes in breast cancer. Histopathology. 1999;35:14–18. doi: 10.1046/j.1365-2559.1999.00667.x. [DOI] [PubMed] [Google Scholar]
- 23.Motomura K, Inaji H, Komoike Y, et al. Intraoperative sentinel lymph node examination by imprint cytology and frozen sectioning during breast surgery. Br J Surg. 2003;87:597–601. doi: 10.1046/j.1365-2168.2000.01423.x. [DOI] [PubMed] [Google Scholar]
- 24.Richter T, Nahrig J, Komminoth P, et al. Protocol for ultrarapid immunostaining of frozen sections. J Clin Pathol. 1999;52:461–463. doi: 10.1136/jcp.52.6.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salem AA, Douglas-Jones AG, Sweetland HM, et al. Intraoperative evaluation of axillary sentinel lymph nodes using touch imprint cytology and immunohistochemistry: I. Protocol of rapid immunostaining of touch imprints. Eur J Surg Oncol. 2003;29:25–28. doi: 10.1053/ejso.2002.1347. [DOI] [PubMed] [Google Scholar]
- 26.Salem AA, Douglas-Jones AG, Sweetland HM, et al. Intra- operative evaluation of axillary sentinel lymph nodes using touch imprint cytology and immunohistochemistry. Part II. Results. Eur J Surg Oncol. 2006;32:484–487. doi: 10.1016/j.ejso.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 27.Khalifa K, Pereira B, Thomas VA, et al. The accuracy of intraoperative frozen section analysis of the sentinel lymph nodes during breast cancer surgery. Int J Fertil Womens Med. 2004;49:208–211. [PubMed] [Google Scholar]
- 28.Aihara T, Munakata S, Morino H, et al. Touch imprint cytology and immunohistochemistry for the assessment of sentinel lymph nodes in patients with breast cancer. Eur J Surg Oncol. 2003;29:845–848. doi: 10.1016/j.ejso.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Johnston EI, Beach RA, Waldrop SM, et al. Rapid intraoperative immunohistochemical evaluation of sentinel lymph nodes for metastatic breast carcinoma. Appl Immunohistochem Mol Morphol. 2006;14:57–62. doi: 10.1097/01.pai.0000153722.21155.5f. [DOI] [PubMed] [Google Scholar]
- 30.Nahrig JM, Richter T, Kuhn W, et al. Intraoperative examination of sentinel lymph nodes by ultrarapid immunohistochemistry. Breast J. 2003;9:277–281. doi: 10.1046/j.1524-4741.2003.09405.x. [DOI] [PubMed] [Google Scholar]
- 31.Weinberg ES, Dickson D, White L, et al. Cytokeratin staining for intraoperative evaluation of sentinel lymph nodes in patients with invasive lobular carcinoma. Am J Surg. 2004;188:419–422. doi: 10.1016/j.amjsurg.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 32.Blumencranz P, Whitworth PW, Deck K, et al. Sentinel node staging for breast cancer: intra operative molecular pathology overcomes conventional histologic sampling errors. Am J Surg. 2007;194:426–432. doi: 10.1016/j.amjsurg.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 33.Tsujimoto M, Nakabayashi K, Yoshidome K, et al. One step nucleic acid amplification for intraoperative detection of lymph node metastasis in breast cancer patients. Clin Cancer Res. 2007;13:4807–4816. doi: 10.1158/1078-0432.CCR-06-2512. [DOI] [PubMed] [Google Scholar]


