Abstract
The mu opioid receptor (MOR) and metabotropic glutamate receptor 5 (mGluR5) are well-established pharmacological targets in the management of chronic pain. Both receptors are expressed in spinal cord. MMG22, a bivalent ligand containing two pharmacophores separated by 22 atoms that simultaneously activates MOR and antagonizes mGluR5 has been shown to produce potent reversal of tactile hypersensitivity in rodent models of LPS- and bone-cancer-induced chronic pain. The present study assessed whether intrathecal MMG22 also is effective in reducing pain of neuropathic origin. Further, we theorized that MMG22 should reduce hyperalgesia in nerve-injured mice in a manner consistent with a synergistic interaction between MOR and mGluR5. Several weeks following spared nerve injury, tactile hypersensitivity was reversed in mice by intrathecal injection of MMG22 (0.01–10 nmol) but also by its shorter spacer analog, MMG10, with similar potency. The potencies of the bivalent ligands were 10-14-fold higher than those of the compounds upon which the bivalent structure was based, the MOR agonist oxymorphone and the mGluR5 antagonist MPEP. Co-administration of oxymorphone and MPEP demonstrated analgesic synergism, an interaction confirmed by isobolographic analysis. The present study indicates that in the spared nerve-injury induced model of neuropathic pain, the two pharmacophores of the bivalent ligands MMG22 and MMG10 target MOR and mGluR5 as separate receptor monomers. The observed increase in potency of MMG22 and MMG10, compared to oxymorphone and MPEP, may reflect the synergistic interaction of the two pharmacophores of the bivalent ligand acting at their respective separate receptor monomers.
Keywords: Bivalent ligand, chronic pain, MOR, mGlur5, neuropathic pain, MPEP
Introduction
The escalation in opioid-induced mortality has highlighted the urgency for development of new strategies for reducing opioid dose requirements and/or novel approaches for chronic pain management [31]. Among a wide range of recent therapeutic targets, a prominent line of development has been the pursuit of antagonists to the mGluR5 receptors, which are known to drive glutamate signaling under conditions of chronic pain. The gold standard mGluR5 antagonist, 6-methyl-2-(phenylethynyl)-pyridine (MPEP) has been shown to reduce manifestations of thermal and tactile hypersensitivity arising from various inflammatory and neuropathic pre-clinical pain models[68]. Further, MPEP has been shown to potentiate the effects of morphine [43; 46], which is suggestive of a positive drug-drug interaction. However, to our knowledge, the combination of MPEP with opioid analgesic agonists has not been previously assessed through isobolographic analysis for synergistic interactions.
Combination drug therapy offers the opportunity to substantially decrease the effective dose for each drug, thereby reducing the total drug requirement for sufficient pain relief [13]. Synergistic interactions have been observed for drugs of many different classes [12; 21; 29; 41; 45] in rodent models of neuropathic pain. The mechanisms by which drugs of distinct classes combine to produce analgesic synergism may involve co-activation of two receptors in different cellular compartments (e.g. pre- and post-synaptic) or co-activation in the same compartment with amplification at the level of downstream signal transduction. Receptors that reside in the same compartment may have the opportunity to physically associate and form heteromeric receptor complexes. Opioid receptors are known to form receptor heteromers with a variety of signaling receptors that offer altered pharmacological properties of opioid ligands following heteromer formation [15]. While there are multiple mechanisms underlying analgesic synergism arising from co-activation of opioid receptors and other receptor classes, heteromer activation may also contribute to the effect.
Targeting putative and known receptor heteromers through bivalent drug design offers an alternative approach to combination therapy for improving the effectiveness of opioid analgesics in the treatment of chronic pain [1; 7; 19; 24; 30; 36]. Receptor heteromers can display distinct pharmacological properties relative to the individual receptors [23; 50], rendering them innovative targets for the development of pain therapeutics. That mu opioid receptors (MORs) and mGluR5 receptors are both co-localized to post-synaptic spinal cord membranes, together with evidence suggesting assembly of MOR-mGluR5 heteromers in vitro[52], supports a proposal for spinal MOR-mGluR5 heteromers. The selective targeting of such heteromers could be effective for reducing chronic pain.
Akgün and colleagues [2] have previously proposed that a bivalent ligand (MMG22) containing both mGluR5 antagonist and MOR agonist pharmacophores could simultaneously antagonize the mGluR5 protomer and activate the MOR protomer of a putative spinal MOR-mGluR5 heteromer. MMG22 was found to produce profound antinociception without tolerance in mouse models of inflammatory pain [2] and bone cancer-induced pain [53]. Here we present data that MMG22 is antiallodynic in nerve injury-induced neuropathic pain, although likely through the targeting of single MOR and mGluR5 receptors rather than MOR-mGluR5 heteromers. Further, co-administration of the pharmacophores upon which the bivalent ligand is designed (oxymorphone and MPEP) demonstrates a synergistic interaction in neuropathic mice.
2. Methods
2.1 Animals
Male ICR-CD1 mice (21–24 g, Harlan) were housed with free access to food and water in a temperature- and humidity-controlled environment. They were housed 4 mice to a cage in a 12 hour light/dark cycle. All experiments were approved by the Institutional Animal Care and Use Committee of the University of Minnesota.
2.2 Chemicals and Reagents
The bivalent ligand MMG22 was synthesized according to previously described protocols[2]. Oxymorphone was from Mallinckrodt and Co (St. Louis, Missouri) and MPEP-HCl was from Tocris. MMG10, MMG22, and the monovalents M19 (oxymorphamine-based) and MG-20 (MPEP-based) and oxymorphamine[51] were synthesized by Dr. Portoghese’s laboratory. Antisera to mu opioid receptor was previously developed in-house.
2.3 Intrathecal injections
All drugs were dissolved in sterile saline and delivered in 5-µl volumes via intrathecal injection in conscious mice[32]. Briefly, the mice were held by the iliac crest and a 30-gauge, 0.5 inch needle attached to a 50-µL Luer-hub Hamilton syringe delivered 5 µL of injectate into the intrathecal space of the mice.
2.4 Spared nerve injury
Tactile hypersensitivity was induced using the spared nerve injury model described by Decosterd and Woolf [18]. Subjects are placed under isoflurane anesthesia and the left sciatic nerve is exposed, along with its three terminal branches. The common peroneal and tibial nerves were ligated with 5.0 silk suture. The nerves were sectioned 2 mm distal to the ligation site. The sural nerve remained uninjured.
2.5 Tactile Hypersensitivity
Mice were placed on a wire mesh grid under a glass enclosure and allowed to acclimate for 30 minutes before testing. Hypersensitivity was tested by using an electronic Von Frey device (Life Sciences, IITC). The tip of the stimulator was pressed to the plantar surface of both the left and the right hind paws with enough force to cause the mouse to withdraw its paw from the tip, typically with a flinching behavior. The amount of force required for the response was recorded in grams. Baseline responses were collected before spared nerve injury surgery. The %MPE was calculated via the following standard formula: [(Experimental Value– Control)/(Cutoff – Control] × 100. In these experiments, these categories corresponded to the following measurements: [(Post-Drug Threshold Value – Pre-Drug Threshold Value)/(Pre-Surgery Baseline Threshold Value – Pre-Drug Value)] × 100. For the experimental comparisons between bivalent analogs of differing spacer lengths, the experimenter was blinded to treatment.
2.6 Immunohistochemistry
Eight weeks following spared nerve ligation surgery, animals were deeply anesthetized (75 mg/kg ketamine, 5 mg/kg xylazine and 1 mg/kg acepromazine, i.m.) and fixed by vascular perfusion as previously described [48]. Spinal cords were removed and placed in 10% sucrose in phosphate-buffered saline overnight. IHC was performed on thaw-mounted cryostat sections (14um). Rabbit anti-mGluR5 (AB AB5675 Millipore [47] was used at a dilution of 1:1000 and guinea-pig anti-MOR antiserum (custom antibody as described previously) [48] was used at a dilution of 1:500 and visualized with Cy3 anti-rabbit and Alexa 488-conjugated secondary antisera (1:200, Jackson ImmunoResearch, West Grove, PA). Sections were imaged with Olympus FluoView FV1000 BX2 Upright Confocal.
2.7 Data analysis
Relative potencies calculated from the dose-response data and the isobolographic analysis for evaluating interactions were calculated as described by the method of Tallarida[56]. Briefly, to test for synergistic interactions, the ED50 values and the 95% confidence intervals of all dose-response curves were arranged around the ED50 value using the equation (ln(10)×A50)×(SE of log A50). Isobolographic analysis, used for evaluating synergistic interactions, necessitates this manipulation. When testing a drug-drug interaction for synergy, additivity, or subadditivity, a theoretical additive ED50 value is calculated for the combination based on the dose-response curves of each drug administered separately. This theoretical value is compared by a t-test (p<0.05) with the observed experimental ED50 values of the combination. These values are based on the total dose of both drugs. An interaction is considered to be synergistic if the observed ED50 value is significantly less (p<0.05) than the calculated theoretical additive ED50 value. Additivity is indicated with the theoretical and experimental ED50 values do not differ. A subadditive interaction is indicated if the observed ED50 value is significantly greater (p<0.05) than the calculated theoretical additive ED50 value [57]. The data were processed using the FlashCalc pharmacological statistics software developed by Dr. Michael Ossipov, University of Arizona-Tucson. Graphs were generated in Graphpad Prism, v. 6.0.
3. Results
3.1 Description of ligands
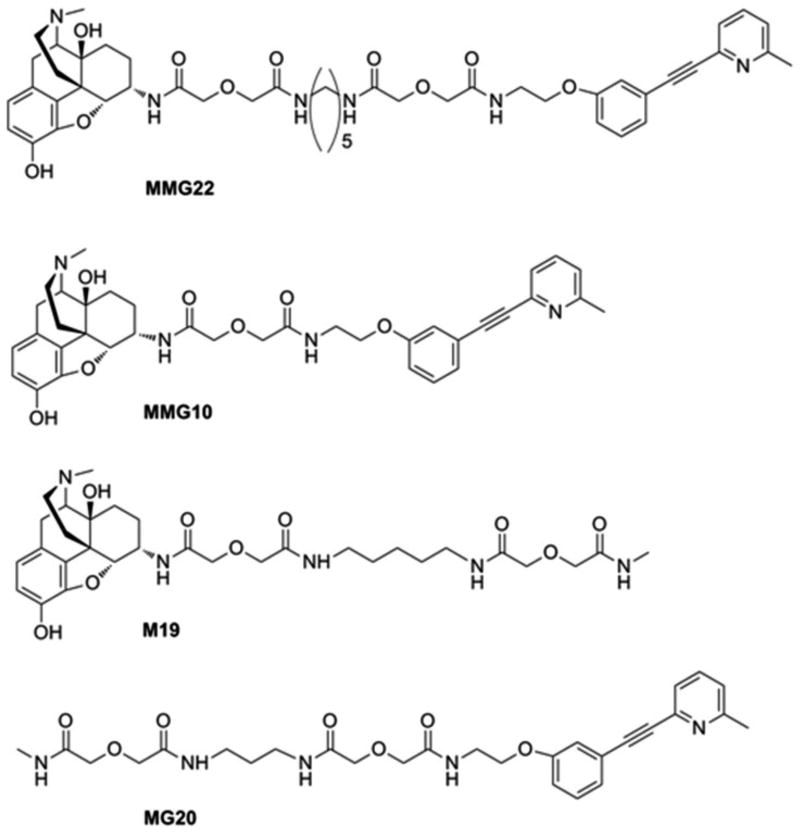
The pharmacophores of ligands were derived from the mu agonist oxymorphone[64], and the mGluR5 antagonist, methoxy-MPEP (M-MPEP)[3]. The phenoxy oxygen of M-MPEP served as the point attachment for a spacer that links the mGluR5 antagonist pharmacophore to the agonist pharmacophore. The methoxy substituent of M-MPEP was replaced by an ethoxyethylamine substituent without compromising the antagonist affinity for the respective receptor. The ligand MMG22 has a 22 atom spacer between the mu and mGluR5 pharmacophores while MMG10 has 10 atoms spacer between the pharmacophores. The monovalent M19 consist of the mu agonist pharmacophore attached to a 19 atom spacer; the monovalent MG20 consists of the mGluR5 pharmacophore attached to 20 atom spacer. These structures are displayed in Fig. 1.
Figure 1. Chemical structures.
The structures of the bivalent ligands MMG22, MMG10, the monovalent ligand M19 containing the opioid pharmacophore with a 19 atom spacer, and MPEP-20 containing the MPEP pharmacophore with a 20 atom spacer.
3.2 MMG22 reverses tactile hypersensitivity in neuropathic mice
Tactile hypersensitivity was induced by spared nerve injury and demonstrated by significantly reduced paw withdrawal thresholds on days 3, 7, and 17 after surgery. These times were selected to be representative of the induction, transition, and maintenance periods of chronic pain that follow spared nerve injury. Intrathecally delivered MMG22 dose-dependently reversed tactile hypersensitivity during the induction, transition, and maintenance periods of neuropathic pain (Fig. 2A, Table 1). The time-effect profiles of intrathecal MMG22 on each day post-injury are depicted in Fig. 2B-D. Intrathecal MMG22 (1, 100, 1,000 pmol) reversed tactile hypersensitivity at 5 and 12 minutes post-injection. However, tactile hypersensitivity returned by 20 minutes post-injection. Higher intrathecal doses of MMG22 (5, 10 nmol) demonstrated reversal of tactile hypersensitivity for a longer duration, out to 2 and 4 hours post-injection (Fig. 2E). Von Frey thresholds were at typical neuropathic levels by 6 hours post injection. In both sets of experiments, a vehicle control (saline) was also tested at these time points and showed no evidence of effects due to injection.
Figure 2. MMG22-Induced Reversal of Tactile Hypersensitivity in Neuropathic Pain.
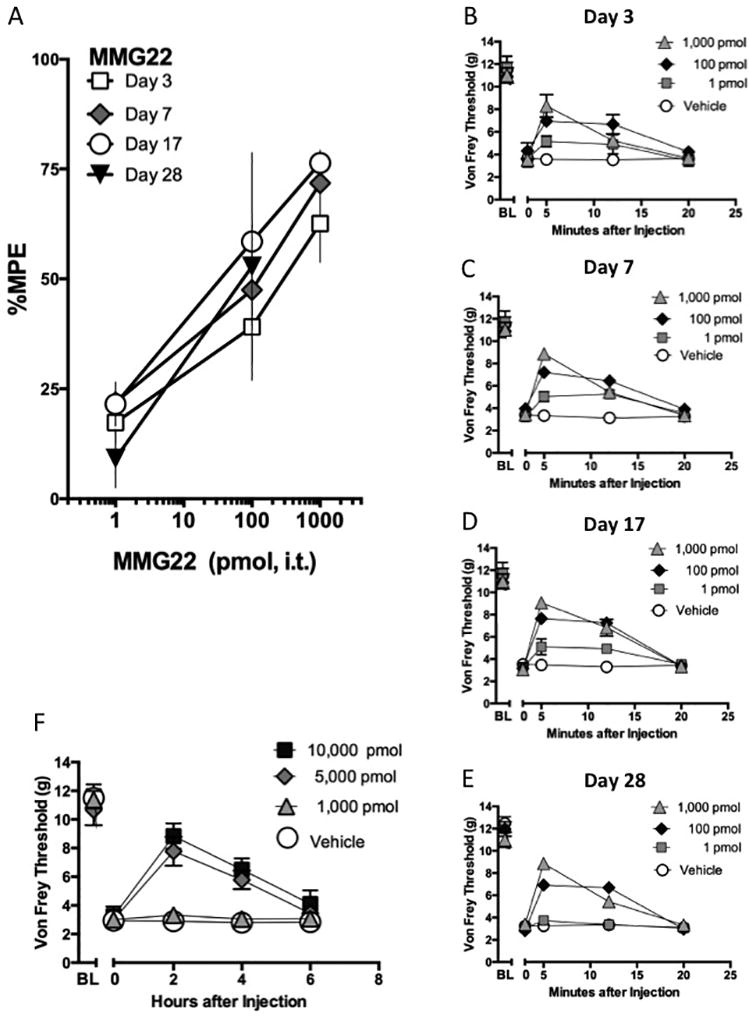
Spared nerve injury surgery was performed to induce tactile hypersensitivity. vF thresholds were measured on days 3, 7, 17, and dose-response curves to intrathecal MMG22 were constructed. (A) Dose-response curves to MMG22 (1, 100, 1000 pmol) were constructed on days 3, 7, and 17 post-surgery, n = 3–4 per dose group (B-D). The duration of action of each dose of MMG22 is shown at 5, 12, and 20 minutes post-injection of MMG22 on days 3 (B), 7 (C) and 17 (D), and 28 (E) post-injury. A saline-treated group was included as control. *Signifies significant differences of the 300 and 1000 pmol dose groups at the specified time points. p <0.05 one way ANOVA, Dunnett’s post-hoc test for comparisons to a control group (vehicle), n = 3–4 subjects per group for A-D, and 8 per group for E. (F) The duration of action of a higher dose range of MMG22 at 2, 4, and 6 hours post-injection of MMG22 on day 21 post-surgery. A saline-treated group was included as control. *Signifies significant differences of the 5000 and 10,000 pmol dose groups at the specified time points. p <0.05 one way ANOVA, Dunnett’s post-hoc test for comparisons to a control group (vehicle), n = 3–5 mice per group.
Table 1. ED50 values of low doses of MMG22.
The ED50 values were calculated from the dose-response curves in Figure 3A representing MMG22 dosing on days 3, 7, 17, and 28
| MMG22 Days Post-Injury |
ED50 value pmol, i.t. (95% C.L.) |
|---|---|
| Day 3 | 310 (20–4900) |
| Day 7 | 60 (19–410) |
| Day 17 | 16 (3.9–71) |
| Day 28 | 270 (121–618) |
3.3 Synergistic Interactions between Opioids and MPEP
The high analgesic potency of the MMG22 bivalent ligand may be explained by a synergistic interaction arising from concurrent activation of MOR with inhibition of the mGluR5 receptor. To test that proposal, we assessed the analgesic interactions of the two monovalent ligands from which the bivalent ligand is derived: the MOR agonist oxymorphone and the mGluR5 antagonist MPEP. Both ligands reverse tactile hypersensitivity in the low nmol range. Mice were intrathecally dosed with 1, 3, and 10 nmol of oxymorphone or MPEP separately and tested at 30 minute increments following each dose to generate a cumulative dose-response curve for each ligand. ED50 values were calculated and the relative potency between the two ligands was determined to be approximately 1.8. We, therefore, used a constant dose-ratio of 1:2 oxymorphone:MPEP (Table 2). A third group of mice was intrathecally dosed with a combination of a constant 1:2 dose ratio of oxymorphone: MPEP (0.1:0.2, 0.3:0.6 and 1:2 nmol). Comparison of the resultant combination dose-response curves (Fig. 3A) illustrates an approximate 8- and 7-fold increase in the potency of oxymorphone and MPEP, respectively, when given in the presence of the other (Table 2). These potency shifts are of a magnitude typical of synergistic interactions. The ED50 values and related confidence intervals were used to construct the associated isobologram (Fig. 3B), which features the significantly lower ED50 values of the observed combination point relative to the theoretical additive combination ED50 value expected were the interaction merely additive. All the dose-response data were analyzed by isobolographic analysis to determine the respective interaction. Statistical comparison (t-test) of the ED50 value of the observed combination to the theoretical additive ED50 value indicates that the interaction is synergistic (Table 2).
Table 2.
Summary of Opioid Agonist-mGluR5 Antagonist Interactions
| Probe Drug, Intrathecal | Opioid Agonist ED50 value (C.I) nmol per 5 µL |
mGluR5 antagonist ED50 value (C.I) nmol/ 5 µL |
Interaction |
|---|---|---|---|
| Oxymorphone + MPEP 1:2 Dose Ratio | |||
| Single Drug | 3.0 (1.8–4.1) | 5.3 (3.0–7.6) | |
| Observed Combination | 0.36 (0.18–0.54)* | 0.72 (0.37–1.1)* | Synergistic |
| Theoretical Additive | 1.4 (0.99–1.8) | 2.8 (2.0–3.6) | |
| Oxymorphamine + MPEP 1:1 Dose Ratio | |||
| Single Drug | 2.2 (1.4–3.0) | 3.1 (2.2–4.0) | |
| Observed Combination | 0.43 (0.26–0.60)* | 0.43 (0.26–0.60)* | Synergistic |
| Theoretical Additive | 1.3 (1.0–1.6) | 1.3 (1.0–1.6) |
Significant difference from theoretical additive by Student t test, p < 0.05
Figure 3. Opioid agonists and MPEP interact synergistically when given spinally to neuropathic mice.
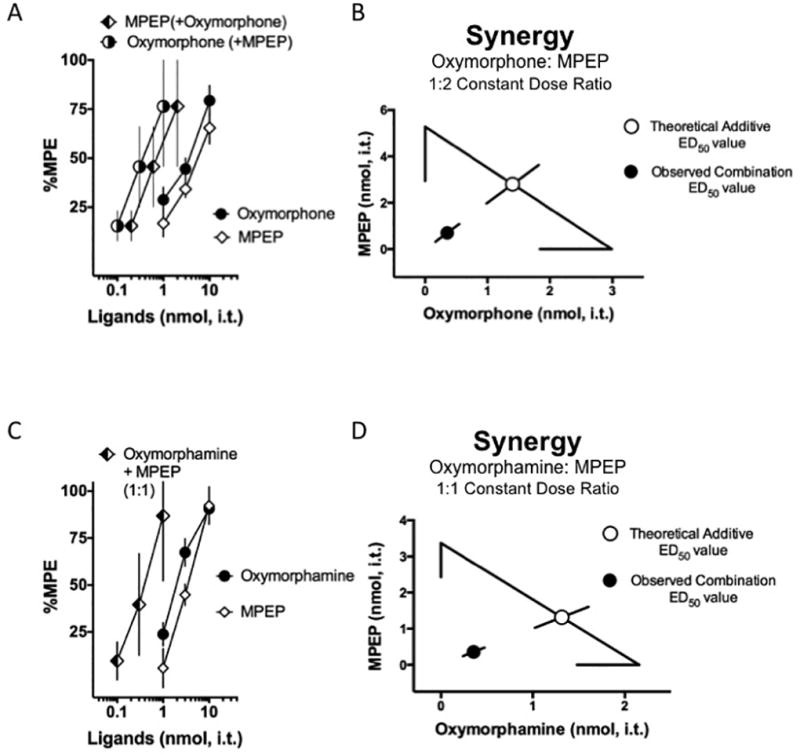
A. Oxymorphone (filled circles) and MPEP (white diamonds) inhibited tactile hypersensitivity in a dose-dependent manner. The ligands were then co-administered at a constant oxymorphone:MPEP dose ratio of 1:2 based on the potency ratio between agonists. Therefore, different doses of each drug are used in the combination. Note that the combination dose-response curves are plotted as the doses of oxymorphone used in the presence of MPEP (half-filled circles) and conversely MPEP in the presence of oxymorphone (half-filled diamonds); these two dose-response curves represent the same data. In order to visually compare each drug given by itself to the increase its potency when given as part of the combination, each drug in the combination is represented individually on the graph. Since there are different doses used in the low, medium, and high efficacy dose combinations for oxymorphone:MPEP, the display results in two separate dose-response curves for the combination, although it is delivered as one single combined drug administration at every point. The doses are separated on the graph to enable direct comparisons to the single ligand dose-response curves.
B. Isobolographic analysis applied to the data from Fig. 3A. The x-intercept represents the ED50 value for oxymorphone and the y-intercept represents the ED50 value for MPEP. The observed combination ED50 values (plotted as the filled circle) was significantly lower (p<0.05; Student’s t-test) than the theoretical additive ED50 value (plotted as the open circle) indicating that the interaction is synergistic in neuropathic mice. C. Oxymorphamine (filled circles) and MPEP (white diamonds) also inhibited tactile hypersensitivity in a dose-dependent manner. In the case of the oxymorphamine:MPEP combination the potencies of each ligand when given individually are comparable and, therefore, the ligands were co-administered (half-filled diamonds) at a constant oxymorphamine:MPEP dose ratio of 1:1. Note that the combination dose-response curves are plotted as the doses of oxymorphone used in the presence of MPEP and conversely MPEP in the presence of oxymorphone, however these are the same data. In other words, the same doses for each drug are given in the combination at the low, medium, and high efficacy points. Consequently the dose-response curve for oxymorphone and the dose-response curve for the MPEP appear on the graph as one single dose-response curve, consistent with the single combined administration at every point. Direct comparisons to the single ligand dose-response curves can be made from the single combination dose-response curve to either of the individual dose-response curves. D. Isobolographic analysis applied to the data from Fig. 3C. The x-intercept represents the ED50 value for oxymorphamine and the y-intercept represents the ED50 value for MPEP. The observed combination ED50 value (plotted as the filled circle) was significantly lower (p<0.05; t-test) than the theoretical additive ED50 value (plotted as the open circle) indicating that the interaction is synergistic in neuropathic mice. See Table 2 for all ED50 values. Group sizes ranged from 5–8 mice.
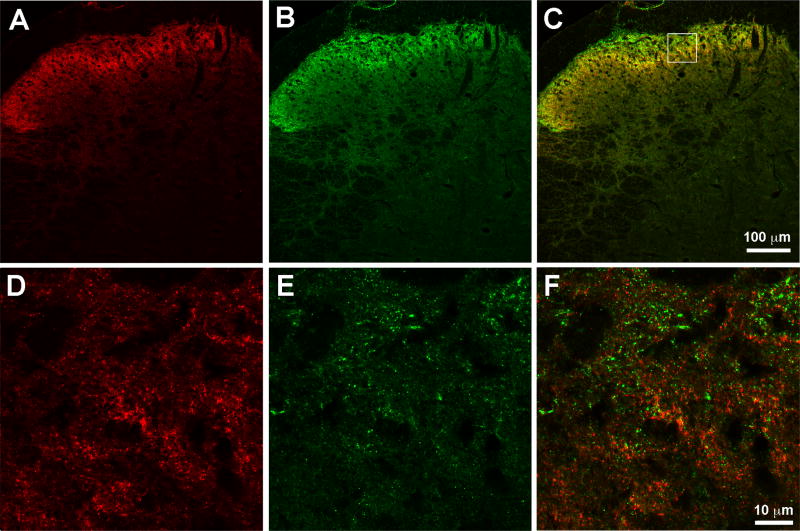
In order to attach the chemical spacers to the mu opioid pharmacophore, oxymorphone must be transformed to oxymorphamine. Therefore, we also conducted an analysis of the interaction between oxymorphamine and MPEP in the same manner as the parent pharmacophore, oxymorphone (Fig. 3C). We observed comparable potency to oxymorphone; the calculated potency ratio with MPEP was 1:1. Analysis was then conducted as previously described on these data to determine whether the effect of MPEP and oxymorphone given at a 1:1 ratio was synergistic, additive, or subadditive. This isobologram is presented in Fig. 3C. As in Fig. 3A, mice were intrathecally dosed with 1, 3, and 10 nmol of oxymorphamine or MPEP separately and tested at 30 minute intervals after each dose resulting in a cumulative dose-response curve for each ligand. The ED50 values were calculated and the relative potency between the two ligands was determined to be 1:4 We used a constant dose-ratio of 1:1 oxymorphamine:MPEP (Table 2). A separate group of mice were intrathecally dosed with the combination of oxymorphamine: MPEP in a constant dose-ratio of 1:1. In the case of oxymorphamine-MPEP, the combination dose-response curves reveal an approximate 5-fold increase in the potencies of oxymorphonamine and MPEP respectively when given in the presence of the other (Table 2). The corresponding isobologram (Fig. 3C) demonstrates the characteristic significantly lower ED50 values of the observed combination point relative to the theoretical additive combination ED50 value and isobolographic analysis confirmed that the interaction between oxymorphamine and MPEP is also synergistic (Table 2). To determine the anatomic relationship of the receptors in spinal cord we co-labeled spinal cord sections prepared from neuropathic mice. Immunoreactivity was observed for both MOR and mGluR5 in superficial dorsal horn as has been previously reported. Although there were instances of co-localization of mGluR5-ir and MOR-ir within the same puncta, the predominant relationship of MOR and mGluR5 was in close-apposition rather than co-localization. (Fig 4)
Figure 4. Mu opioid receptor and mGluR5 are differentially expressed in spinal cord.
Representative sections of mouse spinal cord perfused eight weeks after spared nerve-injury. A) MOR-ir, red B) mGluR5-ir, green C) merged image suggesting expression of the two receptors in different structures. Boxed image represents the section magnified in D-F. D) higher magnification of the MOR-ir, red E) higher magnification of mGluR5-ir, green E) higher magnification of the merged image of both receptors confirming predominantly differential expression of the two receptors.
3.4 Dose-response curves of the monovalent ligands M19 and MG20
Given the synergistic activity of the opioid agonists and MPEP monovalent compounds, it is possible that the chemical spacer is not important for the effect of the bivalent MMG22. To test this hypothesis we evaluated the effects of monovalent ligands M19, which is oxymorphamine with a 19-atom spacer but no MPEP pharmacophore, and MG20, a monovalent ligand with MPEP as the pharmacophore and a 20-atom spacer but no oxymorphamine pharmacophore. Seven days post-surgery, von Frey paw withdrawal thresholds were assessed to confirm induction of tactile hypersensitivity and the subjects were divided into three groups with equivalent responses. The individual groups received intrathecal doses (0.1, 1.0 and 10 nmol) of the oxymorphamine monovalent M19 and the MPEP monovalent, MG20 or a 1:1 combination of the monovalents. Mice were tested 30 minutes following administration and a cumulative dose-response curve was generated and represented in Fig. 5. Intrathecal M19 reversed tactile hypersensitivity with comparable potency (ED50 value 1.1 nmol, 0.45–2.9) to that of oxymorphone and oxymorphamine (Table 2). In contrast, MG20 had no effect. The 1:1 combination of M19 and MG20 yielded an analgesic dose-response curve with the ED50 value very similar to that of M19 when given alone (Fig. 4). Therefore, unlike the MPEP small molecule, the attachment of the spacer rendered the monovalent MG20 ineffective and unable to interact with M19 in the same manner demonstrated by the small molecules oxymorphone or oxymorphamine and MPEP. We speculate that the presence of the opioid pharmacophore constrains the spacer in a manner that enables the MPEP pharmacophore.
Figure 5. The MMG22 monovalents M19 and MG20 do not interact.
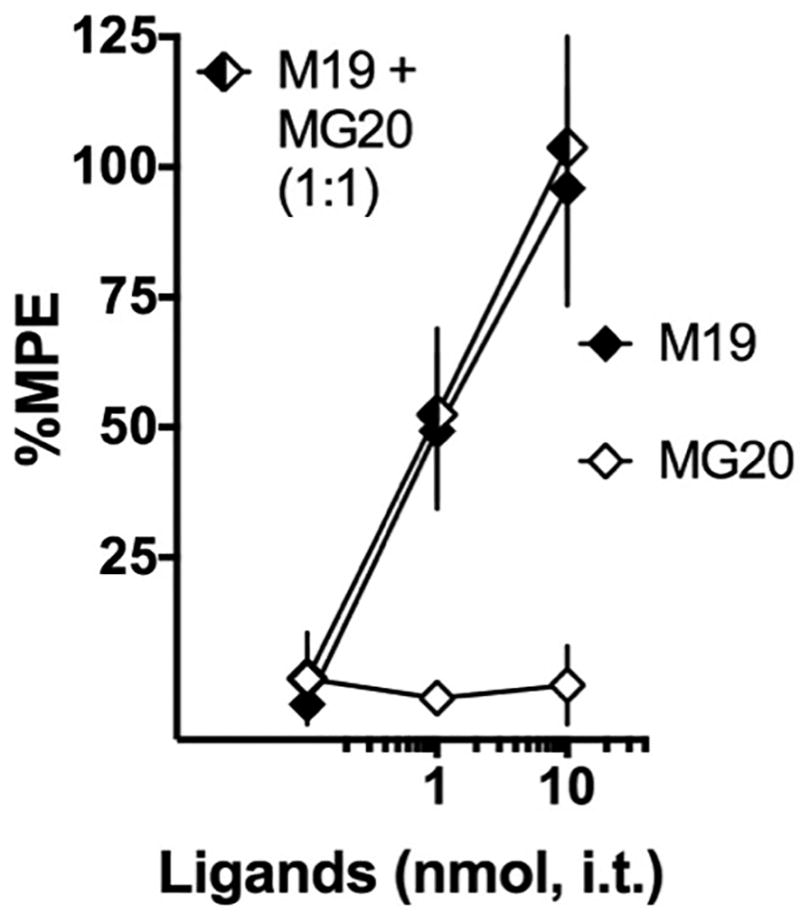
Monovalent ligands were generated for oxymorphamine (M19) and MPEP (MG20). The mu opioid monovalent M19 dose-dependently reversed tactile hypersensitivity (black diamonds, ED50 value 1.1 nmol, 0.45–2.9). However, the MPEP based monovalent MG20 had no effect (white diamonds). When co-administered, MG20 had no effect on the potency of M19 (half-filled diamonds, ED50 value 0.9 nmol, 0.5–1.5). n = 7 mice per group.
3.5 Comparison of Spacer Length on Effects of MMG22 and MMG10
It has been previously shown that the number of atoms that comprise the spacer length between the two pharmacophores can greatly impact the potency of bivalent ligands [16], including those with the oxymorphone and MPEP pharmacophores [53]. Therefore, we conducted a direct side-by-side comparison of dose-effect of MMG22 (spacer length of 22 atoms) and MMG10 (spacer length of 10 atoms). In contrast to previous reports in both LPS-induced inflammation[2] and cancer-induced pain [53], we observed that intrathecally delivered MMG10 reversed nerve injury-induced tactile hypersensitivity with comparable potency to MMG22 (Fig. 6); there was no significant difference between the ED50 values for the two compounds.
Figure 6. Potency comparison of MMG22 and MMG10.
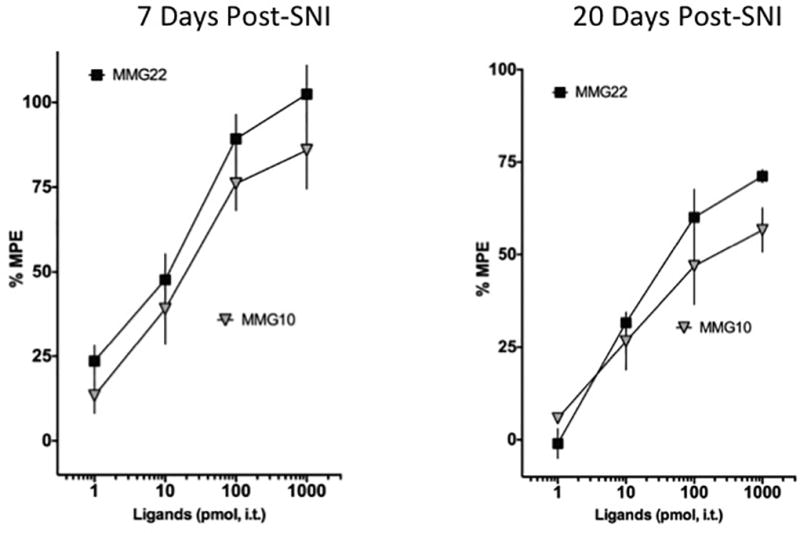
The dose-response curve of a bivalent ligand analog of MMG22 with 10 spacer atoms (MMG10) in reversing tactile hypersensitivity was directly compared in a side-by-side experiment with MMG22. The twenty-two atom spacer bivalent MMG22 dose-dependently reversed tactile hypersensitivity (black squares, ED50 value 8.6 pmol, 4.5–17, n = 11). Similarly, the ten atom spacer bivalent MMG10 reversed neuropathic pain behaviors with comparable potency (grey inverted triangles, ED50 value 23 pmol, 10–51, n =12).
4. Discussion
Chronic neuropathic pain is recognized as important public health challenge carrying significant burden for individuals and society [33]. The prevalence of neuropathic pain is estimated between 7–10% in the population [60]. New mechanism-driven pharmacological treatments [27] have targeted neuropathic pain, but with varying effectiveness [5] and side effect limitations. Support for providing appropriate opioid treatment to pain patients was recently reaffirmed in the National Pain Strategy [31]. However, the risk of addiction for some patients and the diversion to the general population diminishes support for providing opioid medication to non-cancer pain patients [31]. Consensus centers around strategies to optimize therapy while reducing risk, including lowering opioid doses, reducing the duration for opioid treatment, optimizing effective combinations, and identifying new drugs. Through the simultaneous targeting of MOR and mGluR5 via a single bivalent ligand MMG22, a new mechanism-driven combination treatment has emerged.
4.1 Role of mu opioid receptor in neuropathic pain
Varying viewpoints remain regarding the effectiveness of MOR analgesics in neuropathic pain. Arner and Meyerson [4] reported that opioids are less effective for the treatment of neuropathic pain and pre-clinical literature indicated reduced effectiveness of intrathecal morphine in neuropathic rats [9; 38]. Complementarily, MOR expression decreased in dorsal root ganglia following peripheral axotomy [66]. However, in the same pre-clinical studies, intraperitoneally or intracerebroventricularly delivered morphine demonstrated no reduction in opioid potency compared to control [9; 38]. Similarly, intrathecal morphine showed no difference in potency between neuropathic mice and controls [22]. Robust reduction of thermal and tactile hypersensitivity in neuropathic rats was also observed with either oral [17] or intraplantar morphine [42].
MOR agonist medications [35] are thought to be effective in human neuropathic pain when given in sufficient doses and/or in combination with adjuvants [61]. However recent meta-analyses [20; 26; 55] have concluded that the evidence for opioid effectiveness under conditions of neuropathic pain is either moderately supportive or inconclusive due to the broad heterogeneity of neuropathic conditions comprising the clinical trials. The emphasis has shifted to whether opioids should not be given for chronic non-malignant pain due to the risks of addiction and overdose. The neuropharmacology of opioid addiction was defined primarily by decades of pre-clinical studies of subjects with presumptive normal sensory thresholds [6]. The few studies of opioid responding in neuropathic pain subjects demonstrated that establishment of prescription opioid-maintained responding [39] or conditioned place preference [44] is significantly diminished compared to controls [63]. Consistent with those observations, dopamine levels in the ventral striatum are reduced in subjects with neuropathic pain [58]. Still, the increased prevalence of opioid addiction and opioid-induced mortality has resulted in a necessary reprioritization of treatment approaches, including opioid dose-reduction and expanded development of non-opioid analgesics.
4.2 Role of mGluR5 receptor in neuropathic pain
The contribution of mGluR5 to neuropathic pain is well established [25]. mGluR5 is localized to unmyelinated nociceptive peripheral sensory neurons [8] and post-synaptic neurons in spinal cord dorsal horn [34]. Subcellular expression patterns of mGluR5 appear altered under conditions of neuropathic pain; a reduction in plasma membrane and an increase in nuclear membrane expression of mGluR5 following spared nerve injury has recently been observed [62]. MPEP moderately reduces tactile [43; 67; 68] and cold [43] hypersensitivity in nerve-injured mice. Systemically or spinally delivered MPEP reduces spontaneous and evoked responses of wide dynamic range neurons in nerve-injured rodents [54]. MPEP also potentiates morphine inhibition of neuropathic pain [43; 67] and reduces opioid analgesic tolerance [43; 67]. There is evidence for MPEP effects on NR2B-containing NMDA receptors [37], which may explain its inhibition of the development of opioid tolerance. There remains interest in developing therapeutic negative allosteric modulators that selectively target mGluR5 [14; 65]. However, higher doses of systemically delivered MPEP (100 mg/kg) and the analog MTEP (30–100 mg/kg) resulted in reduction of locomotor activity and impairment of rotarod performance[68]. These doses were comparable to or less than an order of magnitude higher than the effective analgesic doses (10–30 mg/kg). Therefore, minimizing the dose-range needed for analgesia may be important for developing mGluR5 antagonists.
4.3 Concurrent Targeting of Mu Opioid Receptor and mGluR5
The present study examined the effect of a bivalent ligand, MMG22, containing the mGluR5 antagonist MPEP and the oxymorphone-derived MOR agonist in chronic neuropathic pain. Previous work with MMG22 has demonstrated inhibition of pain behaviors in models of inflammatory [2] and chronic bone cancer-induced pain [53]. When delivered intrathecally, MMG22 demonstrated the greatest analgesic potency among a series of analogs with variable spacer length, in LPS-treated mice [2]. Furthermore, an approximate 4700-fold increase in MMG22 potency was observed in LPS mice as compared to control mice. Additionally, MMG22 reduced tactile hypersensitivity in a model of bone cancer-induced pain. Interestingly, the potency of MMG22 increased up to 570-fold with progressive increase in bone tumor growth and subsequent hyperalgesia [53]. In contrast to these substantial shifts in MMG22 potency observed in these models, we observed that MMG22 reversed neuropathic pain with a 10-fold greater potency than the individual pharmacophores in the spared nerve injury model of neuropathic pain. The duration of effect was short but was prolonged with increasing dose. To determine whether analgesic synergism could contribute to the effect of MMG22 in neuropathic pain, we examined the interactions between oxymorphone or oxymorphamine and MPEP. Oxymorphone is the MOR agonist upon which the opioid pharmacophore of MMG22 is based. Oxymorphone is converted to oxymorphamine in order to enable attachment of the chemical spacers. Both MOR agonists demonstrated synergistic interactions with MPEP, confirmed by isobolographic analysis (Fig. 4), supporting analgesic synergism as a potential mechanism by which MMG22 exerts its analgesic effect, although likely targeting MOR and mGluR5 receptors separately as monomers rather than as heteromers. Our neuroanatomical analysis of MOR-ir and mGluR5-ir indicates that MOR and mGluR receptors primarily are expressed in separate but proximal puncta in spinal cord superficial dorsal horn (Fig. 4), an expression pattern consistent with the spinal analgesic neuropharmacology presented in Figs. 1–3. We evaluated the necessity of the bivalency of MMG22 by comparing the action of the oxymorphone monovalent M19 and the MPEP monovalent, MG20. These entities contain only one pharmacophore of MMG22 and the corresponding linkers. That MG20 administered alone had no impact on hypersensitivity (Fig. 5) and no impact on the analgesic effect of M19 (Table 2) indicates that the synergistic interaction is dependent on either co-administration of the parent compounds or as the bivalent ligand. Finally, we compared the MMG22 analog MMG10, which has only a 10-atom linker; a single molecule of MMG10 is unlikely to be able to activate both protomers simultaneously because the spacer length is too short. Surprisingly, MMG10 reduced neuropathic pain behaviors with potency comparable to that of the MMG22. While two molecules of MMG10 may activate MOR and antagonize mGluR5 separately as monomers it is unlikely that a single molecule of MMG10 effectively binds to both MOR and mGluR5 simultaneously as a bivalent. The comparable effectiveness of MMG10 suggests that bivalent ligands with shorter spacers (and lower molecular weights) may also be effective therapeutic agents to control neuropathic pain, perhaps through synergistically acting at MOR and mGluR5 monomers.
4.4 Combination Therapy
The present study supports a strategy for opioid agonist-mGluR5 antagonist combinations for the treatment of neuropathic pain. The opportunities of therapeutic synergistic interactions of combined compounds to treat cancer, chronic obstructive pulmonary disease[11], epilepsy[10] to provide immunotherapy[49], and to offer analgesic relief[28] are widely appreciated. Despite clear advantages, subsequent development in terms of appropriate pre-clinical toxicity analysis, optimization of dose ratios[40], and clinical trials with the specific combinations have been limited[33] relative to the extensive pre-clinical characterizations of such synergistic combinations and the common use of polytherapy in practice. This discrepancy may be due to increased complexity associated with development of a drug combination, which requires the optimization of dose ratios, delivery approaches, pharmacokinetics, and assessment of toxicity, all of which must be conducted not only for the agents given singly, but as the intended combination.
In contrast, multi-targeting of receptor heteromers and the rational development of bivalent ligands to target opioid receptors[59] may offer a straightforward approach for capitalizing on synergistic interactions of distinct receptor systems. In the case of the bivalent ligand, development requires characterization of a single chemical entity, albeit one specifically designed to target two receptors. Following establishment of pre-clinical pharmacological efficacy, development should then follow the standard process for a single new chemical entity.
4.5 Summary
These studies provide the first evidence for a reduction of nerve-injury induced neuropathic pain by the bivalent ligand MMG22, which simultaneously activates MOR and antagonizes mGluR5. The report also provides the first demonstration of synergistic analgesic interactions between MOR agonists and an mGluR5 antagonist under conditions of neuropathic pain. Such an interaction is consistent with the proposed dual mechanisms of MMG22 and suggests that co-targeting of the two receptors by MMG22 (or other bivalent analogs such as MMG10) capitalizes upon this particular synergistic receptor combination. It is noteworthy that the present results differ significantly from those of a mouse bone cancer-induced pain model in which the antinociceptive potency of MMG22 is three orders of magnitude greater than its shorter spacer homologues, consistent with MOR-mGluR5 heteromer as a target for bone cancer-induced pain [53]. Given the entirely different profile between spacer length and potency, it may be that the organization of MOR and mGluR5 differs in bone cancer-and nerve injury-induced pain[62]. An expanded understanding of the expression pattern of the monomeric and heteromeric forms of the receptors in the spinal cord and along pain pathways would be beneficial.
Acknowledgments
This work was supported by R01DA030316 (PSP), R01DA035931 (CAF), R01DA015438 (GLW) and NIDA training grant T32-DA007097 supported CDP.
Footnotes
Conflicts of Interest
Drs. Portoghese and Akgün have a related patent pending. The remaining authors have no other conflicts of interest.
References
- 1.Aceto MD, Harris LS, Negus SS, Banks ML, Hughes LD, Akgun E, Portoghese PS. MDAN-21: A Bivalent Opioid Ligand Containing mu-Agonist and Delta-Antagonist Pharmacophores and Its Effects in Rhesus Monkeys. Int J Med Chem. 2012;2012:327257. doi: 10.1155/2012/327257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akgun E, Javed MI, Lunzer MM, Smeester BA, Beitz AJ, Portoghese PS. Ligands that interact with putative MOR-mGluR5 heteromer in mice with inflammatory pain produce potent antinociception. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(28):11595–11599. doi: 10.1073/pnas.1305461110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alagille D, Baldwin RM, Roth BL, Wroblewski JT, Grajkowska E, Tamagnan GD. Functionalization at position 3 of the phenyl ring of the potent mGluR5 noncompetitive antagonists MPEP. Bioorg Med Chem Lett. 2005;15(4):945–949. doi: 10.1016/j.bmcl.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 4.Arner S, Meyerson BA. Lack of analgesic effect of opioids on neuropathic and idiopathic forms of pain. Pain. 1988;33(1):11–23. doi: 10.1016/0304-3959(88)90198-4. [DOI] [PubMed] [Google Scholar]
- 5.Attal N, Bouhassira D. Pharmacotherapy of neuropathic pain: which drugs, which treatment algorithms? Pain. 2015;156(Suppl 1):S104–114. doi: 10.1097/01.j.pain.0000460358.01998.15. [DOI] [PubMed] [Google Scholar]
- 6.Ballantyne JC, LaForge KS. Opioid dependence and addiction during opioid treatment of chronic pain. Pain. 2007;129(3):235–255. doi: 10.1016/j.pain.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 7.Berg KA, Patwardhan AM, Akopian AN. Receptor and channel heteromers as pain targets. Pharmaceuticals (Basel) 2012;5(3):249–278. doi: 10.3390/ph5030249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhave G, Karim F, Carlton SM, Gereau RWt. Peripheral group I metabotropic glutamate receptors modulate nociception in mice. Nat Neurosci. 2001;4(4):417–423. doi: 10.1038/86075. [DOI] [PubMed] [Google Scholar]
- 9.Bian D, Nichols ML, Ossipov MH, Lai J, Porreca F. Characterization of the antiallodynic efficacy of morphine in a model of neuropathic pain in rats. Neuroreport. 1995;6(15):1981–1984. doi: 10.1097/00001756-199510010-00007. [DOI] [PubMed] [Google Scholar]
- 10.Brigo F, Ausserer H, Tezzon F, Nardone R. When one plus one makes three: the quest for rational antiepileptic polytherapy with supraadditive anticonvulsant efficacy. Epilepsy Behav. 2013;27(3):439–442. doi: 10.1016/j.yebeh.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Calzetta L, Matera MG, Cazzola M. Pharmacological interaction between LABAs and LAMAs in the airways: optimizing synergy. European journal of pharmacology. 2015;761:168–173. doi: 10.1016/j.ejphar.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 12.Chabot-Dore AJ, Millecamps M, Naso L, Devost D, Trieu P, Piltonen M, Diatchenko L, Fairbanks CA, Wilcox GL, Hebert TE, Stone LS. Dual allosteric modulation of opioid antinociceptive potency by alpha2A-adrenoceptors. Neuropharmacology. 2015;99:285–300. doi: 10.1016/j.neuropharm.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chabot-Dore AJ, Schuster DJ, Stone LS, Wilcox GL. Analgesic synergy between opioid and alpha2 -adrenoceptors. British journal of pharmacology. 2015;172(2):388–402. doi: 10.1111/bph.12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiechio S. Modulation of Chronic Pain by Metabotropic Glutamate Receptors. Adv Pharmacol. 2016;75:63–89. doi: 10.1016/bs.apha.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Costantino CM, Gomes I, Stockton SD, Lim MP, Devi LA. Opioid receptor heteromers in analgesia. Expert Rev Mol Med. 2012;14:e9. doi: 10.1017/erm.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daniels DJ, Lenard NR, Etienne CL, Law PY, Roerig SC, Portoghese PS. Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(52):19208–19213. doi: 10.1073/pnas.0506627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Vry J, Kuhl E, Franken-Kunkel P, Eckel G. Pharmacological characterization of the chronic constriction injury model of neuropathic pain. European journal of pharmacology. 2004;491(2–3):137–148. doi: 10.1016/j.ejphar.2004.03.051. [DOI] [PubMed] [Google Scholar]
- 18.Decosterd I, Allchorne A, Woolf CJ. Progressive tactile hypersensitivity after a peripheral nerve crush: non-noxious mechanical stimulus-induced neuropathic pain. Pain. 2002;100(1–2):155–162. doi: 10.1016/s0304-3959(02)00275-0. [DOI] [PubMed] [Google Scholar]
- 19.Deekonda S, Wugalter L, Rankin D, Largent-Milnes TM, Davis P, Wang Y, Bassirirad NM, Lai J, Kulkarni V, Vanderah TW, Porreca F, Hruby VJ. Design and synthesis of novel bivalent ligands (MOR and DOR) by conjugation of enkephalin analogues with 4-anilidopiperidine derivatives. Bioorg Med Chem Lett. 2015;25(20):4683–4688. doi: 10.1016/j.bmcl.2015.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Derry S, Stannard C, Cole P, Wiffen PJ, Knaggs R, Aldington D, Moore RA. Fentanyl for neuropathic pain in adults. Cochrane Database Syst Rev. 2016;10:CD011605. doi: 10.1002/14651858.CD011605.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fairbanks CA, Kitto KF, Nguyen HO, Stone LS, Wilcox GL. Clonidine and dexmedetomidine produce antinociceptive synergy in mouse spinal cord. Anesthesiology. 2009;110(3):638–647. doi: 10.1097/ALN.0b013e318195b51d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fairbanks CA, Nguyen HO, Grocholski BM, Wilcox GL. Moxonidine, a selective imidazoline-alpha2 -adrenergic receptor agonist, produces spinal synergistic antihyperalgesia with morphine in nerve-injured mice. Anesthesiology. 2000;93(3):765–773. doi: 10.1097/00000542-200009000-00026. [DOI] [PubMed] [Google Scholar]
- 23.Ferre S, Goldberg SR, Lluis C, Franco R. Looking for the role of cannabinoid receptor heteromers in striatal function. Neuropharmacology. 2009;56(Suppl 1):226–234. doi: 10.1016/j.neuropharm.2008.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujita W, Gomes I, Devi LA. Heteromers of mu-delta opioid receptors: new pharmacology and novel therapeutic possibilities. British journal of pharmacology. 2015;172(2):375–387. doi: 10.1111/bph.12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fundytus ME, Fisher K, Dray A, Henry JL, Coderre TJ. In vivo antinociceptive activity of anti-rat mGluR1 and mGluR5 antibodies in rats. Neuroreport. 1998;9(4):731–735. doi: 10.1097/00001756-199803090-00031. [DOI] [PubMed] [Google Scholar]
- 26.Gaskell H, Derry S, Stannard C, Moore RA. Oxycodone for neuropathic pain in adults. Cochrane Database Syst Rev. 2016;7:CD010692. doi: 10.1002/14651858.CD010692.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilron I, Dickenson AH. Emerging drugs for neuropathic pain. Expert Opin Emerg Drugs. 2014;19(3):329–341. doi: 10.1517/14728214.2014.915025. [DOI] [PubMed] [Google Scholar]
- 28.Gilron I, Max MB. Combination pharmacotherapy for neuropathic pain: current evidence and future directions. Expert Rev Neurother. 2005;5(6):823–830. doi: 10.1586/14737175.5.6.823. [DOI] [PubMed] [Google Scholar]
- 29.Gunduz O, Topuz RD, Karadag CH, Ulugol A. Analysis of the anti-allodynic effects of combination of a synthetic cannabinoid and a selective noradrenaline re-uptake inhibitor in nerve injury-induced neuropathic mice. European journal of pain. 2016;20(3):465–471. doi: 10.1002/ejp.752. [DOI] [PubMed] [Google Scholar]
- 30.Harvey JH, Long DH, England PM, Whistler JL. Tuned-Affinity Bivalent Ligands for the Characterization of Opioid Receptor Heteromers. ACS Med Chem Lett. 2012;3(8):640–644. doi: 10.1021/ml300083p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Health OotASf. National Pain Strategy: A Comprehensive Population Health Level Strategy for Pain US Department of Health and Human Services. 2016 [Google Scholar]
- 32.Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67(2–3):313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- 33.IOM. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. The National Academies Press; Washington, D.C: 2011. [PubMed] [Google Scholar]
- 34.Jia H, Rustioni A, Valtschanoff JG. Metabotropic glutamate receptors in superficial laminae of the rat dorsal horn. The Journal of comparative neurology. 1999;410(4):627–642. [PubMed] [Google Scholar]
- 35.Jones RC, 3rd, Lawson E, Backonja M. Managing Neuropathic Pain. Med Clin North Am. 2016;100(1):151–167. doi: 10.1016/j.mcna.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 36.Le Naour M, Akgun E, Yekkirala A, Lunzer MM, Powers MD, Kalyuzhny AE, Portoghese PS. Bivalent ligands that target mu opioid (MOP) and cannabinoid1 (CB1) receptors are potent analgesics devoid of tolerance. J Med Chem. 2013;56(13):5505–5513. doi: 10.1021/jm4005219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lea PMt, Faden AI. Metabotropic glutamate receptor subtype 5 antagonists MPEP and MTEP. CNS Drug Rev. 2006;12(2):149–166. doi: 10.1111/j.1527-3458.2006.00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee YW, Yaksh TL. Analysis of drug interaction between intrathecal clonidine and MK-801 in peripheral neuropathic pain rat model. Anesthesiology. 1995;82(3):741–748. doi: 10.1097/00000542-199503000-00016. [DOI] [PubMed] [Google Scholar]
- 39.Martin TJ, Kim SA, Buechler NL, Porreca F, Eisenach JC. Opioid self-administration in the nerve-injured rat: relevance of anti-allodynic effects of analgesics. Anesthesiology. 2007;106(2):312–322. doi: 10.1097/00000542-200702000-00020. [DOI] [PubMed] [Google Scholar]
- 40.Mayer LD, Janoff AS. Optimizing combination chemotherapy by controlling drug ratios. Mol Interv. 2007;7(4):216–223. doi: 10.1124/mi.7.4.8. [DOI] [PubMed] [Google Scholar]
- 41.Miranda HF, Noriega V, Zepeda R, Zanetta P, Prieto-Rayo J, Prieto JC, Sierralta F. Antinociceptive synergism of gabapentin and nortriptyline in mice with partial sciatic nerve ligation. Pharmacology. 2015;95(1–2):59–64. doi: 10.1159/000370244. [DOI] [PubMed] [Google Scholar]
- 42.Obara I, Parkitna JR, Korostynski M, Makuch W, Kaminska D, Przewlocka B, Przewlocki R. Local peripheral opioid effects and expression of opioid genes in the spinal cord and dorsal root ganglia in neuropathic and inflammatory pain. Pain. 2009;141(3):283–291. doi: 10.1016/j.pain.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 43.Osikowicz M, Mika J, Makuch W, Przewlocka B. Glutamate receptor ligands attenuate allodynia and hyperalgesia and potentiate morphine effects in a mouse model of neuropathic pain. Pain. 2008;139(1):117–126. doi: 10.1016/j.pain.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 44.Ozaki S, Narita M, Narita M, Ozaki M, Khotib J, Suzuki T. Role of extracellular signal-regulated kinase in the ventral tegmental area in the suppression of the morphine-induced rewarding effect in mice with sciatic nerve ligation. Journal of neurochemistry. 2004;88(6):1389–1397. doi: 10.1046/j.1471-4159.2003.02272.x. [DOI] [PubMed] [Google Scholar]
- 45.Papathanasiou T, Juul RV, Heegaard AM, Kreilgaard M, Lund TM. Co-administration of morphine and gabapentin leads to dose dependent synergistic effects in a rat model of postoperative pain. Eur J Pharm Sci. 2016;82:97–105. doi: 10.1016/j.ejps.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 46.Picker MJ, Daugherty D, Henry FE, Miller LL, Dykstra LA. Metabotropic glutamate antagonists alone and in combination with morphine: comparison across two models of acute pain and a model of persistent, inflammatory pain. Behav Pharmacol. 2011;22(8):785–793. doi: 10.1097/FBP.0b013e32834d13a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pitcher MH, Ribeiro-da-Silva A, Coderre TJ. Effects of inflammation on the ultrastructural localization of spinal cord dorsal horn group I metabotropic glutamate receptors. The Journal of comparative neurology. 2007;505(4):412–423. doi: 10.1002/cne.21506. [DOI] [PubMed] [Google Scholar]
- 48.Riedl MS, Schnell SA, Overland AC, Chabot-Dore AJ, Taylor AM, Ribeiro-da-Silva A, Elde RP, Wilcox GL, Stone LS. Coexpression of alpha 2A-adrenergic and delta-opioid receptors in substance P-containing terminals in rat dorsal horn. The Journal of comparative neurology. 2009;513(4):385–398. doi: 10.1002/cne.21982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rini B. Future approaches in immunotherapy. Semin Oncol. 2014;41(Suppl 5):S30–40. doi: 10.1053/j.seminoncol.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 50.Rozenfeld R, Devi LA. Exploring a role for heteromerization in GPCR signalling specificity. Biochem J. 2011;433(1):11–18. doi: 10.1042/BJ20100458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sayre LM, Portoghese PS. Stereospecific synthesis of the 6alpha- and 6beta-amino derivatives of naltrexone and oxymorphone. J Org Chem. 1980;45(3366-3368) [Google Scholar]
- 52.Schroder H, Wu DF, Seifert A, Rankovic M, Schulz S, Hollt V, Koch T. Allosteric modulation of metabotropic glutamate receptor 5 affects phosphorylation, internalization, and desensitization of the micro-opioid receptor. Neuropharmacology. 2009;56(4):768–778. doi: 10.1016/j.neuropharm.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 53.Smeester BA, Lunzer MM, Akgun E, Beitz AJ, Portoghese PS. Targeting putative mu opioid/metabotropic glutamate receptor-5 heteromers produces potent antinociception in a chronic murine bone cancer model. European journal of pharmacology. 2014;743:48–52. doi: 10.1016/j.ejphar.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sotgiu ML, Bellomi P, Biella GE. The mGluR5 selective antagonist 6-methyl-2-(phenylethynyl)-pyridine reduces the spinal neuron pain-related activity in mononeuropathic rats. Neurosci Lett. 2003;342(1–2):85–88. doi: 10.1016/s0304-3940(03)00259-3. [DOI] [PubMed] [Google Scholar]
- 55.Stannard C, Gaskell H, Derry S, Aldington D, Cole P, Cooper TE, Knaggs R, Wiffen PJ, Moore RA. Hydromorphone for neuropathic pain in adults. Cochrane Database Syst Rev. 2016;(5):CD011604. doi: 10.1002/14651858.CD011604.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tallarida RJ. Statistical analysis of drug combinations for synergism. Pain. 1992;49(1):93–97. doi: 10.1016/0304-3959(92)90193-F. [DOI] [PubMed] [Google Scholar]
- 57.Tallarida RJ, Porreca F, Cowan A. Statistical analysis of drug-drug and site-site interactions with isobolograms. Life Sci. 1989;45(11):947–961. doi: 10.1016/0024-3205(89)90148-3. [DOI] [PubMed] [Google Scholar]
- 58.Taylor AM, Murphy NP, Evans CJ, Cahill CM. Correlation between ventral striatal catecholamine content and nociceptive thresholds in neuropathic mice. J Pain. 2014;15(8):878–885. doi: 10.1016/j.jpain.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Turnaturi R, Arico G, Ronsisvalle G, Pasquinucci L, Parenti C. Multitarget opioid/non-opioid ligands in pain treatment: new players in an old game II. Eur J Med Chem. 2016;108(211–228) doi: 10.1016/j.ejmech.2015.11.028. [DOI] [PubMed] [Google Scholar]
- 60.VanDenKerkhof EG, Mann EG, Torrance N, Smith BH, Johnson A, Gilron I. An Epidemiological Study of Neuropathic Pain Symptoms in Canadian Adults. Pain Res Manag. 2016;2016:9815750. doi: 10.1155/2016/9815750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Varrassi G, Ageletti C, Guetti C, Marinangeli F, Paladini A. Systemic opioid and chronic pain. European Journal of Pain Supplements. 2009;3:77–83. [Google Scholar]
- 62.Vincent K, Cornea VM, Jong YJ, Laferriere A, Kumar N, Mickeviciute A, Fung JS, Bandegi P, Ribeiro-da-Silva A, O'Malley KL, Coderre TJ. Intracellular mGluR5 plays a critical role in neuropathic pain. Nat Commun. 2016;7:10604. doi: 10.1038/ncomms10604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wade CL, Fairbanks CA. The Self-administration of Analgesic Drugs in Experimentally Induced Chronic Pain. Current topics in behavioral neurosciences. 2014;20:217–232. doi: 10.1007/7854_2014_344. [DOI] [PubMed] [Google Scholar]
- 64.Weiss U. Derivatives of morphine. I. 14-Hydroxydihydromorphinone. Journal of the American Chemical Society. 1955;77(5891–5892) [Google Scholar]
- 65.Zhang L, Balan G, Barreiro G, Boscoe BP, Chenard LK, Cianfrogna J, Claffey MM, Chen L, Coffman KJ, Drozda SE, Dunetz JR, Fonseca KR, Galatsis P, Grimwood S, Lazzaro JT, Mancuso JY, Miller EL, Reese MR, Rogers BN, Sakurada I, Skaddan M, Smith DL, Stepan AF, Trapa P, Tuttle JB, Verhoest PR, Walker DP, Wright AS, Zaleska MM, Zasadny K, Shaffer CL. Discovery and preclinical characterization of 1-methyl-3-(4-methylpyridin-3-yl)-6-(pyridin-2-ylmethoxy)-1H-pyrazolo-[3,4-b]pyra zine (PF470): a highly potent, selective, and efficacious metabotropic glutamate receptor 5 (mGluR5) negative allosteric modulator. J Med Chem. 2014;57(3):861–877. doi: 10.1021/jm401622k. [DOI] [PubMed] [Google Scholar]
- 66.Zhang X, Bao L, Shi TJ, Ju G, Elde R, Hokfelt T. Down-regulation of mu-opioid receptors in rat and monkey dorsal root ganglion neurons and spinal cord after peripheral axotomy. Neuroscience. 1998;82(1):223–240. doi: 10.1016/s0306-4522(97)00240-6. [DOI] [PubMed] [Google Scholar]
- 67.Zhou Q, Wang J, Zhang X, Zeng L, Wang L, Jiang W. Effect of metabotropic glutamate 5 receptor antagonists on morphine efficacy and tolerance in rats with neuropathic pain. European journal of pharmacology. 2013;718(1–3):17–23. doi: 10.1016/j.ejphar.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 68.Zhu CZ, Wilson SG, Mikusa JP, Wismer CT, Gauvin DM, Lynch JJ, 3rd, Wade CL, Decker MW, Honore P. Assessing the role of metabotropic glutamate receptor 5 in multiple nociceptive modalities. European journal of pharmacology. 2004;506(2):107–118. doi: 10.1016/j.ejphar.2004.11.005. [DOI] [PubMed] [Google Scholar]