Abstract
Cancer metabolism has emerged as one of the most interesting old ideas being revisited from a new perspective. In the early 20th century Otto Warburg declared metabolism the prime cause in a disease of many secondary causes, and this statement seems more prescient in view of modern expositions into the true nature of tumor evolution. As the complexity of tumor heterogeneity becomes more clear from a genetic perspective, it is important to consider the inevitably heterogeneous metabolic components of the tumor and the tumor microenvironment. High grade gliomas remain one of the most difficult to treat solid tumors, due in part to the highly vascularized nature of the tumor and the maintenance of more resistant stem-like subpopulations within the tumor. Maintenance of glioma stem cells (GSCs) requires specific alterations within the cells and the greater tumor microenvironment with regards to signaling and metabolism. Specific niches within gliomas help foster the survival of stem-like sub-populations of cells with high tumorigenicity and high metabolic plasticity. Understanding these maintenance pathways and the metabolic dependencies within the niche may highlight potential avenues of addressing tumor resistance and recurrence in glioma patients.
Keywords: Cancer Metabolism, Glioblastoma, Glioma Stem Cells, Cancer Stem Cells, Microenvironment
1 Introduction
Glioblastoma represents one of the most lethal solid tumors with one of the highest mortality rates, having an overall survival of approximately 12–14 months with the full complement of treatment [1]. Negligible progress has been made on that survival figure despite advancements in chemotherapy and surgical techniques over the past 30 years. This has required a reassessment of where within our understanding of glioma biology the major gaps in knowledge remain and the sobering reality is that the gaps are not small. The developing view of tumors as heterogeneous disease comprised of many subpopulations with unique properties has forced us to consider more closely the dynamics within a tumor that play a role in not only driving tumor development forward but also in maintaining tumor survival under severe stress. It is important to start to piece together the specifics of the many different networks within a tumor system including interactions between tumor subpopulations, interactions between the tumor and its stroma surrounding, interactions between the tumor and the immune components, and even interactions between the tumor and the local stem compartment. With the need to begin to address these gaps in understanding gliomagenesis, the tumor microenvironment and the complex metabolic networks within these tumors have come into particular focus in recent research.
Tumor metabolism fundamentally discusses two major points of cell behavior: (1) the specific sourcing of macromolecules of metabolites, and (2) the different cellular mechanism used to deal with different nutrients for either anabolic construction or catabolic breakdown. Many tumors have been shown to augment its microenvironment in order to more optimally acquire nutrients, which is of particular importance to solid tumors as the tumor core becomes more isolated from the native vascular infrastructure. Microvascular hyperplasia is one of the important hallmarks in glioma development and in fact most gliomas maintain extensive, proliferative vascular endothelium [2]. Although this vasculature is required for most of the tumor bulk, solid tumors will have variability in access to oxygen and nutrients in different tumor compartments, and adaptations to this variability is also important for tumor growth. As solid glioma bulk grows in mass, the core tumor space will begin to form necrotic and hypoxic regions and a significant amount of necrotic buildup ensues. However, there will also be cellular compartments within the tumor bulk that adapt to the oxygen and glucose gradients and may thrive in this space. Within a single tumor one will find many different cellular compartments with variations in oxygenation and fuel source availability and there will be cells enriched in these compartments that have made the suitable adjustments to accommodate these conditions. This again harkens back to the complex heterogeneity of solid tumors which only gets worse with tumor progression. Furthermore, the body of research over the past decade regarding glioma stem-cell (GSC) populations have indicated certain highly resistant and tumorigenic sub-populations are maintained in specific microevironmental niches, particularly enriched in these perinecrotic/hypoxic/perivascular compartments [3,4,5].
The cellular adaptions that allow for the development of tumor subpopulations is therefore something that not only requires more investigation but also constitutes one of the key elements to understanding cellular resistance and recurrence. Much of research over the past decade have looked at the source of tumor subpopulations and even tumor origin itself and has suggested the existence of tumor progenitors or tumor initiating stem-like populations in many cancers. The cancer stem cell (CSC) hypothesis as it has come to be known proposes that these stem-like subpopulations are maintained across a wide array of tumors and are predominantly aggressive cellular subsets that can be resistant to nutrient stresses as well as treatment stresses. These cancer stem populations maintain unrestricted self-renewal capacity and this allows for the propagation of the tumor even under insult of therapy [6]. GSCs share many of the same biological hallmarks of normal neural stem cells which includes the ability to form neurospheres, express neural stem markers, and differentiate into both astrocytic and neural lineages [6,7]. The most compelling reason to study glioma biology with GSCs is the fact that they have been shown to be very tumorigenic in vivo and form diffuse and invasive tumors that are highly resistant to conventional treatments, indicative of actual patient disease in clinic [8,9]. Therefore, the need to understand how GSCs are maintained and what, if any, contribution comes from the microenvironment is highly relevant. A key feature of many of these progenitor cell populations or cancer stem cells is the metabolic plasticity that has been described in the literature [10]. The ability to modulate key cellular metabolism processes to adapt to changing nutritional climates may in fact describe an important aspect to the resistance phenotype these cells display. Therefore, the metabolic requirements of these GSCs and their microenvironment are very important in understanding how resistance is established in these tumors.
Most cancer cells have been shown to rely on glycolysis instead of oxidative phosphorylation for glucose metabolism, as described by Warburg et al [11]. The Warburg Effect has been a fixture of cancer cell biology for almost a decade now but new research has been able to describe many instances where the Warburg effect is either not observed or observed to only a certain degree [10]. This would make sense considering most tumors represent a mix of cellular pools that could have diverging metabolic requirements. In fact, there has been diverging observations regarding cancer stem cell metabolism across different tumors. GSCs have been reported to have distinctly different metabolic phenotypes compared to more differentiated tumor cells, and appears to be able to easily switch between glycolytic and oxidative metabolism depending on the microenvironment [12]. This suggests that despite differences in basal metabolism, cancer stem metabolism may rely more on the capacity for metabolic adaptability and reprogramming than on a primary metabolic profile across cancer.
This review will focus on the interactions between the tumor microenvironment and GSCs, specifically looking at the metabolic requirements and dependencies of both components. The relationship between GSCs and the specific stem compartments of the tumor and the vascular/hypoxic niches may shed light on an important element to maintaining these cells, and in turn maintaining the greater tumor.
2 Glioma Stem Cells
Through human development most cells in the body mature from stem-like precursors towards more differentiated cellular fates. These differentiation events are functionally important and tend to result in committed cellular steps towards terminal cell states. However, it is an important aspect of tissue homeostasis to maintain certain sub-populations of stem-like precursors that can give rise to functionally mature progeny in the event of cellular turnover or wound healing [13]. In cancer, it has been proposed that elements of this homeostatic mechanism have been hijacked for cancer propagation.
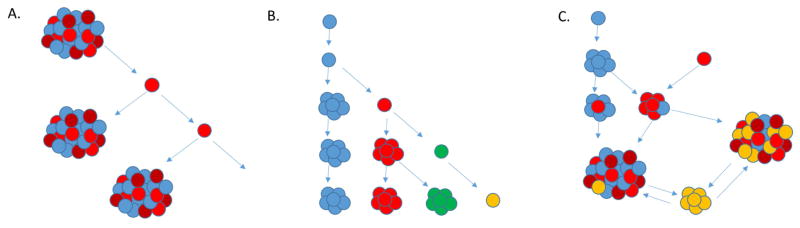
The original cancer stem cell (CSC) hypothesis proposed a model of tumor propagation via stem cell precursors using the hierarchical model of cell division. The traditional hierarchical model of cancer stem cells states distinct stem-like populations exist from the beginning of the tumors inception and are in fact responsible for the propagation of various more differentiated cell populations that will go on to make up the heterogeneous tumor pool [14,15]. In this model, treatment resistance is at least in part explained but the maintenance of the parental cancer stem cells which can then repopulate the tumor bulk once the treatment insult is removed. An alternative idea being developed with regards to cancer stem cell propagation posits the idea of clonal evolution, where the accumulation of a series of mutations, in time, will drive cells away from their assigned cell fates and slowly dedifferentiate into a more progenitor state. In theory, a tumor will eventually develop one or more distinct stem-like clonal populations that have recaptured self-renewal capacity that can then be implemented towards tumor survival and growth. In light of current understandings of tumor heterogeneity and tumor resistance/recurrence, it is more likely that both of these models may in fact describe different elements of a central process and therefore both explain the cancer stem cell model to a point, as some have proposed a hybrid of the two theories to explain the complex dynamics involved (Fig. 1) [13,16,17,18]. The important fact remains that however these cells may have come to be, the elimination of the cancer stem cell population in any tumor model represents one of the most important hurdles to cancer research and treatment today.
Figure 1.
Cancer stem cell hypothesis continues to evolve. (A) traditional cancer stem cell hypothesis suggested hierarchal model where only certain stem-like cell (red) retained capacity to repopulate a tumor and drive continued tumor progression, (B) the clonal model of stem cell propagation suggests many cells within the tumor retains this stem-like repopulation capacity, (C) the newest models suggest a hybrid model that allows for dedifferentiation and transdifferentiation within a tumor from multiple stem precursors in response to intra- and extratumoral pressures, driving tumor recurrence and heterogeneity
Glioma stem cells have been demonstrated in vitro to have self-renewal capacity, differentiate into multiple cell lineages, form neurospheres, and express specific neural stem cell markers such as Nestin, Sox2, Prom1/CD133, and Nanog. Several more markers have been suggested over the years and there is unlikely to be a specific expression profile that encompasses every stem-like glioma subpopulation [19,20]. GSCs have been shown to be more resistant to both chemotherapy and radiation above differentiated tumor cells and several studies have specifically shown GSC ability to repopulate a tumor and drive secondary tumor recurrence post-treatment [9,21,22,23,24]. To further confound things, as with tumors in general, there has been shown to be great heterogeneity even within the GSC pools, which is consistent with the models of CSC maintenance and propagation. Various different expression subtypes have been described in glioma patients (proneural, mesenchymal, classical, and neural) and several of these subtypes have also been attributed to GSCs as well (proneural and mesenchymal) [25]. Distinct GSCs clones even from the same tumor can display variability in gene expression profile and metabolic dependencies [26,27]. There is evidence to suggest that variability in GSC clones is at least in part driven by the tumor microenvironment itself [28] and that all of this transcriptional and metabolic heterogeneity allows cancer stem cells to be very adaptable in order to maintain high rates of self-renewal and differentiation [29].
3 Glioma Stem Cell Metabolism & Maintenance
The plasticity and adaptability of most cancer stem cells to different metabolic conditions and requirements is considered an important hallmark in cancer development [30]. Like the tumor itself, the metabolic landscape of the tumor is very heterogeneous and cells will metabolize differently depending on the environment they are in. CSCs have the ability to generally maintain a very quiescent profile but can rapidly switch into a more proliferative state if there is a need to repopulate the tumor, for instance in response to radiation derived tumor regression. When most cells would die under radiation, the CSCs can remain hidden from the stress and enter the cell cycle afterwards in order to replenish the tumor [31]. This is partly adaptive on the part of the tumor but is in large part allowed by the microenvironmental space.
The Warburg effect describes the predominantly observed behavior of most malignantly transformed cells with respect to their metabolism. While normal cells will largely undergo oxidative phosphorylation in the presence of glucose and oxygen, in many cancer cells the large proportion of glucose is diverted away from mitochondrial oxidation and into glycolysis and the production of lactate by lactate dehydrogenase (LDH) even in the presence of oxygen. This at first seems paradoxical since the mechanism of anaerobic glycolysis, although important in moments of low or no oxygen, is much less efficient than mitochondrial oxidation of glucose. In part this adaptation may provide a sufficient balance between providing the necessary resources for biomass production and growth while allowing plasticity to adapt to various stresses, physiological and clinically driven [32,33]. There have been many described exceptions to the Warburg phenomenon and the importance of this metabolic alteration is yet to be fully elucidated, especially with regards to cancer stem cell metabolism.
The literature on cancer stem cell metabolism is ripe with discrepancies, as there have been nearly as many papers describing glycolytic CSC dependencies as there have been those with oxidative dependencies [34–48]. In all these reports however, there is at least an acknowledgment that the CSC population maintains a distinct metabolic phenotype compared to the tumor bulk, but the exact profile is not known. There have been multiple studies that have described CSCs being more glycolytic than the differentiated progeny across different tumor types [34,35,36,37]. In these studies, glucose uptake, glycolytic enzymes, lactate and ATP production are much higher in CSCs compared to when they were differentiated. These observations corresponded to diminished metabolic contribution from mitochondrial oxidation [36,37,38]. In many cases CSCs were seen to have lower levels of mitochondrial DNA and differentiation was shown to subsequently increase mitochondrial DNA copy number [39,40]. Mitochondrial copy number was also inversely correlated the expression of many genes associated with pluripotency such as Oct4, TERT, and Myc [41]. This however all stands in contrast to a growing body of research that indicates CSCs may in fact rely more on mitochondrial function and metabolism. Studies have shown CSCs actually being less glycolytic than their differentiated counterparts and consume less glucose, produce less lactate, and maintain higher ATP levels. In these studies, CSC mitochondria tend to have larger mass and increased membrane potential, which has been related to increased mitochondrial ROS and enhanced oxygen consumption rates compared to differentiated progeny [42–48]. Mitochondrial mass was particularly important in these studies and correlated highly with metastatic potential and resistance to DNA damage. There is evidence showing mitochondrial biogenesis to be higher in circulating/migratory tumor cells and that inhibition of the transcription co-activator peroxisome proliferator-activated receptor gamma co-activator 1 alpha (PGC1a) can reduce stemness in breast and pancreatic CSCs [42,49,50]. Mitochondria in these CSCs as mentioned before have higher levels of ROS but the total intracellular ROS levels remain lower, suggesting a strong antioxidant mechanism being utilized in these CSCs. This antioxidant mediated ROS buffering not only seems to help maintain stemness in CSCs but promotes treatment resistance, and further underscores the importance of elucidating metabolism in CSCs to help address problems with non-responders in the clinic.
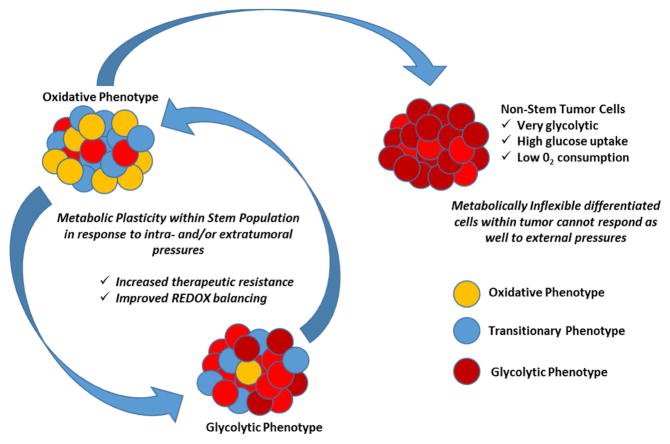
These discrepancies however may not be a problem in our understanding but rather indicative of what CSC metabolism really looks like. Cells undergo metabolic fluctuations during differentiation under hypoxia, going between glycolysis and oxidative phosphorylation to overcome mitochondrial deficiencies [51]. It is quite possible that CSCs are able to similarly modulate redox status in order to regulate their own stem maintenance. CSCs may simply be demonstrating a level of metabolic plasticity that normal cells and more differentiated tumor cells cannot accomplish in response to the microenvironment (Fig 2.). The true metabolic condition of the CSC population would require analysis of cells directly from patients and/or maintained at a very low passage. In these cases, GSCs from patient-derived gliomas tended towards mitochondrial oxidative phosphorylation compared to the differentiated progeny [12]. Furthermore, patient derived GSCs maintained at low passage also maintain very high metabolic plasticity since blocking mitochondrial metabolism simply forces these cells to switch to a more glycolytic profile [52]. This plasticity could allow CSCs to deal with fluctuating conditions and survive in unfavorable conditions whether it be stress from treatment or the harshness of metastatic sites [52,53]. This also presents a new problem wherein single inhibition of one metabolic pathway may not be effective in vivo despite presumably being effective in vitro, since the true CSC population may be able to modify its metabolic preferences depending on what is available to them. Studying CSC metabolism in culture also takes out perhaps the most important aspect of GSC maintenance, metabolism, and plasticity: the tumor microenvironment.
Figure 2.
Cancer stem cell metabolism may demonstrate another element of cellular plasticity that provides a basis for therapeutic resistance and tumor recurrence after treatment interventions. More terminally differentiated tumor cells may not be as metabolically flexible as stem cells that can switch to different metabolic pathways and different fuel sources more readily.
Any discussion of metabolism and gliomas requires a mention of isocitrate dehydrogenase 1/2 (IDH1/2). The IDH1 enzyme is responsible for catalyzing the oxidative decarboxylation of isocitrate into 2-oxoglutarate and mutations in the IDH1 gene is one of the most common in diffuse gliomas, primarily lower grade gliomas [54,55]. The IDH1 mutation and mutations in its homologue IDH2 usually results in loss of normal enzymatic function and the abnormal production of 2-hydroxyglutarate (2-HG) [56]. This consequently reduces the amount of cellular a-ketoglutarate and this hampers the functioning of many a-KG dependent processes. In lower grade gliomas with this mutation, extensive changes in histone and DNA methylation can be found and has been suggested to implicated in tumorigenesis. However, IDH1 mutant patients have higher median overall survival compared to patents with wildtype IDH1 [57]. The significance of these mutational events in the context of GSC maintenance has not been investigated very well but there is certainly reason to believe that any dramatic alteration to the metabolic landscape would have a knock-on effect in the stem compartments.
4 Glioma Stem Cell Microenvironment
GSCs, like most stem cells, demonstrate a very dynamic relationship with their niche, constantly maintaining bi-directional cross-talk with the tumor microenvironment. This interaction is essential in the understanding of gliomagenesis, propagation, and treatment resistance. First it is important to understand the distinct sub-localizations within the tumor space. GSCs have been enriched in the perivascular/proliferative niche of most gliomas, where there is both an abundance of stem maintenance signaling from the surrounding endothelium and exchange of nutrients. This is often specifically located around the subventricular zone (SVZ) and the hippocampus, which has also been described as a stem cell niche for normal neural stem cells [58,59,60].
There are many different soluble factors that play important roles in the vascular niche with respect to GSC maintenance. As mentioned before, glioblastoma is one of the most vascularized of the solid tumors and microvascular hyperplasia has been described as a key feature in glioma initiation and progression [61]. The contribution to this phenotype is no small part attributed to the GSCs and the vascular niche of the tumor microenvironment. GSCs have been shown to highly promote angiogenesis and express factors such as vascular endothelial growth factor (VEGF), attracting endothelial cells to the tumor bulk and driving neovascular growth [59]. These endothelial cells in turn have been shown to express high levels of Sonic Hedghog (SHh), which plays an important role in the recruitment and activation of cancer stem cells and helps maintain GSC self-renewal and growth [62,63]. GSC enriched compartments of gliomas have been seen to co-localize with endothelial cells that express high levels of SHh and in turn drive SHh-GLI1 signaling in these GSCs.
Another important factor found in the vascular niche is the FGF-2, a growth factor commonly used in vitro to help maintain GSCs in culture. FGF-2 helps GSC maintenance in the niche and has also been demonstrated to bolster the blood-brain-barrier function of associated endothelial cells [64]. FGF-2 withdrawal tends to drive GSCs towards differentiation and FGF-2 signaling has also been associated with more robust Nestin expression, further contributing to stemness [65,66]. FGF-2 has also been shown to combine with EGF, another factor used in vitro for GSC propagation, and that FGF-2 and EGF may be involved in an autocrine feedback loop to help retain GSC self-renewal [66].
The general architecture of the glioma tumor space is comprised of normoxic cells that drive the generally drive the tumor at its leading edges along the periphery and most of the hypoxic cells located the poorly oxygenated necrotic core of the tumor. Therefore, most tumors whether they are small or large, display this gradient of oxygenation throughout the tumor and this results in the development of distinct cellular compartments. The plasticity of GSCs allows them to reside in most compartments of the tumor, but as mentioned earlier, the perivascular niche and vascular niche is often enriched for GSCs due to the interaction with the endothelium. Many studies have shown that GSCs may contribute directly to the vasculature and in fact be able to drive endothelial cells through a trans-differentiation process [3,67]. Endothelial cells surrounding GSCs have a been shown to have expression profiles very similar to the cancer cells themselves and have suggested they might in fact be of neoplastic origin [68,69]. This microenvironment therefore maintains the GSCs in order to preserve their potential to proliferate and differentiate, and can protect them from any treatment insults that they may encounter [70]. Cellular responses to hypoxia are often modulated through the family of hypoxia-inducible factors (HIFs). In stem cell biology and GSC biology, HIF1a and HIF2a have been implicated in oxygen dependent signaling and stem cell maintenance, and HIF1/2a expression has been demonstrated to be very high in the hypoxic compartments of gliomas [71]. HIF1a expression is high in GSCs even under mild hypoxia and has been shown to prevent GSC apoptosis through NFκB stabilization and expression of anti-apoptotic NFκB target genes [72]. All these studies demonstrate that the hypoxic microenvironment plays an important function in maintaining GSCs and promoting recruitment of vascular components to the tumor bulk to sustain proliferation and growth.
5 Metabolic Contributions of the Microenvironment
Only more recently has the microenvironmental contribution to CSC metabolism been taken into consideration more carefully. The tumor microenvironment has been shown to not only promote a protective niche for GSC survival via vascular remodeling and hypoxia mediated signaling, but also to allow for a bi-directional metabolic processes. In breast CSCs, a symbiotic metabolic loop has been shown where more differentiated, glycolytic tumor cells in the normoxic edges of the tumor would feed metabolites towards the oxidative stem-like CSCs near the hypoxic cores [73], and similar studies have been reported in glioblastoma. Other studies have focused on CSCs that have already undergone epithelial-to-mesenchymal transition (EMT) being able to source metabolites from the extracellular microenvironment in order to fuel mitochondrial oxidation [74]. Catabolites such as pyruvate, lactate, glutamine, alanine, and/or ketone bodies have been shown to be secreted in the microenvironment from the surrounding endothelium and stroma and provides CSCs with fuel for metabolism, even under starvation conditions. Some studies have even shown exchange of mitochondrial DNA between the tumor microenvironment and tumor cells in order to support CSCs with compromised or diminished respiratory function, and this not only replenishes the mitochondrial function of these cells but also bolsters the self-renewal, tumor-initiating capacity, and treatment resistance of the CSCs [75,76]. This suggests that CSCs can perhaps exchange and/or internalize not only nutrients for metabolism but also energy-producing elements of the mitochondria in order to support its own metabolic requirements. This is a clear example of how the microenvironment supports the dynamic fluctuations of CSC metabolic demands and perhaps disruption of this communication could be one approach to exploiting these metabolic dependencies. This is also a very important aspect of CSC metabolism that would not be addressed in higher-passage, single cell culture of CSCs in vitro.
The metabolic fluctuations can also effect CSC interaction with immune components of the microenvironment. Metabolic stress, whether it be nutrient or treatment derived, has been shown to induce an immunosuppressive phenotype by shifting tumor infiltrating T-cells into tumor protecting Treg cells [77,78]. This could be adopted by CSCs wherein they accommodate a more glycolytic profile or resource energy from non-glucose carbon sources such as palmitate or other fatty acids in order to allow for a more accommodating immune microenvironment. Normal tumor cells may not be able to modulate their metabolism as readily or utilize microenvironmental alterations to glucose or oxygen accessibility to such effect. Additionally, microenvironmental inflammation and subsequent secretion of cytokines such as IL-6 and IL-8 along with NFκB activation has been shown to drive PI3K/Akt mediated glycolytic shifts in CSCs and promote CSC self-renewal [79,80,81]. More work in this area needs to be done but the idea that cytokine modulation in the microenvironment could again promote stemness and metabolic plasticity of CSCs lends more credence to the idea that the microenvironment-CSC crosstalk is a vital component of metabolism and resistance in many tumors.
Hypoxia is also very important in any discussion regarding metabolic reprogramming and plasticity. It has been indicated to promote neurosphere formation in vitro [82] as well as upregulating certain stem cell genes such as Sox2 and Oct4. Studies have shown both HIF1a and HIF2a are critical to proper GSC functioning. HIF1a stabilization was observed to enrich bulk tumors with more GSCs and HIF1a knockdown depleted GSC self-renewal and neurosphere formation [67,83]. This HIF stabilization is modulated in part via PI3K/Akt and ERK1/2 signaling [83]. It should be noted however that HIF1a is also critical for normal neural stem cell functioning so therapeutic inhibition is likely not a useful mechanism of treatment in this case [5,84]. HIF2a however is more specific to GSC maintenance and function. HIF2a specific stabilization was sufficient to increase expression of stem specific factors such as Nanog, Oct4, and Sox2 and studies have shown that despite HIF1/2 expression in the GSC niche, there is a preferential expression of HIF2a over HIF1a in the GSC niche when compared to the normal neural progenitor pools [5,85]. The specific importance of HIF2a in GSC maintenance and survival versus neural stem cell maintenance makes it a better potential therapeutic target for disrupting HIF-mediated signaling in the microenvironment.
Hypoxia also drives tumors towards a glycolytic profile, with most tumor microenvironments more acidic due to high levels of lactate produced and secreted into the tumor space. Recent studies have demonstrated that acidification of the tumor microenvironment by glycolytic osteosarcoma cells induces a negative feedback on glycolytic metabolism, associated with an increase in amino acid catabolism and urea cycle enhancement [86]. In this study, osteosarcoma cells under an acidic microenvironment were more epigenetically stable compared to normal cells and more sensitive to HDAC (histone deacetylase) inhibitors. Others have suggested that pharmacological targeting of specific carbon sources to the cancer cells may in fact cause a specific cellular response inducing resistance. They showed that acquired resistance to 3-bromopyruvate (3-BP), a common antiglycolytic agent, was mediated by hypermethylation of the gene responsible for specific drug transport of 3-BP into the cell [87]. This was one of the first observations of metabolic alterations in the microenvironment leading to epigenetic changes that could affect tumorigenicity and stemness.
Further studies have also shown that chronic acidosis strongly induced altered tumor metabolism via increased dependence on glutamine and fatty acids and decreased focus on glycolysis in CSCs and this shift was associated with global epigenetic changes of mitochondria related proteins relevant in tumor stress response mechanisms. Tumor acidification has been shown to promote GSC marker expression and self-renewal in gliomas with GSCs themselves facilitating a paracrine loop promoting further expression of HIF1/2a in the stem compartments. In vitro studies have also shown that modulating the pH can effect HIF2a expression and subsequently diminish GSC self-renewal capacity, suggesting maintaining a low pH is beneficial for tumors to maintain its pool of GSCs and ultimately maintain tumor growth and resistance.
6 Conclusion
Despite the controversies surrounding the cancer stem cell hypothesis and even specific arguments within the area of glioma stem cells, primarily dealing with expression marker profiles, there is a fundamental concept that should be understood. That concept is that of a more stem-like progenitor segment of the tumor population with more tumorigenic properties helping maintain if not drive tumor development. Many proposals have been presented as to the exact nature and origin of such subpopulations across different tumor types, and although there is a lack of consensus regarding specifics, it should not be surprising that there is great variance in what has been reported. In a disease that is predicated upon the idea of pathological growth via hijacked cellular mechanisms, it stands to reason that many of these mechanisms may be tissue specific, and the alterations tailored to the survival requirements and conditions of the particular localization. This is why any conversation about cancer and metabolism should also strongly consider microenvironmental context. Once these things are separated, isolated observations with respect to cellular stemness, metabolic preferences, and signaling dependencies will inevitably exhibit variability. In light of all this it’s vital to develop protocols and assays that try to reconcile technical artifacts and artificial laboratory changes with the true pathophysiological nature of cancer in the patient.
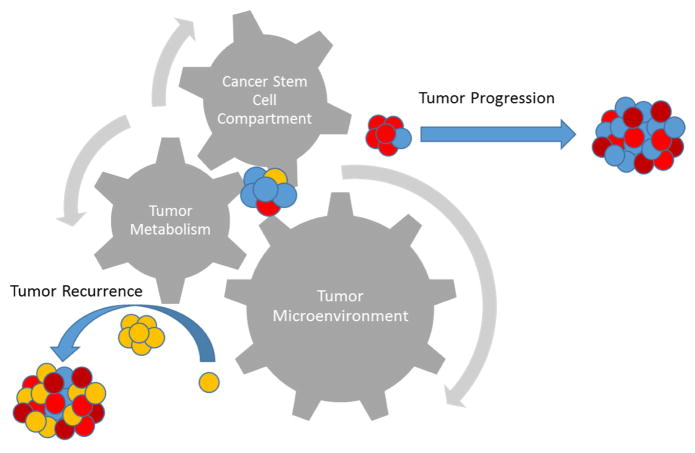
The tumor microenvironment represents a very dynamic space with many crucial cellular and non-cellular factors at play that determine the course of the tumor. It is one more significant part of the mechanism that allows for and helps propagate a highly dynamic and heterogeneous tumor background that further confounds attempts to deliver reliable therapies to treat cancer. The remerging fields of cancer metabolism and cancer stem cells are involved in this tumor microenvironment and the mechanisms described in both altered tumor metabolism and cancer stem cell maintenance cannot be separated from the cross-talk between the tumor and the surrounding microenvironment (Fig 3.). GSCs have been shown to be incredibly metabolically plastic with high adaptive capacity under nutrient and treatment induced stress. This plasticity is maintained and promoted through the microenvironmental cross-talk in the vascular and hypoxic compartments of tumors and studies that do not take this into consideration when attempting to address metabolic vulnerabilities or dependencies in cancer and cancer stem cells is incomplete. GSCs are valuable tools to study the fundamental mechanisms of tumor resistance and recurrence and strategies that could exploit metabolic vulnerabilities and create synthetic lethality present very exciting new prospects. However, in order to fully understand their importance and to translate any of these observations successfully into the clinic it is necessary to further assess what aspects of GSC metabolism require microenvironmental contributions and what these contributions are exactly. With better in vitro modeling of this dynamic system there is reason to be hopeful that clinically relevant targets focused on eradicating GSCs along with traditional treatment modalities will develop in time.
Figure 3.
Interplay between tumor metabolism and the tumor microenvironment as well as its effect on the cancer stem cell compartment is vital to properly understanding the complex dynamics of tumor progression and recurrence
Highlights.
Tumor heterogeneity is a major obstacle in glioma treatment and is partly driven and promoted by glioma stem cells (GSCs)
This heterogeneity constitutes not only genetic variability but also metabolic variability within a solid tumor
Metabolic plasticity of GSCs driven by intrinsic factors as well as extrinsic signaling from the glioma microenvironment
Any therapeutic strategy targeting GSCs requires understanding of metabolic variation and crosstalk between the glioma and its microenvironment
Acknowledgments
Research in the lab related to the contents of this review is supported by a grant from FasterCures and the Milken Family Foundation (to JY) and a grant from the National Institute for Neurological Disorders and Stroke (NIH) (2R01# NS048959 to JY).
Footnotes
8 Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
9 Author Contributions
The majority of content in this review was researched and written by TT with critical review from JY.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stupp Roger, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. The lancet oncology. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 2.Hardee Matthew E, Zagzag David. Mechanisms of glioma-associated neovascularization. The American journal of pathology. 2012;181(4):1126–1141. doi: 10.1016/j.ajpath.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lathia Justin D, et al. Direct in vivo evidence for tumor propagation by glioblastoma cancer stem cells. PloS one. 2011;6(9):e24807. doi: 10.1371/journal.pone.0024807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calabrese Christopher, et al. A perivascular niche for brain tumor stem cells. Cancer cell. 2007;11(1):69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 5.Li Zhizhong, et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer cell. 2009;15(6):501–513. doi: 10.1016/j.ccr.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vescovi Angelo L, Galli Rossella, Reynolds Brent A. Brain tumour stem cells. Nature Reviews Cancer. 2006;6(6):425–436. doi: 10.1038/nrc1889. [DOI] [PubMed] [Google Scholar]
- 7.Schonberg David L, et al. Brain tumor stem cells: Molecular characteristics and their impact on therapy. Molecular aspects of medicine. 2014;39:82–101. doi: 10.1016/j.mam.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beier Dagmar, et al. Efficacy of clinically relevant temozolomide dosing schemes in glioblastoma cancer stem cell lines. Journal of neuro-oncology. 2012;109(1):45–52. doi: 10.1007/s11060-012-0878-4. [DOI] [PubMed] [Google Scholar]
- 9.Chen Jian, et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488(7412):522–526. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peiris-Pagès Maria, et al. Cancer stem cell metabolism. Breast Cancer Research. 2016;18(1):55. doi: 10.1186/s13058-016-0712-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warburg O, Posener K, Negelein E. Ueber den Stoffwechsel der Tumoren. Biochem. 1924;Z152:319–344. [Google Scholar]
- 12.Vlashi Erina, et al. Metabolic state of glioma stem cells and nontumorigenic cells. Proceedings of the National Academy of Sciences. 2011;108(38):16062–16067. doi: 10.1073/pnas.1106704108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aguilar-Gallardo Cristóbal, Simón Carlos. Seminars in reproductive medicine. 01. Vol. 31. Thieme Medical Publishers; 2013. Cells, stem cells, and cancer stem cells. [DOI] [PubMed] [Google Scholar]
- 14.Sell Stewart. Stem cell origin of cancer and differentiation therapy. Critical reviews in oncology/hematology. 2004;51(1):1–28. doi: 10.1016/j.critrevonc.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Wicha Max S, Liu Suling, Dontu Gabriela. Cancer stem cells: an old idea—a paradigm shift. Cancer research. 2006;66(4):1883–1890. doi: 10.1158/0008-5472.CAN-05-3153. [DOI] [PubMed] [Google Scholar]
- 16.Pattabiraman Diwakar R, Weinberg Robert A. Tackling the cancer stem cells—what challenges do they pose? Nature reviews Drug discovery. 2014;13(7):497–512. doi: 10.1038/nrd4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kreso Antonija, Dick John E. Evolution of the cancer stem cell model. Cell stem cell. 2014;14(3):275–291. doi: 10.1016/j.stem.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Wang Weikang, et al. Dynamics between cancer cell subpopulations reveals a model coordinating with both hierarchical and stochastic concepts. PloS one. 2014;9(1):e84654. doi: 10.1371/journal.pone.0084654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh Sheila K, et al. Cancer stem cells in nervous system tumors. Oncogene. 2004;23(43):7267–7273. doi: 10.1038/sj.onc.1207946. [DOI] [PubMed] [Google Scholar]
- 20.Gangemi Rosaria Maria Rita, et al. SOX2 silencing in glioblastoma tumor-initiating cells causes stop of proliferation and loss of tumorigenicity. Stem cells. 2009;27(1):40–48. doi: 10.1634/stemcells.2008-0493. [DOI] [PubMed] [Google Scholar]
- 21.Lathia Justin D, et al. Direct in vivo evidence for tumor propagation by glioblastoma cancer stem cells. PloS one. 2011;6(9):e24807. doi: 10.1371/journal.pone.0024807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Shengwen Calvin, et al. Cancer stem cells from a rare form of glioblastoma multiforme involving the neurogenic ventricular wall. Cancer cell international. 2012;12(1):41. doi: 10.1186/1475-2867-12-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang Qiang, et al. Glioma stem cells are more aggressive in recurrent tumors with malignant progression than in the primary tumor, and both can be maintained long-term in vitro. BMC cancer. 2008;8(1):304. doi: 10.1186/1471-2407-8-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Rong, et al. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010;468(7325):829–833. doi: 10.1038/nature09624. [DOI] [PubMed] [Google Scholar]
- 25.Lottaz Claudio, et al. Transcriptional profiles of CD133+ and CD133- glioblastoma-derived cancer stem cell lines suggest different cells of origin. Cancer research. 2010;70(5):2030–2040. doi: 10.1158/0008-5472.CAN-09-1707. [DOI] [PubMed] [Google Scholar]
- 26.Chandran Uma R, et al. Gene expression profiling distinguishes proneural glioma stem cells from mesenchymal glioma stem cells. Genomics data. 2015;5:333–336. doi: 10.1016/j.gdata.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saga Isako, et al. Integrated analysis identifies different metabolic signatures for tumor-initiating cells in a murine glioblastoma model. Neuro-oncology. 2014;16(8):1048–1056. doi: 10.1093/neuonc/nou096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Endaya Berwini B, et al. Transcriptional profiling of dividing tumor cells detects intratumor heterogeneity linked to cell proliferation in a brain tumor model. Molecular oncology. 2016;10(1):126–137. doi: 10.1016/j.molonc.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sugimori Michiya, et al. Discovery of power-law growth in the self-renewal of heterogeneous glioma stem cell populations. PloS one. 2015;10(8):e0135760. doi: 10.1371/journal.pone.0135760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanahan Douglas, Weinberg Robert A. Hallmarks of cancer: the next generation. cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Lagadec C, et al. TrkA overexpression enhances growth and metastasis of breast cancer cells. Oncogene. 2009;28(18):1960–1970. doi: 10.1038/onc.2009.61. [DOI] [PubMed] [Google Scholar]
- 32.Shlomi Tomer, et al. Genome-scale metabolic modeling elucidates the role of proliferative adaptation in causing the Warburg effect. PLoS computational biology. 2011;7(3):e1002018. doi: 10.1371/journal.pcbi.1002018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keibler Mark A, et al. Metabolic requirements for cancer cell proliferation. Cancer & metabolism. 2016;4(1):16. doi: 10.1186/s40170-016-0156-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ciavardelli D, et al. Breast cancer stem cells rely on fermentative glycolysis and are sensitive to 2-deoxyglucose treatment. Cell death & disease. 2014;5(7):e1336. doi: 10.1038/cddis.2014.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Emmink Benjamin L, et al. The secretome of colon cancer stem cells contains drug-metabolizing enzymes. Journal of proteomics. 2013;91:84–96. doi: 10.1016/j.jprot.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 36.Liao Jianqun, et al. Ovarian cancer spheroid cells with stem cell-like properties contribute to tumor generation, metastasis and chemotherapy resistance through hypoxia-resistant metabolism. PloS one. 2014;9(1):e84941. doi: 10.1371/journal.pone.0084941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palorini Roberta, et al. Energy metabolism characterization of a novel cancer stem cell-like line 3AB-OS. Journal of cellular biochemistry. 2014;115(2):368–379. doi: 10.1002/jcb.24671. [DOI] [PubMed] [Google Scholar]
- 38.Zhou Yunfei, et al. Metabolic alterations in highly tumorigenic glioblastoma cells: preference for hypoxia and high dependency on glycolysis. Journal of Biological Chemistry. 2011;286(37):32843–32853. doi: 10.1074/jbc.M111.260935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Chenguang, et al. Cyclin D1 repression of nuclear respiratory factor 1 integrates nuclear DNA synthesis and mitochondrial function. Proceedings of the National Academy of Sciences. 2006;103(31):11567–11572. doi: 10.1073/pnas.0603363103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakamaki Toshiyuki, et al. Cyclin D1 determines mitochondrial function in vivo. Molecular and cellular biology. 2006;26(14):5449–5469. doi: 10.1128/MCB.02074-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee WT, St John J. The control of mitochondrial DNA replication during development and tumorigenesis. Ann N Y Acad Sci. 2015;1350:95–106. doi: 10.1111/nyas.12873. [DOI] [PubMed] [Google Scholar]
- 42.De Luca Arianna, et al. Mitochondrial biogenesis is required for the anchorage-independent survival and propagation of stem-like cancer cells. Oncotarget. 2015;6(17):14777. doi: 10.18632/oncotarget.4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Janiszewska Michalina, et al. Imp2 controls oxidative phosphorylation and is crucial for preserving glioblastoma cancer stem cells. Genes & development. 2012;26(17):1926–1944. doi: 10.1101/gad.188292.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lagadinou Eleni D, et al. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell stem cell. 2013;12(3):329–341. doi: 10.1016/j.stem.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lamb Rebecca, et al. Mitochondrial mass, a new metabolic biomarker for stem-like cancer cells: Understanding WNT/FGF-driven anabolic signaling. Oncotarget. 2015;6(31):30453. doi: 10.18632/oncotarget.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pastò Anna, et al. Cancer stem cells from epithelial ovarian cancer patients privilege oxidative phosphorylation, and resist glucose deprivation. Oncotarget. 2014;5(12):4305. doi: 10.18632/oncotarget.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sancho Patricia, et al. MYC/PGC-1α balance determines the metabolic phenotype and plasticity of pancreatic cancer stem cells. Cell metabolism. 2015;22(4):590–605. doi: 10.1016/j.cmet.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 48.Vlashi Erina, et al. Metabolic differences in breast cancer stem cells and differentiated progeny. Breast cancer research and treatment. 2014;146(3):525–534. doi: 10.1007/s10549-014-3051-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.LeBleu Valerie S, et al. PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nature cell biology. 2014;16(10):992–1003. doi: 10.1038/ncb3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wey Alice, Knoepfler Paul S. c-myc and N-myc promote active stem cell metabolism and cycling as architects of the developing brain. Oncotarget. 2010;1(2):120. doi: 10.18632/oncotarget.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grazia Cipolleschi Maria, et al. Hypoxia-resistant profile implies vulnerability of cancer stem cells to physiological agents, which suggests new therapeutic targets. Cell cycle. 2014;13(2):268–278. doi: 10.4161/cc.27031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flavahan William A, et al. Brain tumor initiating cells adapt to restricted nutrition through preferential glucose uptake. Nature neuroscience. 2013;16(10):1373–1382. [Google Scholar]
- 53.Dong Chenfang, et al. Loss of FBP1 by Snail-mediated repression provides metabolic advantages in basal-like breast cancer. Cancer cell. 2013;23(3):316–331. doi: 10.1016/j.ccr.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dimitrov Lilia, et al. New developments in the pathogenesis and therapeutic targeting of the IDH1 mutation in glioma. International journal of medical sciences. 2015;12(3):201. doi: 10.7150/ijms.11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Molenaar Remco J, et al. The driver and passenger effects of isocitrate dehydrogenase 1 and 2 mutations in oncogenesis and survival prolongation. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer. 2014;1846(2):326–341. doi: 10.1016/j.bbcan.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 56.Guo Changcun, et al. Isocitrate dehydrogenase mutations in gliomas: mechanisms, biomarkers and therapeutic target. Current opinion in neurology. 2011;24(6):648. doi: 10.1097/WCO.0b013e32834cd415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yan Hai, et al. IDH1 and IDH2 mutations in gliomas. New England Journal of Medicine. 2009;360(8):765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Christensen Karina, Schrøder Henrik Daa, Kristensen Bjarne Winther. CD133+ niches and single cells in glioblastoma have different phenotypes. Journal of neuro-oncology. 2011;104(1):129–143. doi: 10.1007/s11060-010-0488-y. [DOI] [PubMed] [Google Scholar]
- 59.Jain Rakesh K, et al. Angiogenesis in brain tumours. Nature Reviews Neuroscience. 2007;8(8):610–622. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 60.Carmeliet Peter, Jain Rakesh K. Angiogenesis in cancer and other diseases. nature. 2000;407(6801):249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 61.Fidoamore Alessia, et al. Glioblastoma stem cells microenvironment: the paracrine roles of the niche in drug and radioresistance. Stem cells international. 2016;2016 doi: 10.1155/2016/6809105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clement Virginie, et al. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Current biology. 2007;17(2):165–172. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ulasov Ilya V, et al. Inhibition of Sonic hedgehog and Notch pathways enhances sensitivity of CD133+ glioma stem cells to temozolomide therapy. Molecular medicine. 2011;17(1–2):103. doi: 10.2119/molmed.2010.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Toyoda K, Tanaka K, Nakagawa S, et al. Initial contact of glioblastoma cells with existing normal brain endothelial cells strengthen the barrier function via fibroblast growth factor 2 secretion: a new in vitro blood-brain barrier model. Cellular and Molecular Neurobiology. 2013;33(4):489–501. doi: 10.1007/s10571-013-9913-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pollard Steven M, et al. Glioma stem cell lines expanded in adherent culture have tumor-specific phenotypes and are suitable for chemical and genetic screens. Cell stem cell. 2009;4(6):568–580. doi: 10.1016/j.stem.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 66.Chang Kai-Wei, et al. Fibroblast growth factor-2 up-regulates the expression of nestin through the Ras–Raf–ERK–Sp1 signaling axis in C6 glioma cells. Biochemical and biophysical research communications. 2013;434(4):854–860. doi: 10.1016/j.bbrc.2013.04.031. [DOI] [PubMed] [Google Scholar]
- 67.Bar Eli E. Glioblastoma, cancer stem cells and hypoxia. Brain Pathology. 2011;21(2):119–129. doi: 10.1111/j.1750-3639.2010.00460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Rong, et al. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010;468(7325):829–833. doi: 10.1038/nature09624. [DOI] [PubMed] [Google Scholar]
- 69.Ricci-Vitiani Lucia, et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468(7325):824–828. doi: 10.1038/nature09557. [DOI] [PubMed] [Google Scholar]
- 70.Sanai Nader, Alvarez-Buylla Arturo, Berger Mitchel S. Neural stem cells and the origin of gliomas. New England Journal of Medicine. 2005;353(8):811–822. doi: 10.1056/NEJMra043666. [DOI] [PubMed] [Google Scholar]
- 71.Brat Daniel J, et al. Pseudopalisades in glioblastoma are hypoxic, express extracellular matrix proteases, and are formed by an actively migrating cell population. Cancer research. 2004;64(3):920–927. doi: 10.1158/0008-5472.can-03-2073. [DOI] [PubMed] [Google Scholar]
- 72.Rohwer Nadine, Cramer Thorsten. Hypoxia-mediated drug resistance: novel insights on the functional interaction of HIFs and cell death pathways. Drug Resistance Updates. 2011;14(3):191–201. doi: 10.1016/j.drup.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 73.Gordon Nicole, et al. Gene expression signatures of breast cancer stem and progenitor cells do not exhibit features of Warburg metabolism. Stem cell research & therapy. 2015;6(1):157. doi: 10.1186/s13287-015-0153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cuyàs Elisabet, Corominas-Faja Bruna, Menendez Javier A. The nutritional phenome of EMT-induced cancer stem-like cells. Oncotarget. 2014;5(12):3970. doi: 10.18632/oncotarget.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pasquier Jennifer, et al. Preferential transfer of mitochondria from endothelial to cancer cells through tunneling nanotubes modulates chemoresistance. Journal of translational medicine. 2013;11(1):94. doi: 10.1186/1479-5876-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tan An S, et al. Mitochondrial genome acquisition restores respiratory function and tumorigenic potential of cancer cells without mitochondrial DNA. Cell metabolism. 2015;21(1):81–94. doi: 10.1016/j.cmet.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 77.Shi Lewis Z, et al. HIF1α–dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. Journal of Experimental Medicine. 2011;208(7):1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Tingting, Liu Guangwei, Wang Ruoning. The intercellular metabolic interplay between tumor and immune cells. Frontiers in immunology. 2014;5 doi: 10.3389/fimmu.2014.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aguilar-Gallardo Cristóbal, Simón Carlos. Seminars in reproductive medicine. 01. Vol. 31. Thieme Medical Publishers; 2013. Cells, stem cells, and cancer stem cells. [DOI] [PubMed] [Google Scholar]
- 80.Mauer Jan, Denson Jesse L, Brüning Jens C. Versatile functions for IL-6 in metabolism and cancer. Trends in immunology. 2015;36(2):92–101. doi: 10.1016/j.it.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 81.Martinez-Outschoorn Ubaldo E, et al. Cytokine production and inflammation drive autophagy in the tumor microenvironment: role of stromal caveolin-1 as a key regulator. Cell Cycle. 2011;10(11):1784–1793. doi: 10.4161/cc.10.11.15674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McCord Amy M, et al. Physiologic oxygen concentration enhances the stem-like properties of CD133+ human glioblastoma cells in vitro. Molecular Cancer Research. 2009;7(4):489–497. doi: 10.1158/1541-7786.MCR-08-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Soeda A, et al. Hypoxia promotes expansion of the CD133-positive glioma stem cells through activation of HIF-1α. Oncogene. 2009;28(45):3949–3959. doi: 10.1038/onc.2009.252. [DOI] [PubMed] [Google Scholar]
- 84.Zhao Tong, et al. Hypoxia-driven proliferation of embryonic neural stem/progenitor cells–role of hypoxia-inducible transcription factor-1α. The FEBS journal. 2008;275(8):1824–1834. doi: 10.1111/j.1742-4658.2008.06340.x. [DOI] [PubMed] [Google Scholar]
- 85.Seidel Sascha, et al. A hypoxic niche regulates glioblastoma stem cells through hypoxia inducible factor 2α. Brain. 2010;133(4):983–995. doi: 10.1093/brain/awq042. [DOI] [PubMed] [Google Scholar]
- 86.Chano Tokuhiro, et al. Tumour-specific metabolic adaptation to acidosis is coupled to epigenetic stability in osteosarcoma cells. American journal of cancer research. 2016;6(4):859. [PMC free article] [PubMed] [Google Scholar]
- 87.Baldini Nicola, et al. Metabolism and microenvironment in cancer plasticity. Cancer & metabolism. 2016;4(1):1. [Google Scholar]