See Mander et al. (doi:10.1093/awx174) for a scientific commentary on this article.
Acute sleep deprivation increases amyloid-ß, suggesting that this relationship may underlie the observed association between chronic sleep disruption and Alzheimer’s disease. Ju, Ooms et al. report that specific disruption of slow wave sleep increases amyloid-ß in human CSF, while sub-acute sleep disruption is associated with increased CSF tau.
Keywords: slow wave activity, sleep, beta-amyloid, tau, EEG
Abstract
See Mander et al. (doi:10.1093/awx174) for a scientific commentary on this article.
Sleep deprivation increases amyloid-β, suggesting that chronically disrupted sleep may promote amyloid plaques and other downstream Alzheimer’s disease pathologies including tauopathy or inflammation. To date, studies have not examined which aspect of sleep modulates amyloid-β or other Alzheimer’s disease biomarkers. Seventeen healthy adults (age 35–65 years) without sleep disorders underwent 5–14 days of actigraphy, followed by slow wave activity disruption during polysomnogram, and cerebrospinal fluid collection the following morning for measurement of amyloid-β, tau, total protein, YKL-40, and hypocretin. Data were compared to an identical protocol, with a sham condition during polysomnogram. Specific disruption of slow wave activity correlated with an increase in amyloid-β40 (r = 0.610, P = 0.009). This effect was specific for slow wave activity, and not for sleep duration or efficiency. This effect was also specific to amyloid-β, and not total protein, tau, YKL-40, or hypocretin. Additionally, worse home sleep quality, as measured by sleep efficiency by actigraphy in the six nights preceding lumbar punctures, was associated with higher tau (r = 0.543, P = 0.045). Slow wave activity disruption increases amyloid-β levels acutely, and poorer sleep quality over several days increases tau. These effects are specific to neuronally-derived proteins, which suggests they are likely driven by changes in neuronal activity during disrupted sleep.
Introduction
Alzheimer’s disease pathology is associated with sleep disruption, even in the preclinical stages of disease (Ju et al., 2013; Spira et al., 2013). Amyloid plaque formation, an early necessary step in Alzheimer’s disease pathogenesis, is associated with sleep disruption in a bi-directional manner (Ju et al., 2014). Soluble amyloid-β varies diurnally in both mouse and human (Kang et al., 2009; Huang et al., 2012), where sleep is associated with decreased amyloid-β, and wakefulness with increased amyloid-β. Sleep deprivation acutely increases soluble amyloid-β and chronically increases amyloid plaques in mouse (Kang et al., 2009; Roh et al., 2014), and one night of total sleep deprivation increases soluble amyloid-β in humans (Ooms et al., 2014). However, it is unknown which aspect of sleep is responsible for modulation of amyloid-β. Slow wave activity (SWA), which occurs during deep non-REM sleep, is a strong candidate for amyloid-β modulation. The EEG slow waves that characterize SWA represent decreased synaptic activity (Nir et al., 2011), and soluble amyloid-β is released into the interstitial space during neuronal synaptic activity (Cirrito et al., 2005). Furthermore, clearance of solutes from the interstitial space, including exogenous amyloid-β, accelerates during SWA-rich sleep (Xie et al., 2013). These data lead to the hypothesis that disrupted SWA would cause relatively increased neuronal activity, increased amyloid-β release and decreased clearance, resulting in increased amyloid-β levels measurable in the CSF. Indeed, in cross-sectional studies, SWA was negatively correlated with CSF amyloid-β in middle-aged (Ju et al., 2016) and older (Varga et al., 2016) individuals. Furthermore, chronic sleep disruption increases tau levels and tau phosphorylation in mice (Rothman et al., 2013; Qiu et al., 2016). Tau is also released during neuronal activity, although clearance from the brain is slower than for amyloid-β (Yamada et al., 2014).
In this study, we used a novel SWA disruption protocol to test whether specific disruption of SWA leads to increased amyloid-β and tau levels in CSF.
Materials and methods
Participants
We enrolled 22 participants aged 35–65 years from a community-based research registry at Washington University. Participants had no comorbidities except controlled hypertension, took no neuro-active medications, had normal neurological exams, reported ≤14 alcoholic beverages weekly, had body mass index 18–40 kg/m2, and were cognitively normal based on history, neurological examination, and Mini-Mental State Examination (MMSE) ≥27/30 (Folstein et al., 2004). Participants had regular sleep schedules with bedtime 8 pm–12 am and wake time 4 am–8 pm. Participants did not have obstructive sleep apnoea or periodic limb movement disorder, defined as apnoea-hypopnoea index ≥5 or periodic limb movement index ≥15, during a screening polysomnogram. All participants provided informed, written consent. All procedures were approved by the Washington University Human Research Protection Office.
Experimental design
All participants underwent two sets of procedures, ≥28 days apart. Each procedure set consisted of a daytime visit with questionnaires, actigraphy for 5–14 days, overnight polysomnogram with experimental condition, and lumbar puncture the following morning. An automated SWA disruption protocol was applied for one polysomnogram, and a sham condition was used for the other, in random order. SWA disruption was performed with parameters and methods determined to be effective in this age group (Ooms et al., 2017). Briefly, every 10 s, EEG data were extracted from the live recording. If the data were determined be non-artefactual, and 0.5–4 Hz spectral power was >100 μV2×s indicating SWA, a tone was delivered through earphones to the participant. The amplitude of the tones would progressively increase, until delta power decreased, indicating an arousal out of SWA. During sham condition, participants wore earphones but no tones were delivered. The order of the two conditions was random, and participants were blinded to the condition. All analyses were conducted blinded to condition.
Sleep and EEG spectral analysis
Polysomnograms and sleep staging by a registered polysomnographic technologist were performed according to standard criteria (Iber, 2007). Lights out was at 10 pm, lights on at 6 am. SWA was quantified as spectral power in the delta (0.5–4 Hz) band, or ‘delta power’, averaged over all non-REM epochs, from bilateral frontal and central electrodes. EEG collection and analysis parameters are detailed in the Supplementary material.
Actigraphy data were collected using Actiwatch2™ (Philips-Respironics) and scored as previously described (Ju et al., 2013; see Supplementary material). Participants were instructed to keep regular sleep schedules prior to polysomnograms. If actigraphy did not demonstrate ≥6 h in bed and bedtime 8 pm–12 am on the night prior to either polysomnogram, the participant was excluded. This criterion excluded four participants.
To assess home sleep, actigraphy data from six nights (polysomnogram night and five preceding nights) were scored using polysomnographically-validated criteria (Kushida et al., 2001) to derive total sleep time and sleep efficiency (total sleep time divided by time in bed). Time in bed was calculated as minutes between bedtime and wake time, mid-sleep as the halfway-point between bedtime and wake time. Three participants wore a defective actigraph that prevented determination of total sleep time and sleep efficiency.
CSF analytes and APOE genotype
CSF was obtained by lumbar puncture at 9:30–10 am following polysomnography. This time corresponds to the daily nadir of CSF amyloid-β40 (Huang et al., 2012), and follows the sleep mid-point by ∼6 h, the transit time of amyloid-β from CNS to CSF (Bateman et al., 2006). CSF was immediately placed on ice, aliquoted, and frozen at −80°C. Amyloid-β40 was chosen as the primary measure of amyloid-β since it is the most abundant amyloid-β species and less subject to altered levels caused by amyloid plaques. Amyloid-β40, amyloid-β42, and tau, were assessed by INNOTEST® ELISA (Fujirebio). Total protein was assessed by Bradford assay (Thermo Pierce). YKL-40, a glial marker of neuroinflammation, was assessed by MicroVue ELISA (Quidel) (Sutphen et al., 2015). Hypocretin-1 was assessed by radioimmunoassay as previously described (Mignot et al., 2002). All CSF measurements were performed in duplicate or triplicate with several internal control samples.
As existing amyloid plaques abolish the diurnal variation of amyloid-β related to sleep, (Huang et al., 2012; Roh et al., 2012), individuals with abnormally low amyloid-β42 levels (<608 pg/ml) indicating amyloid plaques were excluded. Cut-off level was determined by the Knight Alzheimer’s Disease Research Center Biomarker Core by comparison of amyloid-β42 values and Pittsburgh compound B PET data from large cohorts (Fagan et al., 2006; Schindler et al., 2016). Two participants were excluded based on this criterion; one had already been excluded for inadequate actigraphically-measured sleep.
Fasted blood (20 ml) was drawn following lumbar puncture, and placed on ice until centrifugation and storage at −80°C. The Hope Center DNA/RNA Purification Core performed DNA extraction and APOE genotyping. Genotype was dichotomized as APOE ɛ4 carrier or non-carrier.
Questionnaires
The Epworth Sleepiness Scale (Johns, 1991) and Pittsburgh Sleep Quality Index (Buysse et al., 1989) were completed during the daytime visits 5–14 days prior to each polysomnogram to assess sleepiness and sleep quality, respectively. Immediately following each polysomnogram, participants completed the Stanford Sleepiness Scale (Hoddes et al., 1973), a measure of immediate subjective sleepiness. They also completed a written questionnaire which asked: ‘Do you recall being woken during the night because of the noises through the earphones?’; ‘If yes, how many times do you think you woke up because of the noises?’; estimated sleep time in hours; and Sleep quality on a scale of 1–10 (10 best).
Statistical analysis
All continuous variables were assessed for normal distribution by inspection of histograms and the Kolmogorov-Smirnov test. Levene’s test was used to assess equality of variances. Differences between SWA disruption and sham conditions for continuous variables were calculated using paired t-tests for normally-distributed variables, and related-samples Wilcoxon signed rank test for other variables. Correlations were assessed with Spearman’s correlation coefficient. To compare participants grouped by order of polysomnogram condition, APOE genotype, or response to SWA disruption, unpaired t-tests were used to compare continuous variables and Fisher exact tests were used to compare dichotomous variables. Tests were two-tailed, and α was 0.05. Statistical analyses were performed with SPSS 24 (IBM). Figures were generated in GraphPad Prism (GraphPad Software Inc).
As interindividual amyloid-β variation (∼30%) is much greater than intraindividual amyloid-β variation (∼10%), and may be subject to APOE genotype (Osorio et al., 2014), a repeated-measures design was selected. From preliminary cross-sectional data, the effect size for variance in amyloid-β40 explained by SWA was f = 0.25. Assuming a moderate (0.7) correlation between repeated measures, α 0.05, and power 0.8, a total sample size of 20 was calculated. We stopped enrolment when 20 participants had completed the study, and two others already enrolled were permitted to complete the study, for a total of 22 participants.
Results
Twenty-two participants completed the study; five participants were excluded for predefined exclusion criteria, for a sample size of 17. The study population was middle-aged (54.1 ± 6.7 years) with a slight majority Caucasian race and female sex (65% each), and five (29%) were APOE ɛ4 carriers. Questionnaire-based sleep measures were in the normal range and actigraphy showed normal home sleep patterns (Table 1).
Table 1.
Demographic and sleep characteristics
| Sham condition Mean ± SD | SWA disruption Mean ± SD | P-value | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 54.1 ± 6.7 | n/a | |
| Race White n (%) | 11 (65) | n/a | |
| Sex female n (%) | 11 (65) | n/a | |
| Body mass index (kg/m2) | 28.2 ± 4.3 | n/a | |
| Education (years) | 15.8 ± 1.6 | n/a | |
| Alcohol (drinks/week) | 1.6 ± 2.1 | n/a | |
| MMSE | 29.6 ± 0.5 | n/a | |
| APOE ɛ4 carrier n (%) | 5 (29) | n/a | |
| Polysomnogram | |||
| Total sleep time (min) | 419 ± 47 | 404 ± 51 | 0.160 |
| non-REM sleep (min) | 311 ± 39 | 300 ± 49 | 0.244 |
| N1 (min) | 22 ± 10 | 34 ± 18 | 0.007 |
| N2 (min) | 265 ± 44 | 254 ± 43 | 0.33 |
| N3 (min) | 7 ± 42a | 0 ± 2a | <0.001a |
| REM (min) | 99 ± 52a | 68 ± 38a | 0.001a |
| Sleep efficiency (%) | 85.4 ± 8.5 | 82.4 ± 9.6 | 0.102 |
| Number tones delivered | 0 | 1177 ± 427 | n/a |
| EEG spectral power | |||
| 0.5–4 Hz (Delta) (μV2×s) | 118 ± 51 | 96 ± 43 | <0.001 |
| 4–8 Hz (μV2×s) | 12.8 ± 5.4 | 12.0 ± 5.4 | 0.215 |
| 8–12 Hz (μV2×s) | 6.2 ± 3.0 | 6.4 ± 3.7 | 0.535 |
| 12–18 Hz (μV2×s) | 2.7 ± 1.2 | 3.0 ± 1.3 | 0.005 |
| 18–32 Hz (μV2×s) | 1.7 ± 0.8 | 2.1 ± 0.9 | 0.008 |
| Actigraphy (n = 14) | |||
| Total sleep time (min), mean six nights | 377 ± 56 | 370 ± 43 | 0.445 |
| Total sleep time (min), polysomnogram night | 422 ± 42 | 404 ± 41 | 0.117 |
| Sleep efficiency (%), mean six nights | 82.6 ± 7.5 | 82.0 ± 6.7 | 0.655 |
| Time in bed (min)b | 456 ± 46 | 454 ± 37 | 0.836 |
| Mid-sleep (hh:min)b | 03:48 am ± 00:23 | 03:47 am ± 00:19 | 0.836 |
| Questionnaires | |||
| Pittsburgh Sleep Quality Indexc | 4.2 ± 1.6 | 3.4 ± 1.8 | 0.059 |
| Epworth Sleepiness Scalec | 5.6 ± 3.6 | 6.4 ± 3.4 | 0.133 |
| Awakened by noise? (yes)d | 1/16 | 12/15 | <0.001 |
| Number tones heard?d | 3 (n = 1) | 11.1 ± 8.9 | n/a |
| Estimated sleep hours | 7.3 ± 1.1 | 5.2 ± 2 | <0.001 |
| Estimated sleep quality | 7.4 ± 1.7 | 5.5 ± 2.7 | 0.02 |
| Stanford Sleepiness Scale | 2.0 ± 0.9 | 2.8 ± 1.8 | 0.16 |
aMedian ± interquartile range; P-value is for related-samples Wilcoxon signed rank test.
bn = 17.
cCompleted during daytime 5–14 days prior to polysomnogram nights.
dNot all participants answered this question.
The SWA disruption protocol decreased SWA as measured by delta power by 23 (95% confidence interval 14–32) μV2×s. As expected, there was decreased N3 sleep with a compensatory increase in N1 sleep during the SWA disruption night, and a decrease in REM sleep; otherwise there was no difference in other polysomnographic sleep variables (Table 1). The participants who underwent SWA disruption first were similar to the participants who underwent sham condition first (Supplementary Table 1). Participants estimated sleep was of shorter duration and worse quality with SWA disruption, although subjective sleepiness was no different in the morning (Table 1).
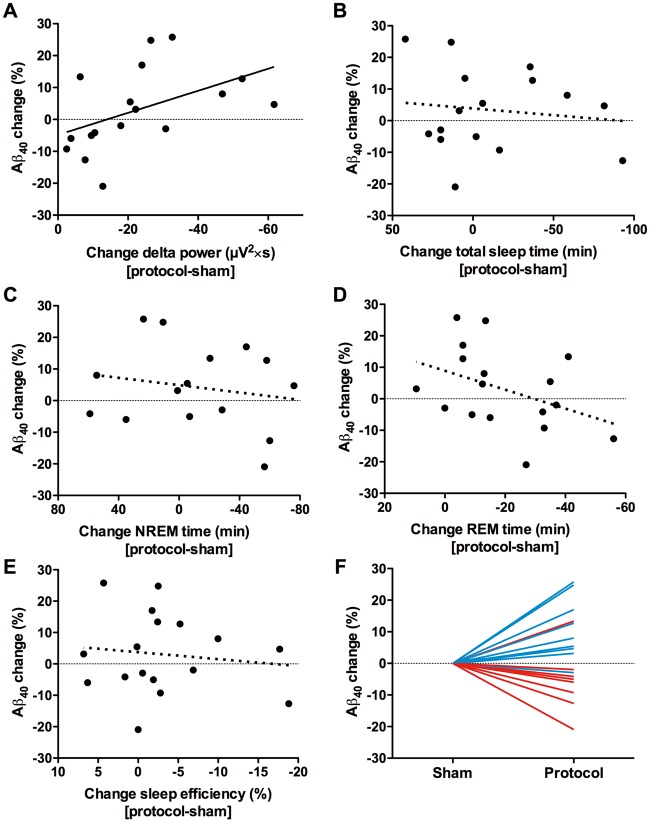
SWA disruption was strongly and significantly correlated with amyloid-β40, such that more SWA disruption was associated with greater increases in amyloid-β40 (Fig. 1A). This effect was specific for SWA disruption, and not for total sleep time, non-REM time, REM time, or sleep efficiency (Fig. 1B–E). While in the entire group there was no significant difference in amyloid-β40 levels between conditions, the subset with sufficient SWA disruption (greater than median, or ≥20 μV2×s decrease) had a significant increase in amyloid-β40 (Fig. 1F). These ‘responders’ to the SWA disruption protocol essentially drove the correlation between SWA disruption and amyloid-β40 change.
Figure 1.
Decreased slow wave activity is associated with increased CSF amyloid-β. (A) Suppression of slow wave activity, as measured by the change in delta spectral power, was strongly correlated with increased amyloid-β40 (r = 0.610, P = 0.009). There was no correlation between change in amyloid-β40 levels and (B) total sleep time (r = −0.075, P = 0.782), (C) time in non-REM sleep (r = −0.156, P = 0.564), (D) time in REM sleep (r = −0.351 P = 0.168), or (E) sleep efficiency (r = −0.007, P = 0.978). (F) When participants are divided at the median (20 μV2×s) amount of slow wave activity disruption, the ‘responders’ to SWA disruption (blue lines) had a significant increase in amyloid-β40 (n = 9, 11562 ± 2603 versus 10562 ± 2868 pg/ml, 95% confidence interval difference 315 to 1686 pg/ml, P = 0.010) while ‘non-responders’ (red lines) did not. X-axes in A–E are more negative to the right, i.e. values to the right indicate more disruption of slow wave activity or less sleep.
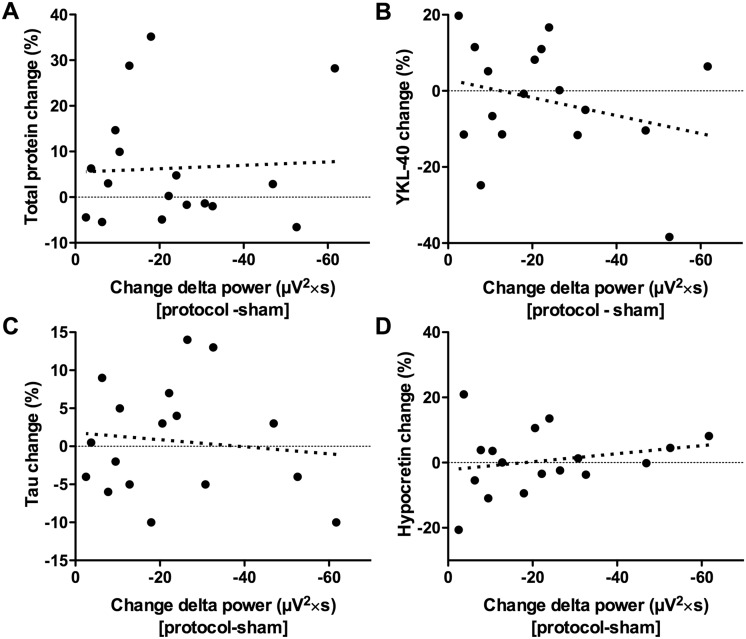
We then assessed whether the effect of SWA on amyloid-β40 was specific to amyloid-β. There was no correlation between SWA disruption and change in total protein (Fig. 2A). YKL-40 (an astrocyte-derived inflammatory protein) and tau (a neuronally-derived protein), both biomarkers increased in Alzheimer’s disease, were not correlated with SWA disruption (Fig. 2B and C). Hypocretin, a wake-promoting peptide that may modulate amyloid-β kinetics and is increased after REM sleep deprivation (Pedrazzoli et al., 2004; Roh et al., 2014), was not correlated with SWA disruption (Fig. 2D). Supporting the relationship between SWA and amyloid-β, in addition to the strong correlation between SWA and amyloid-β40, SWA disruption was also strongly correlated with amyloid-β42 (Supplementary Fig. 1). The CSF amyloid-β42:amyloid-β40 ratio decreased with greater SWA disruption, but this was not significant (Supplementary Fig. 1).
Figure 2.
Slow wave activity disruption is not correlated with change in other CSF proteins. SWA disruption, as measured by the change in delta spectral power, was not correlated with change in (A) total protein (r = 0.098, P = 0.708), (B) YKL-40 (r = −0.199, P = 0.445), (C) tau (r = 0.000, P = 1.000), or (D) hypocretin (r = −0.250, P = 0.333). X-axes are more negative to the right, i.e. values to the right indicate more disruption of slow wave activity.
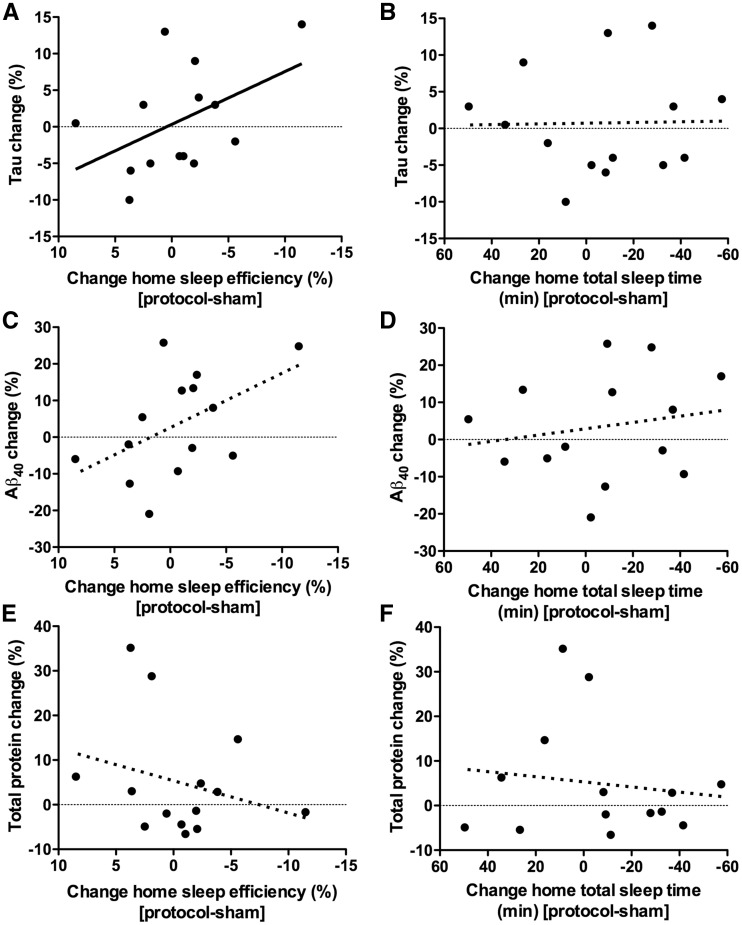
Extracellular levels of tau in the brain, like amyloid-β, are regulated by neuronal activity, but the half-life of tau is longer, 11 days in the mouse brain (Yamada et al., 2014). As SWA disruption did not lead to changes in tau the following morning, we compared actigraphically-measured home sleep over six preceding nights to CSF tau levels. There was a significant negative correlation between home sleep quality and tau, such that worse sleep efficiency was associated with higher tau (Fig. 3A). Sleep quantity was not correlated with tau (Fig. 3B). A strong trend for a correlation between home sleep quality and amyloid-β40 was apparent (Fig. 3C); however, this effect was likely diminished by SWA disruption immediately prior to the lumbar punctures. There was no association between amyloid-β40 and sleep quantity, or between total protein and either sleep quantity or quality (Fig. 3D–F).
Figure 3.
Worse home sleep quality over six nights is associated with increased CSF tau. Home sleep was quantified by actigraphically-measured sleep variables for the six nights prior to lumbar punctures. Valid actigraphy data were available for n = 14 participants. For consistency with other figures, sleep variables are shown as the six nights that included SWA disruption protocol night minus the six nights that included the sham condition night; however, the differences in total sleep time or sleep efficiency reflect variations in home sleep and are not related to the protocol or sham condition. For consistency with other figures, x-axes are more negative to the right, i.e. values to the right indicate less or worse sleep. (A) Worse sleep quality, as measured as the sleep efficiency, was associated with greater tau levels (r = 0.543, P = 0.045). (C) There was a strong but non-significant trend for an association between worse home sleep quality and amyloid-β40 levels (r = 0.481, P = 0.081), but (E) there was no correlation between sleep quality and total protein (r = −0.218, P = 0.455). Change in home sleep quantity, as measured by actigraphically-determined total sleep time, had no correlation with (B) tau (r = 0.103, P = 0.725), (D) amyloid-β40 (r = 0.218, P = 0.455), or (F) total protein (r = −0.103, P = 0.725).
Discussion
Our hypothesis was that one night of selective SWA disruption would lead to increased CSF amyloid-β the following morning. We found a strong association between SWA and amyloid-β, where greater SWA disruption was associated with increased amyloid-β. This effect was specific for SWA and not sleep quantity or quality, and was also specific for amyloid-β but not other CSF proteins. Additionally, we found that worse home sleep quality was associated with increased CSF tau, consistent with sleep disruption affecting CNS tau, but with such changes requiring a longer time to detect likely due to the longer half-life of tau.
Both of these findings support the hypothetical cascade in which decreased SWA leads to increased soluble amyloid-β and tau levels, which eventually increase risk of amyloid plaques (Bero et al., 2011) and tau tangle pathology, and subsequent development of symptomatic Alzheimer’s disease. Furthermore, the specificity of our findings for neuronally-derived proteins (amyloid-β and tau) and not for the glial-derived YKL-40 nor for total CSF protein levels suggests that SWA affects amyloid-β and tau by influencing synaptic activity with decreased release of these proteins into the interstitial space, rather than by affecting general protein clearance mechanisms. That total protein levels in CSF were unaffected suggests there were no global effects of SWA disruption on bulk flow mechanisms by which albumin and other abundant proteins enter and exit the CNS. While the CSF amyloid-β42:amyloid-β40 ratio tended to decrease with greater SWA disruption, this finding was not significant and the study was not powered or designed to assess relative clearance of amyloid-β isoforms. It is possible that following amyloid deposition, the relative clearance of amyloid-β42 from the brain’s interstitial fluid space to the CSF would be retarded by sequestration of monomeric amyloid-β42 into plaques (Potter et al., 2013), which could influence the effect of SWA disruption on CSF amyloid-β42.
A weakness of the study is the relatively small sample size. However, the effect size was stronger than predicted by cross-sectional preliminary data, with a high r of 0.610 for the association between SWA disruption and amyloid-β40 change. In addition, intraindividual analyses strongly increased statistical power. Nevertheless, our study was not a priori powered to assess correlations between SWA disruption and other CSF proteins, and larger studies are necessary to definitively exclude a relationship between SWA and other CSF proteins. Additionally, determining the relative clearance rates of amyloid-β isoforms, and any effect of amyloid plaques, will require larger studies including amyloid-positive individuals. Similarly, due to the small (n = 5) number of APOE ɛ4 carriers, this study was underpowered to assess the effect of APOE genotype on susceptibility to SWA disruption (Supplementary Table 2). Another weakness is that SWA disruption did not increase amyloid-β in some participants. There were no differences between ‘responders’ and ‘non-responders’ (Supplementary Table 3) to the SWA disruption protocol except that ‘non-responders’ had lower baseline SWA during the sham night (delta power 86 versus 147 μV2×s, P = 0.009). Since the same delta power >100 μV2×s cut-off was used in all participants for delivery of tones during SWA disruption, low baseline SWA made it difficult to reduce SWA further, and therefore blunted any effect on amyloid-β. Another minor weakness is that amyloid imaging was not used to exclude individuals with amyloid deposition; however, low CSF amyloid-β42 levels correlate well with amyloid deposition detected by PET (Fagan et al., 2006, 2009). Lastly, this experiment tested only the effect of SWA disruption; we cannot infer any information about the effect of increasing SWA on synaptic activity or protein clearance from this experiment.
Our study supports the hypothesis that SWA and sleep quality modulate amyloid-β and tau levels, respectively. Prospective studies will be required to test whether improving sleep quality and increasing SWA by treating underlying sleep disorders, medications, behavioural interventions, or acoustic enhancement of SWA can reduce amyloid-β levels, long-term risk of amyloid deposition, and progression to Alzheimer’s disease.
Supplementary Material
Acknowledgements
The authors thank the Hope Center DNA/RNA Purification Core at Washington University School of Medicine for APOE genotyping. We truly appreciate the time and effort volunteered by the research participants. We additionally thank the nursing staff in the Washington University Clinical Research Unit and the research coordinators at the Washington University Sleep Medicine Center for their kind support.
Glossary
Abbreviations
- N1/2/3
non-rapid eye movement sleep stage 1/2/3
- SWA
slow wave activity
Funding
Research reported in this publication was supported by National Institutes of Health awards K23-NS089922, UL1RR024992 Sub-Award KL2-TR000450, P01NS074969 (DM Holtzman, PI), P01-AG026276 (JC Morris, PI), P01-NS074969, and P01-AG03991 (JC Morris, PI); the J.P.B Foundation; Alzheimer Nederland grant #15040 (SJO, JAHRC); and the Washington University Institute of Clinical and Translational Sciences grant UL1TR000448 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
Supplementary material
Supplementary material is available at Brain online.
References
- Bateman RJ, Munsell LY, Morris JC, Swarm R, Yarasheski KE, Holtzman DM. Human amyloid-beta synthesis and clearance rates as measured in cerebrospinal fluid in vivo. Nat Med 2006; 12: 856–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichle ME, et al. Neuronal activity regulates the regional vulnerability to amyloid-β deposition. Nat Neurosci 2011; 14: 750–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989; 28: 193–213. [DOI] [PubMed] [Google Scholar]
- Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, et al. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron 2005; 48:913–22. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Mintun MA, Mach RH, Lee S-Y, Dence CS, Shah AR, et al. Inverse relation between in vivo amyloid imaging load and CSF Aβ42 in humans. Ann Neurol 2006; 59: 512–9. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Mintun MA, Shah AR, Aldea P, Roe CM, Mach RH, et al. Cerebrospinal fluid tau and ptau181 increase with cortical amyloid deposition in cognitively normal individuals: Implications for future clinical trials of Alzheimer’s disease. EMBO Mol Med 2009; 1: 371–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini mental state examination. Lutz, FL: Psychological Assessment Resources; 2004. [Google Scholar]
- Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology 1973; 10: 431–6. [DOI] [PubMed] [Google Scholar]
- Huang Y, Potter R, Sigurdson W, Santacruz A, Shih S, Ju YS, et al. Effects of age and amyloid deposition on Aβ dynamics in the human central nervous system. Arch Neurol 2012; 69: 51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iber C. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Darien IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991; 14: 540–5. [DOI] [PubMed] [Google Scholar]
- Ju YS, McLeland JS, Toedebusch CD, Xiong C, Fagan AM, Duntley SP, et al. Sleep quality and preclinical Alzheimer disease. JAMA Neurol 2013; 70: 587–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju YS, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology—a bidirectional relationship. Nat Rev Neurol 2014; 10: 115–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju YS, Finn MB, Sutphen CL, Herries EM, Jerome GM, Ladenson JH, et al. Obstructive sleep apnea decreases central nervous system-derived proteins in the cerebrospinal fluid. Ann Neurol 2016; 80: 154–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science 2009; 326: 1005–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med 2001; 2: 389–96. [DOI] [PubMed] [Google Scholar]
- Mignot E, Lammers GJ, Ripley B, Okun M, Nevsimalova S, Overeem S, et al. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol 2002; 59: 1553–62. [DOI] [PubMed] [Google Scholar]
- Nir Y, Staba RJ, Andrillon T, Vyazovskiy VV, Cirelli C, Fried I, et al. Regional slow waves and spindles in human sleep. Neuron 2011; 70: 153–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooms S, Overeem S, Besse K, Rikkert MO, Verbeek M, Claassen JA. Effect of 1 night of total sleep deprivation on cerebrospinal fluid β-amyloid 42 in healthy middle-aged men: a randomized clinical trial. JAMA Neurol 2014; 71: 971–7. [DOI] [PubMed] [Google Scholar]
- Ooms SJ, Zempel JM, Holtzman DM, Ju YS. February 24, 2017. Automated selective disruption of slow wave sleep. J Neurosci Methods 281; 33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio RS, Ayappa I, Mantua J, Gumb T, Varga A, Mooney AM, et al. The interaction between sleep-disordered breathing and apolipoprotein E genotype on cerebrospinal fluid biomarkers for Alzheimer’s disease in cognitively normal elderly individuals. Neurobiol Aging 2014; 35: 1318–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrazzoli M, D’Almeida V, Martins PJ, Machado RB, Ling L, Nishino S, et al. Increased hypocretin-1 levels in cerebrospinal fluid after REM sleep deprivation. Brain Res 2004; 995: 1–6. [DOI] [PubMed] [Google Scholar]
- Potter R, Patterson BW, Elbert DL, Ovod V, Kasten T, Sigurdson W, et al. Increased in vivo amyloid-β42 production, exchange, and loss in presenilin mutation carriers. Sci Transl Med 2013; 5: 189ra77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu H, Zhong R, Liu H, Zhang F, Li S, Le W. Chronic sleep deprivation exacerbates learning-memory disability and Alzheimer’s disease-like pathologies in AβPP(swe)/PS1(ΔE9) mice. J Alzheimers Dis 2016; 50: 669–85. [DOI] [PubMed] [Google Scholar]
- Roh JH, Huang Y, Bero AW, Kasten T, Stewart FR, Bateman RJ, et al. Disruption of the sleep-wake cycle and diurnal fluctuation of beta-amyloid in mice with Alzheimer’s disease pathology. Sci Transl Med 2012; 4: 150ra122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh JH, Jiang H, Finn MB, Stewart FR, Mahan TE, Cirrito JR, et al. Potential role of orexin and sleep modulation in the pathogenesis of Alzheimer’s disease. J Exp Med 2014; 211: 2487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman SM, Herdener N, Frankola KA, Mughal MR, Mattson MP. Chronic mild sleep restriction accentuates contextual memory impairments, and accumulations of cortical Aβ and pTau in a mouse model of Alzheimer’s disease. Brain Res 2013; 1529: 200–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler SE, Sutphen CL, Morris JC, Fagan AM. Upward drift in CSF Aβ42 values over 10 years. Alzheimers Dement 2016; 12(7, Supplement): P183. [Google Scholar]
- Spira AP, Gamaldo AA, An Y, Wu MN, Simonsick EM, Bilgel M, et al. Self-reported sleep and β-amyloid deposition in community-dwelling older adults. JAMA Neurol 2013; 70: 1537–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutphen CL, Jasielec MS, Shah AR, Macy EM, Xiong C, Vlassenko AG, et al. Longitudinal cerebrospinal fluid biomarker changes in preclinical Alzheimer disease during middle age. JAMA Neurol 2015; 72: 1029–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga AW, Wohlleber ME, Giménez S, Romero S, Alonso JF, Ducca EL, et al. Reduced slow-wave sleep is associated with high cerebrospinal fluid Aβ42 levels in cognitively normal elderly. Sleep 2016; 39: 2041–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep drives metabolite clearance from the adult brain. Science 2013; 342: 373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Holth JK, Liao F, Stewart FR, Mahan TE, Jiang H, et al. Neuronal activity regulates extracellular tau in vivo. J Exp Med 2014; 211: 387–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.