Placental malaria infection results in increased maternal cell and DNA trafficking to the fetus, known as maternal microchimerism, which in turn predicts increased risk of malaria infection but decreased risk of malaria disease in children.
Keywords: Malaria, Placental malaria, Childhood malaria, Microchimerism, Pregnancy
Abstract
Background
A mother’s infection with placental malaria (PM) can affect her child’s susceptibility to malaria, although the mechanism remains unclear. The fetus acquires a small amount of maternal cells and DNA known as maternal microchimerism (MMc), and we hypothesized that PM increases MMc and that MMc alters risk of Plasmodium falciparum malaria during infancy.
Methods
In a nested cohort from Muheza, Tanzania, we evaluated the presence and level of cord blood MMc in offspring of women with and without PM. A maternal-specific polymorphism was identified for each maternal–infant pair, and MMc was assayed by quantitative polymerase chain reaction. The ability of MMc to predict malaria outcomes during early childhood was evaluated in longitudinal models.
Results
Inflammatory PM increased the detection rate of MMc among offspring of primigravidae and secundigravidae, and both noninflammatory and inflammatory PM increased the level of MMc. Detectable MMc predicted increased risk of positive blood smear but, interestingly, decreased risk of symptomatic malaria and malaria hospitalization.
Conclusions
The acquisition of MMc may result in increased malaria infection but protection from malaria disease. Future studies should be directed at the cellular component of MMc, with attention to its ability to directly or indirectly coordinate anti-malarial immune responses in the offspring.
Malaria remains one of the largest killers of children <5 years of age worldwide with nearly half a million preventable deaths each year, the majority of which occur in sub-Saharan Africa [1]. In addition, pregnant women are uniquely susceptible to placental malaria (PM), a condition in which Plasmodium falciparum–infected red blood cells (iRBCs) sequester in the intervillous spaces of the placenta, leading to a dense inflammatory infiltrate of mononuclear cells [2]. PM is associated with miscarriage, stillbirth, and intrauterine growth retardation [3]. First-time mothers are at the greatest risk of PM, and with subsequent pregnancies women develop antibodies that block the adhesion of iRBCs to chondroitin sulfate A expressed in the placenta [4]. PM has also been associated with alterations in the VEGF pathway including elevated placental VEGF and soluble FMS-like tyrosine kinase 1 (sFlt-1) expression in primigravidae [5] and those with inflammatory PM [5, 6].
Multiple studies describe an association between PM and earlier time to first malaria infection as well as increased risk of malaria during childhood after controlling for epidemiologic risk factors [7–10]. The association between PM and malaria in the offspring is thought to be the result of prenatal immune priming against soluble malaria antigens, resulting in a malaria-specific immunotolerant phenotype in the infant [11–13]. The fetus is able to mount malaria-specific T-cell [14, 15] and B-cell responses [15, 16], and cord blood (CB) from infants born to malaria-infected women demonstrated reduced or partial antigen-presenting cell activation [17, 18], interleukin 10 production after parasite stimulation [12, 13, 17, 19], and the presence of regulatory T cells that suppressed reactivity to parasite antigens [19, 20]. This malaria-specific CB tolerance predicted a similar tolerant phenotype at 6 months of age and increased susceptibility to malaria, relative to nonexposed or exposed but sensitized infants [12].
The in utero tolerance generated against malaria mirrors the essential tolerance developed by the fetus against allogeneic antigens expressed by the mother. During pregnancy, fetal tolerance against noninherited maternal antigens develops as a consequence of the acquisition of a small number of maternal cells or DNA known as maternal microchimerism (MMc) [21]. The fetus acquires MMc as early as the second trimester, MMc is persistent in the offspring for decades after birth [22], and children maintain tolerance against maternal, but not paternal, alloantigens [21]. MMc is found in progenitor and differentiated populations [23], including T cells, B cells, monocytes, macrophages [24], and neutrophils [25], as well as nonimmune cells [26]. Limited work in humans suggests that maternal microchimeric cells are functionally active [27]. The mechanism of transfer may involve passive transfer via microtransfusions or active transfer dependent on a VEGF-A gradient across the placenta [28].
We hypothesized that PM, with its associated alterations in the VEGF pathway as well as damage to the trophoblast barrier, increases the prevalence and level of CB MMc and that MMc alters malaria susceptibility in early childhood. Using a nested cohort study, we evaluated the association between PM and CB MMc, as well as the ability of MMc to predict malaria outcomes during early childhood.
MATERIALS AND METHODS
Cohort
Data and samples were utilized from the Mother Offspring Malaria Study (MOMS) prospective birth cohort conducted in Muheza, Tanzania, between 2002 and 2006 [29]. Women and infants were enrolled at the time of delivery after exclusion for probable human immunodeficiency virus (HIV), chronic illness, sickle cell disease (HgbSS), or multiple births, and the infants were followed up to 4 years. Maternal–infant pairs were included in the present study based on availability of placental histology for PM categorization (n = 270), available paired maternal cord samples (n = 77), informative typing for a maternal-specific allele (n = 63), and sufficient CB sample size to assess MMc (n = 53).
Time points for sample collection during early childhood included routine visits (every 2 weeks for the first year of life and every 4 weeks thereafter) and nonroutine visits (village health worker visits, walk-in visits, or hospitalizations). Any non-zero P. falciparum parasite count by microscopy was considered a positive blood smear (BS); non-falciparum malaria was excluded from analysis. Positive smears that occurred within 28 days of an initial positive BS without an intervening negative BS were excluded from analysis. Symptomatic malaria was defined as a positive BS plus any of the following: fever (temperature >38°C), gastrointestinal distress, respiratory distress, anemia (hemoglobin <8 g/dL), convulsions, prostration, or glucose <2.2 mmol/L. Malaria hospitalization was defined as hospitalization in the setting of a positive BS. Maximum parasitemia was defined as the highest BS value within 28 days of an initial positive BS.
The MOMS cohort was approved by both United States (Western Institutional Review Board) and Tanzanian (National Institute for Medical Research, Medical Research Coordinating Committee) ethical review boards. The current study was approved by the Institutional Review Board of the Fred Hutchinson Cancer Research Center.
Diagnosis of Placental Malaria
PM diagnosis was based on microscopy of placental blood film [4], and inflammation was determined by histologic evaluation of placental cryosections [30]. PM negative women were negative by thick smear as well as by histology (no parasites, pigment, or inflammation). A woman was considered positive for PM if the placental thick smear was positive. Inflammatory PM was defined as thick smear positive and any evidence of inflammation by histology. Women with evidence of past infection (pigment or inflammation but no parasites) were excluded.
Cord Blood Collection and Genomic DNA Extraction
To limit the possibility of maternal blood contamination, CB was collected at the time of delivery into EDTA via cross-clamping of the umbilical cord and direct cannulation of the umbilical vessels. CB was frozen and stored at –70°C until genomic DNA (gDNA) extraction. The gDNA was extracted from cord and maternal blood using QiaAMP DNA Micro or Mini Kits (Qiagen) and from filter papers using a modified Chelex-100-based method [31].
Identification of Maternal-Specific Polymorphisms and Microchimerism Evaluation
Maternal and infant samples were genotyped at HLA class II loci DRB1, DQA1, and DQB1 using Luminex-based (One Lambda) polymerase chain reaction (PCR) sequence-specific oligonucleotide probe techniques. Noninherited, nonshared HLA polymorphisms were identified that could be used to evaluate MMc [32]. Maternal–infant pairs with noninformative HLA typing were typed at 4 non-HLA loci: ATIII, TG, GSTT1, and TNN, targeting insertion/deletion/substitution polymorphisms.
The maternal-specific polymorphism for each maternal–infant pair was selectively amplified from CB gDNA using a panel of previously developed quantitative PCR (qPCR) assays [32, 33]. The sensitivity of the qPCR assays is one target genomic equivalent (gEq) in 20000 background gEq [32]. A calibration curve for the polymorphism-specific assay was included to quantify the amount of MMc for each experiment. Every sample was also tested for the nonpolymorphic β-globin gene (HBB), and a HBB calibration curve was concurrently evaluated on each plate to quantify the total number of gEq of DNA tested in each reaction. Only samples with an anticipated total of ≥104 gEq were utilized, and all samples were run in at least 2 separate reactions.
Detectable MMc was defined as any positive maternal-specific qPCR amplification. Level of MMc was determined by comparison of the qPCR amplification against the assay-specific calibration curve and is expressed as the standardized ratio of MMc gEq per 105 gEq tested. To use level of MMc as a predictor, the measured values were transformed as natural logarithm (MMc gEq / 105 gEq tested +1).
Statistical Analysis
Prevalence of CB MMc by PM category was evaluated by logistic regression with adjustment for the total number of gEq tested for each subject. Level of MMc by PM category was evaluated with negative binomial regression, accounting for the number of microchimeric gEq detected as well as the total number of gEq assessed in each sample. This approach accounts for the nonnormal distribution of MMc data as well as the large number of zeros [34]. The output of this model is a detection rate ratio (DRR), which can be interpreted as “X number of microchimeric gEq in group A for every one microchimeric gEq in group B.” Comparison of sFlt-1 level was conducted using the Mann-Whitney U test. Odds of malaria outcomes were assessed using generalized estimating equation (GEE) models with clustering by individual, a binomial outcome structure, and an exchangeable correlation matrix [29]. Odds of positive BS considered all visits as either negative or positive. Odds of symptomatic malaria and malaria hospitalization were restricted to those visits with positive BS. Comparison of maximum parasitemia during infection was assessed using a GEE model with clustering by individual, a Gaussian outcome structure, and an exchangeable correlation matrix. All models utilized robust standard errors.
Covariates considered for inclusion in the adjusted models included sickle cell trait (HgbAA, HgbAS), maternal gravidity (primigravid, secundigravid, or multigravid), village (rural, semiurban), bed net use (no, yes, unknown), malaria transmission season at each visit (low, high), and age of the child at each visit (modeled as a cubic spline with 3 knots at the 10th, 50th, and 90th percentile). Univariate analysis was conducted and each covariate was tested individually in the model. Covariates were included in the final model if they changed the coefficient for detectable MMc by 10% or more (confounding) or were significantly related to the outcome at the P ≤ .10 level (prediction). Reduced GEE models were compared using quasi-likelihood under the independence model criterion to confirm their improved model fit [35]. The reduced model for each outcome is presented.
Final covariates in the GEE model of odds of positive blood smear included maternal gravidity (confounder), bed net (predictor and confounder), malaria transmission season at each visit (predictor), and age of the child (predictor and confounder). Final covariates in the GEE model of maximum parasitemia included sickle cell trait (predictor), maternal gravidity (predictor and confounder), village (confounder), bed net use (predictor and confounder), malaria transmission season at each visit (predictor), and age of the child (confounder). Final covariates in the GEE model of odds of symptomatic malaria included sickle cell trait (predictor), maternal gravidity (predictor and confounder), village (predictor and confounder), bed net (predictor), and age of the child (predictor and confounder). Final covariates in the GEE model of odds of malaria hospitalization included sickle cell trait (predictor), maternal gravidity (confounder), and age of the child (predictor).
To understand whether the effect of MMc to predict malaria outcome was the result of its association with PM, we tested for confounding by PM as well as effect modification by PM. Where the interaction term for PM and MMc was significant at the P ≤ .1 level, we present stratified analyses.
All statistics were conducted in Stata 14 software (StataCorp LP).
RESULTS
Inflammatory Placental Malaria Predicts Increased Cord Blood Maternal Microchimerism
CB MMc was evaluated for 53 infants: 26 born to women without PM, 14 born to women with noninflammatory PM, and 13 born to women with inflammatory PM. The mean number of gEq tested in each group was not significantly different (no PM: 6.7 × 104, noninflammatory PM: 5.9 × 104, inflammatory PM: 8.0 × 104, P = .4). The demographic characteristics of the 3 groups of women did not significantly differ (Table 1).
Table 1.
Descriptive Summary by Maternal Placental Malaria Category
| Covariate | No PM (n = 26) | Noninflammatory PM (n = 14) | Inflammatory PM (n = 13) | P Valuea |
|---|---|---|---|---|
| Maternal age, y | 25.3 | 23.3 | 24.0 | .4 |
| Gravidity | .1 | |||
| 0 | 6 (23) | 4 (29) | 5 (39) | |
| 1 | 5 (19) | 7 (50) | 2 (15) | |
| 2 (+) | 15 (58) | 3 (21) | 6 (46) | |
| Infant sex, female | 13 (50) | 5 (36) | 8 (62) | .4 |
| Sickle cell trait | 7 (27) | 2 (15) | 3 (23) | .7 |
| Birth weight, g | 3063 | 3050 | 2803 | .2 |
| Semiurban | 17 (65) | 4 (29) | 8 (62) | .07 |
| Bed net | .6 | |||
| No | 8 (31) | 6 (43) | 7 (54) | |
| Yes | 16 (61) | 7 (50) | 6 (46) | |
| Unknown | 2 (8) | 1 (7) | 0 (0) | |
| High malaria transmission season at birth | 23 (50) | 8 (57) | 8 (62) | .8 |
Data are presented as No. (%) unless otherwise indicated. Maternal age and birth weight are means.
Abbreviation: PM, placental malaria.
aDifference in means tested using linear regression with PM category modeled as an unordered categorical variable. Difference in distribution tested using χ2 test.
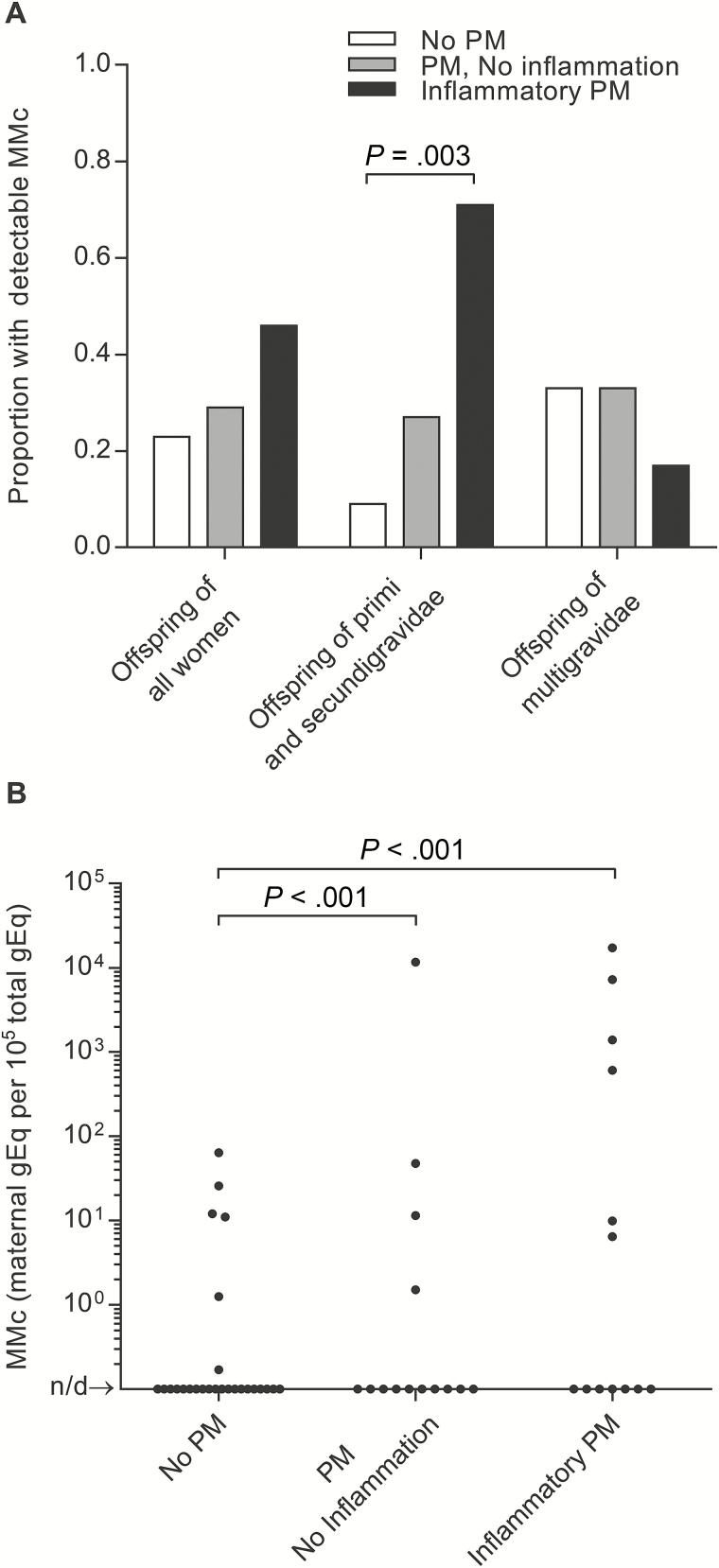
MMc was detected in the CB of 23% of offspring of women without PM, 29% of offspring of women with noninflammatory PM (adjusted odds ratio [AOR], 1.31 [95% confidence interval {CI}, .29–5.92], P = .7), and 46% of offspring of women with inflammatory PM (AOR, 2.93 [95% CI, .68–12.65], P = .2). There was evidence of effect modification by parity where the effect was similar among offspring of primigravidae and secundigravidae but different among offspring of multigravidae (interaction term, P = .04). Among offspring of primigravidae and secundigravidae, detection of MMc was significantly more likely in the setting of inflammatory PM (AOR, 48.43 [95% CI, 3.78–638.26], P = .003) and there was a trend toward increased detection in the setting of noninflammatory PM (AOR, 7.42 [95% CI, .75–72.94], P = .09), whereas no such relationship was evident among offspring of multigravidae (Figure 1A).
Figure 1.
Prevalence and level of cord blood maternal microchimerism (CB MMc) by placental malaria (PM) category. No PM: n = 26; noninflammatory PM: n = 14; inflammatory PM: n = 13. Not detected (n/d). A, Inflammatory PM was associated with increased prevalence of CB MMc among offspring of primigravidae and secundigravidae (adjusted odds ratio, 48.43; P = .003) but not multigravidae. B, Noninflammatory (detection rate ratio [DRR], 199; P < .001) and inflammatory PM (DRR, 466; P < .001) were associated with increased level of CB MMc.
The mean level of MMc was 4 per 105 gEq among offspring of women without PM, 835 per 105 gEq among offspring of women with noninflammatory PM (DRR, 191 [95% CI, 11–3202], P < .001), and 2036 per 105 gEq among offspring of women with inflammatory PM (DRR, 466 [95% CI, 26–8356], P < .001) (Figure 1B). Remarkably, there were 4 CB samples with >1% MMc, all among infants born to women with PM, and the mean level was 8601 per 105 gEq in the offspring of the 2 women with massive intervillositis. sFlt-1, a biomarker of placental dysfunction and preeclampsia [36], was previously measured from maternal peripheral plasma in 15 of the women [37], among whom detection (3859 pg/mL vs 1161 pg/mL, P = .002) and level (R2 = 0.79) of MMc were associated with sFlt-1 level.
Maternal Microchimerism Predicts Increased Risk of Positive Blood Smear During Early Childhood
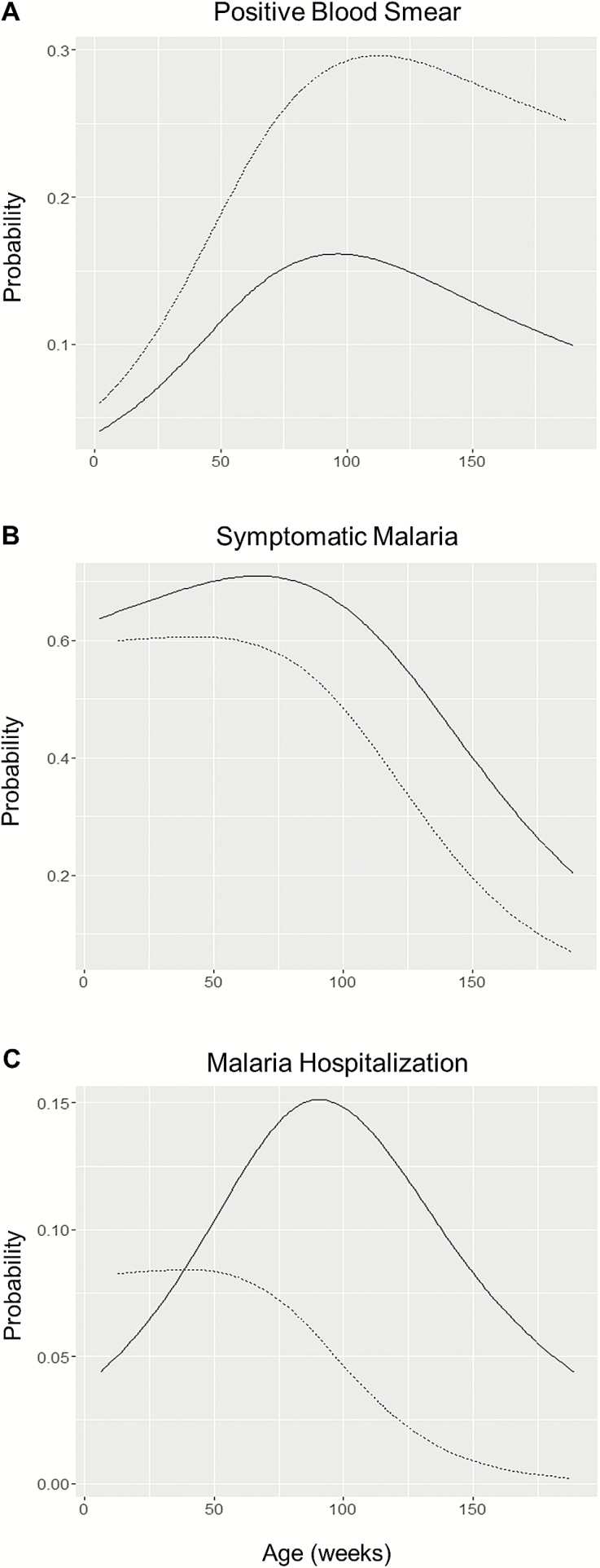
There were 2777 unique BS for the 53 offspring: 243 positive BS from 2000 unique BS for those without detectable MMc and 145 positive BS from 777 unique BS for those with detectable MMc. Detectable MMc predicted increased risk of positive BS during early childhood (AOR, 1.73 [95% CI, 1.06–2.81], P = .03) (Figure 2A). There was no evidence of confounding or effect modification by PM status. Increasing level of MMc predicted increased risk of positive BS (per log increase in MMc: AOR, 1.10 [95% CI, 1.03–1.18], P = .008). During infection, the maximum parasitemia was significantly lower in those infants with detectable MMc vs those without MMc (Δ = –459 iRBCs / 200 white blood cells (WBCs) [95% CI, –708 to –210], P < .001). Increased level of MMc predicted decreased maximum parasitemia (per log increase in MMc: Δ = –59 iRBCs / 200 WBC [95% CI, –90 to –28], P < .001).
Figure 2.
Probability of malaria outcome by cord blood maternal microchimerism (MMc) detection. Offspring with no detectable MMc: n = 37; offspring with detectable MMc: n = 16. Solid line: no detectable MMc; dashed line: Detectable MMc. Age modeled as a cubic spline with 3 knots. A, MMc predicted increased risk of positive blood smear (BS) (adjusted odds ratio [AOR], 1.73; P = .03). B, MMc predicted decreased risk of symptomatic malaria, given a positive BS (AOR, 0.47; P = .001). C, MMc predicted decreased risk of malaria hospitalization, given a positive BS (AOR, 0.41; P = .03).
Maternal Microchimerism Predicts Decreased Risk of Symptomatic Malaria and Malaria Hospitalization
There were 145 symptomatic episodes out of 243 positive BS among those offspring without detectable MMc and 69 symptomatic episodes from 145 positive BS among those offspring with detectable MMc. Children with MMc were significantly less likely to have symptomatic malaria in the setting of a positive BS (AOR, 0.47 [95% CI, .31–.72], P = .001) (Figure 2B). The effect of MMc to predict decreased risk of symptomatic malaria was modified by PM (interaction term: P = .02; among offspring of PM-negative women: AOR, 0.39 [95% CI, .19–.81], P = .01; among offspring of PM-positive women: AOR, 0.73 [95% CI, .48–1.13], P = .2).
There were 26 malaria hospitalizations from 243 positive BS among those offspring without detectable MMc and 8 malaria hospitalizations from 145 positive BS among those offspring with detectable MMc. Similar to our finding with symptomatic malaria, there was a protective effect of MMc on risk of malaria hospitalization (AOR, 0.41 [95% CI, .18–.93], P = .03) (Figure 2C). There was no evidence of confounding or effect modification by PM status. In addition, there was no evidence of effect modification by parity for any malaria outcome.
DISCUSSION
In the present study, we found that inflammatory PM was associated with increased detection and level of CB MMc, most prominently among offspring of primigravidae and secundigravidae. CB from women with noninflammatory PM displayed an intermediate phenotype, suggesting that both malaria infection and inflammation may contribute to the differences we describe. Our results are consistent with prior studies describing increased risk of chronic, inflammatory PM during first and second pregnancies [2, 5], and it may be that the multigravidae in our study developed less placental pathology associated with their infections. The prevalence and level of MMc we found in our PM-negative group were similar to that described in CB from healthy pregnancies, however, the prevalence and level from the PM positive groups were much greater [38]. In addition, the level of MMc was >1% in 4 CB, all from women with PM, nearly 2 orders of magnitude greater than previously described [38]. An association was suggested between sFlt-1 level and MMc level. sFlt-1 secreted by the syncytiotrophoblast during PM may bind VEGF in the maternal intervillous spaces [36], accentuating the VEGF gradient across the placenta [28] and resulting in increased active transport of maternal cells. Alternatively, the increased MM c we detected may represent maternal DNA, for example, in the form of exosomes or microparticles.
We further hypothesized that this increased MMc may in part explain the previously described association between PM and risk of malaria during early childhood [7–10] and investigated a proxy of malaria-specific tolerance, namely, the association between MMc and malaria outcomes during early childhood. Offspring with detectable CB MMc had a greater risk of positive BS during early childhood, however, when infected they were less likely to have symptoms or to require hospitalization. The predictive effect of MMc on risk of positive BS could not be solely explained by the association of MMc and PM as there was no evidence of confounding or effect modification by PM status. In contrast, the effect of MMc to predict risk of symptomatic malaria was greater among the PM-negative group, suggesting that there may be a difference in the effect of “normal” and “abnormal” MMc. Our findings are striking because they dissociate risk of malaria infection from risk of disease. MMc was associated with a greater risk of P. falciparum infection but a lower risk of symptomatic malaria, perhaps due to improved control of maximum parasitemia or a more tempered inflammatory response. These findings may imply a mechanism of natural protection from disease, and we hypothesize that this may be advantageous in malaria-endemic settings.
The acquisition of MMc could play a role in the development of malaria-specific tolerance by a number of different potential mechanisms. First, prior work in mice has demonstrated “cross-tolerance” where antigen-tolerant regulatory T cells are able to suppress reactivity against a novel antigen experienced in conjunction with the primary antigen [39, 40]. These data suggest that the fetal acquisition and maintenance of tolerance to MMc may have the secondary consequence of cross-tolerance to malaria antigens if they are experienced in conjunction. Second, the fetus may acquire a maternal graft specifically enriched for regulatory cells (eg, classical FOXP3+ Tregs or FOXP3– Tr1 cells), which directly modify the antimalarial activity of fetal and infant cells. Maternal FOXP3+ Tregs increase during pregnancy [41], traffic to the placenta [42], and are essential for maintenance of tolerance toward the fetus [41, 42]. Consistent with our epidemiological findings, Tregs may attenuate the risk of immune-mediated pathology during malaria infection by limiting the formation of memory T-cell responses [43]. Similarly, FOXP3– Tr1 cells, associated with chronic antigen exposure [44], predict increased risk of malaria infection [45, 46] but reduced risk of immune-mediated pathology in both mice [47–49] and humans [43]. Third, maternal microchimeric cells may directly present malaria antigens to fetal cells, mimicking the presentation of self-antigens by antigen-presenting cells resulting in clonal deletion, while also potentially inducing peripheral tolerance through antigen contact without expression of inflammation-dependent costimulatory signals. Alternatively, the fetus may develop tolerance to soluble parasite antigens independent of MMc exposure, or MMc may be a marker of altered maternal antimalarial antibody transfer during the pregnancy.
Our study has a number of limitations. First, our sample size was limited by sample availability. Nonetheless, important differences between groups were observed. A second consideration is the possibility of maternal contamination of CB at the time of collection. Arguing against this, all CB samples were collected and processed in the same rigorous manner, with cross-clamping and direct cannulation of umbilical vessels. Third, sample availability did not permit distinguishing between the cellular and genetic components of MMc, although we plan to address this in future studies. Finally, CB and peripheral blood mononuclear cells were not available, precluding direct assessment of maternal or malaria-specific tolerance, although we were able to evaluate clinical outcomes as a proxy.
We investigated a novel mechanism for the association between PM and increased risk of malaria in the offspring, namely the in utero acquisition of maternal cells or DNA. Next steps should address a number of remaining questions including the persistence of MMc during childhood, the phenotype of acquired maternal cells, and the functionality of these cells with regard to their ability to directly affect or coordinate immune responses against malaria. Finally, these observations may have important implications for the role of MMc in other perinatal infections such as CMV or HIV [50], postnatal infections, and response to immunization.
Notes
Acknowledgments. We thank the women and infants from Muheza, Tanzania, for their participation in the MOMS cohort and recognize Dr T. K. Mutabingwa and the MOMS clinical team for their care of study participants.
Financial support. This work was supported by the Thrasher Research Fund; the National Institutes of Health (grant numbers T32 HD007233 and R01 HL117737-15); and the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. Funding for the original MOMS Project was provided by the Bill & Melinda Gates Foundation (Grant 29202), the Foundation for the National Institutes of Health through the Grand Challenges in Global Health Initiative (Grant 1364), the US National Institutes of Health Fogarty International Center (FIC) (Grant D43 TW005509), and the National Institute of Allergy and Infectious Diseases (R01AI52059).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. World malaria report 2015. Geneva, Switzerland: WHO, 2015. [Google Scholar]
- 2. Brabin BJ, Romagosa C, Abdelgalil S, et al. The sick placenta-the role of malaria. Placenta 2004; 25:359–78. [DOI] [PubMed] [Google Scholar]
- 3. Moya-Alvarez V, Abellana R, Cot M. Pregnancy-associated malaria and malaria in infants: an old problem with present consequences. Malar J 2014; 13:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science 1996; 272:1502–4. [DOI] [PubMed] [Google Scholar]
- 5. Muehlenbachs A, Mutabingwa TK, Edmonds S, Fried M, Duffy PE. Hypertension and maternal-fetal conflict during placental malaria. PLoS Med 2006; 3:e446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boeuf P, Tan A, Romagosa C, et al. Placental hypoxia during placental malaria. J Infect Dis 2008; 197:757–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Le Hesran JY, Cot M, Personne P, et al. Maternal placental infection with Plasmodium falciparum and malaria morbidity during the first 2 years of life. Am J Epidemiol 1997; 146:826–31. [DOI] [PubMed] [Google Scholar]
- 8. Le Port A, Watier L, Cottrell G, et al. Infections in infants during the first 12 months of life: role of placental malaria and environmental factors. PLoS One 2011; 6:e27516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mutabingwa TK, Bolla MC, Li JL, et al. Maternal malaria and gravidity interact to modify infant susceptibility to malaria. PLoS Med 2005; 2:e407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schwarz NG, Adegnika AA, Breitling LP, et al. Placental malaria increases malaria risk in the first 30 months of life. Clin Infect Dis 2008; 47:1017–25. [DOI] [PubMed] [Google Scholar]
- 11. Broen K, Brustoski K, Engelmann I, Luty AJ. Placental Plasmodium falciparum infection: causes and consequences of in utero sensitization to parasite antigens. Mol Biochem Parasitol 2007; 151:1–8. [DOI] [PubMed] [Google Scholar]
- 12. Malhotra I, Dent A, Mungai P, et al. Can prenatal malaria exposure produce an immune tolerant phenotype? A prospective birth cohort study in Kenya. PLoS Med 2009; 6:e1000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Malhotra I, Mungai P, Muchiri E, et al. Distinct Th1- and Th2-Type prenatal cytokine responses to Plasmodium falciparum erythrocyte invasion ligands. Infect Immun 2005; 73:3462–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fievet N, Ringwald P, Bickii J, et al. Malaria cellular immune responses in neonates from Cameroon. Parasite Immunol 1996; 18:483–90. [DOI] [PubMed] [Google Scholar]
- 15. Metenou S, Suguitan AL, Jr, Long C, Leke RG, Taylor DW. Fetal immune responses to Plasmodium falciparum antigens in a malaria-endemic region of Cameroon. J Immunol 2007; 178:2770–7. [DOI] [PubMed] [Google Scholar]
- 16. Desowitz RS, Elm J, Alpers MP. Prenatal immune hypersensitization to malaria: Plasmodium falciparum-specific IgE antibody in paired maternal and cord sera from Papua New Guinea. P N G Med J 1992; 35:303–5. [PubMed] [Google Scholar]
- 17. Brustoski K, Möller U, Kramer M, et al. IFN-gamma and IL-10 mediate parasite-specific immune responses of cord blood cells induced by pregnancy-associated Plasmodium falciparum malaria. J Immunol 2005; 174:1738–45. [DOI] [PubMed] [Google Scholar]
- 18. Fievet N, Varani S, Ibitokou S, et al. Plasmodium falciparum exposure in utero, maternal age and parity influence the innate activation of foetal antigen presenting cells. Malar J 2009; 8:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brustoski K, Moller U, Kramer M, et al. Reduced cord blood immune effector-cell responsiveness mediated by CD4+ cells induced in utero as a consequence of placental Plasmodium falciparum infection. J Infect Dis 2006; 193:146–54. [DOI] [PubMed] [Google Scholar]
- 20. Mackroth MS, Malhotra I, Mungai P, Koech D, Muchiri E, King CL. Human cord blood CD4+CD25hi regulatory T cells suppress prenatally acquired T cell responses to Plasmodium falciparum antigens. J Immunol 2011; 186:2780–91. [DOI] [PubMed] [Google Scholar]
- 21. Mold JE, Michaëlsson J, Burt TD, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science 2008; 322:1562–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maloney S, Smith A, Furst DE, et al. Microchimerism of maternal origin persists into adult life. J Clin Invest 1999; 104:41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hall JM, Lingenfelter P, Adams SL, Lasser D, Hansen JA, Bean MA. Detection of maternal cells in human umbilical cord blood using fluorescence in situ hybridization. Blood 1995; 86:2829–32. [PubMed] [Google Scholar]
- 24. Loubière LS, Lambert NC, Flinn LJ, et al. Maternal microchimerism in healthy adults in lymphocytes, monocyte/macrophages and NK cells. Lab Invest 2006; 86:1185–92. [DOI] [PubMed] [Google Scholar]
- 25. Sunku Cuddapah CS, Gadi VK, de Laval de Lacoste B, Guthrie KA, Nelson JL. Maternal and fetal microchimerism in granulocytes. Chimerism 2011; 1:11–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nelson JL. The otherness of self: microchimerism in health and disease. Trends Immunol 2012; 33:421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reed AM, McNallan K, Wettstein P, Vehe R, Ober C. Does HLA-dependent chimerism underlie the pathogenesis of juvenile dermatomyositis? J Immunol 2004; 172:5041–6. [DOI] [PubMed] [Google Scholar]
- 28. Chen CP, Lee MY, Huang JP, et al. Trafficking of multipotent mesenchymal stromal cells from maternal circulation through the placenta involves vascular endothelial growth factor receptor-1 and integrins. Stem Cells 2008; 26:550–61. [DOI] [PubMed] [Google Scholar]
- 29. Harrington WE, Morrison R, Fried M, Duffy PE. Intermittent preventive treatment in pregnant women is associated with increased risk of severe malaria in their offspring. PLoS One 2013; 8:e56183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bulmer JN, Rasheed FN, Francis N, Morrison L, Greenwood BM. Placental malaria. I. Pathological classification. Histopathology 1993; 22:211–8. [DOI] [PubMed] [Google Scholar]
- 31. Plowe CV, Djimde A, Bouare M, Doumbo O, Wellems TE. Pyrimethamine and proguanil resistance-conferring mutations in Plasmodium falciparum dihydrofolate reductase: polymerase chain reaction methods for surveillance in Africa. Am J Trop Med Hyg 1995; 52:565–8. [DOI] [PubMed] [Google Scholar]
- 32. Lambert NC, Erickson TD, Yan Z, et al. Quantification of maternal microchimerism by HLA-specific real-time polymerase chain reaction: studies of healthy women and women with scleroderma. Arthritis Rheum 2004; 50:906–14. [DOI] [PubMed] [Google Scholar]
- 33. Gammill HS, Guthrie KA, Aydelotte TM, Adams Waldorf KM, Nelson JL. Effect of parity on fetal and maternal microchimerism: interaction of grafts within a host? Blood 2010; 116:2706–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guthrie KA, Gammill HS, Kamper-Jorgensen M, et al. Statistical methods for unusual count data: examples from studies of microchimerism. Am J Epidemiol 2016, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pan W. Akaike’s information criterion in generalized estimating equations. Biometrics 2001; 57:120–5. [DOI] [PubMed] [Google Scholar]
- 36. Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 2003; 111:649–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Muehlenbachs A, Fried M, Lachowitzer J, Mutabingwa TK, Duffy PE. Natural selection of FLT1 alleles and their association with malaria resistance in utero. Proc Natl Acad Sci U S A 2008; 105:14488–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lo YM, Lau TK, Chan LY, Leung TN, Chang AM. Quantitative analysis of the bidirectional fetomaternal transfer of nucleated cells and plasma DNA. Clin Chem 2000; 46:1301–9. [PubMed] [Google Scholar]
- 39. Bemelman F, Honey K, Adams E, Cobbold S, Waldmann H. Bone marrow transplantation induces either clonal deletion or infectious tolerance depending on the dose. J Immunol 1998; 160:2645–8. [PubMed] [Google Scholar]
- 40. Dutta P, Molitor-Dart M, Bobadilla JL, et al. Microchimerism is strongly correlated with tolerance to noninherited maternal antigens in mice. Blood 2009; 114:3578–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature 2012; 490:102–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell 2012; 150:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Walther M, Jeffries D, Finney OC, et al. Distinct roles for FOXP3 and FOXP3 CD4 T cells in regulating cellular immunity to uncomplicated and severe Plasmodium falciparum malaria. PLoS Pathog 2009; 5:e1000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zeng H, Zhang R, Jin B, Chen L. Type 1 regulatory T cells: a new mechanism of peripheral immune tolerance. Cell Mol Immunol 2015; 12:566–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jagannathan P, Bowen K, Nankya F, et al. Effective antimalarial chemoprevention in childhood enhances the quality of CD4+ T cells and limits their production of immunoregulatory interleukin 10. J Infect Dis 2016; 214:329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jagannathan P, Eccles-James I, Bowen K, et al. IFNγ/IL-10 co-producing cells dominate the CD4 response to malaria in highly exposed children. PLoS Pathog 2014; 10:e1003864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Couper KN, Blount DG, Wilson MS, et al. IL-10 from CD4CD25Foxp3CD127 adaptive regulatory T cells modulates parasite clearance and pathology during malaria infection. PLoS Pathog 2008; 4:e1000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Freitas do Rosario AP, Lamb T, Spence P, et al. IL-27 promotes IL-10 production by effector Th1 CD4+ T cells: a critical mechanism for protection from severe immunopathology during malaria infection. J Immunol 2012; 188:1178–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Freitas do Rosario AP, Langhorne J. T cell-derived IL-10 and its impact on the regulation of host responses during malaria. Int J Parasitol 2012; 42:549–55. [DOI] [PubMed] [Google Scholar]
- 50. Kwiek JJ, Arney LA, Harawa V, et al. Maternal-fetal DNA admixture is associated with intrapartum mother-to-child transmission of HIV-1 in Blantyre, Malawi. J Infect Dis 2008; 197:1378–81. [DOI] [PMC free article] [PubMed] [Google Scholar]