Abstract
Obesity is a major risk factor for the development of diabetes, insulin resistance, dyslipidemia, cardiovascular disease and other related metabolic conditions. Obesity develops from perturbations in overall cellular bioenergetics when energy intake chronically exceeds total energy expenditure. Lifestyle interventions based on reducing total energy uptake and increasing activities including exercise have proved ineffective in the prevention and treatment of obesity because of poor adherence to such interventions for an extended period of time. Brown adipose tissue (BAT) has an extraordinary metabolic capacity to burn excess stored energy and holds great promise in combating obesity and related diseases. This unique ability to nullify the effects of extra energy intake of these specialized tissues has provided attractive perspectives for the therapeutic potential of BAT in humans. Browning of white adipose tissue by promoting the expression and activity of key mitochondrial uncoupling protein 1 (UCP1) represents an exciting new strategy to combat obesity via enhanced energy dissipation. Members of the transforming growth factor-beta (TGF-β) superfamily including myostatin and follistatin have recently been demonstrated to play a key role in regulating white adipose browning both in in-vitro and in-vivo animal models and thereby present attractive avenues for exploring the therapeutic potential for the treatment of obesity and related metabolic diseases.
Keywords: adipocyte, adipose browning, follistatin, myostatin, transforming growth factor beta
Introduction
Metabolic consequences of obesity represent a significant global problem. Excessive adiposity is responsible for more than three million deaths and a significant cause of disability resulting from perturbed glucose and lipid metabolism [1]. Adipose tissue has traditionally been sub-classified into white adipose tissue (WAT) and brown adipose tissue (BAT), which play opposing roles in regulating energy balance. While WAT acts as the main site of metabolic energy storage, BAT is a specialized thermogenic tissue that burns excess stored energy to produce heat [2]. It was a long-standing, prevailing view that BAT exists only in newborns and small mammals and that adult humans do not have active BAT; the metabolic relevance of the tissue in adult human physiology has long been neglected [3], Landmark papers by several independent groups provided clear functional and molecular evidence for the presence of active BAT depots in adult humans [4], [5], Data from recent population studies demonstrate an inverse relationship between BAT and resting plasma glucose and lipid levels, suggesting a role for BAT in regulating key metabolic aspects in human population [6]. While whole body glucose disposal and insulin sensitivity were enhanced in subjects with significant amounts of BAT, these effects were blunted in obese individuals with undetectable BAT activity [6]. Similar metabolic advantage could also be achieved through a series of genetic and pharmacological manipulations of traditional WAT depots through a process called browning of WAT [7], [8]. Browning of white adipocytes, also called “beige” or “brite” (brown in white) adipocytes, shows a population of cells that exhibit BAT-like characteristics in response to certain external cues [9], [10]. Browning can be achieved in response to various environmental and pharmacological stimuli such as cold exposure, response to β-adrenergic agonists such as CL 316,243, exercise and environmental and endocrine factors, amongst others [8], [11]. These beige or brite cells are capable of expressing uncoupling protein 1 (Ucp1) and displaying thermogenic capacity which is comparable to BAT cells [2], [8]. However, their expression profile is distinctly different and varies substantially depending on fat depot and origin [12]. A strong inverse association between browning propensity in WAT and genetic susceptibility to diet-induced obesity in rodents has been reported, and this genetic susceptibility is linked to the Ucp1 expression in WAT [13]. The browning process is regulated by a complex hormonal interplay and many other browning agents. The list of currently known browning factors range from food compounds, drug substances, metabolites, microbiome, transgenes, gene knockouts and altered living condition [14], [15], [16]. Both brown and beige adipocytes have the potential to be metabolically beneficial due to their unique ability to burn excess stored energy and alter the balance between energy intake and energy expenditure. As several excellent reviews have highlighted the importance of white adipose browning in regulating key metabolic aspects that has the potential to combat obesity and related diseases [11], [17], [18], [19], [20], we will briefly examine the emerging role of two key members of the transforming growth factor-β (TGF-β) superfamily, namely myostatin (Mst) and follistatin (Fst).
Plasticity, biogenesis and molecular gene signature of brown/beige adipocytes
White and brown adipocytes represent two major types of adipocytes that have fat droplets, but they differ significantly in morphology, function and developmental origin [2]. White adipocytes that mainly store energy have unilocular fat droplets and have relatively fewer mitochondria. In contrast, brown adipocytes that are specialized adipose tissues responsible for burning excess energy have abundant mitochondria expressing high levels of UCP1 and have small multilocular fat droplets [3]. Beige adipocytes represent yet another kind of inducible form of thermogenic adipocytes that have low UCP1 expression at the basal conditions but could be induced by several factors to express high levels of UCP1 and promote energy consumption [1], [2]. The expression level of PR domain containing 16 (PRDM16), a zinc finger-containing transcription factor is high in mouse BAT compared to visceral WAT [21]. A knock-down of PRDM16 in brown fat cells leads to an increase in white adipose and muscle-specific genes, suggesting that it acts as a key regulator of brown fat cell fate [21], During embryonic development, BAT is known to be one of the earliest fat depots to form [9], Interscapular BAT is the most prominent depot that plays a major role In maintaining infant body temperature during very early years of life and is known to regress progressively with age [22], [23]. Experimental findings from the lineage tracing of adipocytes in vivo have clearly demonstrated that BAT arises from Myf5-expressing (Myf5+) precursors, a key myogenic regulatory factor expressed in committed skeletal muscle precursors [24]. Pulse-chase experiments using another myogenic marker Pax7 showed that the divergence of brown adipocyte precursors and skeletal muscle occurred between embryonic days 9.5 and 11.5 in mice [25]. In epididymal (Epi) WAT, UCP1-expressing beige adipocytes arise through the proliferation and differentiation of precursors that express platelet-derived growth factor receptor α (PDGFRα), CD44, and SC1 [26]. Beige adipocytes in the inguinal WAT are reported to arise from Myf5-negative (Myf5−) cells, although this concept has recently been challenged. Lineage analysis studies have suggested that subsets of white adipocytes originate from both Myf5+ and Myf5− precursors and respond to β3-adrenergic stimulus [27], Another recent report showed that a subset of UCP1-positive beige adipocytes arise from precursors that express Myh11, a selective marker for smooth muscle cells [28]. These findings, therefore, collectively suggest that beige adipocytes may have distinct cellular origins from classical BAT and are composed of heterogeneous cell populations. Trans-differentiation has also been suggested to allow efficient conversion of white adipocytes to brown adipocytes and vice versa [29], Warm adaptations are also reported to convert beige adipocytes into matured white adipocytes [30]. Similar conversion of beige to white adipocytes has also been speculated to result from aging [23]. In spite of all these recent findings supporting the evidence for trans-differentiation, this hypothesis still needs to be critically analyzed by studying the life-cycle of beige adipocytes.
Although beige and brown adipocytes share some of the same key markers including UCP1 and PRDM16 at differential levels under basal conditions, analysis of clonal cell lines demonstrate that beige and brown fat cells have related but distinctly different gene expression profiles [31]. Despite the heterogeneity of fat tissue and clonal cells, the expression levels of TMEM26, CD40 and TBX1 were reported to be highly enriched in beige adipocytes [31]. Gene expression analysis in adipose tissues isolated from inguinal fat and interscapular BAT from 129SVE mice identified several other beige-selective genes including Klhl13, CD40, Ear2, Sp100 and Slc27a1 [31], Microarray and histological analyses of human BAT that possesses a molecular signature resembling beige cells identified Cited1, HoxC8 and HoxC9 [32] and Shox2 [33] as additional beige-selective markers. Molecular profiling of embryonic brown preadipocyte cells identified Early B-cell factor 2 (Ebf2) as one of the most selective marker for brown and beige adipogenic precursor cells [34]. On the other hand, classical brown adipocyte cells selectively express Eva1 [31], Zic1 [35], Lhx8 [35], [36], and Epsti1 [32], Additional brown-selective genes including Fbxo31, Pdk4, Acot2, Ebf3, Hspb7, Slc29a1 and Oplah were reported using adipose tissues isolated from WAT and interscapular BAT isolated from 129SVE mice [31].
Differences in the molecular signature of microRNA (miRNA) between brown and beige adipocytes have recently emerged. By comparing the genome-wide miRNA expression patterns of mouse WAT and BAT using miRNA microarrays, Sun et al. [37] identified brown-fat-enriched miRNA cluster Mir193b-365 as a key regulator of brown fat development. Subsequent studies using comparative analysis of miRNA expression profiling in mouse and human BAT and cells identified several other miRNAs including miRNA182 and miRNA203 that are required for maintenance and differentiation of brown adipocytes [38], [39]. MiRNA106b and miRNA 93 have been identified as negative regulators of brown fat differentiation [40], Similar positive (Mir196a) and negative (Mir133) regulators of beige adipogenesis have also been reported [41], [42]. Thus, available data on molecular expression profiles suggest a clear difference between BAT and beige fat miRNA gene signatures in mouse and human tissues and cells.
Adipose browning and metabolic health
Obesity and associated metabolic disorders represent major health problems not only in the United States, but also all around the world [43], According to the World Health Organization report, more than one billion adults are overweight. The population of essentially obese [body mass index (BMI) >30] people extends beyond 300 million and this number is predicted to increase by more than 50% by the year 2025 [1]. These alarming numbers suggest that the burden of these metabolic diseases have a profound global economic impact. The balance between the WAT and BAT affects systemic energy balance and is widely believed to be the key determinant during the development of such metabolic diseases [44]. Apart from its role in promoting energy expenditure and body weight regulation, activation of BAT may contribute to whole body physiologic and metabolic processes unrelated to weight change [45]. BAT is reported to be the most active tissue type for promoting triglyceride (TG) clearance [46] and it regulates glucose homeostasis and insulin sensitivity [47].
Metabolic consequences of adipose browning in animal models
Adipose browning in animal models either through genetic or pharmacological manipulation or transplantation of BAT is extensively being investigated to explore possible metabolic benefits. The targeted disruption of Cidea inhibits the uncoupling activity of UCP1 and results in lean mice that are resistant to diet-induced obesity [48]. Ectopic expression of BAT in mouse muscle protects from high-fat-diet-induced obesity, hyperglycemia and insulin resistance [49]. Recent studies using BAT transplants have shown highly promising results in animal models in modulating metabolic outcomes [50], [51]. Subcutaneous (SC) transplants of BAT could correct type 1 diabetes in streptozotocin-treated mice with severely impaired glucose tolerance and a significant loss of adipose tissue [50], BAT transplants resulted in euglycemia, normalized glucose tolerance, reduced tissue inflammation and the reversal of clinical diabetes markers [50]. In another study, BAT transplantation from male donor mice into the recipient mice resulted in improved glucose tolerance, increased insulin sensitivity, lower body weight, decreased fat mass and a complete reversal of high-fat-diet-induced insulin resistance [52]. This improved metabolic advantage in recipient mice was lost when the transplanted BAT was obtained from IL6-knockout mice [52]. BAT transplanted from C57/BL6 mice into the dorsal SC region of age- and sex-matched leptin-deficient Ob/Ob mice led to a significant reduction of body weight gain, increased oxygen consumption and decreased total body fat mass, resulting in the improvement of insulin resistance and liver steatosis [52]. Most recently, activation of BAT has also shown to reduce hypercholesterolemia and protect mice from atherosclerosis development by enhancing the selective uptake of fatty acids from TG-rich lipoproteins into BAT and accelerating the hepatic clearance of cholesterol rich remnants [53].
Adipose browning in humans
Several lines of evidence from clinical studies in human adults suggest that BAT has an anti-obesity function and it protects from metabolic syndrome. Yoneshiro et al. [54] showed that a daily 2-h cold exposure at 17 °C for 6 weeks resulted in increased BAT activity, increase in energy expenditure and concomitant decrease in body fat mass. Induction of beige/brown fat in humans has also been shown to reduce glucose levels along with increase in insulin sensitivity [6]. These effects may be due to the ability of BAT to channel glucose and lipids towards oxidation or secretion of novel adipokines that target important metabolic tissues. One such protein is FGF21, which is largely upregulated in both BAT and beige during prolonged cold-exposure and is a well-recognized metabolic regulator owing to its glucose lowering effects [55]. Although in human studies, the effect of BAT activation has mainly focused on glucose metabolism, recent data also suggest that it contributes to TG metabolism. BAT-positive subjects have lower plasma TG and higher high-density lipoprotein-cholesterol (HDL-C) levels [56]. Furthermore, lipoprotein lipase (LPL) expression in perivascular BAT depots surrounding the aorta is negatively correlated with plasma TG levels [57]. Stem cells derived from human WAT treated with with peroxisome proliferator-activated receptor γ (PPARγ) agonist rosiglitazone or bone morphogenic protein 7 (BMP7) easily differentiate into beige adipocytes and have markedly enhanced oxygen consumption [58], [59]. In a recent report, thermogenic adipocytes were reported to promote HDL lipidome remodeling in humans after cold exposure irrespective of total plasma HDL-C concentrations [60]. The amount of metabolically active BAT is reported to be particularly low in patients with obesity. Several critical processes might contribute to the loss of BAT mass and activity in humans during obesity or even aging; the functional decline might start with impaired responsiveness due to insulin resistance that may promote apoptosis of mature brown and beige adipocytes [61]. The presence of BAT is also associated with a lower risk of nonalcoholic fatty liver disease (NAFLD) in adult humans. Analysis of whole body positron emission tomography-computed tomography (PET-CT) scans of 1832 patients found that the odds ratio for having NAFLD was significantly higher in subjects with significantly low or undetectable BAT [62]. In light of these findings, it is obvious that increased brown/beige adipose activity represents one important promising approach for the therapy of various metabolic diseases in the future.
Transforming growth factor-β (TGF-β) superfamily and adipose browning
Members of TGF-β and related factors play important roles in growth, development and regulation of diverse cellular functions in various cell types. Yadav et al. [63] elegantly demonstrated the importance of TGF-β signaling in controlling the appearance of brown adipocytes within the WAT and regulating glucose and energy- homeostasis. Using Samd3−/− mice, this group demonstrated that Smad3 loss induced WAT browning and promoted mitochondrial biogenesis and function, and protected mice from diet-induced obesity and insulin resistance. These Smad3−/− mice were also protected from hepatic steatosis generally characterized by ectopic fat deposition in liver tissues after consuming a high fat diet. Further support for the role of TGF-β in the etiology of obesity-associated metabolic diseases came from studies that demonstrated a critical role for extracellular matrix protein microfibril-associated glycoprotein 1 (MAGP1) in supporting thermogenesis and protection against obesity and diabetes through regulation of TGF-β [64]. MAGP1 expression is significantly altered in obese humans, and inactivation of the MAGP1 gene (Mfap2−/−) is reported to result in adipocyte hypertrophy and predisposition to metabolic dysfunction. Mfap2−/− mice display defective adaptation to cold challenge, have lower levels of UCP1 expression in BAT and reduced browning of the SC WAT [64], Treatment of these mice with TGF-β neutralizing antibody improved their body temperature and prevented increased adiposity [64]. Mice lacking MAGP1 have phenotypes consistent with altered TGF-β activity [65]. These results provided novel insight into the role of TGF-β with regard to activation of a brown adipocyte program within white fat and an opportunity to explore the therapeutic potential of targeting this pathway for the treatment of obesity and metabolic diseases. Pharmacological inhibition of activin receptor IIB (ActRIIB) in mice using neutralizing antibodies showed increased amounts of BAT without directly affecting WAT [66]. Gene set enrichment analysis following ActRIIB antibody treatment demonstrated significantly induced expression of genes regulating oxidative metabolism and mitochondrial function [66]. In another interesting study, Koncarevic et al. [67] administered a novel ActRIIB decoy receptor comprising a form of the extracellular domain of ActRIIB fused to human Fc (ActRIIB-Fc) that resulted in suppression of diet-induced obesity and related linked metabolic dysfunctions in mice on high fat diet along with increased muscle mass. While this treatment with the ActRIIB decoy receptor improved lipid profiles, prevented hepatic steatosis and increased energy expenditure, it did not affect total food intake, providing a strong argument in favor of increased energy expenditure following the treatment. Although this treatment led to significant increase in muscle mass in mice that could significantly contribute to increased energy expenditure, ActRIIB-Fc was also able to promote browning of WAT and upregulated UCP1 expression in WAT of ActRIIB-Fc-treated mice. In conclusion, these reports identify therapeutic potential of blocking TGF-βSmad3/ActRIIB signaling for obesity-related metabolic disorders.
Mst, irisin and white adipose browning
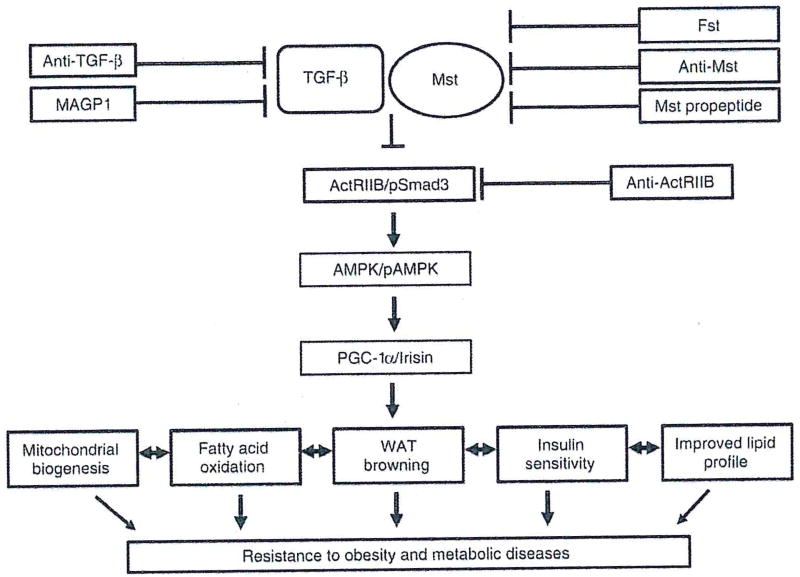
Mst, a secreted growth factor and a key member of the TGF-β superfamily has emerged as an important regulator of lean muscle mass as well as body fat content. Mst-knockout (Mst-KO) mice display a significant increase in skeletal muscle mass [68], decreased fat deposition, enhanced fatty acid oxidation, improved insulin sensitivity and increased resistance to diet-induced obesity [7], [69]. Although Mst expression is low in adipose tissues compared to muscle, its role in regulating adipogenesis is well documented [70], [71]. Mst inhibits brown adipogenic differentiation in mouse brown preadipocytes in vitro [72], Braga et al. [73] reported significant induction of PRDM16 and UCP1 expression in two white adipose depots (Epi and SC) isolated from Mst-KO compared to the wild type (WT) mice. Using primary cultures of differentiating mouse embryonic fibroblasts (MEFs) of WT and Mst-KO mice, the authors further confirmed the induction of key brown adipose markers in Mst-KO mice compared to the WT mice. This induction of key brown adipose differentiation markers were significantly blocked in the presence of exogenous recombinant Mst protein. They also demonstrated significant induction of adipocyte-specific marker adiponectin and energy-sensing AMP-activated protein kinase (AMPK) in differentiating MEF primary cultures isolated from Mst-KO mice compared to the WT mice. Subsequent studies published from several laboratories have confirmed that Mst inhibition promotes white adipose browning and improves insulin sensitivity [74], [75], [76]. Shan et al. [77] further confirmed that loss of Mst results in numerous small-sized brown adipocyte-like cells filled with multilocular small lipid droplets, representing key features of WAT browning and upregulation of a beige-specific gene signature. This upregulation of beige-specific markers was observed only in the mature adipocytes of Mst-KO and it was not due to increased beige cell progenitors present in WAT [77]. These authors further demonstrated that plasma extracted from Mst-KO mice significantly upregulated Ucp1 gene expression in adipocytes differentiated from the stromal vascular fraction of WAT, suggesting the possibility of non-cell autonomous effect of Mst on WAT browning [77]. In the same study, irisin, a protein product of the Fndc5 gene, was identified as a key mediator of WAT browning via activation of the AMPK-PGC1α-Fndc5 pathway. The absence of Mst has also been shown to improve insulin sensitivity in several in vitro and in vivo models [74], [76], Using an anti-Mst peptibody in C57BL/6 mice, Dong et al. [74] reported an increased expression of irisin in skeletal muscle, which contributed to WAT browning. Loss-of-function Mst mutation in Meishan pigs resulted in increased insulin sensitivity and browning of WAT [76]. Key browning genes Cd137 and Tmem26 were significantly upregulated in these Mst-deficient pigs. Levels of insulin receptor (IR) and insulin receptor substrate (IRS) proteins were significantly upregulated in the skeletal muscle of these Mst-deficient pigs. In a recent report, Mst was also shown to modulate post-transcriptional expression of FNDC5 and white adipose browning via a miR 34a-dependent mechanism [75]. Adeno-associated virus (AAV9)-mediated administration of Mst pro-peptide in adult low-density lipoprotein receptor null (Ldlr−/−) mice reduced diet-induced hepatosteatosis and progression of atherosclerosis [78]. Although the authors did not analyze the effect of this Mst pro-peptide on brown adipose activity/expression in this study, recent findings suggest a strong correlation between adipose browning, lipoprotein metabolism and progression of atherosclerosis [53], [79]. It is possible that the observed beneficial effects of Mst inhibition on the attenuation of atherosclerotic lesion, plasma lipid profiles and insulin sensitivity in Ldlr−/− mice in this study may have been mediated at least in part via adipose browning. In summary, Mst inhibition promotes white adipose browning, improves insulin sensitivity and protects experimental animals from diet-induced obesity besides its well-known effect on building muscle mass. Figure 1 briefly summarizes the beneficial effects of antagonizing the components of TGF-β and related pathways including Mst during the progression of obesity and related metabolic diseases via induction of white adipose browning.
Figure 1.
Schematic diagram showing that antagonizing TGF-β/myostatin (Mst) signaling components lead to activation of adipose browning and provides resistance from obesity and metabolic diseases.
Fst, adipose browning and energy metabolism
Fst binds and inhibits the activity of the TGF-β superfamily members in a variety of cell lines [80], [81], [82], [83], Pioneering experiments by Lee and McPherron [84] demonstrated that inhibition of Mst either by genetic elimination or increasing the amount of Fst resulted in greatly increased muscle mass. In spite of abundant literature demonstrating the role of Fst in the regulation of muscle mass and function, its role in regulation of energy and lipid metabolism remains largely unknown. Based on Fst’s established inhibitory actions on Mst/TGF-β signaling and its induction during negative energy balance, it was hypothesized that Fst may regulate adipose browning and energy metabolism [85].
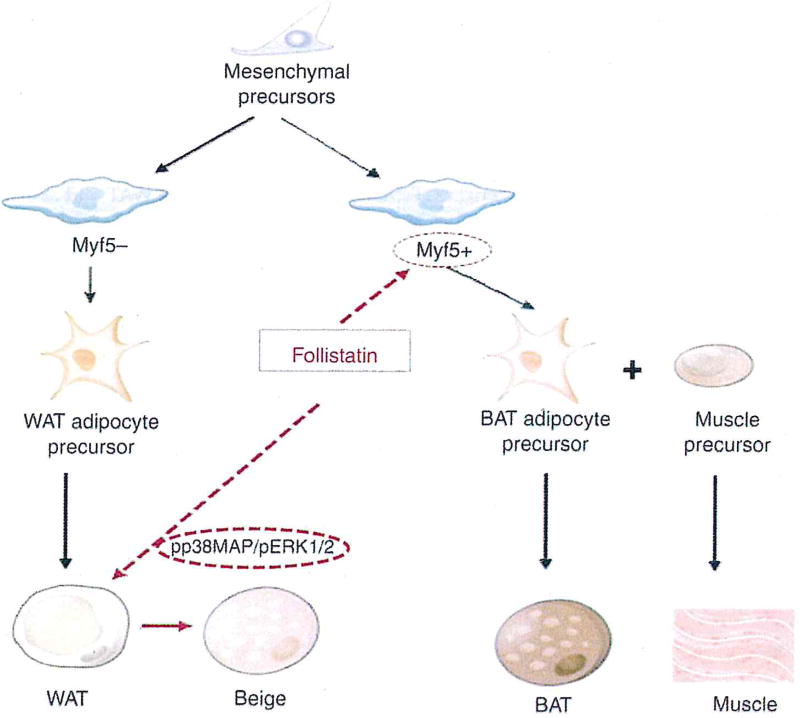
Braga et al. [85] provided the first evidence that Fst promotes adipocyte differentiation, white adipose browning and influences overall energy metabolism. These initial findings provided a rationale to explore the functional role of Fst in the regulation of adipose tissue metabolism in subsequent studies and identify the molecular targets responsible for Fst action on both metabolically active WAT and BAT depots. Using transgenic mice expressing Fst under myosin light chain promoter (Fst-Tg) [84], Singh et al. [86] analyzed tire in vivo actions of Fst in both WAT and BAT. The group demonstrated for the first time that Fst promotes brown adipose function in beige and BAT via targeting distinct molecular pathways (Figure 2). Increased levels of circulating Fst in the transgenic mice were associated with increased (~70%) interscapular BAT mass and upregulation of key brown adipocyte-specific markers Zic1, Lhx8 and Myf5 along with increased levels of Ucp1, Prdm16 and Glnt4 gene and protein expression. Gene and protein expression analysis of Epi and SC WAT depots showed significant upregulation of Ucp1, Prdm16, Pgc-la and Glut4 expression levels [86]. Immunohistological staining of both WAT with UCP1 antibody showed more multilocular adipocytes in SC tissues compared to the Epi fat in both WT and Fst-Tg mice [86]. These depot-specific differences in Epi and SC WAT are consistent with the published literature [89]. Importantly, UCP1 immunostaining was significantly higher in Fst-Tg compared to WT groups [86]. Several key markers involved in thermogenesis, fatty acid oxidation and mitochondrial biogenesis were significantly elevated in both WAT depots obtained from Fst-Tg mice compared to the WT mice [86]. Notably, the expression level of key beige-specific marker Cd137 was also found to be elevated in the Fst-Tg group, suggesting that Fst is promoting browning of both Epi and SC WAT depots [86]. Further analysis of possible molecular targets for Fst in WAT and BAT identified involvement of two distinct molecular pathways that were responsible for promoting brown adipose characteristics in these two adipose tissues [86]. In Epi and SC WAT, Fst promotes increased phosphorylation of p38 MAP kinase and ERK1/2, while it increased Myf5 expression and brown adipose precursors in BAT [86]. The involvement of these distinct molecular targets were further confirmed using in vitro models of differentiating 3T3-L1 and brown pre-adipocyte cells [86]. Furthermore, significantly decreased levels of Myf5 expression in primary cultures of Fst-KO MEF were rescued by exogenous recombinant Fst treatment, reinforcing the importance of Myf5 during Fst signaling in these cells [86]. Thus, the process of adipose browning could be regulated by several compounds [87].
Figure 2.
Follistatin regulation of white adipose tissue (WAT) browning and induction of classical brown adipose tissue (BAT) via targeting Myf5− and Myf5+ precursor pools, respectively.
Fst-induced inhibition of pSmad3/ActRIIB signaling in both adipose depots suggests the involvement of the TGF-β pathway in regulating adipose browning characteristics. Smad3 was previously reported to be essential for the inhibition of myogenic transcription factor Myf5 in skeletal muscle [88], [89], As both skeletal muscle and BAT share the same Myf5-expressing progenitor cells, it is possible that Fst promotes muscle growth and BAT activation by induction of Myf5 expression and expansion of the precursor cell pool [90], [91]. While the authors provide ample in vitro and in vivo evidence for the involvement of p38 MAPK/ERK1/2 in WAT browning, the involvement of other potential extrinsic factors may not be ruled out. Fst and Fndc5-encoded Irisin levels increase following exercise [77], [92]. Recombinant Fst treatment has been reported to upregulate Fndc5 gene expression in muscle [93]. It is, therefore, possible that increased secretion of irisin by skeletal muscle from Fst-Tg mice may induce phosphorylation of p38 MAPK/ERK1/2 to promote white adipose browning [94]. Fst-Tg mice were able to elicit an enhanced response in UCP1 protein levels following CL 316,243 treatment, suggesting the possible involvement of β3-drenergic receptor (β3-AR) signaling, a well-defined activator of p38 MAPK during white adipose browning and non-shivering thermogenesis in BAT [95], [96]. In conclusion, this study demonstrated for the first time that Fst targets both browning of WAT and activation of classical BAT along with its previously reported role in promoting skeletal muscle growth and, therefore, provides a rationale for exploring the therapeutic potential of Fst for the treatment of obesity and related metabolic diseases.
Conclusion
Emerging evidence supports the view that activation of BAT and beige adipocyte characteristics may contribute to the regulation of glucose and lipid homeostasis and prevent the development and progression of obesity and related metabolic syndrome. The possible mechanisms for these beneficial effects of BAT/beige activation are linked to their ability to promote energy expenditure, regulate whole blood glucose and lipid levels, increase insulin sensitivity and prevent liver steatosis and cardiovascular diseases. Therefore, exciting new strategies are being extensively explored to promote browning to combat obesity and several cardiovascular diseases. Inhibition of TGF-β/Mst/pSmad3 signaling either by pharmacological inhibition or genetic manipulation has shown promising results in several in vitro and animal studies. Accordingly, an efficient blockade of this pathway could open avenues for exploring the therapeutic potential of novel class of antagonists. Inhibition of Mst, a key member of the TGF-β superfamily has been shown to improve insulin sensitivity and prevent diet-induced obesity and other related metabolic dysfunction. It was presumed that these protective metabolic effects exclusively result from enlarged muscle mass resulting from Mst inactivation. However, several lines of evidence available in the literature now collectively demonstrate that Mst inactivation also significantly impacts the phenotype of WAT resulting in adipose browning. Fst, a known antagonist of Mst, has recently been identified as a novel regulator of brown adipose characteristics. Fst targets both browning of WAT and activation of classical BAT through distinct mechanisms. As Fst has previously been shown to induce skeletal muscle growth and development, it could be envisioned that this novel TGF-β/Mst antagonist could be used not only for the treatment of aging-related muscle dysfunction but also for obesity and related metabolic syndromes.
Acknowledgments
Research funding: This work was supported by National Institute of Health Grants SC1AG049682 (RS), and SC1CA1658650 (SP) and in part by National Institute on Minority Health and Health Disparity S21MD000103 (Charles R. Drew University).
Footnotes
Conflict of interest: The authors state no conflict of interest.
Informed consent: Informed consent is not applicable.
Ethical approval: The conducted research is not related to either human or animal use.
References
- 1.Tseng YH, Cypess AM, Kahn CR. Cellular bioenergetics as a target for obesity therapy. Nat Rev Drug Discov. 2010;9:465–82. doi: 10.1038/nrd3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 3.Betz MJ, Enerbäck S. Human brown adipose tissue: what we have learned so far. Diabetes. 2015;64:2352–60. doi: 10.2337/db15-0146. [DOI] [PubMed] [Google Scholar]
- 4.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–25. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 5.Cypess AM, Lehman S, Williams C, Tal I, Rodman D, Goldfine AB, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–17. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chondronikola M, Volpi E, Børsheim E, Porter C, Annamalai P, Enerbäck S, et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes. 2014;63:4089–99. doi: 10.2337/db14-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lebrasseur NK. Building muscle, browning fat and preventing obesity by inhibiting myostatin. Diabetologia. 2012;55:13–7. doi: 10.1007/s00125-011-2361-8. [DOI] [PubMed] [Google Scholar]
- 8.Cao L, Choi EY, Liu X, Martin A, Wang C, Xu X, et al. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab. 2011;14:324–38. doi: 10.1016/j.cmet.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wankhade LID, Shen M, Yadav H, Thakali KM. Novel browning agents, mechanisms, and therapeutic potentials of brown adipose tissue. Biomed Res Int. 2016;2016:2365609. doi: 10.1155/2016/2365609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kajimura S, Spiegelman BM, Seale P. Brown and beige fat: physiological roles beyond heat generation. Cell Metab. 2015;22:546–59. doi: 10.1016/j.cmet.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartelt A, Heeren J. Adipose tissue browning and metabolic health. Nat Rev Endocrinol. 2014;10:24–36. doi: 10.1038/nrendo.2013.204. [DOI] [PubMed] [Google Scholar]
- 12.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19:1252–63. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 13.Kozak LP. The genetics of brown adipocyte induction in white fat depots. Front Endocrinol (Lausanne) 2011;2:64. doi: 10.3389/fendo.2011.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang QA, Scherer PE, Gupta RK. Improved methodologies for the study of adipose biology: insights gained and opportunities ahead. J Lipid Res. 2014;55:605–24. doi: 10.1194/jlr.R046441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azhar Y, Parmar A, Miller CN, Samuels JS, Rayalam S. Phytochemicals as novel agents for the induction of browning in white adipose tissue. Nutr Metab (Lond) 2016;13:89. doi: 10.1186/s12986-016-0150-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mestdagh R, Dumas ME, Rezzi S, Kochhar S, Holmes E, Claus SR, et al. Gut microbiota modulate the metabolism of brown adipose tissue in mice. J Proteome Res. 2012;11:620–30. doi: 10.1021/pr200938v. [DOI] [PubMed] [Google Scholar]
- 17.Sidossis L, Kajimura S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J. Clin Invest. 2015;125:478–86. doi: 10.1172/JCI78362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porter C, Chondronikola M, Sidossis LS. The therapeutic potential of brown adipocytes in humans. Front Endocrinol (Lausanne) 2015;6:156. doi: 10.3389/fendo.2015.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodríguez A, Becerril S, Ezquerro S, Méndez-Giménez L, Frühbeck G. Crosstalk between adipokines and myokines in fat browning. Acta Physiol (Oxf) 2017;219:362–81. doi: 10.1111/apha.12686. [DOI] [PubMed] [Google Scholar]
- 20.Abdullahi A, Jeschke MC. White adipose tissue browning: a double-edged sword. Trends Endocrinol Metab. 2016;27:542–52. doi: 10.1016/j.tem.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, et al. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007;6:38–54. doi: 10.1016/j.cmet.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilsanz V, Hu HH, Kajimura S. Relevance of brown adipose tissue in infancy and adolescence. Pediatr Res. 2013;73:3–9. doi: 10.1038/pr.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palmer AK, Kirkland JL. Aging and adipose tissue: potential interventions for diabetes and regenerative medicine. Exp Gerontol. 2016;86:97–105. doi: 10.1016/j.exger.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez-Gurmaches J, Guertin DA. Adipocyte lineages: tracing back the origins of fat. Biochim Biophys Acta. 2014;1842:340–51. doi: 10.1016/j.bbadis.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lepper C, Fan CM. Inducible lineage tracing of Pax7-descendant cells reveals embryonic origin of adult satellite cells. Genesis. 2010;48:424–36. doi: 10.1002/dvg.20630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang W, Seale P. Control of brown and beige fat development. Nat Rev Mol Cell Biol. 2016;17:691–702. doi: 10.1038/nrm.2016.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanchez-Gurmaches J, Hung CM, Sparks CA, Tang Y, Li H, Guertin DA. PTEN loss in the myfs lineage redistributes body fat and reveals subsets of white adipocytes that arise from myfs precursors. Cell Metab. 2012;16:348–62. doi: 10.1016/j.cmet.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Long JZ, Svensson KJ, Tsai L, Zeng X, Roh HC, Kong X, et al. A smooth muscle-like origin for beige adipocytes. Cell Metab. 2014;19:810–20. doi: 10.1016/j.cmet.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frontini A, Cinti S. Distribution and development of brown adipocytes in the murine and human adipose organ. Cell Metab. 2010;11:253–6. doi: 10.1016/j.cmet.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Rosenwald M, Perdikari A, Rülicke T, Wolfrum C. Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol. 2013;15:659–67. doi: 10.1038/ncb2740. [DOI] [PubMed] [Google Scholar]
- 31.Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–76. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L, et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One. 2012;7:e49452. doi: 10.1371/journal.pone.0049452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lidell ME, Betz MJ, Dahlqvist Leinhard O, Heglind M, Elander L, Slawik M, et al. Evidence for two types of brown adipose tissue in humans. Nat Med. 2013;19:631–4. doi: 10.1038/nm.3017. [DOI] [PubMed] [Google Scholar]
- 34.Wang W, Kissig M, Rajakumari S, Huang L, Lim HW, Won KJ, et al. Ebfz is a selective marker of brown and beige adipogenic precursor cells. Proc Natl Acad Sci U S A. 2014;111:14466–71. doi: 10.1073/pnas.1412685111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem. 2010;285:7153–64. doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jespersen NZ, Larsen TJ, Peijs L, Daugaard S, Homøe P, Loft A, et al. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab. 2013;17:798–805. doi: 10.1016/j.cmet.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 37.Sun L, Xie H, Mori MA, Alexander R, Yuan B, Hattangadi SM, et al. Mir193b-365 is essential for brown fat differentiation. Nat Cell Biol. 2011;13:958–65. doi: 10.1038/ncb2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Güller I, McNaughton S, Crowley T, Gilsanz V, Kajimura S, Watt M, et al. Comparative analysis of microRNA expression in mouse and human brown adipose tissue. BMC Genomics. 2015;16:820. doi: 10.1186/s12864-015-2045-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim HJ, Cho H, Alexander R, Patterson HC, Gu M, Lo KA, et al. MicroRNAs are required for the feature maintenance and differentiation of brown adipocytes. Diabetes. 2014;63:4045–56. doi: 10.2337/db14-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu Y, Zuo J, Zhang Y, Xie Y, Hu F, Chen L, et al. Identification of miR-106b-93 as a negative regulator of brown adipocyte differentiation. Biochem Biophys Res Commun. 2013;438:575–80. doi: 10.1016/j.bbrc.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 41.Mori M, Nakagami H, Rodriguez-Araujo G, Nimura K, Kaneda Y. Essential role for miR-196a in brown adipogenesis of white fat progenitor cells. PLoS Biol. 2012:e100131410. doi: 10.1371/journal.pbio.1001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu W, Bi P, Shan T, Yang X, Yin H, Wang YX, et al. miR-133a regulates adipocyte browning in vivo. PLoS Genet. 2013:e10036269. doi: 10.1371/journal.pgen.1003626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hurt RT, Kulisek C, Buchanan LA, McClave SA. The obesity epidemic: challenges, health initiatives, and implications for gastroenterologists. Gastroenterol Hepatol (N Y) 2010;6:780–92. [PMC free article] [PubMed] [Google Scholar]
- 44.Kajimura S, Seale P, Spiegelman BM. Transcriptional control of brown fat development. Cell Metab. 2010;11:257–62. doi: 10.1016/j.cmet.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galgani J, Ravussin E. Energy metabolism, fuel selection and body weight regulation. Int J Obes (Lond) 2008;32(Suppl 7):S109–19. doi: 10.1038/ijo.2008.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, et al. Brown adipose tissue activity controls triglyceride clearance. Nat Med. 2011;17:200–5. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- 47.Stanford Kl, Middelbeek RJ, Townsend KL, An D, Nygaard EB, Hitchcox KM, et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest. 2013;123:215–23. doi: 10.1172/JCI62308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou Z, Yon Toh S, Chen Z, Guo K, Ng CP, Ponniah S, et al. Cidea-deficient mice have lean phenotype and are resistant co obesity. Nat Genet. 2003;35:49–56. doi: 10.1038/ng1225. [DOI] [PubMed] [Google Scholar]
- 49.Almind K, Manieri M, Sivitz WI, Cinti S, Kahn CR. Ectopic brown adipose tissue in muscle provides a mechanism for differences in risk of metabolic syndrome in mice. Proc Natl Acad Sci U S A. 2007;104:2366–71. doi: 10.1073/pnas.0610416104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gunawardana SC, Piston DW. Reversal of type 1 diabetes in mice by brown adipose tissue transplant. Diabetes. 2012;61:674–82. doi: 10.2337/db11-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Villarroya F, Giralt M. The beneficial effects of brown fat transplantation: further evidence of an endocrine role of brown adipose tissue. Endocrinology. 2015;156:2368–70. doi: 10.1210/en.2015-1423. [DOI] [PubMed] [Google Scholar]
- 52.Liu X, Wang S, You Y, Meng M, Zheng Z, Dong M, et al. Brown adipose tissue transplantation reverses obesity in ob/ob mice. Endocrinology. 2015;156:2461–9. doi: 10.1210/en.2014-1598. [DOI] [PubMed] [Google Scholar]
- 53.Berbée JF, Boon MR, Khedoe PR, Bartelt A, Schlein C, Worthmann A, et al. Brown fat activation reduces hypercholesterolemia and protects from atherosclerosis development. Nat Commun. 2015;6:6356. doi: 10.1038/ncomms7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoneshiro T, Aita S, Matsushita M, Kameya T, Nakada K, Kawai Y, et al. Brown adipose tissue, whole-body energy expenditure, and thermogenesis in healthy adult men. Obesity (Silver Spring) 2011;19:13–6. doi: 10.1038/oby.2010.105. [DOI] [PubMed] [Google Scholar]
- 55.Poher AL, Altirriba J, Veyrat-Durebex C, Rohner-Jeanrenaud F. Brown adipose tissue activity as a target for the treatment of obesity/insulin resistance. Front Physiol. 2015;6:4. doi: 10.3389/fphys.2015.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Q, Zhang M, Xu M, Gu W, Xi Y, Qi L, et al. Brown adipose tissue activation is inversely related to central obesity and metabolic parameters in adult human. PLoS One. 2015:e012379510. doi: 10.1371/journal.pone.0123795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chechi K, Blanchard PG, Mathieu P, Deshaies Y, Richard D. Brown fat like gene expression in the epicardial fat depot correlates with circulating HDL-cholesterol and triglycerides in patients with coronary artery disease. Int J Cardiol. 2013;167:2264–70. doi: 10.1016/j.ijcard.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 58.Bartesaghi S, Hallen S, Huang L, Svensson PA, Momo RA, Wallin S, et al. Thermogenic activity of UCP1 in human white fat-derived beige adipocytes. Mol Endocrinol. 2015;29:130–9. doi: 10.1210/me.2014-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Okla M, Ha JH, Temel RE, Chung S. BMP7 drives human adipogenic stem cells into metabolically active beige adipocytes. Lipids. 2015;50:111–20. doi: 10.1007/s11745-014-3981-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bartelt A, John C, Schaltenberg N, Berbée JF, Worthmann A, Cherradi ML, et al. Thermogenic adipocytes promote HDL turnover and reverse cholesterol transport. Nat Commun. 2017;8 doi: 10.1038/ncomms15010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vijgen GH, Bouvy ND, Teule GJ, Brans B, Hoeks J, Schrauwen P, et al. Increase in brown adipose tissue activity after weight loss in morbidly obese subjects. J Clin Endocrinol Metab. 2012;97:E1229–33. doi: 10.1210/jc.2012-1289. [DOI] [PubMed] [Google Scholar]
- 62.Yilmaz Y, Ones T, Purnak T, Ozguven S, Kurt R, Atug O, et al. Association between the presence of brown adipose tissue and non-alcoholic fatty liver disease in adult humans. Aliment Pharmacol Ther. 2011;34:318–23. doi: 10.1111/j.1365-2036.2011.04723.x. [DOI] [PubMed] [Google Scholar]
- 63.Yadav H, Quijano C, Kamaraju AK, Gavrilova O, Malek R, Chen W, et al. Protection from obesity and diabetes by blockade of TGF-β/Smad3 signaling. Cell Metab. 2011;14:67–79. doi: 10.1016/j.cmet.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Craft CS, Pietka TA, Schappe T, Coleman T, Combs MD, Klein S, et al. The extracellular matrix protein MACP1 supports thermogenesis and protects against obesity and diabetes through regulation of TGF-β. Diabetes. 2014;63:1920–32. doi: 10.2337/db13-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weinbaum JS, Broekelmann TJ, Pierce RA, Werneck CC, Segade F, Craft CS, et al. Deficiency in microfibril-associated glycoprotein-1 leads to complex phenotypes in multiple organ systems. J Biol Chem. 2008;283:25533–43. doi: 10.1074/jbc.M709962200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fournier B, Murray B, Gutzwiller S, Marcaletti S, Marcellin D, Bergling S, et al. Blockade of the activin receptor lib activates functional brown adipogenesis and thermogenesis by inducing mitochondrial oxidative metabolism. Mol Cell Biol. 2012;32:2871–9. doi: 10.1128/MCB.06575-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koncarevic A, Kajimura S, Cornwall-Brady M, Andreucci A, Pullen A, Sako D, et al. A novel therapeutic approach to treating obesity through modulation of TGFβ signaling. Endocrinology. 2012;153:3133–46. doi: 10.1210/en.2012-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 69.Bernardo BL, Wachtmann TS, Cosgrove PC, Kuhn M, Opsahl AC, Judkins KM, et al. Postnatal PPARdelta activation and myostatin inhibition exert distinct yet complimentary effects on the metabolic profile of obese insulin-resistant mice. PLoS One. 2010:e113075. doi: 10.1371/journal.pone.0011307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Feldman BJ, Streeper RS, Farese RV, Yamamoto KR. Myostatin modulates adipogenesis to generate adipocytes with favorable metabolic effects. Proc Natl Acad Sci U S A. 2006;103:15675–80. doi: 10.1073/pnas.0607501103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Artaza JN, Bhasin S, Magee TR, Reisz-Porszasz S, Shen R, Groome NP, et al. Myostatin inhibits myogenesis and promotes adipogenesis in C3H10T(1/2) mesenchymal multipotent cells. Endocrinology. 2005;146:3547–57. doi: 10.1210/en.2005-0362. [DOI] [PubMed] [Google Scholar]
- 72.Kim WK, Choi HR, Park SG, Ko Y, Bae KH, Lee SC. Myostatin inhibits brown adipocyte differentiation via regulation of Smad3-mediated β-catenin stabilization. Int J Blochem Cell Biol. 2012;44:327–34. doi: 10.1016/j.biocel.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 73.Braga M, Pervin S, Norris K, Bhasin S, Singh R. Inhibition of in vitro and in vivo brown fat differentiation program by myostatin. Obesity (Silver Spring) 2013;21:1180–8. doi: 10.1002/oby.20117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dong J, Dong Y, Dong Y, Chen F, Mitch WE, Zhang L. Inhibition of myostatin in mice improves insulin sensitivity via irisin-mediated cross talk between muscle and adipose tissues. Int J Obes (Lond) 2016;40:434–42. doi: 10.1038/ijo.2015.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ge X, Sathiakumar D, Lua BJ, Kukreti H, Lee M, McFarlane C. Myostatin signals through miR-34a to regulate Fndc5 expression and browning of white adipocytes. Int J Obes (Lond) 2017;41:137–48. doi: 10.1038/ijo.2016.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cai C, Qian L, Jiang S, Sun Y, Wang Q, Ma D, et al. Loss-of-function myostatin mutation increases insulin sensitivity and browning of white fat in Meishan pigs. Oncotarget. 2017;8:34911–22. doi: 10.18632/oncotarget.16822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shan T, Liang X, Bi P, Kuang S. Myostatin knockout drives browning of white adipose tissue through activating the AMPK-PGC1α-Fndc5 pathway in muscle. FASEB J. 2013;27:1981–9. doi: 10.1096/fj.12-225755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guo W, Wong S, Bhasin S. AAV-mediated administration of myostatin pro-peptide mutant in adult Ldlr null mice reduces diet-induced hepatosteatosis and arteriosclerosis. PLoS One. 2013;8:e71017. doi: 10.1371/journal.pone.0071017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hoeke G, Kooijman S, Boon MR, Rensen PC, Berbée JF. Role of brown fat in lipoprotein metabolism and atherosclerosis. Circ Res. 2016;118:173–82. doi: 10.1161/CIRCRESAHA.115.306647. [DOI] [PubMed] [Google Scholar]
- 80.Amthor H, Nicholas G, McKinnell I, Kemp CF, Sharma M, Kambadur R, et al. Follistatin complexes myostatin and antagonises myostatin-mediated inhibition of myogenesis. Dev Biol. 2004;270:19–30. doi: 10.1016/j.ydbio.2004.01.046. [DOI] [PubMed] [Google Scholar]
- 81.Hill JJ, Davies MV, Pearson AA, Wang JH, Hewick RM, Wolfman NM, et al. The myostatin propeptide and the follistatin-related gene are inhibitory binding proteins of myostatin in normal serum. J Biol Chem. 2002;277:40735–41. doi: 10.1074/jbc.M206379200. [DOI] [PubMed] [Google Scholar]
- 82.Singh R, Bhasin S, Braga M, Artaza JN, Pervin S, Taylor WE, et al. Regulation of myogenic differentiation by androgens: crosstalk between androgen receptor/beta-catenin and follistatin/transforming growth factor-beta signaling pathways. Endocrinology. 2009;150:1259–68. doi: 10.1210/en.2008-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Braga M, Bhasin S, Jasuja R, Pervin S, Singh R. Testosterone inhibits transforming growth factor-β signaling during myogenic differentiation and proliferation of mouse satellite cells: potential role of follistatin in mediating testosterone action. Mol Cell Endocrinol. 2012;350:39–52. doi: 10.1016/j.mce.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci U S A. 2001;98:9306–11. doi: 10.1073/pnas.151270098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Braga M, Reddy ST, Vergnes L, Pervin S, Grijalva V, Stout D, et al. Follistatin promotes adipocyte differentiation, browning, and energy metabolism. J Lipid Res. 2014;55:375–84. doi: 10.1194/jlr.M039719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Singh R, Braga M, Reddy ST, Lee SJ, Parveen M, Grijalva V, et al. Follistatin targets distinct pathways to promote brown adipocyte characteristics in brown and white adipose tissues. Endocrinology. 2017;158:1217–30. doi: 10.1210/en.2016-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lo KA, Sun L. Turning WAT into BAT: a review on regulators controlling the browning of white adipocytes. Biosci Rep. 2013:e0006533. doi: 10.1042/BSR20130046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu D, Black BL, Derynck R. TGF-beta inhibits muscle differentiation through functional repression of myogenic transcription factors by Smad3. Genes Dev. 2001;15:2950–66. doi: 10.1101/gad.925901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kollias HD, McDermott JC. Transforming growth factor-beta and myostatin signaling in skeletal muscle. J Appl Physiol (1985) 2008;104:579–87. doi: 10.1152/japplphysiol.01091.2007. [DOI] [PubMed] [Google Scholar]
- 90.Li J-X, Cummins CL. Getting the skinny on follistatin and fat. Endocrinology. 2017:1109–12. doi: 10.1210/en.2017-00223. [DOI] [PubMed] [Google Scholar]
- 91.Singh R, Braga M, Pervin S. Regulation of brown adipocyte metabolism by myostatin/follistatin signaling. Front Cell Dev Biol. 2014;2:60. doi: 10.3389/fcell.2014.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hansen J, Brandt C, Nielsen AR, Hojman P, Whitham M, Febbraio MA, et al. Exercise induces a marked increase in plasma follistatin: evidence that follistatin is a contraction-induced hepatokine. Endocrinology. 2011;152:164–71. doi: 10.1210/en.2010-0868. [DOI] [PubMed] [Google Scholar]
- 93.Tiano JP, Springer DA, Rane SG. SMAD3 negatively regulates serum irisin and skeletal muscle FNDC5 and peroxisome proliferator-activated receptor γ coactivator 1-α (PGC-1α) during exercise. J Biol Chem. 2015;290:7671–84. doi: 10.1074/jbc.M114.617399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang Y, Li R, Meng Y, Li S, Donelan W, Zhao Y, et al. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes. 2014;63:514–25. doi: 10.2337/db13-1106. [DOI] [PubMed] [Google Scholar]
- 95.Collins S, Surwit RS. The beta-adrenergic receptors and the control of adipose tissue metabolism and thermogenesis. Recent Prog Horm Res. 2001;56:309–28. doi: 10.1210/rp.56.1.309. [DOI] [PubMed] [Google Scholar]
- 96.Zheng Z, Liu X, Zhao Q, Zhang L, Li C, Xue Y. Regulation of UCP1 in the browning of epididymal adipose tissue by β3-adrenergic agonist: a role for microRNAs. Int J Endocrinol. 2014;2014:530636. doi: 10.1155/2014/530636. [DOI] [PMC free article] [PubMed] [Google Scholar]