Abstract
Plant-feeding herbivores can generate complex patterns of foliar wounding, but it is unclear how wounding-elicited volatile emissions scale with the severity of different wounding types, and there is no common protocol for wounding experiments. We investigated the rapid initial response to wounding damage generated by different numbers of straight cuts and punctures through leaf lamina as well as varying area of lamina squeezing in the temperate deciduous tree Populus tremula Wounding-induced volatile emission time-courses were continuously recorded by a proton-transfer-reaction time-of-flight mass-spectrometer. After the mechanical wounding, an emission cascade was rapidly elicited resulting in sequential emissions of key stress volatiles methanol, acetaldehyde, and volatiles of the lipoxygenase pathway, collectively constituting more than 97% of the total emission. The maximum emission rates, reached after one to three minutes after wounding, and integrated emissions during the burst were strongly correlated with the severity in all damage treatments. For straight cuts and punch hole treatments, the emissions per cut edge length were constant, indicating a direct proportionality. Our results are useful for screening wounding-dependent emission capacities.
Keywords: Abiotic stress, green volatiles, hexenal, LOX products, mass spectrometry, proton-transfer-reaction
Introduction
Plants emit biogenic volatile organic compounds (BVOCs) constitutively, but also in response to biotic and abiotic stress situations (Loreto and Schnitzler 2010; Monson 2013; Niinemets and Monson 2013; Niinemets et al. 2010). BVOCs have been observed to protect plants from herbivores by decreasing herbivore performance (Ament et al. 2004), to play a role in plant-to-plant (Arimura et al. 2005), plant-to-microbe (Paiva 2000), and plant-to-insect communication, including tritrophic interactions by attracting herbivore predators or parasites (Ponzio et al. 2014).
In nature, plants are exposed to various forms of mechanical stress such as rain, snow, wind, animals, pathogens, or plants themselves (Benikhlef et al. 2013). When leaf damage happens, it generates a rapid release of free fatty acids from plant membranes that triggers the lipoxygenase (LOX) chain reaction and leads to emissions of volatile LOX products, mainly C6 aldehydes and alcohols (see explanatory schemes in Fall et al. 1999, 2011), often called green leaf volatiles (GLV) (Brilli et al. 2011). The rapid burst of volatile emission upon wounding lasts for several minutes until emissions return to the pre-stress level (Brilli et al. 2012). However, later responses to wounding through emission of volatiles may last hours to days (Niinemets and Monson 2013). The GLV blend protects against microbial infection of the tissues (Croft et al. 1993; Matsui et al. 2012) and plays a role in inducing defense responses in non-wounded tissues (Niinemets et al. 2013). For example, the infestation by piercing-sucking or chewing insects such as aphids, beetles, and caterpillars enhances the release of LOX products, and it further induces terpenoid emissions (Copolovici et al. 2011). Moreover, BVOC emission contributes to atmospheric chemistry because they serve as a sink for OH radicals (Arneth and Niinemets 2010).
Leaf mechanical damage or wounding under lab conditions provides an opportunity to study the mechanisms of physical stress, such as that inflicted by herbivory, under controlled conditions (Brilli et al. 2011; Fall et al. 1999). However, standardization of wounding to mechanical damage only, ignores the proven metabolic effects of herbivore oral secretions, which can alter response to herbivory (Mithöfer and Boland 2008).
In leaf wounding experiments, volatile and non-volatile LOX products, including jasmonic acid, salicylic acid, and their methylated forms, are of special interest because they regulate the expression of defense genes (Copolovici et al. 2014b) and the downstream synthesis of volatile and non-volatile metabolites (Farag et al. 2005), which may have a role in coping with biotic and abiotic stresses (Filella et al. 2006).
From experiments using linear cuts of different length, the rate of emission of LOX products has been observed to be quantitatively associated with the degree of damage (Brilli et al. 2011, 2012). However, different types of damage can induce emissions of different strength and dynamics (Smith and Beck 2013). Therefore, it is relevant to test different types of mechanical damage to replicate the highly complex patterns of wounding stress that occur in nature.
We studied the wound-elicited emissions in European aspen (Populus tremula L.) to improve knowledge of how wound-induced BVOC emissions vary with the type of wounding and the overall degree of damage. The goal of our experiments was to develop a robust laboratory screening protocol for quantitative assessment of wounding effects on BVOC emissions, and to provide model information for prediction of wound-induced emissions in plants exposed to multiple wounding agents under field conditions. We focused on the short-term BVOC emissions that occur during the first minutes after leaf wounding, and we addressed the following questions: (1) What are the overall magnitude and kinetics of GLV emissions after leaf wounding by different wounding procedures? (2) Do different leaf wounding methods change the composition of emitted BVOCs?
We used a proton-transfer-reaction time-of-flight mass spectrometer (PTR-TOF-MS), making it possible to track the entire suite of BVOCs emitted in real-time (Brilli et al. 2011; Schaub et al. 2010), which is especially relevant for tracing plant responses to rapidly occurring stresses such as wounding. This particular instrument goes one step beyond its quadrupole-based predecessor because it instantaneously samples all the emitted masses, and can identify different compounds within a given nominal mass, sorting out the signal of single compounds in multi peaked-shaped spectra (Brilli et al. 2011; Portillo-Estrada 2013).
Methods and Materials
Plant Material
The study was carried out in late summer using root suckers of 15-20 leaves from mature naturally-established Populus tremula trees on the campus of the Estonian University of Life Sciences (Tartu, Estonia, 58.39° N, 26.70° E, elevation 41 m). We chose this species because it has been widely used as a model plant in herbivory studies (Brilli et al. 2011; Fall et al. 1999) and in the study of BVOC emissions (Brilli et al. 2007; Ghirardo et al. 2011), and for its importance to human economy. Poplar trees are increasingly used in short-rotation cultures as a source for biofuel production (Verlinden et al. 2015).
The shoots were harvested, placed in a bucket with water, re-cut under water, and transported to the laboratory where they were kept at a room temperature of 22 °C beneath a 500 W halogen lamp model J-118 providing a quantum flux density of ca. 350 μmol m-2 s-1 at the shoot level to acclimate the leaves to the measurement conditions.
The average leaf dry mass (± SE) (7th to 9th leaves from the shoot apex) was 0.258 ± 0.015 g, and the leaf water content was 0.599 ± 0.006%. Average leaf area was 40.7 ± 2.0 cm2, and leaf dry mass per area unit was 63.4 ± 2.3 g m-2.
Gas Exchange Measurements
Leaf photosynthetic activity, stomatal conductance, and volatile compound emissions were measured online using a GFS-3000 gas-exchange system (Walz GmbH, Effeltrich, Germany) combined with a PTR-TOF-MS (proton-transfer-reaction time-of-flight mass spectrometer) model 8000 (Ionicon Analytic GmbH, Innsbruck, Austria). See a scheme of the experimental setup in Figure 1a. We used the standard 8 cm2 leaf cuvette (3010-S of Walz GFS-3000) with a LED array/PAM-fluorometer 3055-FL for leaf illumination. During the measurements, the light intensity was kept at a saturating level of 500 μmol m-2 s-1. The cuvette was flushed with ambient air with a flow rate of 750 μmol s-1. A constant leaf temperature of 25 °C, a constant air humidity (16000 ppm H2O, approx. 60% relative humidity) entering the cuvette, and a constant CO2 concentration of 400 μmol mol-1 in the ingoing air were maintained. A flow of 74 μmol s-1 from the Walz gas exchange system outlet was used for PTR-TOF-MS measurements. This rate of flow was maintained constant by the built-in mass flow controller in the PTR-TOF-MS instrument.
Fig. 1.
Simplified scheme of (a) the experimental setup, and greyscale pictures of the damage introduced by different leaf wounding methods in aspen (Populus tremula): (b) straight penetrating cut through the leaf lamina with a razor, (c) hole punch in the leaf lamina, and (d) a controlled squeeze of the leaf lamina.
Net CO2 assimilation rate (A), leaf transpiration rate (T), and stomatal conductance (gs) were calculated from measured concentrations of CO2 and H2O in in- and outgoing air, leaf temperature and one-sided leaf area using the built-in equations of the Walz system. The experimental leaves covered completely the cuvette window area (8 cm2) in all cases. After leaf wounding, the intercellular space and cytoplasmic content becomes exposed at the cut edge, temporarily generating a free liquid surface. Thus, changes in calculated transpiration rate and stomatal conductance upon wounding might partly reflect evaporation from that free liquid surface. Therefore, we refer to the stomatal conductance and transpiration rate in wounded leaves as apparent estimates.
PTR-TOF-MS Analytical Procedures
In the PTR-TOF-MS instrument, the BVOCs in the air sample are detected in real-time through proton reactions occurring between the hydronium ions (H3O+) produced within the discharge ion source and the air sample. The air exiting the cuvette was continuously analyzed with the PTR-TOF-MS, averaging the data of 31250 spectra (m/z 0-316) per second. The drift tube was operated at 600 V drift voltage, 2.3 mbar pressure, and 60 °C temperature, resulting in a field density ratio (E/N) of ≈130 Td. The resulting protonated ions were extracted from the drift tube and pulsed every 32 μsec to the time-of-flight region, and separated by their m/z ratio. The detection was done by a MCP (multi-channel-plate) and a TDC (time-to-digital converter) (Burle Industries Inc., Lancaster, PA, USA). For further details of the measuring principle, we refer to Graus et al. (2010).
The raw data generated by the PTR-TOF-MS was acquired by TofDaq software (Tofwerk AG, Switzerland). The sensitivity of our specific PTR-TOF-MS instrument to the measured volatiles (dependent on the ion mass) was derived from the reaction rate constant calculated prior to the experiment. For these calculations, a commercial gas mixture containing eight pure compounds ranging from m+/z 21 to 181 with known concentrations was used to generate a calibration curve (known as transmission curve), which was further used to calculate the concentration of the other measured BVOCs (Jordan et al. 2009).
The raw data post-processing by routine functions was done in PTR-MS Viewer v3.1 (Tofwerk AG, Switzerland). The BVOCs peaks in the spectrum were identified by their time of flight from the pulser to the detector (proportional to the molecular mass) compared to the time performed by known standard compounds. The peak area (i.e., compound abundance) for each compound was integrated through the built-in software tools. The PTR-TOF-MS recorded the continuous range of molecular masses. Therefore, within one nominal mass, the signal of several compounds could be recorded, sometimes appearing as overlapping peaks. Resolving overlapping peaks was an important task, and the signals of adjacent peaks that interfered with the signal of the compound of interest were discarded. The concentration for each compound was calculated after integrating the peak area in each spectrum (1 Hz data), and related to the concentration of H3O+ (using the isotopomer H318O+, m+/z 21.0221). The detection limit for all measured volatiles was approximately 7 pptv (parts per trillion by volume).
Volatile Organic Compounds Monitored
Emissions of 26 relevant compounds on wound-induced BVOC emissions were tracked (Table 1). These compounds were divided among six major compound classes: lightweight oxygenated compounds (LOCs, compounds between C1-C3); pentenyl family LOX products; hexenal family LOX products; hexanal family LOX products; benzenoids and jasmonates; and induced isoprenoids (isoprenoids other than isoprene) (Table 1). The compounds from the latter two classes included volatiles and semi-volatiles known for their role in plant protection, communication and stress signaling.
Table 1.
List of selected biogenic volatile organic compounds (BVOCs) analyzed by a proton-transfer-reaction time-of-flight mass spectrometer (PTR-TOF-MS) in aspen (Populus tremula) leaf wounding experiments
| Compound | Molecular formula of the protonated compound | Protonated molecular mass | Molecular mass of major fragments | Average abundance in the wound-induced volatile blend (parts per million) | Explained variance (r2) for the linear correlation with wound length a |
|---|---|---|---|---|---|
| Lightweight oxygenated compounds (LOCs) | 430000 | 0.66*** | |||
| formaldehyde | (CH2O)H+ | 31.018 | 1000 | 0.54*** | |
| methanol | (CH4O)H+ | 33.034 | 150000 | 0.71*** | |
| acetaldehyde | (C2H4O)H+ | 45.034 | 260000 | 0.63*** | |
| formic acid | (CH2O2)H+ | 47.013 | 2700 | 0.70*** | |
| ethanol | (C2H6O)H+ | 47.049 | 3100 | 0.43*** | |
| acetone | (C3H6O)H+ | 59.049 | 4400 | 0.61*** | |
| acetic acid | (C2H4O2)H+ | 61.028 | 2500 | 0.57*** | |
| Pentenyl family LOX products | 6000 | 0.78*** | |||
| pentenal | (C5H8O)H+ | 85.065 | 71.086 | 0.77*** | |
| pentenone | (C5H8O)H+ | 85.065 | 0.81*** | ||
| pentanal | (C5H10O)H+ | 87.080 | 71.086 | 0.70*** | |
| pentenol | (C5H10O)H+ | 87.080 | 69.070 | 0.70*** | |
| Hexenal family LOX products | 560000 | 0.80*** | |||
| hexenal | (C6H10O)H+ | 99.080 | 81.070 | 560000 | 0.77*** |
| hexenol | (C6H12O)H+ | 101.096 | 83.085 | 2200 | 0.68*** |
| hexenyl acetate | (C8H14O2)H+ | 143.107 | 140 | 0.60*** | |
| Hexanal family LOX products | 800 | 0.76*** | |||
| hexanal | (C6H12O)H+ | 101.096 | 83.085 | 700 | 0.68*** |
| hexanol | (C6H14O)H+ | 103.112 | 85.101 | 47 | 0.80*** |
| hexyl acetate | (C8H16O2)H+ | 145.122 | 17 | 0.42*** | |
| Benzenoids and jasmonates | 60 | 0.52*** | |||
| methyl benzoate b | (C8H8O2)H+ | 137.060 | 28 | 0.33** | |
| methyl salicylate b | (C8H8O3)H+ | 153.055 | 15 | 0.64*** | |
| jasmonic acid c | (C12H18O3)H+ | 211.133 | 10 | 0.15* | |
| methyl jasmonate c | (C13H20O3)H+ | 225.146 | 8 | 0.16* | |
| Induced isoprenoids (isoprenoids other than isoprene) | 180 | 0.51*** | |||
| Σ monoterpenes | (C10H16)H+ | 137.133 | 81.070 | 90 | 0.40** |
| DMNT d | (C11H18)H+ | 151.148 | 22 | 0.36*** | |
| Linalool | (C10H20O)H+ | 155.143 | 27 | 0.29** | |
| Σ sesquiterpenes | (C15H24)H+ | 205.195 | 22 | 0.37*** | |
| TMTT e | (C16H26)H+ | 219.211 | 14 | 0.14* | |
The explained variances are given for the correlations of the wound length with the emitted volatiles during 7 min after wounding. Experiments involving linear cuts with razor blade and leaf punctures were pooled
Phenylpropanoid compound; (c) LOX products; (d) (E)-4,8-dimethyl-1,3,7-nonatriene; (e) (E,E)-4,8,12-trimethyl-1,3,7,11-tridecatetraene
LOX products
(E)-4,8-dimethyl-1,3,7-nonatriene
(E,E)-4,8,12-trimethyl-1,3,7,11-tridecatetraene
Asterisks denote the statistical significance: (*) P<0.05; (**) P<0.01; (***) P<0.001
Experimental Protocol
The measurement routine was as follows: (1) empty cuvette (background) measurement during 5 min, followed by (2) careful clamping of the selected leaf into the cuvette. The borders of the leaf surface inserted in the cuvette were marked to allow for clamping exactly the same portion of leaf area upon repeated insertion. After insertion, (3) the leaf was stabilized under the measurement conditions until steady-state values of stomatal conductance (gs), net CO2 assimilation rate (A), and isoprene emission rate (m+/z = 69.070) were reached. The leaf stabilization to the cuvette conditions (light, humidity, air flow, etc.) usually took 30 to 40 min. (4) The leaf was rapidly removed from the cuvette and the wounding treatment was performed within a few seconds (at most for 15 s for the greatest number of leaf punches, see below). (5) The leaf was immediately re-inserted in the cuvette, clamping exactly the same part of the leaf measured previously in step 2. Thereafter, (6) BVOC release was recorded for at least 7 min by PTR-TOF-MS. Finally, (7) the leaf was removed and the empty cuvette measurements were taken again.
As it has been demonstrated previously that reduction in light intensity reduces isoprene emission rate, and that darkening can result in acetaldehyde and LOX product emission burst (Brilli et al. 2011; Graus et al. 2004; Karl et al. 2002), we tested the effects of temporary leaf removal under our conditions (moderately high light outside the cuvette) on BVOC emissions. When the leaf was taken out of the cuvette for 40 sec and re-inserted without leaf wounding, acetaldehyde and LOX emissions remained at the baseline level, while isoprene emission returned quickly, within a minute to the previous value. Thus, we conclude that effects of leaf wounding on BVOC emissions demonstrated in our study are associated with leaf wounding rather than with leaf removal and re-insertion.
In mature leaves, three types of leaf wounding were tested (Fig. 1) including simple cuts, punches, and squeezes that simulate different types of herbivore damage by insects and mechanical damage, tear and shear, by wind and large herbivores. In each case, the degree of damage was varied to test for the quantitative relationships between the severity of damage and BVOC emission response. Each mechanical damage was replicated from four to eight times on different leaves from different shoots.
— Simple cut (Fig. 1b) was done using a razor blade through the leaf lamina, trying to avoid major leaf veins. The cuts were linear, and the lengths tested were 7, 15, 30, and 45 mm.
— Puncture or punch (Fig. 1c) type of lamina damage was achieved by removing leaf disc(s) of 19.07 ± 0.15 mm2 by a paper punch. The perimeter (wound edge) created by one punch was 15.48 ± 0.06 mm. The number of punctures tested in a single leaf varied from one to four.
— Leaf lamina squeeze (Fig. 1d) was the damage resulting from application of a pressure of 90 N cm-2 by stainless steel combination pliers. Operators were trained to exercise the required pressure with a high degree of repeatability (± 5 N cm-2). The area squeezed varied from 33 to 99 mm2.
Although all applied types of mechanical stress result in cellular damage, the types of damage applied differ in several important respects. When a razor cut or a leaf puncture is performed, it results in a direct exposure of the broken cell wall and the cytoplasmic and vacuole content to oxidation by atmospheric air. Both wounding methods generate the conditions for triggering the reactions of the LOX pathways and the emission of its products and for the emission of volatile compounds from possible reservoirs. However, for an equivalent cut surface length, the puncture constitutes a more localized damage, such as that generated by hole feeders or window feeders, while straight cuts resemble the damage by free feeders or skeletonizers. Squeezing the leaf lamina, such as that occurring during browsing by large herbivores and upon lamina mechanical bending or elastic buckling under strong winds, differs from the two other mechanical stresses in that this type of damage results in rupturing of parenchyma cell walls and vacuoles with consequent release of cytoplasmic contents to the intercellular air space. The damage also results in the activation of the LOX pathway, but there is no free liquid surface generated, and the release of volatiles generated occurs through the stomata. The squeeze damage that occurs inside the leaves is also somewhat analogous with the damage generated by leaf miners that feed inside the leaves leaving leaf epidermal structures intact.
Data Analysis
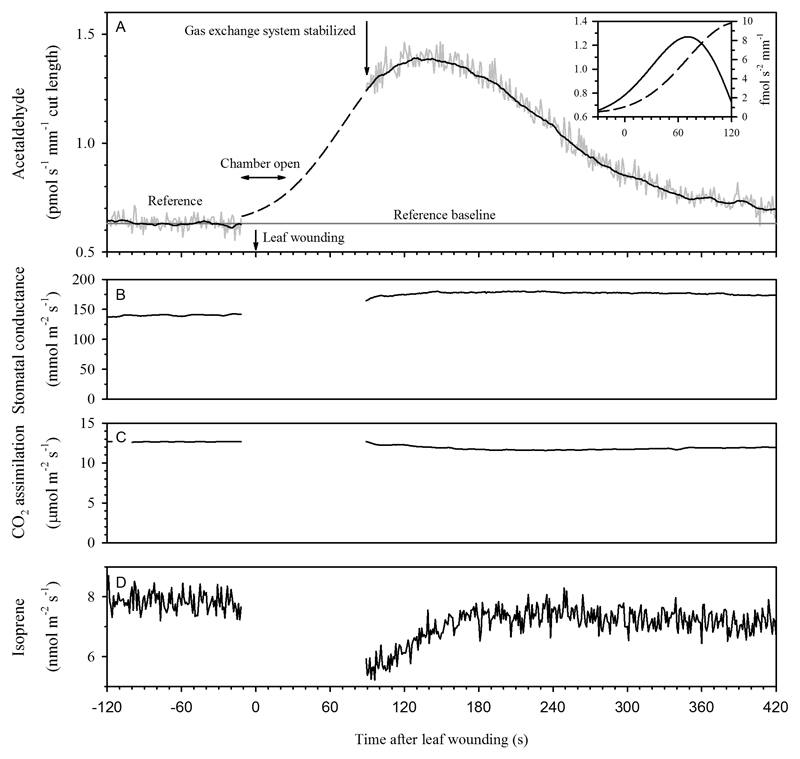
In order to identify the wound-related volatile emissions, a pre-wounding baseline value was calculated for each compound before the fitting (Fig. 2a), and it was later subtracted from the data obtained after the wounding. The available emission time response kinetic curves were smoothed using a Fast Fourier Transform (FFT) with 51 points filter size. Since the leaf had to be removed from the cuvette to perform leaf wounding, gap-filling during the period between wounding and re-insertion into the cuvette was necessary (Fig. 2a). The ‘missing data’ period corresponded to the time between the leaf wounding and the time when the outlet cuvette gas concentrations had stabilized after re-inserting the leaf in the cuvette (ca. 20-30 s, see (Sun et al. 2012) for the Walz GFS-3000 leaf chamber dynamics and the black dashed line; Fig. 2a). We used a bi-Gaussian fitting (Origin v8.0, OriginLab, Northampton, MA, USA) from the time of leaf wounding (at baseline concentration) to the emission peak maximum for the gap-filling (black dashed line; Fig. 2a):
| (Eqn 1) |
where y0 is the reference baseline, H is the outlet concentration over the reference at the peak maximum (smoothed data), xc is the time (s) corresponding to the peak maximum, w1 is the time (s) between xc and the time at For each curve, w1 was adjusted to achieve the best fitting. We note that in the case of compounds, emissions of which were elicited after the stabilization of gas flows, gap-filling is not necessary. Although when routinely applied to all compounds, ”gap-filling” would generate near-to-baseline values of these compounds for the missing data period. The goodness of the Gaussian fitting during the gap-filling period was carefully evaluated for each sample and compound. Among different functions tested, the bi-Gaussian fitting provided the best data extrapolation for all compounds in our dataset.
Fig. 2.
Example of the experimental routine of the leaf wounding experiment demonstrating changes in induced emissions of (a) acetaldehyde measured by a proton-transfer-reaction time-of-flight mass spectrometer (PTR-TOF-MS) with 1 s resolution, (b) apparent stomatal conductance and (c) net assimilation rate, and (d) alterations in constitutive isoprene emission rate in Populus tremula. The data presented correspond to a 15 mm razor cut through the leaf lamina. In (a), the raw recorded data are shown by a grey line and the smoothed data by a continuous line. The reference baseline is an average of the reference measurement period prior to the leaf wounding. The ‘missing data period’ corresponds to the time where the leaf had to be removed from the chamber to perform the mechanical damage at time zero as well as the unreliable data recording during the stabilization of gas flow. The raw data of the emission peak was fitted to a Gaussian function. The extrapolation of the fitting to time zero corresponding to the missing data period is represented with a dashed line. The inset in (a) illustrates the procedure to determine the maximum slope of volatile organic compound emission. The dashed line (left y-axis) is the Gaussian fit, and the solid line (right y-axis) is the first derivative of the data.
The first derivative of the emission curve, f, was calculated by averaging the slopes of two adjacent data points as follows (inset in Fig. 2a):
| (Eqn 2) |
The maximal slope of the emission curve was calculated as the maximal magnitude (amplitude) of the first derivative. The total amount of the compound released during the emission pulse was obtained by integrating the emission rate from the start of wounding (including the interpolated data from the fitting) until seven min after leaf wounding (black colored lines; Fig. 2a).
The example in Fig. 2a was intentionally selected to represent one of the most problematic cases where the ‘missing data’ period was relatively long. Nevertheless, even for this dataset, the compounds emission peak maxima were correctly detected. In fact, BVOC emission maxima for all compounds occurred later than 100 s after leaf wounding (Fig. 3a). Given that the mechanical wounding procedure, leaf enclosure in the cuvette, and stabilization lasted at most 50-60 s, we conclude that the wounding procedure was short enough for accurate peak detection.
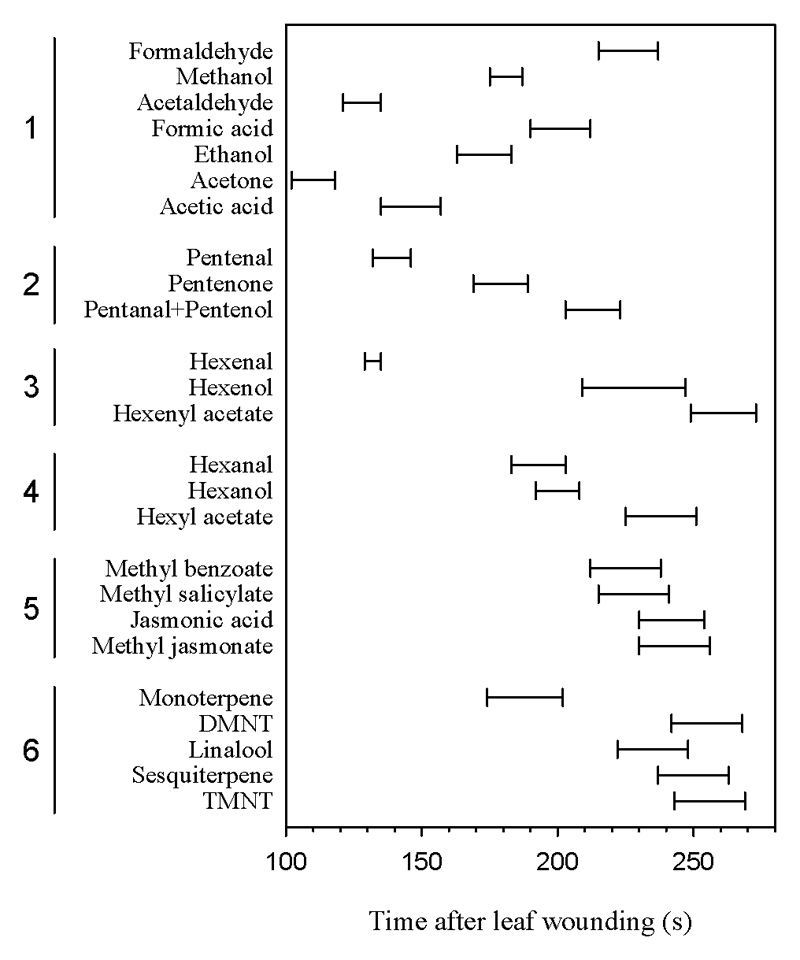
Fig. 3.
Timetable of occurrence of maximum emissions of volatile compound species after Populus tremula leaf wounding grouped by their molecular size and formation pathway (Table 1) in Populus tremula: (1) lightweight oxygenated compounds, (2) pentenyl family, (3) hexenal family, (4) hexanal family, (5) benzenoids and jasmonates, and (6) induced isoprenoids. Groups 2, 3 and 4 correspond to lipoxygenase (LOX) pathway products. DMNT [(E)-4,8-dimethyl-1,3,7-nonatriene] and TMNT [(E,E)-4,8,12-trimethyl-1,3,7,11-tridecatetraene] are homoterpenes. Error bars correspond to standard errors (n = 78).
Results
Photosynthetic and Isoprene Emission Responses to Wounding
After leaf wounding, the apparent stomatal conductance increased (Fig. 2b), and CO2 assimilation rate (Fig. 2c) and constitutive isoprene emission rate (Fig. 2d) dropped slightly. Pre-stress steady-state levels in net assimilation and isoprene emission rates were not achieved even for several hours after wounding (data not shown). Across the wounding treatments, isoprene emission rate decreased on average (± SE) by 7.4 ± 2.3%, and leaf CO2 assimilation rate by 9.2 ± 1.6% (for both traits, P<0.001 for the comparisons of the trait values before and after the treatment by paired t-tests). The apparent stomatal conductance changed differently for each wounding type, depending on the wound length and type, and no evidence of coupling stomatal conductance and BVOC emissions was found.
General Characteristics and Temporal Dynamics of Wound-Elicited BVOC Emissions
Among the 26 wound-induced volatiles studied (Table 1), the major wound-induced compounds emitted were hexenal (m+/z 99.080 + m+/z 81.070), acetaldehyde (m+/z 45.034), and methanol (m+/z 33.034) (Table 1). The lightweight oxygenated compounds (LOCs, C1-C3) and the hexenal family contributed ca. 99% to the total emissions (Tables 1, 2).
Table 2.
Average (± SE) estimates of characteristics describing integrated amount and timing of total biogenic volatile organic compound (BVOC) emissions from Populus tremula leaves upon different wounding treatments
| Lightweight oxygenated compounds | LOX pathway products |
Benzenoids and jasmonates | Induced isoprenoids | |||
|---|---|---|---|---|---|---|
| Pentenyl family | Hexanal family | Hexenal family | ||||
| Integrated BVOC emission (pmol mm-1 cut length) * |
229±27 a | 3.01±0.28 b | 0.351±0.030 bc | 280±29 a | 0.038±0.004 c | 0.118±0.009 c |
| Maximum emission rate (pmol s-1 mm-1 cut length) * |
1.160±0.100 a | 0.020±0.001 b | 0.003±0.000 c | 1.563±0.153 a | 0.002±0.000 c | 0.003±0.000 c |
| Time of peak maximum (s) | 166±38 a | 167±42 a | 210±60 b | 184±35 ab | 230±50 b | 210±60 b |
Leaf squeeze damage treatments excluded
For the integrated BVOC emission, emission rate was integrated for a period of 7 min after leaf damage. The compound families as defined in Table 1. Letters denote significant differences between BVOC families (Tukey's HSD test; P<0.001)
The studied volatiles exhibited different temporal emission characteristics with the time of peak emission varying between 100 and 280 s after wounding (Fig. 3). As a general trend, molecules with a larger number of carbon atoms were emitted later after the leaf wounding than were molecules with lower number of C atoms.
The integrated emission of all BVOCs during the burst, Ei (nmol), and the maximum BVOC emission rate, Emax (pmol s-1), were highly correlated (Pearson’s linear correlation; r2 = 0.979; P<0.001; N = 78) by the following relationship:
| (Eqn 3) |
meaning that the emission dynamic was constant for all the wounding types and throughout a large range of Ei values (from 0.4 to 56.2 nmol).
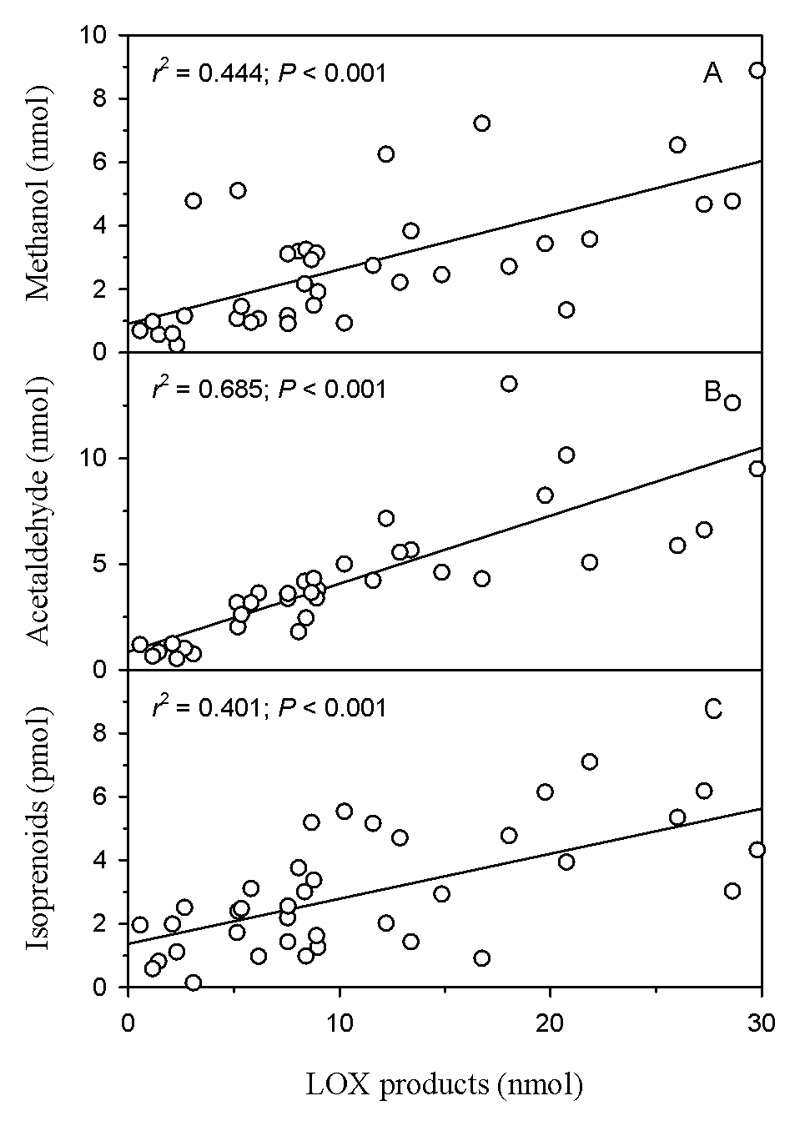
Across the BVOC groups, the emissions of C5 and C6 LOX products and LOCs were correlated significantly with each other (Table 3). Additionally, the emission of benzenoids and jasmonates were significantly correlated with the emission of LOX product families and induced isoprenoids (Table 3). Analogous correlations also were observed among the main compounds emitted. In particular, the emissions of methanol and acetaldehyde were positively correlated with the emission of LOX products (Fig. 4a, b; all data pooled), and the emission of LOX products were correlated with emissions of induced isoprenoids (Fig. 4c). Because the LOX product blend was dominated by hexenal emission (Table 1), the emissions of methanol, acetaldehyde, and induced isoprenoids were also significantly correlated (P<0.001) to hexenal emission, similarly as shown in Figure 4.
Table 3.
Correlations (r2) between different volatile compound families (Table 1) in the amount of biogenic volatile organic compounds (BVOCs) emitted during seven minutes after leaf mechanical wounding by razor cuts and punch holes in Populus tremula
| Pentenyl family | Hexanal family | Hexenal family | Benzenoids and jasmonates | Induced isoprenoids | Total BVOC emission | |
|---|---|---|---|---|---|---|
| Lightweight oxygenated compounds | 0.705*** | 0.642*** | 0.682*** | 0.073* | 0.0075 | 0.872*** |
| Pentenyl family | 0.878*** | 0.969*** | 0.187*** | 0.059* | 0.938*** | |
| Hexanal family | 0.765*** | 0.164*** | 0.033 | 0.781*** | ||
| Hexenal family | 0.156*** | 0.053 | 0.947*** | |||
| Benzenoids and jasmonates | 0.383*** | 0.131** | ||||
| Induced isoprenoids | 0.033 |
Relationships based on 70 observations.
Asterisks denote the statistical significance: (*) P<0.05; (**) P<0.01; (***) P<0.001
Fig. 4.
Correlations between the total emissions of (a) methanol, (b) acetaldehyde, and (c) induced isoprenoids with lipoxygenase (LOX) pathway products (pentenyl, hexenal, and hexanal compounds; see Table 1) in Populus tremula. The total emission is the integrated amount of given volatile over the 7 min. period from leaf wounding. All experiments through different wounding treatments were pooled (N = 78).
Wound-Related Volatile Emissions
The relative amounts of key induced volatiles from LOCs and hexenal families did not depend on the wounding type (Table 4), but different wounding methods were associated with several differences in relative amounts of the minor compounds. Leaf punctures produced higher relative amounts of benzenoids and jasmonates and induced isoprenoids than the two other treatments, and also higher relative amounts of pentenyls than the linear cut treatment (Table 4). In addition, the squeeze treatment resulted in a greater share of hexanal family compounds than the linear cut treatment (Table 4).
Table 4.
Average (± SE) relative amounts (%) of wound-related biogenic volatile organic compounds (BVOCs) grouped by family (Table 1) in relation to wounding in Populus tremula
| Lightweight oxygenated compounds | LOX pathway products |
Benzenoids and jasmonates | Induced isoprenoids | ||||
|---|---|---|---|---|---|---|---|
| Pentenyl family | Hexanal family | Hexenal family | |||||
| Wounding type * | cut | 44.6±1.8 a | 0.532±0.023 a | 0.060±0.004 a | 54.8±1.7 a | 0.004±0.001 a | 0.015±0.002 a |
| punch | 40.2±2.9 a | 0.705±0.042 b | 0.077±0.005 ab | 58.9±2.8 a | 0.010±0.002 b | 0.027±0.004 b | |
| squeeze | 49.1±4.0 a | 0.618±0.046 ab | 0.096±0.011 b | 50.2±4.0 a | 0.003±0.001 a | 0.005±0.001 a | |
Significant differences (P<0.05) after Tukey's HSD test among wounding types are denoted by different lowercase letters
Correlations of Volatile Emissions with the Cut Length
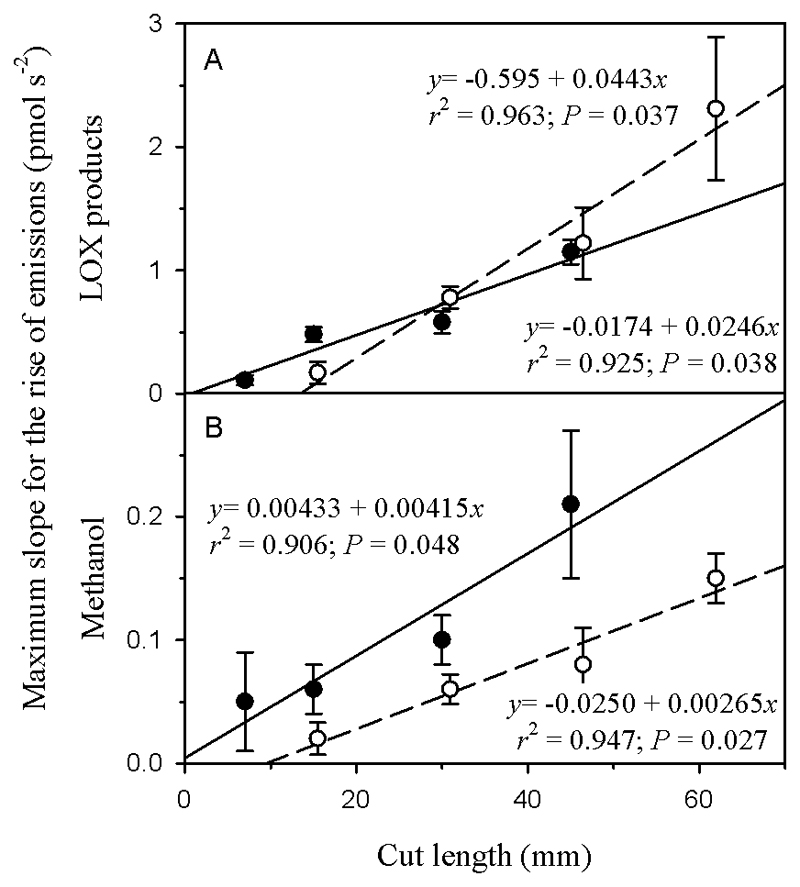
For mature leaves, the average (± SE) total integrated BVOC emission during 7 min following leaf wounding, expressed on the basis of millimeter of cut length (pooling all cutting and punching data) was 506 ± 46 pmol mm-1. In most cases, the emission of individual BVOCs was highly correlated with the wound length when the lamina cut and leaf puncture data were pooled (Table 1). A greater degree of damage was associated with a maximum slope (before the peak maximum) of the emission peak (Fig. 5a for the sum of LOX products and Fig. 5b for methanol), greater total emission during the emission burst (Fig. 6a, b), and maximum emission rate (the rate at the emission peak; Fig. 6c, d). Only in a few cases (i.e., jasmonic acid, methyl jasmonate, and TMTT), the emissions were weakly correlated with wound length (r2 < 0.16, P<0.05) (Table 1).
Fig. 5.
Relationships between the wound length and the average (± SE) maximum slope for the rise of emissions after leaf wounding for (a) lipoxygenase (LOX) pathway products and (b) methanol in Populus tremula (for the slope estimation see inset in Fig. 2a). Black filled symbols correspond to lamina straight cuts and white filled symbols to leaf punctures.
Fig. 6.
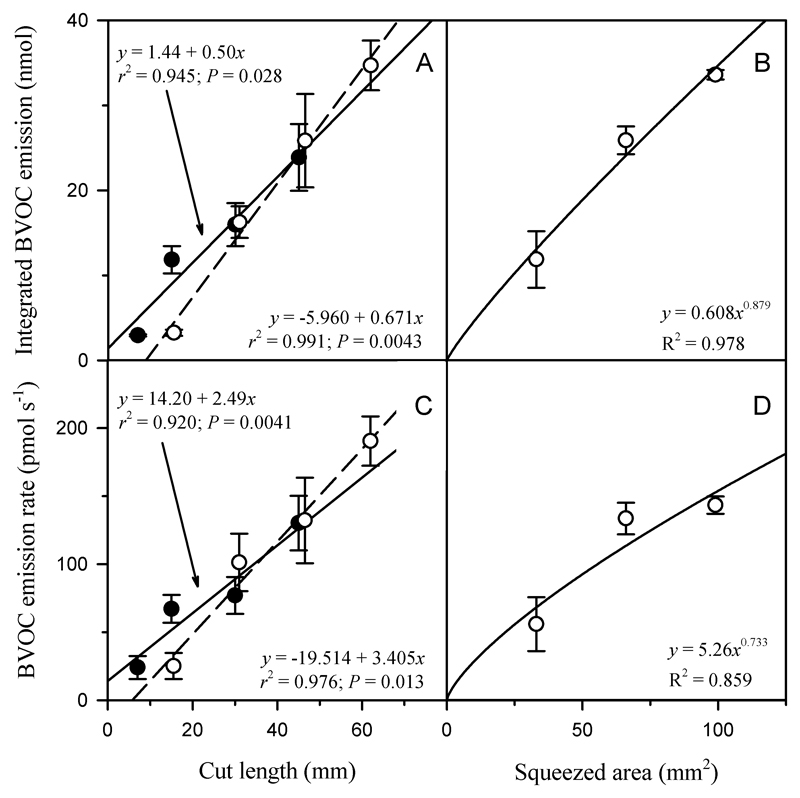
Quantitative relationships between (a, b) the degree of leaf wounding and resulting average (± SE) integrated total BVOC (biogenic volatile organic compound) emission, and (c, d) maximum emission rate after leaf damage by (a, c) cutting or puncturing and (b, d) squeezing in Populus tremula. In (a) and (b), the integrated BVOC emission is the integrated estimate for 7 minutes after leaf wounding. In (a) and (c), black filled symbols correspond to lamina straight cuts and white filled symbols to leaf punctures. Total BVOC emission is the sum of emissions of all the 26 compounds measured (Table 1).
The integrated BVOC emission and the maximum emission rate generated by cutting the lamina with a razor blade and by leaf punctures were similarly related to the cut length (i.e., the length of the wound produced) (Fig. 6a, c). In addition, for the sum of BVOCs, the total emissions and the maximum emission rate increased with the squeeze area (Fig. 6b, d). However, for methanol, the rate of rise of emission (the maximum slope of emission vs. time relationship) was greater at given cut length in the case of linear cuts than in the case of leaf punches (Fig. 5b).
Discussion
We did not perceive any alteration in volatile emissions or photosynthesis rate due to cutting shoots from trees in nature or under water feeding in the lab during the experiment. We believe the cut shoots were long enough to prevent water from reaching the experimental leaves through the xylem before the experiment ended. The transport would last for several hours at an estimated sap flow velocity of 9 cm h-1 (Balek and Pavlík 1977).
Wounding Effects on Foliage Physiological Characteristics
The decrease of photosynthetic activity and constitutive isoprene emission could be a result of within-plant tissue electrical signaling upon leaf wounding (Galle et al. 2013; Lautner et al. 2005). According to this hypothesis, wounding can generate a variation potential (VP) that triggers membrane depolarization and retards repolarization, temporally depressing photosynthetic activity. Leaf wounding also resulted in an apparent moderate (ca. 10-15%) increase in stomatal conductance, as seen by Brilli et al. (2011). This probably reflected the generation of a free liquid surface for evaporation from the open wound and the Ivanov effect, i.e., loss of turgor of epidermal cells and associated temporal opening of stomata (Moldau et al. 1993). The rapid initial reduction of constitutive isoprene emissions by ca. 10% in our study might indicate reduction of the dimethylallyl diphosphate pool size due to reduced input of photosynthetic carbon during stomatal closure and delayed activation of alternative carbon sources (Brilli et al. 2007; Rasulov et al. 2009).
The Nature of Wounding-dependent BVOC Emissions
Methanol, hexenal, and acetaldehyde also have been found to be major constituents of leaf wounding volatile emissions in previous studies (Brilli et al. 2011; Karl et al. 2001; Loreto et al. 2006). However, the level of acetaldehyde emission in our experiments was about 10-fold greater, and methanol emission also was much greater than observed by Brilli et al. (2011).
Although there has been recent progress in understanding the biosynthesis and emission of lightweight oxygenated volatile organic compounds (LOC, C1-C3) (Graus et al. 2004; Jardine et al. 2012; Karl et al. 2002; Wildt et al. 2003), the biochemical emission mechanisms of several widespread compounds such as acetone or acetaldehyde is still unclear. In addition to wound-induced elicitation of LOCs release, several LOCs also are products of different biochemical pathways occurring during “normal” non-stressed metabolic processes taking place in different parts of the plant. Many of these compounds are highly water-soluble (e.g., methanol). Methanol, that is produced as a result of pectin demethylation in cell walls by pectin methylesterases (Fall and Benson 1996; Fall et al. 2001; Micheli 2001; Ridley et al. 2001), can accumulate in the leaf liquid phase (Niinemets et al. 2004), and thus, one might conclude that enhanced emissions after leaf damage partly result from the release of methanol synthesized prior to leaf wounding. However, activation of pectin methylesterases with concomitant release of methanol is one of the first wound stress reactions (Baluška et al. 2005; Ridley et al. 2001).
Other LOC volatiles, such as acetaldehyde or LOX products, are less water-soluble than methanol (Niinemets and Reichstein 2003), and thus, their release should reflect primarily de novo synthesis. Formation of C5 and C6 molecules (Fig. 3a; groups 2, 3, and 4) has been attributed to the oxidation of C18 fatty acids present in cell membranes by the lipoxygenase enzyme (LOX) after leaf wounding (Brilli et al. 2012; Loreto et al. 2006). The release of acetaldehyde from wounded leaves also has been associated with LOX pathway, in particular, with pyruvate formed down the reaction cascade (Graus et al. 2004; Monson 2013). Recent work further suggests that both the acetate and hexenyl parts of hexenyl acetate generated upon wounding (Table 1) and dark-light transitions result from oxidation of fatty acids (Jardine et al. 2009, 2012). The presence of strong correlations among families of LOX products (Table 3, Fig. 4) further indicates strong coordination of reactions along the stress response cascade.
The benzenoids, jasmonates, and isoprenoids induced in response to herbivory damage generally are considered as infochemicals for predators and parasites of herbivores (Kessler and Baldwin 2002). However, their synthesis is induced over a period of hours to days after the stress event (Niinemets et al. 2013) (e.g., jasmonic acid (Lee et al. 2004), methyl salicylate (Beauchamp et al. 2005), DMNT and linalool (Vuorinen et al. 2007)). In addition, the production of infochemicals such as jasmonic acid has been demonstrated to be induced upon actual herbivory damage by caterpillars while simple mechanical wounding appears to be less effective (Scala et al. 2013), thus indicating that herbivore-specific elicitors might be needed to start the gene expression level response downstream of LOX product formation (Tian et al. 2012). We observed emissions of key signaling compounds in our wounding experiments (Table 1), likely indicating the presence of a certain basal level synthesis activity. Nevertheless, although detectable in our wounding experiments, these compounds contributed only a small fraction to the total emissions (Table 1).
Timetable of BVOC Elicitation After Wounding
In our study, the initial emission burst of most of the studied volatiles occurred within seven minutes after leaf wounding (Fig. 3). This is somewhat faster than the time-period of 10-12 min. observed by Brilli et al. (2011), and much faster than the period of 20 min. to more than 100 min. quantified by Fall et al. (1999). We believe that the differences in system response time are important, as the slower the system response time, the more the emission signal smeared out in time, as reviewed recently (Niinemets 2012; Niinemets et al. 2011). The gas-exchange cuvette used in our study had a 5-fold smaller volume than the conifer cuvette used by Brilli et al. (2011), and a 75-fold smaller volume than the Teflon bag used by Fall et al. (1999). Considering further the gas flow rates, the cuvette response time was ca. 2.5-fold greater in our study than observed by Brilli et al. (2011), and at least 75-fold greater than in the study of Fall et al. (1999). Further, given the low turbulence in the Teflon bag system of Fall et al. (1999), the emission signal could be smeared out even more. Consequently, we chose a mechanical wounding system not built into the chamber, but the short period lost by removing and re-clamping the leaf during the wounding and the fast chamber flow stabilization (given by the high cuvette response time) allowed the identification of the peak maximum for every volatile compound in every treatment.
Despite differences in system response time, the sequence of emission of different LOC and LOX compounds in our study is broadly in agreement with previous observations (Brilli et al. 2011; Fall et al. 1999, 2001). Analysis of all data collectively indicates that during the initial rapid stress response, simple molecules containing fewer carbon atoms are emitted sooner (Table 1, Fig. 3) and are elicited to a greater degree (Table 1) after wounding than more complex and heavier molecules. There is a key sequence of C1 compound methanol, followed by C2 acetaldehyde and C6 hexenal and C10 monoterpene, indicating clear time-dependent elicitation of distinct pathways.
Early release of acetaldehyde, almost simultaneously with hexenal (Fig. 3), is somewhat puzzling. This result differs from the observations of Graus et al. (2004) in isoprene-emitting grey poplar (Populus x canescens) where wounding-driven acetaldehyde emissions peaked later than emissions of hexenal, and are believed also to originate from the free fatty acids released from membranes (Jardine et al. 2009; Monson 2013). In fact, Fall et al. (1999) observed in P. tremula that wounding-dependent acetaldehyde release was independent of the presence of oxygen, contrary to the LOX pathway. Thus, the fast release of acetaldehyde might indicate formation of acetaldehyde as the result of a pyruvate overflow mechanism in conditions of suppressed isoprene emission after wounding (Karl et al. 2002). However, in the non-isoprene-emitting herb Dactylis glomerata, timing of wounding-dependent acetaldehyde and hexenal emissions also coincided (Brilli et al. 2011) as in our study. These puzzling pieces of evidence call for further studies on the origin of wounding-dependent release of acetaldehyde across different species.
In the case of LOX products, we argue that the sequence of compounds emitted is in agreement with the reaction pathways previously postulated (Brilli et al. 2011; Fall et al. 1999, 2001). Thus, the products formed first were detected earlier followed by products that are formed subsequently downstream in the pathway. The first LOX products released were the hexenal and pentenyl families: hexenal (m+/z 99.080 + m+/z 81.070) emission peaks occurred on average around 132 s after leaf wounding, and subsequent derivatization products in the hexenal family pathway (Fall et al. 1999) like hexenol (m+/z 101.096 + m+/z 83.085) requiring NADPH-dependent reduction (Matsui et al. 2012), and the esterified compound hexenyl acetate (m+/z 143.107) occurred later on at 228 ± 19 s and 261 ± 12 s, respectively. As for the pentenyl family, the emission of pentenal (m+/z 85.065 + 71.086) was followed by pentenone (m+/z 85.065) and pentanal + pentenol (m+/z 87.080), which have the same mass and cannot be distinguished by PTR-TOF-MS. Emission of pentenal occurred almost simultaneously with hexenal in accordance with the evidence that the initial step of synthesis of both aldehydes is catalyzed by the same 13-lipoxygenase (Fisher et al. 2003; Shen et al. 2014). Later, after the wounding, the timing of emission of the hexanal family species also followed the formation sequence demonstrated by Fall et al. (1999): hexanal (m+/z 101.096 + m+/z 83.085) and hexanol (m+/z 103.112 + m+/z 85.101) peaked at 193 ± 10 s and 200 ± 8 s, respectively, and are precursors of hexyl acetate (m+/z 145.122), which peaked later at 238 ± 13 s.
Quantitative Relationships Between Degree of Damage and Wounding-induced Volatile Emissions
We observed important quantitative scaling of the degree of leaf damage and the rate of elicitation of volatile emission (Fig. 5, Table 1) and the total amount of volatiles released and the maximum emission rate (Fig. 6; Eqn 3). Other studies indicate that LOX emissions scale with the severity of stress (Copolovici et al. 2014b; Fall et al. 1999; Niinemets et al. 2013) as well as mono-, sesqui-, and homoterpene emissions in herbivory studies (Copolovici et al. 2011, 2014a).
The magnitude and duration of the burst phenomenon will contribute to generating the final atmospheric concentrations and achieve the threshold needed to repel herbivores or to signal neighboring leaves, plants, and herbivore predators and parasitoids (Blande et al. 2014).
What Is the Best Method for Routine Wounding Treatments?
Comparison of linear cuts and leaf punctures is straightforward based on the wound edge (mm). Highly repetitive emission rates per unit cut edge length were observed across both wounding treatments (statistically zero intercept and common slope in Fig. 6a, b). In addition, total BVOC emission peak shape (peak maximum to peak area ratio) was constant for all the wounding types and wounding lengths (Eqn 3). Thus, the temporal dimension of emission (peak shape) did not depend on the cut edge length.
From a practical perspective, if choosing a reproducible method across many species with different foliage structure, squeeze wounding seems not to be suitable since the actual damage is dependent on leaf toughness, thickness, and squeezing force. In addition, the degree of squeeze wounding is given in surface units (mm2), and is, therefore, not directly comparable to the other two treatments. On the other hand, lamina cuts generally were easier to perform, and it was easy to avoid veins or achieving long wounds by making parallel cuts. These characteristics make this method easier to replicate. However, because the cuts were done manually, replicating cuts of a given length may be less accurate than applying a leaf puncher. In addition to better reproducibility, leaf puncture wounding also was a faster method than others. We recommend acquiring different sizes and shapes of leaf punchers in order to replicate the wounding in several species (e.g., narrower leaves where veins have to be avoided). Alternatively, in needle-like leaves, using the lamina cut method appears to be more convenient if leaf punchers exceed the width of the leaf lamina.
In summary, this study demonstrated rapid and highly coordinated sequence of volatile release in response to wounding, and also that different types of wounding lead to similar emission blends. The BVOC emissions per wound length that resulted from straight cuts or leaf punctures were essentially constant for mature leaves, and thus, the emissions scaled linearly with the degree of lamina damage. We recommend the punch hole technique for further routine experiments that investigate the quantitative relationships between the degree of damage and plant emissions due to its high replicability, accuracy, and ease of performance. Although both the maximum wounding-induced BVOC emission rate and the integrated amount of BVOC released during the emission burst were strongly correlated, for comparisons among studies, we believe that the integrated BVOC emission is a more reliable measure because it is less affected by the measurement system response time than the maximum emission rate.
Acknowledgements
We thank Peter C. Harley for insightful comments on the MS. We thank the two reviewers and Editors for helpful advice that significantly improved the manuscript. This work was supported by the Estonian Ministry of Science and Education [institutional grant IUT-8-3], Estonian Science Foundation [grant 9253], the European Commission through the European Regional Fund [Center of Excellence in Environmental Adaptation] and Marie Curie [grant ERMOS73] and through the Transnational Access to Research Infrastructures activity [ExpeER], the European Research Council [advanced grant 322603, SIP-VOL+] and the European Social fund ESF [MJD 438].
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- Ament K, Kant MR, Sabelis MW, Haring MA, Schuurink RC. Jasmonic acid is a key regulator of spider mite-induced volatile terpenoid and methyl salicylate emission in tomato. Plant Physiol. 2004;135:2025–2037. doi: 10.1104/pp.104.048694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura G, Kost C, Boland W. Herbivore-induced, indirect plant defences. Bba-Mol Cell Biol L. 2005;1734:91–111. doi: 10.1016/j.bbalip.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Arneth A, Niinemets Ü. Induced BVOCs: How to bug our models? Trends Plant Sci. 2010;15:118–125. doi: 10.1016/j.tplants.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Balek J, Pavlík O. Sap stream velocity as an indicator of the transpirational process. J Hydrol. 1977;34:193–200. [Google Scholar]
- Baluška F, Liners F, Hlavačka A, Schlicht M, Van Cutsem P, McCurdy DW, Menzel D. Cell wall pectins and xyloglucans are internalized into dividing root cells and accumulate within cell plates during cytokinesis. Protoplasma. 2005;225:141–155. doi: 10.1007/s00709-005-0095-5. [DOI] [PubMed] [Google Scholar]
- Beauchamp J, et al. Ozone induced emissions of biogenic VOC from tobacco: Relations between ozone uptake and emission of LOX products. Plant Cell Environ. 2005;28:1334–1343. [Google Scholar]
- Benikhlef L, et al. Perception of soft mechanical stress in Arabidopsis leaves activates disease resistance. BMC Plant Biol. 2013:13. doi: 10.1186/1471-2229-13-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blande JD, Holopainen JK, Niinemets Ü. Plant volatiles in polluted atmospheres: Stress responses and signal degradation. Plant Cell Environ. 2014;37:1892–1904. doi: 10.1111/pce.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brilli F, Barta C, Fortunati A, Lerdau M, Loreto F, Centritto M. Response of isoprene emission and carbon metabolism to drought in white poplar (Populus alba) saplings New Phytol. 2007;175:244–254. doi: 10.1111/j.1469-8137.2007.02094.x. [DOI] [PubMed] [Google Scholar]
- Brilli F, et al. Qualitative and quantitative characterization of volatile organic compound emissions from cut grass. Environ Sci Technol. 2012;46:3859–3865. doi: 10.1021/es204025y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brilli F, et al. Detection of plant volatiles after leaf wounding and darkening by proton transfer reaction "time-of-flight" mass spectrometry (PTR-TOF) Plos One. 2011;6:e20419. doi: 10.1371/journal.pone.0020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copolovici L, Kännaste A, Remmel T, Niinemets Ü. Volatile organic compound emissions from Alnus glutinosa under interacting drought and herbivory stresses. Environ Exp Bot. 2014a;100:55–63. doi: 10.1016/j.envexpbot.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copolovici L, Kännaste A, Remmel T, Vislap V, Niinemets Ü. Volatile emissions from Alnus glutinosa induced by herbivory are quantitatively related to the extent of damage. J Chem Ecol. 2011;37:18–28. doi: 10.1007/s10886-010-9897-9. [DOI] [PubMed] [Google Scholar]
- Copolovici L, Väärtnõu F, Portillo Estrada M, Niinemets Ü. Oak powdery mildew (Erysiphe alphitoides)-induced volatile emissions scale with the degree of infection in Quercus robur. Tree Physiol. 2014b;34:1399–1410. doi: 10.1093/treephys/tpu091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft KPC, Juttner F, Slusarenko AJ. Volatile products of the lipoxygenase pathway evolved from Phaseolus vulgaris (L.) leaves inoculated with Pseudomonas Syringae pv. phaseolicola. Plant Physiol. 1993;101:13–24. doi: 10.1104/pp.101.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fall R, Benson AA. Leaf methanol - The simplest natural product from plants. Trends Plant Sci. 1996;1:296–301. [Google Scholar]
- Fall R, Karl T, Hansel A, Jordan A, Lindinger W. Volatile organic compounds emitted after leaf wounding: On-line analysis by proton-transfer-reaction mass spectrometry. J Geophys Res-Atmos. 1999;104:15963–15974. [Google Scholar]
- Fall R, Karl T, Jordon A, Lindinger W. Biogenic C5 VOCs: release from leaves after freeze-thaw wounding and occurrence in air at a high mountain observatory. Atmos Environ. 2001;35:3905–3916. [Google Scholar]
- Farag MA, Fokar M, Zhang HA, Allen RD, Pare PW. (Z)-3-Hexenol induces defense genes and downstream metabolites in maize. Planta. 2005;220:900–909. doi: 10.1007/s00425-004-1404-5. [DOI] [PubMed] [Google Scholar]
- Filella I, Peñuelas J, Llusià J. Dynamics of the enhanced emissions of monoterpenes and methyl salicylate, and decreased uptake of formaldehyde, by Quercus ilex leaves after application of jasmonic acid. New Phytol. 2006;169:135–144. doi: 10.1111/j.1469-8137.2005.01570.x. [DOI] [PubMed] [Google Scholar]
- Fisher AJ, Grimes HD, Fall R. The biochemical origin of pentenol emissions from wounded leaves. Phytochemistry. 2003;62:159–163. doi: 10.1016/s0031-9422(02)00521-6. [DOI] [PubMed] [Google Scholar]
- Galle A, Lautner S, Flexas J, Ribas-Carbo M, Hanson D, Roesgen J, Fromm J. Photosynthetic responses of soybean (Glycine max L.) to heat-induced electrical signalling are predominantly governed by modifications of mesophyll conductance for CO2. Plant Cell Environ. 2013;36:542–552. doi: 10.1111/j.1365-3040.2012.02594.x. [DOI] [PubMed] [Google Scholar]
- Ghirardo A, Gutknecht J, Zimmer I, Brüggemann N, Schnitzler J-P. Biogenic volatile organic compound and respiratory CO2 emissions after 13C-labeling: online tracing of C translocation dynamics in poplar plants. Plos One. 2011;6:e17393. doi: 10.1371/journal.pone.0017393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graus M, Müller M, Hansel A. High Resolution PTR-TOF: Quantification and formula confirmation of VOC in real time. J Am Soc Mass Spectrom. 2010;21:1037–1044. doi: 10.1016/j.jasms.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Graus M, Schnitzler JP, Hansel A, Cojocariu C, Rennenberg H, Wisthaler A, Kreuzwieser J. Transient release of oxygenated volatile organic compounds during light-dark transitions in grey poplar leaves. Plant Physiol. 2004;135:1967–1975. doi: 10.1104/pp.104.043240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardine K, et al. Green leaf volatiles and oxygenated metabolite emission bursts from mesquite branches following light-dark transitions. Photosynthesis Res. 2012;113:321–333. doi: 10.1007/s11120-012-9746-5. [DOI] [PubMed] [Google Scholar]
- Jardine K, Karl T, Lerdau M, Harley P, Guenther A, Mak JE. Carbon isotope analysis of acetaldehyde emitted from leaves following mechanical stress and anoxia. Plant Biol. 2009;11:591–597. doi: 10.1111/j.1438-8677.2008.00155.x. [DOI] [PubMed] [Google Scholar]
- Jordan A, et al. A high resolution and high sensitivity proton-transfer-reaction time-of-flight mass spectrometer (PTR-TOF-MS) Int J Mass Spectrom. 2009;286:122–128. [Google Scholar]
- Karl T, Curtis AJ, Rosenstiel TN, Monson RK, Fall R. Transient releases of acetaldehyde from tree leaves - products of a pyruvate overflow mechanism. Plant Cell Environ. 2002;25:1121–1131. [Google Scholar]
- Karl T, Fall R, Jordan A, Lindinger W. On-line analysis of reactive VOCs from urban lawn mowing. Environ Sci Technol. 2001;35:2926–2931. doi: 10.1021/es010637y. [DOI] [PubMed] [Google Scholar]
- Kessler A, Baldwin IT. Plant responses to insect herbivory: The emerging molecular analysis. Annu Rev Plant Biol. 2002;53:299–328. doi: 10.1146/annurev.arplant.53.100301.135207. [DOI] [PubMed] [Google Scholar]
- Lautner S, Grams TEE, Matyssek R, Fromm J. Characteristics of electrical signals in poplar and responses in photosynthesis. Plant Physiol. 2005;138:2200–2209. doi: 10.1104/pp.105.064196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, et al. Inverse correlation between jasmonic acid and salicylic acid during early wound response in rice. Biochem Biophys Res Commun. 2004;318:734–738. doi: 10.1016/j.bbrc.2004.04.095. [DOI] [PubMed] [Google Scholar]
- Loreto F, Barta C, Brilli F, Nogues I. On the induction of volatile organic compound emissions by plants as consequence of wounding or fluctuations of light and temperature. Plant Cell Environ. 2006;29:1820–1828. doi: 10.1111/j.1365-3040.2006.01561.x. [DOI] [PubMed] [Google Scholar]
- Loreto F, Schnitzler J-P. Abiotic stresses and induced BVOCs. Trends Plant Sci. 2010;15:154–166. doi: 10.1016/j.tplants.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Matsui K, Sugimoto K, Mano Ji, Ozawa R, Takabayashi J. Differential metabolisms of green leaf volatiles in injured and intact parts of a wounded leaf meet distinct ecophysiological requirements. Plos One. 2012;7:e36433. doi: 10.1371/journal.pone.0036433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheli F. Pectin methylesterases: cell wall enzymes with important roles in plant physiology. Trends Plant Sci. 2001;6:414–419. doi: 10.1016/s1360-1385(01)02045-3. [DOI] [PubMed] [Google Scholar]
- Mithöfer A, Boland W. Recognition of herbivory-associated molecular patterns. Plant Physiol. 2008;146:825–831. doi: 10.1104/pp.107.113118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldau H, Wong S-C, Osmond CB. Transient depression of photosynthesis in bean leaves during rapid water loss. Aust J Plant Physiol. 1993;20:45–54. [Google Scholar]
- Monson RK. Metabolic and gene expression controls on the production of biogenic volatile organic compounds. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Tree Physiology. Vol. 5. Springer; Berlin: 2013. pp. 153–179. [Google Scholar]
- Niinemets Ü. Whole plant photosynthesis. In: Flexas J, Loreto F, Medrano H, editors. Terrestrial photosynthesis in a changing environment. A molecular, physiological and ecological approach. Cambridge University Press; Cambridge: 2012. pp. 399–423. [Google Scholar]
- Niinemets Ü, Kännaste A, Copolovici L. Quantitative patterns between plant volatile emissions induced by biotic stresses and the degree of damage. Front Plant Sci. 2013;4:262. doi: 10.3389/fpls.2013.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinemets Ü, et al. Estimations of isoprenoid emission capacity from enclosure studies: Measurements, data processing, quality and standardized measurement protocols. Biogeosciences. 2011;8:2209–2246. [Google Scholar]
- Niinemets Ü, Loreto F, Reichstein M. Physiological and physicochemical controls on foliar volatile organic compound emissions. Trends Plant Sci. 2004;9:180–186. doi: 10.1016/j.tplants.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü, Monson RK. State-of-the-art of BVOC research: What do we have and what have we missed? A synthesis. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Tree Physiology. Vol. 5. Springer; Berlin: 2013. pp. 509–528. [Google Scholar]
- Niinemets Ü, et al. The leaf-level emission factor of volatile isoprenoids: Caveats, model algorithms, response shapes and scaling. Biogeosciences. 2010;7:1809–1832. [Google Scholar]
- Niinemets Ü, Reichstein M. Controls on the emission of plant volatiles through stomata: Sensitivity or insensitivity of the emission rates to stomatal closure explained. J Geophys Res-Atmos. 2003;108:4208. [Google Scholar]
- Paiva NL. An introduction to the biosynthesis of chemicals used in plant-microbe communication. J Plant Growth Reg. 2000;19:131–143. doi: 10.1007/s003440000016. [DOI] [PubMed] [Google Scholar]
- Ponzio C, Gols R, Weldegergis BT, Dicke M. Caterpillar-induced plant volatiles remain a reliable signal for foraging wasps during dual attack with a plant pathogen or non-host insect herbivore. Plant Cell Environ. 2014;37:1924–1935. doi: 10.1111/pce.12301. [DOI] [PubMed] [Google Scholar]
- Portillo-Estrada M. Advantages of PTR-MS and PTR-TOF-MS techniques for measuring volatile organic compounds (VOCs) Sci Bull Escorena. 2013;8:65–67. [Google Scholar]
- Rasulov B, Hüve K, Välbe M, Laisk A, Niinemets Ü. Evidence that light, carbon dioxide and oxygen dependencies of leaf isoprene emission are driven by energy status in hybrid aspen. Plant Physiol. 2009;151:448–460. doi: 10.1104/pp.109.141978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley BL, O’Neill MA, Mohnen D. Pectins: Structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry. 2001;57:929–967. doi: 10.1016/s0031-9422(01)00113-3. [DOI] [PubMed] [Google Scholar]
- Scala A, Allmann S, Mirabella R, Haring MA, Schuurink RC. Green leaf volatiles: A plant's multifunctional weapon against herbivores and pathogens. Int J Mol Sci. 2013;14:17781–17811. doi: 10.3390/ijms140917781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub A, Blande JD, Graus M, Oksanen E, Holopainen JK, Hansel A. Real-time monitoring of herbivore induced volatile emissions in the field. Physiol Plant. 2010;138:123–133. doi: 10.1111/j.1399-3054.2009.01322.x. [DOI] [PubMed] [Google Scholar]
- Shen J, et al. A 13-lipoxygenase, TomloxC, is essential for synthesis of C5 flavour volatiles in tomato. J Exp Bot. 2014;65:519–428. doi: 10.1093/jxb/ert382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L, Beck JJ. Effect of mechanical damage on emission of volatile organic compounds from plant leaves and implications for evaluation of host plant specificity of prospective biological control agents of weeds. Biocontrol Sci Technol. 2013;23:880–907. [Google Scholar]
- Sun Z, Niinemets Ü, Hüve K, Noe SM, Rasulov B, Copolovici L, Vislap V. Enhanced isoprene emission capacity and altered light responsiveness in aspen grown under elevated atmospheric CO2 concentration. Global Change Biol. 2012;18:3423–3440. [Google Scholar]
- Tian DL, et al. Salivary glucose oxidase from caterpillars mediates the induction of rapid and delayed-induced defenses in the tomato plant. Plos One. 2012;7:e36168. doi: 10.1371/journal.pone.0036168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlinden MS, Broeckx LS, Ceulemans R. First vs. second rotation of a poplar short rotation coppice: Above-ground biomass productivity and shoot dynamics. Biomass Bioenerg. 2015;73:174–185. [Google Scholar]
- Vuorinen T, Nerg AM, Syrjala L, Peltonen P, Holopainen JK. Epirrita autumnata induced VOC emission of silver birch differ from emission induced by leaf fungal pathogen. Arthropod-Plant Inte. 2007;1:159–165. [Google Scholar]
- Wildt J, Kobel K, Schuh-Thomas G, Heiden AC. Emissions of oxygenated volatile organic compounds from plants. Part II: Emissions of saturated aldehydes. J Atmos Chem. 2003;45:173–196. [Google Scholar]