Abstract
Objective
To examine the effect that neoadjuvant axitinib for the treatment of localized renal cell carcinoma has on body compartment composition.
Patients and Methods
The study was based on a single-institution, single-arm clinical trial that enrolled 24 patients with locally advanced non-metastatic biopsy-proven clear cell renal cell carcinoma. Patients received axitinib orally for up to 12 weeks. Computed tomography scans were completed before the start of treatment, after 7 weeks of treatment and at the completion of 12 weeks of treatment. Patients underwent nephrectomy after axitinib treatment. The primary outcome of the current study was change in body compartment composition. Secondary outcomes included development of new-onset sarcopenia and changes in body weight.
Results
A total of 23 patients had a complete set of imaging for evaluation, of which 19 (82.6%) lost weight. Median weight loss was 4.5 kg (p < 0.001). Seven patients (30.4%) had sarcopenia prior to treatment, with an additional 5 (21.7%) developing sarcopenia during treatment. Median decrease in skeletal muscle was 2.9 cm2/m2 (p < 0.001), visceral adipose tissue was 4.9 cm2/m2 (p = 0.132), and subcutaneous adipose tissue was 1.0 cm2/m2 (p = 0.043). Ten(62.5%) of the 16 patients without baseline sarcopenia achieved a partial response, while only one(14.3%) of the 7 patients with baseline pretreatment sarcopenia achieved a partial response (p=0.069).
Conclusions
Neoadjuvant axitinib resulted in a decrease in skeletal muscle and subcutaneous adipose tissue, as well as weight loss. Patients with baseline sarcopenia tended to have a lower response rate to neoadjuvant axitinib.
Keywords: renal cell carcinoma, sarcopenia, axitinib, neoadjuvant therapy, nephrectomy
INTRODUCTION
The advent of targeted therapy for the treatment of renal cell carcinoma [RCC] began around 2005 with the use of sunitinib and sorafenib in patients with metastatic renal cell carcinoma.1, 2 Since then, the number of vascular endothelial growth factor receptor [VEGFR] and mammalian target of rapamycin [mTOR] inhibitors used to treat RCC has grown to include several other drugs, including axitinib. Axitinib is an oral VEGFR tyrosine kinase inhibitor [TKI] targeting mostly VEGFR-1, VEGFR-2 and VEGFR-3.3 In addition to using axitinib to treat patients with metastatic RCC, this drug has been used in the neoadjuvant or preoperative setting, mostly in the context of clinical trials4.
The use of neoadjuvant therapy prior to surgery is a familiar paradigm in diseases such as breast5, esophageal6 and pancreatic cancer7, among others. These treatments can be associated with changes in body composition, such as loss of lean muscle mass, also known as sarcopenia. Alterations to visceral adipose and subcutaneous adipose tissue can also occur. These changes have been shown to have an impact on clinical outcome. For example, patients with breast cancer who received preoperative therapy were more likely to have a pathologic complete response if they had sarcopenia.8 On the other hand, sarcopenia was associated with an inferior median overall survival in patients receiving neoadjuvant chemotherapy for gastroesophageal cancer.9 Similarly, sarcopenia was associated with worse overall survival in patients with pancreatic cancer who received neoadjuvant therapy.10 In RCC, sarcopenia has been recently studied, in patients with localized11, and metastatic disease.12, 13
To our knowledge, changes in body composition due to the effect of neoadjuvant targeted therapy in patients with localized RCC have not been studied. In addition, minimal data exist on predictors of response to neoadjuvant targeted therapy for RCC. Our primary aim was to determine what changes in body composition occurred in patients with non-metastatic clear cell RCC treated with neoadjuvant axitinib in the context of a prospective clinical trial. Our secondary aim was to investigate if these changes were associated with differences in the response to axitinib treatment.
PATIENTS AND METHODS
Patient selection
The present study is a post hoc investigation based on laboratory and imaging data obtained from a published phase II clinical trial of neoadjuvant axitinib in patients with locally advanced non-metastatic clear cell renal cell carcinoma (NCT01263769). The original inclusion/exclusion criteria, dosing regimen and response to treatment have been previously reported.4 Radiographic and laboratory data were prospectively collected during the course of the clinical trial at similar time points. Patients received axitinib at a minimum dose of 5mg twice daily. Computed tomography (CT) scans and laboratory studies were performed before the initiation of axitinib, after 7 weeks of treatment and after 12 weeks of treatment. The Institutional Review Board at The University of Texas MD Anderson Cancer Center approved the current study.
Anthropometric analysis
The cross sectional area of the skeletal muscle [SM], subcutaneous adipose [SA] and visceral adipose [VA] compartments were determined by a single observer using CT scans taken before initiation of treatment, after 7 weeks of treatment and after completion of 12 weeks of treatment. DICOM images that corresponded to the midpoint of the L3 vertebral body were analyzed using SliceOMatic software (Figures 1 and 2, Tomovision, Magog, Canada). Cross-sectional areas were then standardized to the square of height in meters.14 Sarcopenia was defined as SM ≤ 38.9 cm2/m2 for women and SM ≤ 55.4 cm2/m2 for men.15 Patient body mass index [BMI] was calculated as weight in kilograms divided by the square of the height in meters. BMI was classified according to World Health Organization classification16: underweight: < 18.5 kg/m2, normal range: 18.5 – 24.99 kg/m2, overweight: ≥ 25 kg/m2, obese: ≥ 30 kg/m2.
Figure 1. Change in Skeletal Muscle Compartment during 12 weeks of neoadjuvant treatment with axitinib.

Panel A – pre-treatment CT scan
Skeletal muscle 51.93 cm2/m2
Subcutaneous adipose 36.89 cm2/m2
Visceral adipose 87.37 cm2/m2
Panel B – 7 week CT scan
Skeletal muscle 47.31 cm2/m2
Subcutaneous adipose 36.32 cm2/m2
Visceral adipose 71.64 cm2/m2
Panel C – 12 week CT scan
Skeletal muscle 42.69 cm2/m2
Subcutaneous adipose 29.61 cm2/m2
Visceral adipose 63.34 cm2/m2
Dark blue = visceral adipose tissue, red = skeletal muscle, light blue = subcutaneous adipose tissue, green = intramuscular adipose tissue, orange = skin
Figure 2. Change in Subcutaneous Adipose Compartment during 12 weeks of neoadjuvant treatment with axitinib.
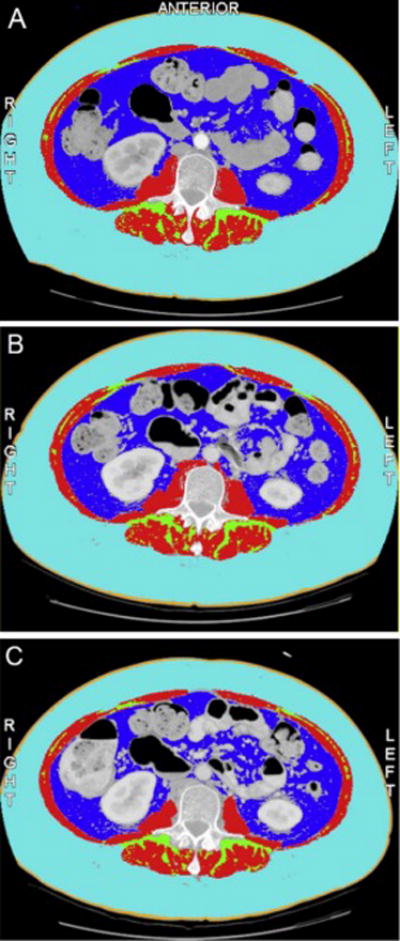
Panel A – pre-treatment CT scan
Skeletal muscle 36.25 cm2/m2
Subcutaneous adipose 161.07 cm2/m2
Visceral adipose 58.23 cm2/m2
Panel B – 7 week CT scan
Skeletal muscle 37.36 cm2/m2
Subcutaneous adipose 143.93 cm2/m2
Visceral adipose 45.25 cm2/m2
Panel C – 12 week CT scan
Skeletal muscle 33.39 cm2/m2
Subcutaneous adipose 123.60 cm2/m2
Visceral adipose 38.65 cm2/m2
Dark blue = visceral adipose tissue, red = skeletal muscle, light blue = subcutaneous adipose tissue, green = intramuscular adipose tissue, orange = skin
Statistical Analysis
Summary statistics were calculated for body compositions outcomes by treatment time. Summary statistics on the difference between the 12-week assessment and the pretreatment assessment were also calculated by initial sarcopenia status. Baseline characteristics and responses were compared by initial sarcopenia status using Fisher’s exact test, chi-squared test, or rank-sum test. A longitudinal mixed-effect linear regression model was conducted to assess change in body composition results over time. The model included a random intercept to account for multiple measurements within subject along with a term for initial sarcopenia status and time. An interaction term with assessment time and sarcopenia status was examined to see if changes in body composition over time differed by initial sarcopenia status. This term was excluded if non-significant. A logistic regression model was conducted to assess initial sarcopenia status (yes/no) on predicting response status (stable disease vs. partial response). We performed statistical analysis with Stata version 14 (College Station, TX). P < 0.05 was considered statistically significant.
RESULTS
Patient characteristics
The original phase II study enrolled 24 patients, all of whom had biopsy-proven clear cell RCC. Of the original 24 patients, 12 presented with symptoms: 9 symptomatic patients were in the non-sarcopenic group and 3 were in the sarcopenic group. Nine patients presented with hematuria, 2 patients with flank pain, 2 patients with paraneoplastic syndrome, and 2 patients with weight loss (only 1 was sarcopenic at baseline). One patient stopped treatment at week 7 and was excluded from the current analysis, as this patient did not receive the full 12-week dose of axitinib. The current study therefore consisted of the 23 patients who received at least 11 weeks of treatment and had pre-operative, 7-week, and 12-week imaging and labs available for analysis. Table 1 displays the baseline patient characteristics of the 23 patients who were included in the study. The most common grade 3 adverse events4 while on therapy were: Hypertension in 10 patients, AST/ALT increase in 2, abdominal pain in 2, fatigue in 1, oral mucositis in 1, hand-foot syndrome in 1, acute kidney injury in 1, and thrombocytopenia in 1.
Table 1.
Patient characteristics
| N | % | |
|---|---|---|
|
| ||
| Number of patients | 23 | 100 |
|
| ||
| Median Age, years (IQR) | 60 (51.5 – 66) | |
|
| ||
| Gender | ||
| Female | 5 | 21.7 |
| Male | 18 | 78.3 |
|
| ||
| ECOG performance status | ||
| 0 | 19 | 82.6 |
| 1 | 4 | 17.4 |
|
| ||
| Clinical stage | ||
| T3a | 23 | 100 |
|
| ||
| Pathologic stage | ||
| 1a | 3 | 13.0 |
| 1b | 1 | 4.3 |
| 2a | 0 | 0 |
| 2b | 1 | 4.3 |
| 3a | 17 | 73.9 |
| 3b | 0 | 0 |
| 3c | 0 | 0 |
| 4 | 1 | 4.3 |
|
| ||
| Pathologic grade | ||
| 1 | 0 | 0 |
| 2 | 11 | 47.8 |
| 3 | 11 | 47.8 |
| 4 | 1 | 4.3 |
|
| ||
| Clear cell RCC (biopsy) | 23 | 100 |
|
| ||
| Median pretreatment BMI, kg/m2 (IQR) | 30.1 (27.8 – 31.9) | |
|
| ||
| Pretreatment BMI | ||
| < 18.5 – underweight | 0 | 0 |
| 18.5 – 24.9 – normal | 2 | 8.7 |
| 25 – 29.9 – overweight | 9 | 39.1 |
| >29.9 – obese | 12 | 52.2 |
|
| ||
| Pretreatment sarcopenia | ||
| Yes | 7 | 30.4 |
| No | 16 | 69.6 |
ECOG = Eastern Cooperative Oncology Group; RCC = renal cell carcinoma; BMI = body mass index; IQR = interquartile range
Anthropometric changes during neoadjuvant axitinib treatment
At baseline, the median body mass index [BMI] of the study population prior to the start of treatment was 30.9 kg/m2 (Interquartile range [IQR] 28.3 to 32.3 kg/m2). Two (8.7%) patients had a normal BMI, 9 (39.1%) patients were overweight and 12 (52.2%) patients were obese. Median serum albumin prior to treatment was 4.5 g/dL (IQR 4.1 to 4.7 g/dL). Prior to initiation of axitinib, 7 (30.4%) patients had sarcopenia, of which 1 had a normal BMI, 4 were overweight and 2 were obese. The median serum albumin of these 7 patients with sarcopenia was 4.6 g/dL (IQR 4.0 to 4.9 g/dL).
After 7 weeks of treatment, 20 of 23 (87.0%) patients had lost weight, with a median weight loss of 3.1 kg (IQR 1.8 to 5.6 kg) (Figure 3a). The median BMI after 7 weeks of treatment was 28.7 kg/m2 (IQR 27.4 to 30.7 kg/m2) (Figure 3b). After 7 weeks of treatment, 2 (8.7%) patients had a normal BMI, 12 (52.2%) patients were overweight and 9 (39.1%) were obese. Three patients went from obese to overweight. After 7 weeks of treatment, the median decrease in SM was 1.7 cm2/m2 (IQR 0.56 to 3.72 cm2/m2) (p < 0.001), the median decrease in VA was 2.1 cm2/m2 (IQR −3.6 to 10.6 cm2/m2) (p = 0.270), and the median decrease in SA was 1.1 cm2/m2 (IQR −1.9 to 10.9 cm2/m2) (p = 0.039). Median albumin decreased by 0.2 g/dL (IQR −0.2 to 0.4 g/dL) (p = 0.224). There was no difference in change of body compartment composition based on initial sarcopenia status. Two patients who did not initially have sarcopenia developed sarcopenia by week 7.
Figure 3.
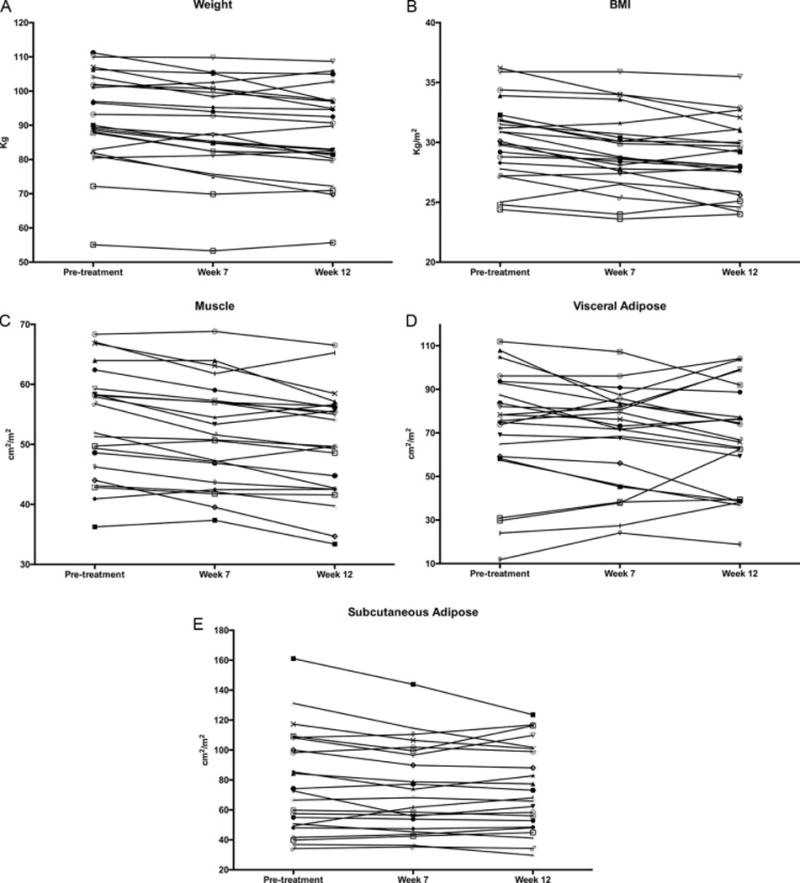
a – Change in weight during 12 weeks of neoadjuvant treatment with axitinib
b – Change in body mass index during 12 weeks of neoadjuvant treatment with axitinib
c – Change in skeletal muscle compartment during 12 weeks of neoadjuvant treatment with axitinib
d – Change in visceral adipose compartment during 12 weeks of neoadjuvant treatment with axitinib
e – Change in subcutaneous adipose compartment during 12 weeks of neoadjuvant treatment with axitinib
After 12 weeks of treatment, compared to pretreatment, 19 of 23 (82.6%) patients lost weight, with a median weight loss of 4.5 kg (IQR 1.3 to 8.4 kg) (Figure 3a p < 0.001). The median BMI after treatment was 28.0 kg/m2 (IQR 25.9 to 31 kg/m2). Three (13.0%) patients had a normal BMI, 13 (56.6%) 11patients were overweight and 7 (30.4%) patients were obese. One patient went from normal BMI to overweight, 2 patients went from overweight to normal, and 5 patients went from obese to overweight. Between pretreatment and 12 weeks of treatment, the median decrease in SM was 2.9 cm2/m2 (IQR 1.7 to 6.2 cm2/m2) (p < 0.001), the median decrease in VA was 4.9 cm2/m2 (IQR −7.9 to 19.6 cm2/m2) (p = 0.132), and the median decrease in SA was 1.0 cm2/m2 (IQR −1.7 to 9.6 cm2/m2) (p = 0.043) (Figures 3c-e). Median albumin decreased by 0.2 g/dL (IQR −0.1 to 0.5 g/dL) (p = 0.005). There was no difference in change of body compartment composition based on initial sarcopenia status. Five patients who did not initially have sarcopenia, developed sarcopenia by week 12. Table 2 displays changes in serum albumin and body composition during the course of treatment.
Table 2.
Serum albumin and body composition changes during neoadjuvant treatment with axitinib (N=23)
| Variable | Time | Media | IQR | p-value |
|---|---|---|---|---|
| Albumin (g/dL) | Pre-treatment | 4.5 | 4.1 – 4.7 | |
| 7 weeks | 4.3 | 4.0 – 4.5 | ||
| 12 weeks | 4.3 | 4.0 – 4.5 | 0.005 | |
| Skeletal Muscle (cm2/m2) | Pre-treatment | 56.8 | 46.2 – 59.3 | |
| 7 weeks | 51.6 | 43.6 – 57.3 | ||
| 12 weeks | 49.7 | 42.5 – 56.4 | <0.001 | |
| Visceral adipose (cm2/m2) | Pre-treatment | 75.5 | 58.2 – 92.9 | |
| 7 weeks | 73.2 | 46.0 – 83.7 | ||
| 12 weeks | 66.8 | 39.4 – 88.7 | 0.132 | |
| Subcutaneous adipose (cm2/m2) | Pre-treatment | 72.7 | 49.4 – 108.2 | |
| 7 weeks | 68.1 | 47.4 – 99.5 | ||
| 12 weeks | 68.1 | 48.8 – 100.8 | 0.043 | |
| Weight (kg) | Pre-treatment | 92.0 | 82.8 – 102.0 | |
| 7 weeks | 89.3 | 82.4 – 100.7 | ||
| 12 weeks | 87.7 | 80.3 – 97.0 | <0.001 | |
| BMI (kg/m2) | Pre-treatment | 30.9 | 28.3 – 32.3 | |
| 7 weeks | 28.7 | 27.4 – 30.7 | ||
| 12 weeks | 28.0 | 25.9 – 31.0 | <0.001 |
BMI= Body Mass Index
Treatment response according to sarcopenia status
Ten (62.5%) of the 16 patients without baseline sarcopenia achieved a partial response according to RECIST17, while only one (14.3%) of the 7 patients with baseline pretreatment sarcopenia achieved a partial response (p=0.069). There were no instances of complete response or progressive disease in either group. Patients without baseline sarcopenia had a median decrease in tumor size of 30.85%, while those with baseline sarcopenia had a median decrease in tumor size of 20.9% (p=0.053). The odds ratio of a patient without initial sarcopenia having a partial response to treatment, compared to a patient with initial sarcopenia, was 10, with a 95% confidence internal [CI] of 0.96 – 104.49.
There were 5 patients who developed sarcopenia during treatment. Three (60%) of these patients had a partial response. The odds ratio of a patient who developed sarcopenia having a partial response to treatment was 0.86, with a 95% CI of 0.10 – 7.51. Table 3 summarizes response to treatment according to sarcopenia status.
Table 3.
Response to treatment stratified by sarcopenia status
| Sarcopenia | SD N (%) |
PR N (%) |
OR of having a PR | 95% CI | p-value | |
|---|---|---|---|---|---|---|
| Initial status | Yes | 6 (50.0) | 1 (9.1) | Reference | ||
| No | 6 (50.0) | 10 (90.0) | 10.00 | 0.96 – 104.49 | 0.054 | |
| Over course of treatment* | Never | 4 (33.3) | 7 (63.6) | Reference | ||
| Initial | 6 (50.0) | 1 (9.1) | 0.10 | 0.01 – 1.10 | 0.060 | |
| Developed | 2 (16.7) | 3 (27.3) | 0.86 | 0.10 – 7.51 | 0.889 |
SD = stable disease; PR = partial response; OR = odds ratio; CI = confidence interval
We did not find an association between sarcopenia and postoperative complications. Two non-sarcopenic patients without sarcopenia experienced a grade 3 complication: one requiring a drain placement by interventional radiology for chylous ascites, and one requiring takeback on the same day of surgery for hemorrhage with no obvious source of bleeding found on re-exploration. There were no grade 4 or 5 complications. Two non-sarcopenic patients required re-admission.
COMMENT
Our study shows that neoadjuvant axitinib used for 12 weeks in patients with non-metastatic clear cell renal cell carcinoma was associated with a decrease in skeletal muscle, subcutaneous adipose tissue, body weight and serum albumin, without a significant change in visceral adipose tissue. There was a trend towards a lower rate of partial response to axitinib treatment in patients with baseline sarcopenia, but this did not reach statistical significance. To our knowledge, this is the first study to examine changes in body composition resulting from neoadjuvant targeted therapy in patients with non-metastatic RCC, and it is the first study to examine changes in body composition due to axitinib in general.
Targeted therapy, particularly tyrosine kinase inhibitors (TKIs), has been shown to cause weight loss in several types of cancer. The use of sorafenib in patients with metastatic medullary thyroid cancer was shown to result in weight loss in up to 58% of patients.18, 19 Weight loss has also been reported with the use of sorafenib in advanced hepatocellular carcinoma.20 On the other hand, the use of TKIs has been associated with reversal of sarcopenia in advanced gastrointestinal stromal tumors.21 Weight loss or anorexia has been reported with the use of TKIs in patients with RCC. For instance, 22% of patients with metastatic RCC treated with pazopanib reported anorexia as a side effect.22 Antoun et al studied the development of sarcopenia in 48 patients with metastatic RCC treated with sorafenib,13 and noted that 52% of patients had sarcopenia before initiation of treatment with sorafenib, and that after one year of treatment, 71% of patients had sarcopenia, with an average weight loss of 4.2 kg.
In patients with RCC, sarcopenia has been primarily studied in the metastatic setting. Sarcopenia has been shown to be predictive of treatment related toxicity in patients with metastatic RCC receiving sunitinib or sorafenib.23–25 Dose limiting toxicities have also been observed in sarcopenic patients with hepatocellular carcinoma receiving sorafenib.26 Sharma et al. demonstrated that patients with metastatic RCC and sarcopenia had a worse overall survival after cytoreductive nephrectomy than patients without sarcopenia [7 months vs. 23 months].27 On a multivariable analysis adjusted for BMI, performance status, Charlson comorbidity index, albumin level, IMDC score, clinical stage, number of metastatic sites and receipt of neoadjuvant or adjuvant therapy, only number of metastatic sites [hazard ratio 2.094] and sarcopenia [hazard ratio 2.127] were predictive of worse overall survival. Fukushima et al also showed that sarcopenia was associated with worse overall survival in patients with metastatic RCC.12 The 3-year overall survival for patients without sarcopenia was 73%, compared with 31% for patients with sarcopenia [p<0.001]. A multivariable analysis identified sarcopenia, prior nephrectomy, number of metastatic sites, corrected calcium and LDH as independent predictors of overall survival in that study. To date, only one study examined baseline sarcopenia in patients with localized RCC. Psutka et al found that sarcopenia was associated with inferior outcomes in patients with localized RCC undergoing radical nephrectomy.11 Patients with sarcopenia had a 5-year cancer specific survival of 79%, compared to 85% for patients without sarcopenia [p=0.05]. The 5-year overall survival was 65% for patients with sarcopenia and 74% for patients without sarcopenia [p=0.005]. While the current study did not investigate the mechanisms of the association between baseline sarcopenia and lower responses to axitinib, the presence of elevated VEGF-A levels in patients with cancer-related weight loss28 could partially explain the lower response rates to axitinib in the sarcopenic setting.
Our study has limitations worth discussing. This was a single-institution clinical trial with no control arm and a small number of patients. However, it is unlikely that non-metastatic RCC would result in significant changes in muscle mass or a median weight loss of 4.5 kg in 12 weeks. Two patients presented with weight loss, one in each group, both of whom had stable disease. One could argue that these patients may have lost weight due to worrying about their diagnosis of cancer. However, in our original clinical trial, we used FKSI-1529 – a kidney cancer validated questionnaire that includes a worry assessment – and showed that patients were most worried at the pre-treatment assessment and there was actually an improvement in their worry score with each subsequent visit.4 Therefore it is unlikely that worrying would be responsible for the sustained weight loss from pre-treatment to week 7 to week 12, as patients were in fact worrying less during that period of time. In addition, the small number of patients makes the study underpowered to make an assessment of the relationship between sarcopenia and response to treatment. We did not attempt to correlate sarcopenia with survival outcomes, as there have not been enough events for this type of analysis. Postoperative body composition (after axitinib was stopped) was not included in our analysis, as this will likely be confounded by the surgery itself and postoperative recovery.
The current study differs from the current literature in several ways. First, patients were enrolled in this trial in a prospective fashion, with all patients having similar histology, treatment duration, and imaging intervals. Second, this is the first study to examine the relationship between sarcopenia and targeted therapy in non-metastatic renal cell carcinoma. As none of the patients in this study had metastatic disease or previous treatment for RCC, this allowed us to isolate the effects of axitinib and to attribute the changes in body composition to axitinib alone. Third, this is the first study to examine this relationship in general with axitinib in any tumor.
In conclusion, we report the first study that examines the effect of axitinib on body compartment composition in patients with cancer in general, and in patients treated for non-metastatic clear cell RCC in particular. Our patients experienced a median weight loss of 4.5 kg after 12 weeks of treatment, with significant decline in skeletal muscle and subcutaneous adipose tissue. There was a trend towards a lower rate of response to axitinib in patients with baseline sarcopenia, but this was not statistically significant. Future larger studies in this setting are needed to confirm the current findings and to investigate the relation between baseline sarcopenia and response to targeted therapy.
Supplementary Material
Acknowledgments
The Biostatistics Resource Group is supported by the National Institutes of Health/National Cancer Institute (NIH/NCI) under award number P30CA016672.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: Jose A. Karam has acted as a consultant/advisory board member for Pfizer, Novartis and EMD Serono. Christopher G. Wood has received research funding from Pfizer and served as a consultant and on its advisory board. None of these disclosures by both authors are related to the current manuscript.
References
- 1.Motzer RJ, Michaelson MD, Redman BG, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 2.Ratain MJ, Eisen T, Stadler WM, et al. Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:2505–2512. doi: 10.1200/JCO.2005.03.6723. [DOI] [PubMed] [Google Scholar]
- 3.Rugo HS, Herbst RS, Liu G, et al. Phase I trial of the oral antiangiogenesis agent AG-013736 in patients with advanced solid tumors: pharmacokinetic and clinical results. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:5474–5483. doi: 10.1200/JCO.2005.04.192. [DOI] [PubMed] [Google Scholar]
- 4.Karam JA, Devine CE, Urbauer DL, et al. Phase 2 trial of neoadjuvant axitinib in patients with locally advanced nonmetastatic clear cell renal cell carcinoma. European urology. 2014;66:874–880. doi: 10.1016/j.eururo.2014.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:778–785. doi: 10.1200/JCO.2007.15.0235. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. The New England journal of medicine. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 7.Tempero MA, Malafa MP, Behrman SW, et al. Pancreatic adenocarcinoma, version 2.2014: featured updates to the NCCN guidelines. Journal of the National Comprehensive Cancer Network : JNCCN. 2014;12:1083–1093. doi: 10.6004/jnccn.2014.0106. [DOI] [PubMed] [Google Scholar]
- 8.Del Fabbro E, Parsons H, Warneke CL, et al. The relationship between body composition and response to neoadjuvant chemotherapy in women with operable breast cancer. The oncologist. 2012;17:1240–1245. doi: 10.1634/theoncologist.2012-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan BH, Brammer K, Randhawa N, et al. Sarcopenia is associated with toxicity in patients undergoing neo-adjuvant chemotherapy for oesophago-gastric cancer. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2015;41:333–338. doi: 10.1016/j.ejso.2014.11.040. [DOI] [PubMed] [Google Scholar]
- 10.Cooper AB, Slack R, Fogelman D, et al. Characterization of Anthropometric Changes that Occur During Neoadjuvant Therapy for Potentially Resectable Pancreatic Cancer. Annals of surgical oncology. 2015;22:2416–2423. doi: 10.1245/s10434-014-4285-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Psutka SP, Boorjian SA, Moynagh MR, et al. Decreased Skeletal Muscle Mass is Associated with an Increased Risk of Mortality after Radical Nephrectomy for Localized Renal Cell Cancer. The Journal of urology. 2016;195:270–276. doi: 10.1016/j.juro.2015.08.072. [DOI] [PubMed] [Google Scholar]
- 12.Fukushima H, Nakanishi Y, Kataoka M, Tobisu K, Koga F. Prognostic Significance of Sarcopenia in Patients with Metastatic Renal Cell Carcinoma. The Journal of urology. 2016;195:26–32. doi: 10.1016/j.juro.2015.08.071. [DOI] [PubMed] [Google Scholar]
- 13.Antoun S, Birdsell L, Sawyer MB, Venner P, Escudier B, Baracos VE. Association of skeletal muscle wasting with treatment with sorafenib in patients with advanced renal cell carcinoma: results from a placebo-controlled study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:1054–1060. doi: 10.1200/JCO.2009.24.9730. [DOI] [PubMed] [Google Scholar]
- 14.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. American journal of epidemiology. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 15.Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2008;33:997–1006. doi: 10.1139/H08-075. [DOI] [PubMed] [Google Scholar]
- 16.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organization technical report series. 2000;894:i–xii. 1–253. [PubMed] [Google Scholar]
- 17.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Schneider TC, Abdulrahman RM, Corssmit EP, Morreau H, Smit JW, Kapiteijn E. Long-term analysis of the efficacy and tolerability of sorafenib in advanced radioiodine refractory differentiated thyroid carcinoma: final results of a phase II trial. European journal of endocrinology/European Federation of Endocrine Societies. 2012;167:643–650. doi: 10.1530/EJE-12-0405. [DOI] [PubMed] [Google Scholar]
- 19.Castroneves LA, Negrao MV, Freitas RM, et al. Sorafenib for the treatment of Progressive Metastatic Medullary Thyroid Cancer: Efficacy and Safety Analysis. Thyroid. 2015 doi: 10.1089/thy.2015.0334. [DOI] [PubMed] [Google Scholar]
- 20.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. The New England journal of medicine. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 21.Moryoussef F, Dhooge M, Volet J, et al. Reversible sarcopenia in patients with gastrointestinal stromal tumor treated with imatinib. Journal of cachexia, sarcopenia and muscle. 2015;6:343–350. doi: 10.1002/jcsm.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 23.Antoun S, Baracos VE, Birdsell L, Escudier B, Sawyer MB. Low body mass index and sarcopenia associated with dose-limiting toxicity of sorafenib in patients with renal cell carcinoma. Annals of oncology : official journal of the European Society for Medical Oncology/ESMO. 2010;21:1594–1598. doi: 10.1093/annonc/mdp605. [DOI] [PubMed] [Google Scholar]
- 24.Huillard O, Mir O, Peyromaure M, et al. Sarcopenia and body mass index predict sunitinib-induced early dose-limiting toxicities in renal cancer patients. British journal of cancer. 2013;108:1034–1041. doi: 10.1038/bjc.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cushen SJ, Power DG, Teo MY, et al. Body Composition by Computed Tomography as a Predictor of Toxicity in Patients With Renal Cell Carcinoma Treated With Sunitinib. American journal of clinical oncology. 2014 doi: 10.1097/COC.0000000000000061. [DOI] [PubMed] [Google Scholar]
- 26.Mir O, Coriat R, Blanchet B, et al. Sarcopenia predicts early dose-limiting toxicities and pharmacokinetics of sorafenib in patients with hepatocellular carcinoma. PloS one. 2012;7:e37563. doi: 10.1371/journal.pone.0037563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma P, Zargar-Shoshtari K, Caracciolo JT, et al. Sarcopenia as a predictor of overall survival after cytoreductive nephrectomy for metastatic renal cell carcinoma. Urologic oncology. 2015;33:339 e317–323. doi: 10.1016/j.urolonc.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 28.Lerner L, Hayes TG, Tao N, et al. Plasma growth differentiation factor 15 is associated with weight loss and mortality in cancer patients. Journal of cachexia, sarcopenia and muscle. 2015;6:317–324. doi: 10.1002/jcsm.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cella D, Yount S, Du H, et al. Development and validation of the Functional Assessment of Cancer Therapy-Kidney Symptom Index (FKSI) The journal of supportive oncology. 2006;4:191–199. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


