Abstract
Context
Human papillomavirus (HPV) infection is the principal cause of a distinct form of oropharyngeal squamous cell carcinoma that is increasing in incidence among men in the United States. However, little is known about the epidemiology of oral HPV infection.
Objective
To determine the prevalence of oral HPV infection in the United States.
Design, Setting, and Participants
A cross-sectional study was conducted as part of the National Health and Nutrition Examination Survey (NHANES) 2009–2010, a statistically representative sample of the civilian noninstitutionalized US population. Men and women aged 14 to 69 years examined at mobile examination centers were eligible. Participants (N=5579) provided a 30-second oral rinse and gargle with mouthwash. For detection of HPV types, DNA purified from oral exfoliated cells was evaluated by polymerase chain reaction and type-specific hybridization. Demographic and behavioral data were obtained by standardized interview. Statistical analyses used NHANES sample weights to provide weighted prevalence estimates for the US population.
Main Outcome Measures
Prevalence of oral HPV infection.
Results
The prevalence of oral HPV infection among men and women aged 14 to 69 years was 6.9% (95% CI, 5.7%–8.3%) and of HPV type 16 was 1.0% (95% CI, 0.7%–1.3%). Oral HPV infection followed a bimodal pattern with respect to age, with peak prevalence among individuals aged 30 to 34 years (7.3%; 95% CI, 4.6%–11.4%) and 60 to 64 years (11.4%; 95% CI, 8.5%–15.1%). Men had a significantly higher prevalence than women for any oral HPV infection (10.1% [95% CI, 8.3%–12.3%] vs 3.6% [95% CI, 2.6%–5.0%], P<.001; unadjusted prevalence ratio [PR], 2.80 [95% CI, 2.02–3.88]). Infection was less common among those without vs those with a history of any type of sexual contact (0.9% [95% CI, 0.4%–1.8%] vs 7.5% [95% CI, 6.1%–9.1%], P<.001; PR, 8.69 [95% CI, 3.91–19.31]) and increased with number of sexual partners (P<.001 for trend) and cigarettes smoked per day (P<.001 for trend). Associations with age, sex, number of sexual partners, and current number of cigarettes smoked per day were independently associated with oral HPV infection in multivariable models.
Conclusion
Among men and women aged 14 to 69 years in the United States, the overall prevalence of oral HPV infection was 6.9%, and the prevalence was higher among men than among women.
Oral human papillomavirus (HPV) infection is the cause of a subset of oropharyngeal squamous cell carcinomas (OSCCs).1 Human papillomavirus–positive OSCCs are associated with sexual behavior in contrast to HPV-negative OSCCs that are associated with chronic tobacco and alcohol use.2 At least 90% of HPV-positive OSCCs are associated with high-risk (or oncogenic) HPV type 16 (HPV-16),3 and oral infection confers an approximate 50-fold increase in risk for HPV-positive OSCC.2
The incidence of OSCC has significantly increased over the last 3 decades in several countries, and HPV has been directly implicated as the underlying cause.4–6 Although the incidence of HPV-negative OSCC declined by 50% in the United States from 1988 to 2004 (from 2.0 cases per 100 000 population to 1.0 per 100 000), the incidence of HPV-positive OSCC increased by 225% (from 0.8 per 100 000 to 2.6 per 100 000), predominantly among young individuals, men, and white individuals.6 The increase among the young is consistent with reported sexual behavioral changes by birth cohort since the 1950s in the United States.7 However, the reasons for the predominant increase among men are unclear.
Although oral HPV infection is the cause of a cancer that is increasing in incidence in the United States,6 little is known regarding the epidemiology of infection. In this study, we report the first population-based study of oral HPV prevalence, conducted within the US National Health and Nutrition Examination Survey (NHANES).
METHODS
The NHANES is conducted by the National Center for Health Statistics (NCHS) to monitor the health and nutritional status of the US population. The design for NHANES 2009–2010 was a stratified multistage probability sample of the civilian, noninstitutionalized US population. Race and ethnicity were self-selected by NHANES participants from a card that displayed categories defined by the NCHS. Black individuals, Hispanic individuals, persons aged 60 years and older, and low-income persons were oversampled to improve the accuracy of the statistical estimates.
The survey consisted of a household interview followed by physical examination and interviews at a mobile examination center (MEC). All males and females aged 14 to 69 years who were examined at the MEC were eligible. The protocol was approved by the NCHS institutional review board, and written informed consent was obtained from all participants. Further information regarding study design is available on the NHANES Web site.8
Demographic and Behavioral Data
The NHANES in-home, interviewer-administered survey was used to obtain sociodemographic data. Self-reported data on substance use (eg, tobacco, alcohol, and marijuana) and sexual behavior were obtained by audio, computer-assisted self-interview at the MEC. For individuals aged 14 to 69 years, sexual behavioral data included ever having had sex; age at first sex; number of partners inclusive of vaginal, oral (performing or receiving), and anal sex; and ever performance of oral sex. Data on same-sex partners for men included number of lifetime and recent (defined as prior 12 months) partners for performing oral or anal sex; for women, data included only lifetime and recent oral sex partners. Additional sexual behavioral data obtained from individuals aged 14 to 59 years included number of lifetime and recent same- and opposite-sex partners for vaginal or performing oral sex, age at first performance of oral sex, time since performing oral sex on a new partner, frequency of barrier use for performing oral sex in the prior 12 months, having had sex (vaginal, oral [performing], or anal sex) with a new partner in the prior 12 months, sexual orientation, and history of sexually transmitted infection.
Oral Samples and DNA
Oral rinse samples were collected by means of a 30-second oral rinse and gargle of 10 mL of Scope mouthwash (or saline). Participants alternated swish and gargle every 5 seconds and expectorated the sample into a sterile collection cup. Deidentified samples were stored at 4°C and shipped weekly to Ohio State University for HPV detection. Detailed quality control procedures are available on the NHANES Web site.9
From oral exfoliated cells, DNA was purified after centrifugation and sequential digestion with DNase-free RNase A and proteinase K using the Qiagen Virus/Bacteria Midi kit and Pathogen Complex 800 program on the Qiasymphony SP instrument (Qiagen) and then eluted in 60 μL of AVE buffer (as described).10
HPV Detection and Genotyping
Detection of 37 HPV types within the Alphapapillomavirus genera was performed by multiplex polymerase chain reaction (PCR) with PGMY primer pools and primers for β-globin followed by line-blot hybridization (Roche Linear Array HPV Genotyping Test; Roche Molecular Systems).11 Twelve microliters of DNA was included in a 100-μL reaction. β-Globin–positive samples were considered evaluable. Samples were reported as HPV positive if any of the 37 HPV DNA types were detected, including high-risk (16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 82) and low-risk (6, 11, 40, 42, 54, 55, 61, 62, 64, 67, 69, 70, 71, 72, 81, 82 subtype IS39, 83, 84, 89 [cp6108]) types.12 Samples positive for HPV-33, -35, -52, or -58 were evaluated for HPV-52 by use of a type-specific TaqMan real-time PCR because of the inability of the Roche Linear Array HPV Genotyping Test to discriminate HPV-52 infections.13 Samples with an HPV-52 copy number above the lower limit of reproducibility (≥3 copies) as derived from a reference standard curve were considered positive (as previously described).13
Statistical Analysis
All analyses were conducted using NHANES 2009–2010 MEC sample weights to account for the complex survey design and to provide unbiased estimates of HPV prevalence and predictors for the total US population. Prevalence estimates of HPV are reported as percentages with 95% confidence intervals. Confidence intervals for HPV type-specific prevalence were estimated using methods for sparse sample sizes.14 Bivariate analyses with demographic and behavioral characteristics were conducted using the survey design-adjusted Wald F test. Associations with oral HPV prevalence were evaluated and reported as adjusted prevalence ratios (PRs) and 95% CIs using binary logistic regression with significance assessed through the Wald F P value. Variables for adjustment in logistic regression models were selected based on bivariate associations as well as a priori. Nonlinear patterns in prevalence of any HPV infection across age, treated as a continuous variable, were evaluated using restricted cubic splines with 5 knots (placed at the 5th, 25th, 50th, 75th, and 95th percentiles of age distribution).15
Given high colinearity across sexual behaviors, each behavior was evaluated in separate models. Multiplicative statistical interactions were evaluated through product terms. Goodness of fit of all models was assessed using the Hosmer-Lemeshow Satterthwaite adjusted F test. In addition to associations with any HPV infection, we simultaneously evaluated and compared factors associated with high-risk and low-risk HPV infections using multinomial logistic regression.
We observed significant differences in demographic characteristics between participants with evaluable samples and nonparticipants in the Oral HPV Protocol (n = 5501 and 486, respectively) and responders and nonresponders to the MEC interview (n=4964 and 537, respectively) (eTable 1, available at http://www.jama.com). Therefore, we conducted analyses using 2 sets of poststratification weights—one to account for nonparticipation in the protocol and another also for non-participation in the MEC interview. These analyses did not materially influence results, and therefore all results are reported using the NHANES sample weights.
All analyses (code available on request) were conducted using SAS-callable SUDAAN version 10.0.1 (RTI).16 Two-sided P values less than .05 were considered statistically significant. No adjustments for multiple comparisons were performed.
RESULTS
Analyses of HPV prevalence overall and across demographic characteristics included 5501 of 5579 participants. Seventy-six samples were damaged during shipping and 2 were β-globin negative. Analyses of behavioral predictors included 4964 participants aged 14 to 69 years who responded to the computer-assisted interview at the MEC (eFigure 1).
HPV Prevalence
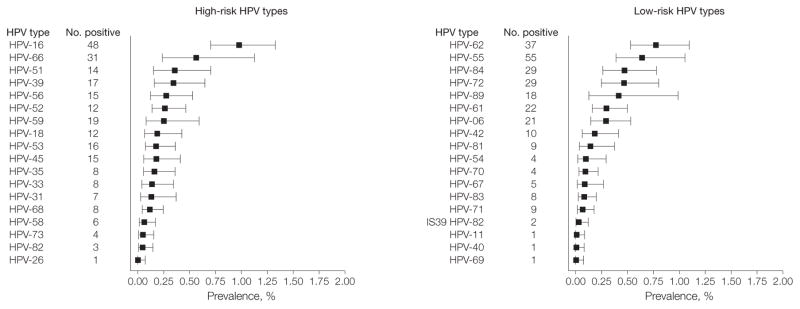
The overall prevalence of oral HPV infection was 6.9% (95% CI, 5.7%–8.3%). The prevalence of high-risk HPV infections was 3.7% (95% CI, 3.0%–4.6%) and for low-risk HPV infections was 3.1% (95% CI, 2.5%–3.9%). The type-specific prevalence estimates for the 37 HPV types evaluated are shown in Figure 1. The most prevalent HPV type detected was HPV-16 (1.0%; 95% CI, 0.7%–1.3%).
Figure 1. Prevalence of Oral HPV Infection by Individual Genotypes in the US Population Aged 14 to 69 Years.
The weighted prevalence (and 95% CI) for each of the 37 human papillomavirus (HPV) types evaluated is stratified by classification as high-risk or low-risk HPV types based on epidemiological associations with cervical cancer. HPV type 64 was not detected. The data are derived from the 5501 NHANES participants with evaluable samples. Error bars indicate 95% CIs.
Factors Associated With Prevalent HPV Infection in Univariate Analysis
Several demographic characteristics were associated with prevalent oral HPV infection, including age and sex (Table 1). The prevalence of oral HPV infection followed a bimodal pattern with age (Table 1 and Figure 2), with a first peak in prevalence observed among those aged 30 to 34 years (7.3%; 95% CI, 4.6%–11.4%) and a second, higher peak among those aged 60 to 64 years (11.4%; 95% CI, 8.5%–15.1%). Men had a significantly higher prevalence than women for overall oral HPV infection (10.1% vs 3.6%, P <.001; unadjusted PR, 2.80, 95% CI, 2.02–3.88) and for oral HPV-16 infection (1.6% vs 0.3%, P <.001; PR, 5.41, 95% CI, 2.12–13.83). Although the prevalence among black participants was higher than among white participants (10.5% vs 6.5%), this difference did not reach statistical significance (P = .06). Prevalence of HPV was higher among current smokers and heavy alcohol drinkers (and increased with intensity for both) and among former and current marijuana users (Table 1).
Table 1.
Prevalence of HPV Infection in the US Population by Demographic Characteristics in NHANES 2009–2010
| Characteristic | Total No. (No. With HPV Infection) | HPV Prevalence, % (95% CI) | Unadjusted PR (95% CI) |
|---|---|---|---|
| Age, ya | |||
| 14–17 | 656 (16) | 1.7 (1.0–3.1) | 1 [Reference] |
|
| |||
| 18–24 | 792 (45) | 5.6 (4.1–7.5) | 3.21 (1.54–6.71) |
|
| |||
| 25–29 | 463 (32) | 7.1 (4.3–11.6) | 4.11 (1.89–8.93) |
|
| |||
| 30–34 | 436 (39) | 7.3 (4.6–11.4) | 4.21 (2.11–8.41) |
|
| |||
| 35–39 | 461 (31) | 5.4 (3.4–8.4) | 3.11 (1.60–6.02) |
|
| |||
| 40–44 | 495 (30) | 6.3 (4.4–8.9) | 3.64 (1.75–7.58) |
|
| |||
| 45–49 | 482 (37) | 7.3 (4.4–12.0) | 4.22 (1.87–9.55) |
|
| |||
| 50–54 | 474 (50) | 8.3 (5.5–12.3) | 4.80 (2.33–9.88) |
|
| |||
| 55–59 | 381 (47) | 11.2 (6.5–18.5) | 6.46 (2.70–15.43) |
|
| |||
| 60–64 | 498 (55) | 11.4 (8.5–15.1) | 6.57 (3.38–12.77) |
|
| |||
| 65–69 | 363 (26) | 4.2 (2.4–7.2) | 2.44 (0.95–6.23) |
|
| |||
| P value (P value for trend)b | .02 (.009) | ||
|
| |||
| Sexa | |||
| Male | 2748 (295) | 10.1 (8.3–12.3) | 2.80 (2.02–3.88) |
|
| |||
| Female | 2753 (113) | 3.6 (2.6–5.0) | 1 [Reference] |
|
| |||
| P value | <.001 | ||
|
| |||
| Race/ethnicitya | |||
| Mexican American | 1189 (76) | 6.2 (4.8–7.9) | 0.95 (0.63–1.42) |
|
| |||
| Other Hispanic | 624 (48) | 7.8 (5.6–10.5) | 1.20 (0.74–1.95) |
|
| |||
| White, non-Hispanic | 2282 (154) | 6.5 (4.7–9.0) | 1 [Reference] |
|
| |||
| Black, non-Hispanic | 1081 (117) | 10.5 (7.9–13.8) | 1.61 (1.01–2.57) |
|
| |||
| Other race | 325 (13) | 4.3 (2.4–7.2) | 0.67 (0.35–1.29) |
|
| |||
| P value | .06 | ||
|
| |||
| Educationa | |||
| Less than high school | 1987 (139) | 6.3 (5.1–7.7) | 1 [Reference] |
|
| |||
| High school or equivalent | 1126 (106) | 8.8 (6.7–11.5) | 1.41 (0.95–2.07) |
|
| |||
| Some college or greater | 2379 (163) | 6.4 (4.7–8.7) | 1.02 (0.67–1.56) |
|
| |||
| Missing | 9 (0) | 0.0 | |
|
| |||
| P value | .11 | ||
|
| |||
| Income-to-poverty ratioa,c | |||
| <1.0 | 1263 (118) | 8.9 (7.4–10.5) | 1 [Reference] |
|
| |||
| ≥1.0 to <2.0 | 1349 (107) | 7.6 (6.1–9.4) | 0.86 (0.64–1.13) |
|
| |||
| ≥2.0 to <3.0 | 652 (45) | 6.7 (5.4–8.3) | 0.75 (0.58–0.98) |
|
| |||
| ≥3.0 | 1719 (102) | 6.1 (4.2–8.8) | 0.69 (0.48–0.98) |
|
| |||
| Missing | 518 (36) | 6.4 (4.4–9.3) | |
|
| |||
| P value (P value for trend) | .10 (.06) | ||
|
| |||
| Marital statusa | |||
| Never married | 1892 (117) | 6.3 (5.0–7.8) | 1 [Reference] |
|
| |||
| Married/living with partner | 2771 (203) | 6.7 (5.1–8.7) | 1.07 (0.76–1.50) |
|
| |||
| Widowed/divorced/separated | 834 (88) | 9.1 (6.9–12.0) | 1.46 (0.99–2.14) |
|
| |||
| Missing | 4 (0) | 0.0 | |
|
| |||
| P value | .11 | ||
|
| |||
| Cigarette usea,d | |||
| Never/former | 4163 (237) | 5.4 (4.2–7.1) | 1 [Reference] |
|
| |||
| Current, <10 cigarettes/d | 805 (94) | 9.7 (7.4–12.5) | 1.78 (1.25–2.54) |
|
| |||
| Current, 11–20 cigarettes/d | 363 (55) | 14.7 (9.6–21.8) | 2.70 (1.69–4.32) |
|
| |||
| Current, >20 cigarettes/d | 96 (17) | 20.7 (12.6–32.0) | 3.80 (2.27–6.36) |
|
| |||
| Missing | 74 (5) | 3.2 (0.9–10.9) | |
|
| |||
| P value (P value for trend) | <.001 (<.001) | ||
|
| |||
| Alcohol use in past 12 mo, average No. of drinks/wke | |||
| 0 | 1420 (109) | 7.2 (5.0–10.4) | 1 [Reference] |
|
| |||
| <1 | 904 (55) | 5.2 (3.4–7.8) | 0.71 (0.42–1.20) |
|
| |||
| 1–7 | 1212 (89) | 6.6 (4.9–8.8) | 0.91 (0.61–1.36) |
|
| |||
| 8–14 | 349 (55) | 12.1 (8.8–16.3) | 1.67 (1.03–2.71) |
|
| |||
| >14 | 291 (33) | 12.3 (7.3–19.8) | 1.70 (1.13–2.54) |
|
| |||
| Missing | 386 (41) | 7.8 (5.0–11.9) | |
|
| |||
| P value (P value for trend) | .08 (.007) | ||
|
| |||
| Marijuana usef,g | |||
| Never | 2164 (103) | 4.1 (3.2–5.3) | 1 [Reference] |
|
| |||
| Former | 1491 (124) | 7.8 (5.9–10.3) | 1.90 (1.27–2.85) |
|
| |||
| Current | 510 (57) | 11.8 (8.6–15.9) | 2.87 (1.85–4.46) |
|
| |||
| Missing | 26 (2) | 13.6 (2.4–50.2) | |
|
| |||
| P value (P value for trend) | .005 (<.001) | ||
|
| |||
| Current use of birth control pills/hormonesh | |||
| No | 1808 (7) | 3.7 (2.5–5.3) | 1 [Reference] |
|
| |||
| Yes | 233 (69) | 1.4 (0.4–5.2) | 0.39 (0.10–1.53) |
|
| |||
| Missing | 233 (18) | 5.7 (3.0–10.6) | |
|
| |||
| P value | .09 | ||
|
| |||
| HPV vaccinationi | |||
| No | 1985 (10) | 0.5 (0.2–1.7) | |
|
| |||
| Yes | 290 (0) | 0.0 | |
Abbreviations: HPV, human papillomavirus; PR, prevalence ratio.
Analyses based on all participants aged 14–69 years with evaluable oral rinse specimens (n=5501).
Adjusted Wald F P values for heterogeneity. P values for trend shown in parentheses were calculated using the Cochran-Mantel-Haenszel test. Subjects with missing data were excluded from all P value and PR calculations.
Income-to-poverty ratio is the ratio of the family income to the federal poverty threshold, taking into account family size and updated annually for inflation. A ratio below 1 indicates family income is below the federal poverty threshold.
Ever use of cigarettes was defined for individuals aged 14–19 years as ever having tried cigarettes and for those aged 20–69 years as lifetime use of ≥100 cigarettes. A current smoker was defined as someone who had smoked a cigarette in the prior 30 days. A former smoker was defined as an ever user who had not smoked a cigarette in the prior 30 days.
Analyses for alcohol use were restricted to individuals aged 20 years and older.
Analyses were restricted to individuals aged 14–59 years who responded to the audio, computer-assisted self-interview (n=4191).
Current marijuana use was defined as use of marijuana at least once within the past 30 days.
Analyses for current use of birth control pills/hormones were restricted to women aged 20 years and older.
Analyses of HPV vaccination were restricted to women aged 14–59 years and to HPV types 16 and 18.
Figure 2. Association of Age With Oral HPV Prevalence in the US Population Aged 14 to 69 Years.
Observed and modeled human papillomavirus (HPV) prevalence by individual age as well as 95% CI for each age. To evaluate the influence of covariates on HPV prevalence across age, the model was adjusted for sex, race, smoking, marital status, and lifetime number of (any) sex partners. Given the standardization for covariates included in the multivariate model, the HPV prevalence curve obtained from the adjusted model is presented at the mean levels of the covariates (sex, race, smoking, marital status, and lifetime number of any sex partners, as appropriate) and aligned with the HPV prevalence curve in the unadjusted model to enable visual display of the data. P<.001 for unadjusted model; P=.02 for adjusted. P values less than .05 for spline terms denote statistical evidence for bimodality in the data.
Oral HPV prevalence was associated with several measures of sexual behavior (Table 2). Oral HPV prevalence was more than 8-fold higher among individuals who reported ever having had sex vs not (7.5% vs 0.9%, P <.001; PR, 8.69, 95% CI, 3.91–19.31). Prevalence of HPV increased with lifetime or recent number of partners for any kind of sex, vaginal sex, or oral sex (all P <.001 for trend). One in 5 individuals with more than 20 lifetime sexual partners was infected. Prevalence was higher among individuals who first performed oral sex at 18 years or younger (Table 2).
Table 2.
Prevalence of HPV Infection in the US Population by Behavioral Characteristics in NHANES 2009–2010
| Characteristic | Total No. (No. With HPV Infection) | HPV Prevalence, % (95% CI) | Unadjusted PR (95% CI) |
|---|---|---|---|
| Ever had sex (vaginal, anal, or oral)a | |||
| No | 592 (8) | 0.9 (0.4–1.8) | 1 [Reference] |
|
| |||
| Yes | 4346 (350) | 7.5 (6.1–9.1) | 8.69 (3.91–19.31) |
|
| |||
| Missing | 26 (3) | 7.0 (1.7–24.8) | |
|
| |||
| P value | <.001 | ||
|
| |||
| Ever performed oral sexa | |||
| No | 1473 (59) | 3.5 (2.3–5.4) | 1 [Reference] |
|
| |||
| Yes | 3452 (300) | 7.8 (6.2–9.8) | 2.21 (1.25–3.92) |
|
| |||
| Missing | 39 (2) | 3.8 (0.8–16.2) | |
|
| |||
| P value | .007 | ||
|
| |||
| Ever performed vaginal sexa | |||
| No | 691 (14) | 1.4 (0.9–2.3) | 1 [Reference] |
|
| |||
| Yes | 4249 (345) | 7.5 (6.1–9.2) | 5.36 (3.00–9.58) |
|
| |||
| Missing | 24 (2) | 5.7 (1.1–24.9) | |
|
| |||
| P value | <.001 | ||
|
| |||
| Ever had anal sexa | |||
| No | 3257 (194) | 5.3 (4.2–6.7) | 1 [Reference] |
|
| |||
| Yes | 1683 (166) | 9.5 (7.5–12.1) | 1.80 (1.34–2.41) |
|
| |||
| Missing | 24 (1) | 1.3 (0.1–10.5) | |
|
| |||
| P value | .003 | ||
|
| |||
| Sexual orientationa | |||
| Never had sex | 592 (8) | 0.9 (0.4–1.8) | 0.12 (0.04–0.33) |
|
| |||
| Homosexual/bisexual | 297 (29) | 7.1 (4.0–12.2) | 1.05 (0.62–0.79) |
|
| |||
| Heterosexual | 4049 (321) | 7.5 (6.1–9.1) | 1 [Reference] |
|
| |||
| Missing | 26 (3) | 7.0 (1.7–24.8) | |
|
| |||
| P value | <.001 | ||
|
| |||
| No. of lifetime oral sex partnersb,c | |||
| 0 | 1190 (38) | 3.5 (2.2–5.5) | 1 [Reference] |
|
| |||
| 1 | 770 (31) | 3.3 (2.1–5.1) | 0.93 (0.44–1.97) |
|
| |||
| 2–5 | 1442 (102) | 6.0 (4.5–8.1) | 1.72 (0.99–2.99) |
|
| |||
| 6–10 | 379 (40) | 9.6 (6.8–13.3) | 2.72 (1.52–4.89) |
|
| |||
| 11–20 | 211 (31) | 15.4 (9.0–25.2) | 4.40 (1.88–10.31) |
|
| |||
| ≥21 | 166 (41) | 21.5 (12.9–33.5) | 6.12 (2.89–12.98) |
|
| |||
| Missing | 33 (3) | 5.2 (1.3–18.3) | |
|
| |||
| P value (P value for trend) | <.001 (<.001) | ||
|
| |||
| No. of oral sex partners in past 12 mob | |||
| 0 | 1848 (76) | 4.0 (3.1–5.0) | 1 [Reference] |
|
| |||
| 1 | 1818 (144) | 7.6 (6.3–9.2) | 1.93 (1.44–2.59) |
|
| |||
| ≥2 | 496 (64) | 12.0 (7.6–18.2) | 3.03 (1.76–5.20) |
|
| |||
| Missing | 29 (2) | 3.9 (0.8–16.0) | |
|
| |||
| P value (P value for trend) | .004 (<.001) | ||
|
| |||
| Age first performed oral sexb | |||
| Never performed oral sex | 1190 (38) | 3.5 (2.2–5.5) | 0.39 (0.20–0.76) |
|
| |||
| ≤18 y | 1525 (151) | 9.0 (6.7–11.9) | 1 [Reference] |
|
| |||
| >18 y | 1445 (95) | 5.9 (4.8–7.3) | 0.66 (0.49–0.89) |
|
| |||
| Missing | 31 (2) | 3.4 (0.7–14.3) | |
|
| |||
| P value | .04 | ||
|
| |||
| Time since new oral sex partnerb | |||
| Never performed oral sex | 1190 (38) | 3.5 (2.2–5.5) | 0.36 (0.19–0.70) |
|
| |||
| >24 mo | 1567 (124) | 5.8 (4.3–7.7) | 0.60 (0.45–0.79) |
|
| |||
| 12–24 mo | 217 (23) | 11.5 (7.3–17.8) | 1.20 (0.70–2.04) |
|
| |||
| ≤12 mo | 1189 (99) | 9.6 (7.3–12.6) | 1 [Reference] |
|
| |||
| Missing | 28 (2) | 4.1 (0.9–17.1) | |
|
| |||
| P value | .02 | ||
|
| |||
| Barrier use during oral sex in past 12 mob | |||
| No oral sex in past year | 1848 (76) | 4.0 (3.1–5.0) | 0.47 (0.34–0.66) |
|
| |||
| Never/rarely | 1973 (178) | 8.4 (6.7–10.5) | 1 [Reference] |
|
| |||
| Usually/always | 297 (26) | 9.2 (5.5–15.0) | 1.10 (0.69–1.73) |
|
| |||
| Missing | 73 (6) | 5.1 (1.9–13.1) | |
|
| |||
| P value | .007 | ||
|
| |||
| No. of lifetime sex partners (any sex)a,c | |||
| 0 | 620 (10) | 1.1 (0.6–2.3) | 1 [Reference] |
|
| |||
| 1 | 687 (17) | 2.0 (1.0–4.1) | 1.79 (0.59–5.44) |
|
| |||
| 2–5 | 1489 (77) | 4.0 (2.8–5.7) | 3.53 (1.53–8.12) |
|
| |||
| 6–10 | 952 (78) | 8.3 (6.2–11.1) | 7.30 (3.40–15.64) |
|
| |||
| 11–20 | 564 (60) | 9.0 (6.4–12.5) | 7.90 (3.54–17.63) |
|
| |||
| ≥21 | 570 (112) | 20.5 (17.4–23.9) | 17.93 (8.45–38.06) |
|
| |||
| Missing | 82 (7) | 5.3 (2.4–11.2) | |
|
| |||
| P value (P value for trend) | <.001 (<.001) | ||
|
| |||
| No. of sex partners (any sex) in past 12 mob,c | |||
| 0 | 1012 (43) | 3.4 (2.3–5.0) | 1 [Reference] |
|
| |||
| 1 | 2425 (168) | 6.9 (5.6–8.4) | 2.03 (1.32–3.12) |
|
| |||
| ≥2 | 728 (73) | 10.1 (7.8–13.1) | 3.00 (1.78–5.04) |
|
| |||
| Missing | 26 (2) | 5.3 (1.1–22.1) | |
|
| |||
| P value (P value for trend) | .004 (<.001) | ||
|
| |||
| No. of lifetime vaginal sex partnersb,c | |||
| 0 | 644 (13) | 1.4 (0.9–2.3) | 1 [Reference] |
|
| |||
| 1 | 647 (20) | 2.4 (1.2–4.6) | 1.67 (0.65–4.25) |
|
| |||
| 2–5 | 1227 (65) | 4.4 (3.4–5.7) | 3.10 (1.67–5.74) |
|
| |||
| 6–10 | 772 (56) | 8.1 (6.1–10.7) | 5.72 (3.22–10.17) |
|
| |||
| 11–20 | 442 (45) | 8.7 (6.1–12.3) | 6.13 (3.25–11.53) |
|
| |||
| ≥21 | 417 (84) | 20.7 (16.4–25.8) | 14.58 (8.47–25.11) |
|
| |||
| Missing | 42 (3) | 4.9 (1.1–19.9) | |
|
| |||
| P value (P value for trend) | <.001 (<.001) | ||
|
| |||
| No. of vaginal sex partners in past 12 mob,c | |||
| 0 | 1023 (40) | 3.4 (2.3–5.1) | 1 [Reference] |
|
| |||
| 1 | 2293 (161) | 7.0 (5.6–8.7) | 2.06 (1.32–3.26) |
|
| |||
| ≥2 | 844 (83) | 9.2 (7.3–11.5) | 2.70 (1.69–4.31) |
|
| |||
| Missing | 31 (2) | 5.4 (1.2–21.6) | |
|
| |||
| P value | .003 (<.001) | ||
|
| |||
| New sex partner (any sex) in past 12 mob,c | |||
| No | 3430 (210) | 5.9 (5.0–7.1) | 1 [Reference] |
|
| |||
| Yes | 679 (70) | 10.7 (6.9–16.2) | 1.81 (1.18–2.77) |
|
| |||
| Missing | 82 (6) | 4.8 (1.6–13.8) | |
|
| |||
| P value | .04 | ||
|
| |||
| Frequency of vaginal/anal sex in past 12 mob | |||
| None | 777 (24) | 2.7 (1.5–4.6) | 1 [Reference] |
|
| |||
| Once | 193 (14) | 8.0 (4.8–13.0) | 2.99 (1.36–6.54) |
|
| |||
| 2–11 times | 785 (66) | 8.9 (6.9–11.4) | 3.32 (1.74–6.37) |
|
| |||
| 12–51 times | 911 (66) | 6.4 (4.9–8.4) | 2.40 (1.26–4.59) |
|
| |||
| 52–103 times | 609 (43) | 6.6 (4.7–9.2) | 2.47 (1.26–4.84) |
|
| |||
| 104–364 times | 486 (45) | 9.2 (6.4–13.0) | 3.43 (1.70–6.91) |
|
| |||
| ≥365 times | 61 (6) | 7.7 (2.5–21.6) | 2.89 (0.84–9.86) |
|
| |||
| Missing | 369 (22) | 5.0 (3.3–7.7) | |
|
| |||
| P value (P value for trend) | .16 (.02) | ||
|
| |||
| History of genital herpes/genital wartsb | |||
| No | 3967 (262) | 6.4 (5.2–7.9) | 1 [Reference] |
|
| |||
| Yes | 208 (23) | 10.4 (5.5–18.9) | 1.62 (0.76–3.45) |
|
| |||
| Missing | 16 (1) | 4.5 (0.5–29.8) | |
|
| |||
| P value | .28 | ||
Abbreviations: HPV, human papillomavirus; PR, prevalence ratio.
Analyses were restricted to individuals aged 14–69 years who responded to the audio, computer-assisted self-interview (n=4964).
Analyses were restricted to individuals aged 14–59 years who responded to the audio, computer-assisted self-interview (n=4191).
Lifetime number of any sex partners (vaginal/anal/oral sex combined) included the number of opposite-sex partners. Lifetime and recent number of oral or vaginal sex partners included the sum of same- and opposite-sex partners.
Factors Independently Associated With Oral HPV Infection
In multivariable analysis inclusive of individuals aged 14 to 69 years, factors independently associated with prevalent oral HPV included age, sex, lifetime number of sexual partners, and current smoking intensity (Table 3). Although adjustment for other factors dampened the first age-related peak in oral HPV prevalence, the bimodal age pattern remained statistically significant (P =.02) (Figure 2). Prevalence increased with number of lifetime sexual partners (P <.001 for trend) and number of cigarettes smoked per day (P = .001 for trend).
Table 3.
Adjusted Prevalence Ratios for Demographic and Behavioral Associations With Oral HPV Infection in the US Population Aged 14 to 69 Yearsa
| Characteristic | Total No. (No. With HPV Infection) | Adjusted PR (95% CI)
|
P Value for Interaction by Sexb | |||
|---|---|---|---|---|---|---|
| Overallb | Menc | Womend | ||||
| Sex | ||||||
| Male | 2483 (264) | 2.33 (1.66–3.26) | ||||
|
| ||||||
| Female | 2385 (88) | 1 [Reference] | ||||
|
| ||||||
| P value | <.001 | |||||
|
| ||||||
| Race/ethnicity | ||||||
| Mexican American | 1029 (62) | 1.16 (0.77–1.74) | 1.01 (0.68–1.51) | 1.48 (0.66–3.32) |
|
.23 |
|
| ||||||
| Other Hispanic | 549 (37) | 1.14 (0.76–1.72) | 0.99 (0.64–1.54) | 1.52 (0.67–3.48) | ||
|
| ||||||
| White, non-Hispanic | 2085 (144) | 1 [Reference] | 1 [Reference] | 1 [Reference] | ||
|
| ||||||
| Black, non-Hispanic | 950 (97) | 1.35 (0.91–2.02) | 1.42 (0.99–2.04) | 1.05 (0.42–2.60) | ||
|
| ||||||
| Other race | 255 (12) | 1.12 (0.48–2.62) | 1.28 (0.47–3.50) | 0.78 (0.15–3.97) | ||
|
| ||||||
| P value | .67 | .46 | .63 | |||
|
| ||||||
| Marital status | ||||||
| Never married | 1691 (97) | 1 [Reference] | 1 [Reference] | 1 [Reference] |
|
.01 |
|
| ||||||
| Married/living with partner | 2445 (175) | 0.74 (0.55–1.01) | 0.81 (0.56–1.16) | 0.44 (0.23–0.84) | ||
|
| ||||||
| Widowed/divorced/separated | 732 (80) | 0.84 (0.56–1.27) | 0.96 (0.61–1.52) | 0.42 (0.21–0.83) | ||
|
| ||||||
| P value | .12 | .26 | .03 | |||
|
| ||||||
| Cigarette usee | ||||||
| Never/former | 3728 (207) | 1 [Reference] | 1 [Reference] | 1 [Reference] |
|
.02 |
|
| ||||||
| Current, < 10 cigarettes/d | 718 (79) | 1.33 (0.95–1.87) | 1.45 (1.00–2.09) | 1.14 (0.54–2.42) | ||
|
| ||||||
| Current, 10–20 cigarettes/d | 338 (51) | 1.79 (1.14–2.81) | 1.29 (0.75–2.22) | 4.23 (2.27–7.88) | ||
|
| ||||||
| Current, > 20 cigarettes/d | 84 (15) | 2.09 (1.23–3.57) | 1.58 (0.93–2.66) | 5.92 (1.56–22.47) | ||
|
| ||||||
| P value (P value for trend)f | .01 (.001) | .12 (.03) | .002 (<.001) | |||
|
| ||||||
| No. of lifetime sex partners (any sex)g | ||||||
| 0 | 619 (10) | 1 [Reference] | 1 [Reference] | 1 [Reference] |
|
.30 (ordinal) .12 (categorical) |
|
| ||||||
| 1 | 686 (17) | 1.82 (0.63–5.31) | 2.37 (0.55–10.25) | 1.29 (0.35–4.70) | ||
|
| ||||||
| 2–5 | 1485 (77) | 3.23 (1.30–8.04) | 3.11 (1.05–9.19) | 3.17 (0.78–12.98) | ||
|
| ||||||
| 6–10 | 951 (78) | 5.92 (2.51–13.94) | 8.63 (2.91–25.63) | 2.80 (0.76–10.35) | ||
|
| ||||||
| 11–20 | 560 (58) | 5.42 (2.31–12.75) | 7.47 (2.56–21.75) | 2.43 (0.61–9.64) | ||
|
| ||||||
| ≥21 | 567 (112) | 10.65 (4.65–24.42) | 12.86 (4.16–39.71) | 7.62 (2.12–27.37) | ||
|
| ||||||
| P value (P value for trend)f | <.001 (<.001) | <.001 (<.001) | .09 (.04) | |||
Abbreviations: HPV, human papillomavirus; PR, prevalence ratio.
Analyses were conducted among 4964 participants aged 14–69 years who responded to the audio, computer-assisted self-interview.
Prevalence ratios and 95% CIs were estimated from binary logistic regression models with simultaneous adjustment for all variables in the table as well as age modeled as restricted cubic splines with 5 knots (age and 3 spline terms). P values for interaction of sex with each term were evaluated in separate models using product terms.
Models were restricted to male participants and included age modeled as restricted cubic splines with 5 knots (age and 3 spline terms), race, marital status, smoking, and lifetime number of any sex partners.
Models were restricted to female participants and included age (as a linear term), race, marital status, smoking, and lifetime number of any sex partners.
Ever use of cigarettes was defined for individuals aged 14–19 years as ever having tried cigarettes and for ages 20–69 years as lifetime use of ≥100 cigarettes. A current smoker was defined as someone who had smoked a cigarette in the prior 30 days. A former smoker was defined as an ever user who had not smoked a cigarette in the prior 30 days.
P values for trend across categories were calculated through the use of ordinal variables modeled with 1 df.
Lifetime number of any sex partners included the number of opposite-sex oral, anal, or vaginal sex partners.
The prevalence of oral HPV infection was significantly higher among men than women (adjusted PR, 2.33; 95% CI, 1.66–3.26), even after accounting for higher-risk behaviors reported by men (eTable 2 and eTable 3). Significant interactions were observed between sex and age (P = .05 for interaction), smoking (P =.02 for interaction), and marital status (P = .01 for interaction). Therefore, multivariable analyses were performed stratified by sex (Table 3). A significant bimodal distribution across age was observed for men but not women (Figure 3). The association of smoking with oral HPV prevalence was stronger among women than men. Additionally, oral HPV prevalence was significantly higher among never-married women but not among never-married men (Table 3).
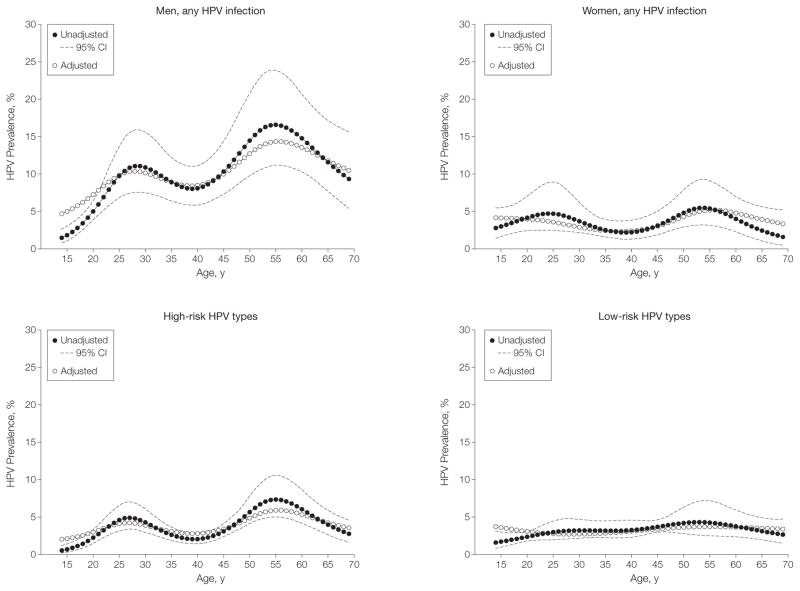
Figure 3. Modeled HPV Prevalence Across Age in the US Population Aged 14 to 69 Years by Sex and HPV Types.
Age was modeled using restricted cubic splines with 5 knots (age and 3 spline terms). To evaluate the influence of covariates on human papillomavirus (HPV) prevalence across age, models were adjusted for sex, race, smoking, marital status, and lifetime number of (any) sex partners, as appropriate. Given the standardization for covariates included in the multivariate models, the HPV prevalence curves obtained from the adjusted models are presented at the mean levels of the covariates (sex, race, smoking, marital status, and lifetime number of any sex partners, as appropriate) and aligned with the HPV prevalence curve in the unadjusted models to enable visual display of the data. For men, unadjusted P<.001; adjusted P=.002. For women, unadjusted P=.07; adjusted P=.14. For high-risk HPV, unadjusted P<.001; adjusted P<.001. For low-risk HPV, unadjusted P=.10; adjusted P=.64. P values less than .05 for spline terms denote statistical evidence for bimodality in the data.
In contrast to sex, race was not independently associated with oral HPV infection after accounting for differences in sexual behavior (Table 3). Although oral sexual behaviors were more common among white participants, number of vaginal sexual partners was higher among black participants than other races, as was unmarried status (eTable 4). Observed associations with alcohol and marijuana use were no longer significant after introduction of sexual behavior variables into multivariable models (eTable 5).
A multivariable analysis was performed restricted to individuals aged 14 to 59 years, for whom detailed sexual behavioral data were available. Strong associations were observed between lifetime as well as recent number of vaginal or oral sexual partners and oral HPV prevalence (Figure 4 and eFigure 2). Associations with sex, race, marital status, smoking, and oral HPV infection remained unchanged in these analyses. However, oral HPV prevalence increased in a linear relationship with age (with peak prevalence among 55- to 59-year-olds) and not in a bimodal pattern, perhaps reflecting the exclusion of 60- to 69-year-old individuals. This was despite the fact that several relevant sexual behaviors either declined with age (number of recent partners for any sex) or increased with age (time since performing oral sex on a new partner and age at oral sexual debut) (eFigure 3).
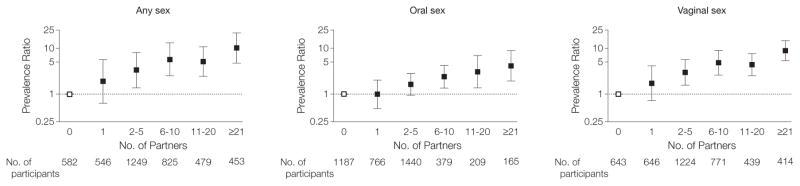
Figure 4. Association of Number of Lifetime Sexual Partners With Prevalent Oral HPV Infection in the US Population Aged 14 to 59 Years.
Human papillomavirus (HPV) prevalence ratio was adjusted for age (as a linear term), sex, race/ethnicity, marital status, and cigarette use. The analysis was restricted to individuals aged 14 to 59 years, for whom detailed sexual behavior data were available. Given high colinearity across sexual behaviors, each behavior was evaluated in separate models. P<.001 for trend in all 3 categories of sexual partner. Lifetime number of partners for performing oral sex included the sum of same- and opposite-sex partners; the reference category was 0 lifetime partners. Error bars indicate 95% CIs.
Predictors of High-Risk and Low-Risk Infections
When multivariable analysis was performed separately for an outcome of high-risk or low-risk HPV infections, associations with age and race differed: a bimodal pattern across age was observed only for high-risk HPV infections (Figure 3) and the higher prevalence among black vs white participants was restricted to low-risk infections. Additionally, associations with lifetime number of any sex partners appeared stronger for high-risk than low-risk HPV infections (eTable 6).
COMMENT
The prevalence of oral HPV infection among men and women aged 14 to 69 years in the United States is approximately 7%, substantially lower than the reported prevalence of genital HPV infection.17,18 Infection with HPV-16 was detected in 1% of men and women, corresponding to an estimated 2.13 million infected individuals in the United States. We identified sexual behavior and current smoking, including intensity, to be potentially modifiable risk factors for oral HPV infection. Notably, prevalence was as high as 20% among those with more than 20 lifetime sexual partners or among current smokers of more than 20 cigarettes per day. Prevalence of HPV had a striking bimodal pattern with age among men and was significantly higher among men than women, consistent with higher rates of HPV-positive OSCC among individuals aged 50 to 64 years and among men.
Our data provide evidence that oral HPV infection is predominantly sexually transmitted. Infection was uncommon among sexually inexperienced individuals, was 8-fold higher among sexually experienced individuals, and increased significantly with number of sexual partners. Taken together, these data indicate that transmission by casual, nonsexual contact is likely to be unusual. Although risk of HPV-positive OSCC has previously been associated with oral sexual behaviors,2,19,20 the colinearity of sexual behaviors precluded associating infection with any particular behavior. Oral HPV infection was more common among sexually experienced individuals who did not report performing oral sex than among sexually inexperienced individuals, consistent with transmission by other sexually associated contact (eg, deep kissing). The NHANES does not collect data relevant to nonsexual means of transmission.
Oral HPV infection has a bimodal distribution with age, with peak prevalence among individuals aged 55 to 64 years. A similar, albeit lower, peak in cervical HPV prevalence among older age groups is also observed in some populations and remains unexplained.21,22 The second peak in oral HPV prevalence we observed was not entirely explained by participants’ sexual behaviors or other factors and could have arisen from a combination of increased incidence, reactivation of latent infections due to age-related loss of immunity,23 differences in sexual behaviors across birth cohorts,7 or increased persistence among older individuals.24 The bimodal age pattern was evident primarily for high-risk but not low-risk infections, suggesting an association of age with oral HPV persistence (given higher rates of persistence for high-risk infections). Prior studies, however, demonstrate that incident cervical HPV infections have similar persistence rates regardless of age.25 Nonetheless, given the absence of screening and treatment for high-risk oral HPV infections, the cumulative prevalence of chronic, persistent infections would be expected to increase with age.
To our knowledge, this is the first population-based study to concurrently examine the epidemiology of HPV infection among men and women. The prevalence of oral HPV infection overall was approximately 3-fold higher in men than women, and the prevalence of HPV-16 was more than 5-fold higher, a difference that likely explains the higher incidence of HPV-positive OSCC among men. The age-specific differences in HPV prevalence between men (bimodal) and women (unrelated with age) could also explain the predominant increase in incidence of HPV-positive OSCC over the last 3 decades among men in the United States,6,26 assuming birth cohort effects explain a large component of the bimodal age pattern. Should this be the case, a plateau in incidence rates might be anticipated within a decade or more.
Although men had a higher number of sexual partners than women, only approximately 16% of the difference in prevalence between sexes could be explained by sexual behavior and other covariates. One explanation for higher prevalence among men could be higher probability of HPV transmission through oral sex on women vs men.27 Indeed, oral HPV prevalence increased more sharply with number of sexual partners for men than women. Hormonal differences between sexes could affect the duration of oral HPV infection, given hormonal influences on cervical HPV natural history (eg, contraceptive use).28 Higher seroconversion rates among women in response to genital infection29 could also, in theory, confer greater protection against subsequent oral infection.30
Current smoking (and intensity) was independently associated with oral HPV infection. Interestingly, the effect was stronger among women than men. Current smoking is associated with a higher prevalence, viral load, and risk of progression to cervical precancer among women infected with HPV,28 consistent with immunosuppressive effects of smoking.31 Smoking has been associated with lower rates of seroconversion in response to cervical HPV infection,32 albeit inconsistently.33 Assuming humoral immunity is important for protection against oral HPV, smoking may work against the benefits of enhanced seroconversion rates among women in comparison with men. Whether smoking does or does not increase risk for HPV-positive OSCC is a matter of ongoing debate, as published studies have had contradictory conclusions.2,34,35 Our data indicate that smoking may be a modifiable risk factor for HPV-positive OSCC in addition to HPV-negative OSCC, in the former case, perhaps as a mediating factor by promoting persistence of oral HPV infection in addition to possible mutagenic effects.
This study has several potential limitations. By restricting our analysis to the Alphapapillomaviruses, we have underestimated the true prevalence of oral HPV infection.36 However, only the Alphapapillomavirus genera are established as human carcinogens. Sexual behaviors are difficult to measure, and therefore, we cannot exclude residual confounding from selective misreporting as an explanation for observed differences in oral HPV prevalence across age and sex. Given the cross-sectional nature of this study, observed associations cannot be interpreted as temporally linked with infection. Additionally, the low prevalence of HPV infection precluded detailed analysis of some outcomes, for instance, factors associated with HPV-16 infection. Additional data collected through NHANES 2013–2014 may allow such analyses.
Our results have important research as well as public health implications. Natural history studies of cervical HPV infection were essential for the development of public health interventions, such as HPV vaccination to prevent and HPV detection to screen for cervical cancer. The precise reasons for a bimodal age pattern and higher oral HPV prevalence among men are largely unknown and difficult to discern in a cross-sectional study, and explanations discussed herein are admittedly speculative. Natural history studies of oral HPV infection are therefore necessary to understand the effects of age, sex, and modifiable risk factors (eg, smoking and sexual behavior) on the incidence and duration of oral HPV infection.
The oral HPV prevalence across age and sex observed in this study appears consistent with differences in incidence of HPV-positive OSCC across these subgroups, raising the question as to whether targeted secondary prevention strategies are feasible.
The US Centers for Disease Control and Prevention currently recommends routine HPV vaccination for females aged 9 to 26 years and males aged 9 to 21 years for the prevention of genital warts and anogenital cancers based on demonstrated efficacy in randomized clinical trials.37–40 By contrast, vaccine efficacy against oral HPV infection is unknown, and therefore vaccination cannot currently be recommended for the primary prevention of oropharyngeal cancer. Given an analysis of US cancer registry data recently projected that the number of HPV-positive oropharyngeal cancers diagnosed each year will surpass that of invasive cervical cancers by the year 2020,6 perhaps such vaccine trials are warranted. Such trials could inform ongoing discussions regarding the benefits of HPV vaccination for males, given the higher prevalence of oral HPV infection demonstrated here as well as higher incidence of HPV-positive OSCC among men.
Acknowledgments
Funding/Support: This study was supported by the Ohio State University Comprehensive Cancer Center, Merck, John and Nina Cassils, and the Intramural Research Program of the National Cancer Institute.
Footnotes
Role of the Sponsors: Industry and philanthropic sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript. Several authors are employees of Ohio State University or the National Cancer Institute.
Online-Only Material: The 6 eTables and 3 eFigures are available at http://www.jama.com.
Additional Contributions: We thank Brenda G. Lewis, MPH, National Center for Health Statistics (NCHS), and other staff members of NHANES/NCHS for their assistance in accomplishing the aims of the Oral HPV Protocol. None received compensation for the contributions besides salary from the federal government.
Author Contributions: Drs Gillison and Chaturvedi had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Gillison.
Acquisition of data: Gillison, Broutian, Pickard, Tong, Xiao.
Analysis and interpretation of data: Gillison, Broutian, Pickard, Tong, Xiao, Kahle, Graubard, Chaturvedi.
Drafting of the manuscript: Gillison, Broutian, Pickard, Tong, Xiao, Kahle, Chaturvedi.
Critical revision of the manuscript for important intellectual content: Gillison, Graubard, Chaturvedi.
Statistical analysis: Gillison, Pickard, Kahle, Graubard, Chaturvedi.
Obtaining funding: Gillison, Chaturvedi.
Administrative, technical or material support: Gillison, Broutian, Xiao.
Study supervision: Gillison.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Gillison is the principal investigator of the unrestricted grant from Merck in support of this study and has been a consultant to Merck and GlaxoSmithKline. No other disclosures were reported.
References
- 1.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92(9):709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 2.Gillison ML, D’Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100(6):407–420. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 3.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14(2):467–475. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 4.Näsman A, Attner P, Hammarstedt L, et al. Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: an epidemic of viral-induced carcinoma? Int J Cancer. 2009;125(2):362–366. doi: 10.1002/ijc.24339. [DOI] [PubMed] [Google Scholar]
- 5.Hong AM, Grulich AE, Jones D, et al. Squamous cell carcinoma of the oropharynx in Australian males induced by human papillomavirus vaccine targets. Vaccine. 2010;28(19):3269–3272. doi: 10.1016/j.vaccine.2010.02.098. [DOI] [PubMed] [Google Scholar]
- 6.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finer LB. Trends in premarital sex in the United States, 1954–2003. Public Health Rep. 2007;122(1):73–78. doi: 10.1177/003335490712200110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Health and Nutrition Examination Survey. Centers for Disease Control and Prevention; [Accessed January 11, 2012]. http://www.cdc.gov/nchs/nhanes.htm. [Google Scholar]
- 9.2009–2010 Data documentation, codebook, and frequencies. [Accessed January 11, 2012];National Health and Nutrition Examination Survey. http://www.cdc.gov/nchs/nhanes/nhanes2009-2010/ORHPV_F.htm.
- 10.Broutian TR, He X, Gillison ML. Automated high throughput DNA isolation for detection of human papillomavirus in oral rinse samples. J Clin Virol. 2011;50(4):270–275. doi: 10.1016/j.jcv.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gravitt PE, Peyton CL, Alessi TQ, et al. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000;38(1):357–361. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muñoz N, Bosch FX, de Sanjosé S, et al. International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348(6):518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 13.Marks M, Gupta SB, Liaw KL, et al. Confirmation and quantitation of human papillomavirus type 52 by Roche Linear Array using HPV52-specific Taq-Man E6/E7 quantitative real-time PCR. J Virol Methods. 2009;156(1–2):152–156. doi: 10.1016/j.jviromet.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Korn E, Graubard B. Confidence intervals for proportions with small expected number of positive counts estimated from survey data. Surv Methodol. 1998;24(2):193–201. [Google Scholar]
- 15.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 16.SUDAAN Language Manual, Release 10.0. Research Triangle Park, NC: Research Triangle Institute; 2008. [Google Scholar]
- 17.Hariri S, Unger ER, Sternberg M, et al. Prevalence of genital human papillomavirus among females in the United States, the National Health and Nutrition Examination Survey, 2003–2006. J Infect Dis. 2011;204(4):566–573. doi: 10.1093/infdis/jir341. [DOI] [PubMed] [Google Scholar]
- 18.Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007;297(8):813–819. doi: 10.1001/jama.297.8.813. [DOI] [PubMed] [Google Scholar]
- 19.D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356(19):1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz SM, Daling JR, Doody DR, et al. Oral cancer risk in relation to sexual history and evidence of human papillomavirus infection. J Natl Cancer Inst. 1998;90(21):1626–1636. doi: 10.1093/jnci/90.21.1626. [DOI] [PubMed] [Google Scholar]
- 21.Gravitt PE. The known unknowns of HPV natural history. J Clin Invest. 2011;121(12):4593–4599. doi: 10.1172/JCI57149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith JS, Melendy A, Rana RK, Pimenta JM. Age-specific prevalence of infection with human papillomavirus in females: a global review. J Adolesc Health. 2008;43(4) suppl:S5–S25. doi: 10.1016/j.jadohealth.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 23.García-Piñeres AJ, Hildesheim A, Herrero R, et al. Persistent human papillomavirus infection is associated with a generalized decrease in immune responsiveness in older women. Cancer Res. 2006;66(22):11070–11076. doi: 10.1158/0008-5472.CAN-06-2034. [DOI] [PubMed] [Google Scholar]
- 24.Castle PE, Schiffman M, Herrero R, et al. A prospective study of age trends in cervical human papillomavirus acquisition and persistence in Guanacaste, Costa Rica. J Infect Dis. 2005;191(11):1808–1816. doi: 10.1086/428779. [DOI] [PubMed] [Google Scholar]
- 25.Rodríguez AC, Schiffman M, Herrero R, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst. 2010;102(5):315–324. doi: 10.1093/jnci/djq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaturvedi A, Engels E, Anderson W, Gillison ML. Incidence trends for human papillomavirus-related and unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26(4):612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 27.Hernandez BY, Wilkens LR, Zhu X, et al. Transmission of human papillomavirus in heterosexual couples. Emerg Infect Dis. 2008;14(6):888–894. doi: 10.3201/eid1406.070616.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castellsagué X, Muñoz N. Chapter 3: cofactors in human papillomavirus carcinogenesis: role of parity, oral contraceptives, and tobacco smoking. J Natl Cancer Inst Monogr. 2003;(31):20–28. [PubMed] [Google Scholar]
- 29.Markowitz LE, Sternberg M, Dunne EF, McQuillan G, Unger ER. Seroprevalence of human papillomavirus types 6, 11, 16, and 18 in the United States: National Health and Nutrition Examination Survey 2003–2004. J Infect Dis. 2009;200(7):1059–1067. doi: 10.1086/604729. [DOI] [PubMed] [Google Scholar]
- 30.Safaeian M, Porras C, Schiffman M, et al. Costa Rican Vaccine Trial Group. Epidemiological study of anti-HPV16/18 seropositivity and subsequent risk of HPV16 and -18 infections. J Natl Cancer Inst. 2010;102(21):1653–1662. doi: 10.1093/jnci/djq384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnson Y, Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun. 2010;34(3):J258–J265. doi: 10.1016/j.jaut.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Wiley DJ, Wiesmeier E, Masongsong E, et al. Proof of Principle Study Investigative Group. Smokers at higher risk for undetected antibody for oncogenic human papillomavirus type 16 infection. Cancer Epidemiol Biomarkers Prev. 2006;15(5):915–920. doi: 10.1158/1055-9965.EPI-05-0963. [DOI] [PubMed] [Google Scholar]
- 33.Wang SS, Schiffman M, Herrero R, et al. Determinants of human papillomavirus 16 serological conversion and persistence in a population-based cohort of 10 000 women in Costa Rica. Br J Cancer. 2004;91(7):1269–1274. doi: 10.1038/sj.bjc.6602088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith EM, Rubenstein LM, Haugen TH, Hamsikova E, Turek LP. Tobacco and alcohol use increases the risk of both HPV-associated and HPV-independent head and neck cancers. Cancer Causes Control. 2010;21(9):1369–1378. doi: 10.1007/s10552-010-9564-z. [DOI] [PubMed] [Google Scholar]
- 35.Applebaum KM, Furniss CS, Zeka A, et al. Lack of association of alcohol and tobacco with HPV16-associated head and neck cancer. J Natl Cancer Inst. 2007;99(23):1801–1810. doi: 10.1093/jnci/djm233. [DOI] [PubMed] [Google Scholar]
- 36.Bottalico D, Chen Z, Dunne A, et al. The oral cavity contains abundant known and novel human papillomaviruses from the Betapapillomavirus and Gammapapillomavirus genera. J Infect Dis. 2011;204(5):787–792. doi: 10.1093/infdis/jir383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paavonen J, Naud P, Salmerón J, et al. HPV PATRICIA Study Group. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374(9686):301–314. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 38.Garland SM, Hernandez-Avila M, Wheeler CM, et al. Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928–1943. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 39.Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N Engl J Med. 2011;364(5):401–411. doi: 10.1056/NEJMoa0909537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palefsky JM, Giuliano AR, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365(17):1576–1585. doi: 10.1056/NEJMoa1010971. [DOI] [PubMed] [Google Scholar]