Abstract
HPV vaccination is recommended for all female survivors of childhood cancer; yet, it is underutilized. Parent-child sexual communication and healthcare provider recommendation for HPV vaccination influence familial vaccination decisions. However, caregivers may be less likely to discuss sexual health issues with survivors as compared to healthy peers. Therefore, this study compared mothers of daughters with/without history of childhood cancer on measures of sexual communication, HPV-specific communication, and healthcare provider recommendation for HPV vaccination, and examined the effects of sociodemographic and medical factors on these measures. There were no differences between mothers of survivors/non-cancer survivors on the outcomes (ps > .05). Among all mothers, daughter’s age was associated with sexual communication (ps < .05). Household income and daughter’s age were associated with healthcare provider recommendation for vaccination (ps < .05). Among mothers of survivors, daughter’s age at diagnosis was associated with sexual communication, HPV-specific communication, and healthcare provider recommendation for vaccination (ps < .05). Findings have implications for the role of healthcare providers as advocates for mother-daughter sexual communication and HPV vaccination, especially among survivors of childhood cancer.
Keywords: HPV vaccination, cancer survivorship, mother-daughter sexual communication, provider recommendation
Introduction
Human papillomavirus (HPV) is the most common sexually transmitted infection (Centers for Disease Control and Prevention [CDC], 2011). Epidemiological data suggest that up to 80% of sexually active women will contract HPV in their lifetime, with prevalence rates ranging from 40% (among 14-19 year-olds) to 49% (among 20-24 year-olds; Dunne et al., 2007). HPV infection increases following the median age of first sexual activity (16.9 years for U.S. females; Wulf, 2002). As such, women engaging in sex at younger ages may have more opportunities for risk of HPV exposure. This is concerning, as data from the 2013 United States Youth Risk Behavior Surveillance (YRBS), a national school-based survey measuring risky behavior in adolescents, found that 48% of high school students reported a history of sexual intercourse (Kann et al., 2014). Research also suggests that 60% of female adolescents will be sexually active by age 18 (Cox, Faslino, & Tavakoli, 2008). Therefore, a significant proportion of adolescent females are at risk for contracting HPV.
HPV is problematic for women due to its causal role in cervical, vaginal, vulvar, and oral cancers (CDC, 2010). Cervical cancer is the second most common cancer among women worldwide (World Health Organization [WHO], 2013) and is particularly problematic in immunocompromised populations, including women surviving childhood cancer (Hagensee, Cameron, Leigh, & Clark, 2004; Klosky et al., 2012). Considering that one in 10 survivors of childhood cancer develop a second malignant neoplasm by young adulthood (Armstrong et al., 2011), efforts to protect this group from preventable HPV-associated malignancies and related complications becomes a priority.
Fortunately, vaccines protecting against specific types of HPV associated with cervical and other HPV-related cancers are currently available, safe, and clinically effective (CDC, 2010). The Advisory Committee on Immunization Practices (ACIP) recommends that all adolescent girls be vaccinated against HPV between the ages of 11 and 12 years, and the vaccines are approved for females between the ages of 9 and 26 years (CDC, 2010). Given the increased risk of HPV-related complications among survivors, Version 3.0 of the Children’s Oncology Group Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent and Young Adult Cancer recommends that all eligible females receive the HPV vaccine (Children’s Oncology Group [COG], 2008). However, vaccination rates among survivors are unacceptably low (Hoffman et al., 2012; Klosky et al., 2013).
Poor vaccination rates in the survivorship population may be influenced by several factors. Studies have identified correlates of parental intentionality toward vaccination and/or decisions to vaccine their daughters, including parental communication regarding sexual behavior, parental communication about the HPV vaccination, and healthcare provider’s recommendation for the HPV vaccination (Allen et al., 2010; Brabin, Roberts, Farzaneh, & Kitchener, 2006; Gamble, Klosky, Parra, & Randolph, 2010; Peasant, Foster, Gamble, Rao, & Klosky, 2012). However, research has suggested that parents and healthcare providers may not adequately communicate sexual health concerns such as HPV transmission with survivors (Murphy, Klosky, Termuhlem, Sawczyn, & Quinn, 2012). Parents may avoid discussing sexual topics with survivors because they believe that their children are unable to understand issues surrounding sex and sexuality or because they believe that their children are unlikely to engage in sexual behavior (Murphy et al., 2012; Klosky et al., 2013). Providers may fail to recommend the vaccine for these survivors because they do not anticipate that a survivor will engage in sexual activity or because they believe that the sexual development of the survivor is delayed (Murphy et al., 2012). However, failure by oncology and other medical teams to recommend the vaccine to parents is problematic, as parents often make vaccine decisions for their children based on providers’ recommendations (Gamble et al., 2010). If HPV vaccination is not being discussed in the survivorship context, children and adolescents may not be vaccinated.
Given the influence of parental sexual communication and provider recommendation on HPV vaccination, it is imperative to understand how discussions about sexual health concerns may differ between caregivers of survivors and non-cancer survivors. It is also essential to understand how, and if, medical teams recommend the HPV vaccine to childhood cancer survivors, who have am increased risk secondary malignancies, including HPV-associated malignancies (Henderson et al., 2007; Meadows et al., 2009) . Therefore the purpose of this study was twofold. Aim 1 of this study was to compare mothers of daughters with/without a history of childhood cancer on measures of sexual communication, HPV-specific communication, and reports of healthcare provider recommendation. Aim 2 was to examine how measures of sexual communication, HPV-specific communication, and healthcare provider recommendation differ as a function of sociodemographic and cancer-specific characteristics of mothers, their daughters, and survivors of childhood cancer. By understanding the differences in communication patterns and identifying factors contributing to sexual communication and HPV vaccine recommendation, providers can develop and implement targeted strategies to foster parent-child sexual health discussions and increase recommendation for vaccination in order to increase vaccination rates within the survivorship population.
Methods
Participants
Maternal primary caregivers with daughters surviving childhood cancer were recruited from the After Completion of Treatment (ACT) clinic at St. Jude Children’s Research Hospital. The ACT Clinic is an outpatient survivorship resource providing follow-up care for survivors of childhood cancer who are more than five years post diagnosis and at least two years disease free. Caregivers were eligible to participate if they were: 1) the mother/female primary caregiver of a female ACT patient aged 9-17 years, 2) proficient in reading/writing English, and 3) cognitively able to understand/complete the questionnaires.
Control participants were acquaintances of ACT families. Mothers with daughters aged 9-17 years were referred for study participation by participants from the ACT clinic. This acquaintance control technique allowed for better restriction of possible demographic differences among ACT and control families (e.g., daughter’s age, race, maternal education), with the distinguishing feature among participants being daughter’s history/no history of childhood cancer. Control participants were eligible if they met criteria 1-3 as listed in the preceding paragraph and were: 1) referred by a participating survivor family and 2) had no children or relatives with a history of childhood cancer.
Of the 392 mothers who were eligible, 305 mothers consented to participate in the study (see Figure 1). Seventy- seven percent (n = 235) were mothers of survivors of childhood cancer, and 23.0% (n = 70) were mothers of daughters without a history of cancer. Among mothers of survivors, 75 (32.6%) had initiated HPV vaccination for their daughters, whereas 155 (67.4%) had not. Twenty-four (34.3%) mothers of daughters without a history of childhood cancer had initiated HPV vaccination for their daughters, whereas 46 (65.7%) had not done so. Eighty-nine (37.9%), 45 (19.1%), and 101 (43.0%) mothers of survivors reported that their daughter had a history leukemia or lymphoma, brain or central nervous system tumor, or solid tumor, respectively; all survivors were less than 12 years of age at diagnosis. A full description of the demographic and medical factors is presented in Table 1.
Figure 1.
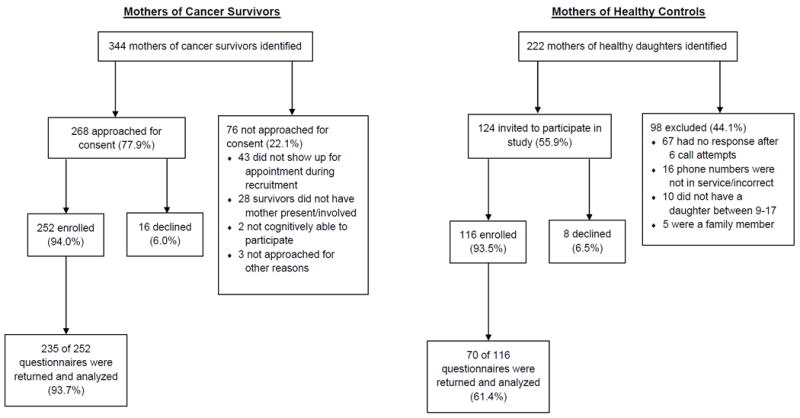
Flowchart depicting recruitment and questionnaire completion for mothers of cancer survivors and mothers of healthy controls.
Table 1.
Demographic and Medical Characteristics of Cancer Survivor Group, Control Group, and Combined Sample
| Cancer Survivors | Controls | Combined | |
|---|---|---|---|
| n = 235 | n = 70 | n = 305 | |
| Freq (%) | Freq (%) | Freq (%) | |
| Race/Ethnicity of Maternal Caregiver | |||
| White | 177 (75.30) | 54 (77.14) | 231 (75.74) |
| Non-White | 58 (24.70) | 16 (22.86) | 74 (24.26) |
| Marital Status of Maternal Caregiver | |||
| Married | 163 (69.36) | 54 (77.14) | 217 (71.15) |
| Divorced/Separated/Widowed | 39 (16.60) | 12 (17.14) | 51 (16.72) |
| Other | 29 (12.34) | 4 (5.71) | 33 (10.82) |
| Missing | 4 (1.70) | 0 (0.00) | 4 (1.31) |
| Education Level of Maternal Caregiver | |||
| Less than College Degree | 154 (65.53) | 44 (62.86) | 198 (64.92) |
| College Degree or more | 73 (31.06) | 26 (37.14) | 99 (32.46) |
| Missing | 8 (3.40) | 0 (0.00) | 8 (2.62) |
| Household Income | |||
| Less than $20,000 | 38 (16.17) | 5 (7.14) | 43 (14.1) |
| $20,000 to $59,999 | 75 (31.91) | 24 (34.29) | 99 (32.46) |
| $60,000 and above | 109 (46.38) | 36 (51.43) | 145 (47.54) |
| Missing | 13 (5.53) | 5 (7.14) | 18 (5.90) |
| Age of Maternal Caregiver (in Years) | |||
| 18-40 | 108 (45.96) | 31 (44.29) | 139 (45.57) |
| 41-62 | 125 (53.19) | 39 (55.71) | 164 (53.77) |
| Missing | 2 (0.85) | 0 (0.00) | 2 (0.66) |
| Age of Daughter (in Years) | |||
| 9-13 | 117 (49.79) | 38 (54.29) | 155 (50.82) |
| 14-17 | 118 (50.21) | 32 (45.71) | 150 (49.18) |
| Cancer Diagnosis of Daughter | |||
| Leukemia/Lymphoma | 89 (37.87) | -- | -- |
| Brain/CNS Tumors | 45 (19.15) | -- | -- |
| Solid Tumors | 101 (42.98) | -- | -- |
| Age at Diagnosis (in Years) | |||
| 0-4.9 | 178 (58.40) | -- | -- |
| 5.0-11.9 | 57 (18.7) | -- | -- |
Procedure
Mothers were recruited during their daughter’s scheduled ACT clinic visit. Study aims were explained by a member of the research team, verbal informed consent was obtained, and questionnaires were completed. Afterword, families were thanked, debriefed, and provided with an information sheet on HPV and vaccination. For mothers who did not return questionnaires during the clinic visit, additional surveys were mailed to their home. The acquaintance control participants were identified by mothers of cancer survivors, and their contact information was provided to the research team. Potential participants were contacted via telephone. If a potential participant could not be reached, was a family member of the referring participant, or declined to participate, research staff discontinued contact with the potential participant and did not follow up with the referral source for another acquaintance control. Following consent, the acquaintance participants were mailed (or emailed a link) the same questionnaires that were completed by the mothers of the survivors. Paper and pencil questionnaires were returned in a preaddressed, stamped envelope. See Figure 1 for a detailed CONSORT diagram.
Measures
Demographic and Medical Factors
Demographic and medical information was collected from mothers. Demographic information collected included race/ethnicity (of caregiver and daughter), maternal age, marital status, annual income, educational level of caregiver, age of daughter, and religious affiliation. Additional cancer-related information was obtained from mothers of survivors and from survivors’ medical records. This information included type of cancer diagnosis, time from diagnosis, age at diagnosis, and intensity of treatment (Kazak et al., 2012; Werba, Hobbie, Kazak, Ittenbach, Reily, & Meadows, 2007). Based on medical information extracted from each survivors’ medical chart, intensity of treatment ratings were calculated for each survivor according to her treatment modality (i.e., surgery, chemotherapy, radiation) and the stage of disease progression.
Mother–Adolescent Sexual Communication
The Mother-Adolescent Sexual Communication Questionnaire (MASC; Cox et al., 2008) is an 18-item measure used to assess maternal adolescent communication related to sexual behavior and development. The MASC is comprised of 4 subscales: 1) Content, 2) Context, 3) Timing, and 4) Style. Content describes the extent to which different sexual topics (e.g., pregnancy, sexually transmitted infections) were discussed between mothers and daughters. Context describes mothers’ beliefs that sexual communication should occur within the context of physical and emotional maturation (e.g., menstruation, emotional readiness). Timing describes the extent to which mothers took advantage of opportunities for sexual communication (e.g., when mothers think that daughters may be sexually active). Style describes the extent to which mothers took a more authoritative approach when discussing sex and sexuality (e.g., mothers using their sexual experiences during conversations about sex and sexuality, allowing daughters to talk freely about sex and sexuality) with their daughters. Participants responded on a 5-point Likert scale, from 1 = Strongly Disagree to 5 = Strongly Agree. The reliability coefficients of the content, context, timing, and style subscales were .90, .27, .70, and .59, respectively.
HPV-Specific Communication
HPV-specific communication is the extent to which mothers provided detailed information about the HPV vaccine and its purpose to their daughters. A three-item scale was constructed to assess the content of HPV-specific communication. An example item was, “Regarding the HPV vaccine, I have told/will tell my daughter it is a shot to keep girls healthy.” Responses were provided on a 4-point Likert-type scale from 1 = Untrue to 4 = True. The Cronbach alpha coefficient for this measure was 0.52.
HPV Recommendation
Healthcare provider recommendation refers to whether or not mothers received a recommendation from their daughter’s healthcare provider for the HPV vaccine. Healthcare provider recommendation was assessed with a single yes/no item: “Has your daughter’s doctor ever recommended that she receive the HPV vaccine?” Of note, this item asked about a recommendation from a doctor. However, mothers also interacted with nurse practitioners and other healthcare providers and it is likely that mothers also reported whether their daughters had received a recommendation for vaccination from any of their healthcare providers.
Analytic Approach
Descriptive statistics were conducted to examine demographic and cancer-related characteristics of the sample. Comparisons were made between mothers of daughters with/without a history of cancer on measures of maternal-adolescent sexual communication and HPV-specific communication using multivariate analysis of variance (MANOVA). A univariate chi-square test was conducted to examine group differences as a function of healthcare provider recommendation for/endorsement of the HPV vaccine.
MANOVA was utilized to explore demographic differences in sexual and HPV-specific communication. Univariate chi-square tests were used to examine the demographic differences in healthcare provider recommendation for the HPV vaccine. Variables with p-values less than .10 in the univariate analysis were included in subsequent multivariate logistic regression models.
Among mothers of survivors, differences in sexual communication and HPV-specific communication as a function of cancer-related factors were assessed using MANOVA. Univariate chi-square tests were conducted to examine differences in healthcare provider recommendation for the HPV vaccine by cancer-related factors. Factors with a p-value less than .10 were included in the multivariate logistic regression model.
Results
Differences between Mothers of Daughters with/without a History of Cancer
Differences between mothers of daughters with/without a history of cancer were examined on measures of mother-adolescent sexual communication, HPV-specific communication, and healthcare provider recommendation. The overall model was not significant for any of the four measures of sexual communication, HPV-specific communication, or healthcare provider recommendation, F(5, 257) = 1.68, p =.153.
Because no significant differences were identified, mothers of daughters with/without a history of childhood cancer were combined into a single group for subsequent analyses examining sexual communication, HPV-specific communication, and healthcare provider recommendation as a function of demographic characteristics. However, only mothers of cancer survivors were included in analyses accessing cancer-related differences in sexual communication, HPV-specific communication, and healthcare provider recommendation.
Multivariate Models of Sexual and HPV Communication and Healthcare Provider Recommendation
Demographic differences among all Mothers
Demographic differences in sexual communication, HPV-specific communication, and healthcare provider recommendation were assessed among all mothers. There was a significant overall effect for daughter’s age, F(5, 231) = 6.54, p <.001, and maternal education level, F (5, 231) = 2.31, p =.045. Those with older daughters (14-17 years) had higher MASC content subscale scores than those with younger daughters (9-13 years; F(1, 235) = 20.87, p <.001). Mothers with older daughters also reported a higher style subscale, F(1, 235) = 6.13, p =.014, and timing subscale scores, F(1, 235) = 12.56, p <.001, than mothers with younger daughters (Table 2). There were no demographic differences in HPV-specific communication scale scores. Mothers with daughters between 14 -17 years of age were over 3 times more likely to report a healthcare provider recommendation to vaccinate their daughters (OR = 3.37, 95% CI: 1.98–5.74, p <.001) than mothers with daughters between 9-13 years of age. Mothers reporting an income between $20,000- $59, 999 were less likely to report a healthcare provider recommendation for the HPV vaccination (OR =.49, 95% CI: .28-.86, p =.014) than those who reported an annual income of $60,000 or more (Table 3).
Table 2.
Daughter’s age at interview (all mothers) and daughter’s age at diagnosis (mothers of cancer survivors) as predictors of communication.
| Daughter’s Age
|
Daughter’s Age at Diagnosis
|
|||||
|---|---|---|---|---|---|---|
| 9-13 years | 14-17 years | 0-4.9 years | 5.0-11.9 years | |||
| n = 155 | n = 150 | n = 177 | n = 58 | |||
| Mean (SD) | Mean (SD) | p | Mean (SD) | Mean (SD) | p | |
| Sexual Communication | ||||||
| Content | 29.13 (7.30) | 33.08 (5.69) | <.001 | 30.94 (5.83) | 33.20 (5.84) | .003 |
| Context | 9.91 (2.09) | 9.58 (2.27) | .282 | 9.76 (2.02) | 10.09 (2.29) | .049 |
| Timing | 13.47 (3.66) | 14.86 (3.27) | <.001 | 14.12 (3.18) | 14.92 (3.16) | .046 |
| Style | 11.08 (2.56) | 11.87 (2.06) | .014 | 11.54 (2.14) | 11.94 (1.91) | .162 |
| HPV-Specific Communication | 13.79 (2.24) | 13.43 (2.42) | .452 | 13.33 (2.32) | 14.10 (2.21) | .029 |
Table 3.
Daughter’s age at interview and household annual income (all mothers) and daughter’s age at diagnosis (mothers of cancer survivors) as predictors of physician recommendation.
| Physician Recommendation | |||||
|---|---|---|---|---|---|
| Yes | No | ||||
| Freq (%) | Freq (%) | OR | 95% CI | p | |
| Daughter’s Age | |||||
| 9-13 years | 54 (36.7) | 93 (63.3) | 1.00 | ||
| 14-17 years | 88 (63.3) | 51 (36.7) | 3.37 | 1.98 – 5.74 | <.001 |
| Annual Household Income | |||||
| $60,000 and above | 74 (54.4) | 62 (45.6) | 1.00 | ||
| $20,000 to $59,999 | 39 (40.2) | 58 (59.8) | .49 | .280 - .864 | .014 |
| Daughter’s Age at Diagnosis | |||||
| 0-4.9 years | 68 (41.2) | 97 (58.8) | 1.00 | ||
| 5.0-11.9 years | 14 (26.9) | 38 (73.1) | 4.21 | 2.09 – 8.50 | <.001 |
Medical differences among mothers of cancer survivors
Among mothers of survivors, there were significant differences in sexual and HPV-related communication between mothers whose daughter were diagnosed between ages 0-4.9 and 5.0-11.9, F(5, 187) = 2.93, p =.014. Mothers of survivors diagnosed between ages 5.0-11.9 reported higher content scores than those daughters diagnosed between ages 0-4.9, F(1, 191) = 9.16, p =.003. There were similar findings for context (F(1, 191) = 3.94, p =.049) and timing (F(1, 191) = 4.04, p =.046) subscale scores. Mothers with daughters who were older at diagnosis reported providing more detailed information about the HPV vaccine to their daughters compared to those whose daughters were younger at diagnosis, F(1, 191) = 4.83, p =.029 (Table 2). Mothers whose daughters were older at diagnosis were four times more likely to receive a healthcare provider recommendation to vaccinate than patients diagnosed at a younger age (OR = 4.21, 95% CI: 2.09–8.50, p <.001; Table 3).
Discussion
Parental sexual and HPV-specific communication, along with healthcare provider recommendation, have been implicated in parental decision-making to vaccinate their daughters (Gamble et al., 2010; Peasant et al., 2012). However, research has suggested that mothers and healthcare providers may be less attentive to sexual health concerns of survivors relative to healthy peers (Murphy et al., 2012). Based on these findings, we examined differences between mothers of survivors/non-survivors on the quality of maternal-daughter sexual communication, delivery and accuracy of HPV-related information provided, and healthcare provider recommendation rates for HPV vaccination. This study also evaluated influences of sociodemographic and medical factors on sexual and HPV communication and healthcare provider recommendation.
Mothers approached discussions of sexual health with their children similarly, regardless of their daughters’ cancer history. Although unexpected, these results were consistent with research demonstrating that adolescent and young adult survivors of childhood cancer engaged in equivalent risky sexual activity as compared to healthy siblings (Klosky et al., 2012) suggesting that there may be more similarities than differences across survivors/non-survivors in terms of sexual history/behaviors. Furthermore, these findings provide evidence for the assertion that the sexual communication among survivors of childhood cancer follows a similar trajectory to non-survivors. Future research should examine other aspects of sexual communication and development among survivors to establish clear patterns of similarities or differences between these groups and evaluate how these patterns may influence future sexual outcomes.
The presented findings also indicated that healthcare providers recommend the HPV vaccination to mothers at the same rate, regardless of daughter’s cancer history. This is contradictory to previous research (Murphy et al., 2012), which suggested discrepancies between survivors/non-survivors in the amount of attention paid to sexual health issues by their healthcare providers. Some researchers posit that sexual health concerns may be overshadowed by more acute oncology concerns or that these concerns may be ignored because of perceptions that childhood cancer patients and survivors’ sexual development is thwarted (Murphy et al., 2012). Our findings suggested that this position may not translate to healthcare providers’ HPV vaccination recommendations. One explanation for these conflicting findings is that there may be different rates of recommendation based on healthcare provider specialty. Rates of HPV vaccination recommendation may differ among oncology healthcare providers and internal medicine healthcare providers. Future research should better identify which specialists are recommending the HPV vaccine to survivors and if there are differences in recommendation rates.
While it is encouraging that mothers and healthcare providers are talking about sex and the HPV vaccination with this vulnerable population, rates of communication are troubling. Given their increased risk of HPV-related complications, healthcare providers should recommend the HPV vaccination as standard care practice. Only 50% of mothers of survivors in this study received a recommendation for the HPV vaccination, with fewer patients having initiated the vaccine. This is concerning based on COG (2008) recommendations that all survivors of childhood cancer receive the HPV vaccination. One possibility for suboptimal recommendation rates is a diffusion of responsibility between survivors’ primary care clinics and their oncology teams. Research suggests that long-term follow-up clinics have difficulty communicating with community-based healthcare providers treating survivors (Aziz, Oeffinger, Brooks, & Turoff, 2006), leaving healthcare providers uncertain of who is responsible for the patient’s vaccine management. Having multiple teams involved in care may lead each team to believe that the other team will address the topic of HPV vaccination (Klosky et al., 2013). Unfortunately, this may result in neither team addressing vaccination, leading to lower vaccination rates. Research also indicates that primary care healthcare providers providing follow-up care to survivors may not be familiar with the recommendations and guidelines for long-term care (Nathan et al., 2013). These findings point to a need for better communication between oncology and primary care healthcare providers, which may bridge gaps in HPV vaccination recommendations.
Regardless of cancer history, mothers were more likely to discuss various sexual topics, take advantage of salient opportunities to discuss sex, and take an authoritative approach to sex-related discussions with older daughters. While it is promising that mothers are open to discussing sexual topics with older adolescent daughters, research suggests that parents should initiate dialogue regarding sex with their daughters before sexual debut (Hutchinson, 2002). Hutchinson (2002) found that adolescent girls who reported discussing sex-related topics with their parents were less likely to initiate sexual intercourse. Furthermore, research suggests that those who initiate sexual intercourse prior to age 16 are more likely to engage in risky sexual behavior (Sandfort, Orr, Hirsch, & Santelli, 2008). This is especially problematic for survivors of childhood cancer who are already at risk for HPV-related complications. Therefore, it is important for healthcare providers to encourage, and perhaps even model, appropriate sexual communication among mothers of younger girls.
Although this study did not find any demographic differences that related to HPV-specific communication, younger patients were less likely to receive a recommendation for the HPV vaccine by their medical teams. These findings are consistent with previous research (CDC, 2014), and present a vaccination challenge. The ACIP recommends that females be vaccinated before exposure to the virus through sexual contact (CDC, 2010). Healthcare providers who wait until girls are older to recommend the vaccine may be missing a critical window of opportunity for HPV protection.
Mothers reporting annual household incomes of greater than $60,000 were more likely to receive a recommendation than mothers reporting incomes between $20,000 and $59,999. This finding suggests that working and middle class families are “falling through the cracks.” Initiatives such as the Vaccines for Children program have been developed to increase the accessibility of vaccines such as the HPV vaccine to low-income families (Pourat & Jones, 2012). Many insurance companies provide coverage for higher-income, insured families desiring the vaccine (Brown et al., 1996). However, provisions for HPV vaccination may be limited for middle class families, who are often at greatest risk for being uninsured or underinsured. This gap in resources may be reflected in healthcare providers’ recommendation patterns. Therefore, healthcare providers should be cognizant of the recommendation gap and provide any available resources for families who have difficulty financing their daughter’s vaccination.
Among cancer survivors, mothers were more likely to discuss a variety of different sexual topics, to talk more frequently, and to take more developmental opportunities to discuss sex if their daughters were diagnosed with cancer at an older age. Older age at diagnosis was also associated with increased HPV-specific communication among mothers and daughters and increased likelihood that families would receive healthcare provider recommendations for the HPV vaccine. These findings are novel and highlight the influence of female survivors’ age of diagnosis on several aspects of sexual and HPV-related communication. Research on the neurodevelopmental late effects of childhood cancer treatment suggests that age of diagnosis is a critical factor to consider in the context of sexual health among survivors (Flory et al., 2006).
Research suggests that children who are diagnosed with cancer at younger ages may be more susceptible to negative cognitive late effects, or complications that develop after treatment (Flory et al., 2006). Patients who are treated with central nervous system irradiation and/or chemotherapies may experience inattention, poorer executive functioning, hyperactivity, and emotional regulation deficits that develop over the course of their childhood and adolescence (Flory et al., 2006). Parents may shy away from discussing sexual topics with these survivors because of their perceived inability to understand the subject matter. Alternatively, parents may avoid discussing sexuality and HPV-related topics because they do not think that their children will engage in sexual behavior (Murphy et al., 2012; Klosky et al., 2013). Similarly, healthcare providers may perceive delayed sexual development and fail to recommend the vaccine for these survivors because of the lack of perceived risk for engaging in sexual behavior (Murphy et al., 2012). However, survivors who experience cognitive late effects may be more likely to engage in risky sexual behavior and/or be victims of sexual assault (Schofield, Bierman, Heinrichs, & Nix, 2008). Therefore, it is imperative that healthcare providers and parents of survivors communicate with their children about sex, HPV, and the HPV vaccination.
Conclusion
These findings highlight needs for interventions among mothers and healthcare providers to increase sexual communication among young girls, middle class families, and survivors diagnosed at a young age. Interventions exist that enhance parents’ comfort regarding how to introduce and openly discuss sexual topics (Barr, Johnson Moore, & Howard, 2012). Similar interventions can be integrated into pediatric primary care and oncology clinics. Research has also demonstrated the effectiveness of provider-targeted interventions increasing knowledge and immunization practices for vaccine-preventable diseases (Gonik, Jones, Fasano, Contreras, & Roberts, 2001). These interventions should be extended to address healthcare providers’ self-efficacy toward recommending the HPV vaccine for younger adolescents. Future research should also continue to evaluate HPV recommendation and uptake rates among middle class families to inform policy to implement programs to provide access to the HPV vaccine.
For survivors, communication among oncologists, primary care, and long-term healthcare providers may serve as effective mechanisms to ensure survivors and parents receive vaccine recommendations. Oncology healthcare providers should also discuss the HPV vaccine with the parents of younger survivors and active patients. Healthcare providers should take the opportunity to integrate HPV vaccination recommendations into patients’ long-term care plan during treatment, even when too young to receive the vaccine. By incorporating HPV vaccination into survivorship discussions, parents may be more likely to continue to conversation with their child and, ultimately, vaccinate their child against HPV.
Contributor Information
Courtney Peasant, Department of Psychology, St. Jude Children’s Research Hospital; The University of Memphis; Yale School of Public Health at Yale University
Rebecca H. Foster, Department of Psychology, St. Louis Children’s Hospital; University of Washington, St. Louis
Kathryn M. Russell, Department of Psychology, St. Jude Children’s Research Hospital
Brianne E. Favaro, St. Jude Children’s Research Hospital
James L. Klosky, Department of Psychology, St. Jude Children’s Research Hospital
References
- Allen JD, Othus MK, Shelton RC, Li Y, Norman N, Tom L, del Carmen MG. Parental decision making about the HPV vaccine. Cancer Epidemiology Biomarkers & Prevention. 2010;19(9):2187–2198. doi: 10.1158/1055-9965.EPI-10-0217. [DOI] [PubMed] [Google Scholar]
- Armstrong GT, Liu W, Leisenring W, Yasui Y, Hammond S, Bhatia S, Robison LL, et al. Occurrence of multiple subsequent neoplasms in long-term survivors of childhood cancer: A report from the childhood cancer survivor study. Journal of Clinical Oncology. 2011;29(22):3056–3064. doi: 10.1200/JCO.2011.34.6585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz NM, Oeffinger KC, Brooks S, Turoff AJ. Comprehensive long-term follow-up programs for pediatric cancer survivors. Cancer. 2006;107(4):841–848. doi: 10.1002/cncr.22096. [DOI] [PubMed] [Google Scholar]
- Barr EM, Johnson Moore M, Howard A. A pilot project to increase parent comfort communicating with their children about sexual health. American Journal of Sexuality Education. 2012;7(3):253–266. [Google Scholar]
- Brabin L, Roberts SA, Farzaneh F, Kitchener HC. Future acceptance of adolescent human papillomavirus vaccination: A survey of parental attitudes. Vaccine. 2006;24(16):3087–3094. doi: 10.1016/j.vaccine.2006.01.048. [DOI] [PubMed] [Google Scholar]
- Brown RT, Sawyer MB, Antoniou G, Toogood I, Rice M, Thompson N, Madan-Swain A. A 3-year follow-up of the intellectual and academic functioning of children receiving central nervous system prophylactic chemotherapy for leukemia. Journal of Developmental & Behavioral Pediatrics. 1996;17(6):392–398. doi: 10.1097/00004703-199612000-00004. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the advisory committee on immunization practices (ACIP) Morbidity and Mortality Weekly Report. 2010;59(20):626–629. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. [October 1, 2014];Genital HPV infection - fact sheet. 2013 Retrieved from http://www.cdc.gov/std/HPV/STDFact-HPV.htm.
- Centers for Disease Control and Prevention. [September 24, 2014];Vaccines for Children Program (VFC) 2014 Retrieved from http://www.cdc.gov/vaccines/programs/vfc/about/index.html Published April 24, 2013.
- Children’s Oncology Group. Children’s Oncology Group Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers 2008 [Google Scholar]
- Cox MF, Fasolino TK, Tavakoli AS. Factor analysis and psychometric properties of the mother-adolescent sexual communication (MASC) instrument for sexual risk behavior. Journal of Nursing Measurement. 2008;16(3):171–183. doi: 10.1891/1061-3749.16.3.171. [DOI] [PubMed] [Google Scholar]
- Dunne EF, Unger ER, Sternberg M, McQuillan G, Swan DC, Patel SS, Markowitz LE. Prevalence of HPV infection among females in the United States. JAMA: The Journal of the American Medical Association. 2007;297(8):813–819. doi: 10.1001/jama.297.8.813. [DOI] [PubMed] [Google Scholar]
- Flory K, Molina BS, Pelham J, William E, Gnagy E, Smith B. Childhood ADHD predicts risky sexual behavior in young adulthood. Journal of Clinical Child and Adolescent Psychology. 2006;35(4):571–577. doi: 10.1207/s15374424jccp3504_8. [DOI] [PubMed] [Google Scholar]
- Gamble HL, Klosky JL, Parra GR, Randolph ME. Factors influencing familial decision-making regarding human papillomavirus vaccination. Journal of Pediatric Psychology. 2010;35(7):704–715. doi: 10.1093/jpepsy/jsp108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonik B, Jones T, Fasano N, Contreras D, Roberts C. 300 Vaccine-preventable diseases (VPD): Improving the obstetrician/gynecologist’s knowledge and immunization practice patterns. American Journal of Obstetrics and Gynecology. 2001;185(6):S162. doi: 10.1016/s0029-7844(00)00860-7. [DOI] [PubMed] [Google Scholar]
- Hagensee ME, Cameron JE, Leigh JE, Clark RA. Human papillomavirus infection and disease in HIV-infected individuals. The American Journal of the Medical Sciences. 2004;328(1):57. doi: 10.1097/00000441-200407000-00008. [DOI] [PubMed] [Google Scholar]
- Henderson TO, Whitton J, Stovall M, Mertens AC, Mitby P, Friedman D, Diller L, et al. Secondary sarcomas in childhood cancer survivors: a report from the Childhood Cancer Survivor Study. Journal of the National Cancer Institute. 2007;99(4):300–308. doi: 10.1093/jnci/djk052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman L, Okcu MF, Dreyer ZE, Suzawa H, Bryant R, Middleman AB. Human papillomavirus vaccination in female pediatric cancer survivors. Journal of Pediatric and Adolescent Gynecology. 2012;25(5):305–307. doi: 10.1016/j.jpag.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Hutchinson MK. The influence of sexual risk communication between parents and daughters on sexual risk behaviors. Family Relations. 2002;51(3):238–247. [Google Scholar]
- Kann L, Kinchen S, Shanklin SL, Flint KH, Hawkins J, Harris WA, Zaza S, et al. Youth risk behavior surveillance-United States, 2013. Morbidity and Mortality Weekly Report. 2014;63(4):1–162. [PubMed] [Google Scholar]
- Kazak AE, Hocking MC, Ittenbach RF, Meadows AT, Hobbie W, DeRosa BW, Reilly A, et al. A revision of the intensity of treatment rating scale: Classifying the intensity of pediatric cancer treatment. Pediatric Blood & Cancer. 2012;59(1):96–99. doi: 10.1002/pbc.23320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klosky JL, Foster RH, Hodges J, Peasant C, Gamble H, McDermott MJ, Rao KP. Human papillomavirus vaccination and the primary prevention of cancer: Implications for survivors of childhood cancer. In: Quintana Y, editor. Studies in Health Technology and informatics. Washington, D.C: IOS Press; 2012. pp. 33–42. [DOI] [PubMed] [Google Scholar]
- Klosky JL, Howell CR, Li Z, Foster RH, Mertens AC, Robison LL, Ness KK. Risky health behavior among adolescents in the childhood cancer survivor study cohort. Journal of Pediatric Psychology. 2012;37(6):634–646. doi: 10.1093/jpepsy/jss046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klosky JL, Russell KM, Canavera KE, Gammel HL, Hodges JR, Foster RH, Hudson MM, et al. Risk factors for non-initiation of the human papillomavirus vaccine among adolescent survivors of childhood cancer. Cancer Prevention Research. 2013;6(10):1101–1110. doi: 10.1158/1940-6207.CAPR-13-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows AT, Friedman DL, Neglia JP, Mertens AC, Donaldson SS, Stovall M, Inskip PD, et al. Second neoplasms in survivors of childhood cancer: findings from the Childhood Cancer Survivor Study cohort. Journal of Clinical Oncology. 2009;27(14):2356–2362. doi: 10.1200/JCO.2008.21.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D, Klosky JL, Termuhlen A, Sawczyn KK, Quinn GP. The need for reproductive and sexual health discussions with adolescent and young adult cancer patients. Contraception. 2012;88(2):215–220. doi: 10.1016/j.contraception.2012.08.041. [DOI] [PubMed] [Google Scholar]
- Nathan PC, Daugherty CK, Wroblewski KE, Kigin ML, Stewart TV, Hlubocky FJ, Galliher JM, et al. Family physician preferences and knowledge gaps regarding the care of adolescent and young adult survivors of childhood cancer. Journal of Cancer Survivorship. 2013;7(3):1–8. doi: 10.1007/s11764-013-0271-0. [DOI] [PubMed] [Google Scholar]
- Peasant C, Foster RH, Gamble HL, Rao K, Klosky JL. The role of sexual commmunication on parental intentions for HPV vaccination among daughters surviving childhood cancer. Annals of Behavioral Medicine. 2012;43:S149–S149. [Google Scholar]
- Sandfort TGM, Orr M, Hirsch JS, Santelli J. Long-term health correlates of timing of sexual debut: Results from a national US study. American Journal of Public Health. 2008;98(1):155. doi: 10.2105/AJPH.2006.097444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield HT, Bierman KL, Heinrichs B, Nix RL. Predicting early sexual activity with behavior problems exhibited at school entry and in early adolescence. Journal of Abnormal Child Psychology. 2008;36(8):1175–1188. doi: 10.1007/s10802-008-9252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werba BE, Hobbie W, Kazak AE, Ittenbach RF, Reilly AF, Meadows AT. Classifying the intensity of pediatric cancer treatment protocols: The intensity of treatment rating scale 2.0 (ITR-2) Pediatric Blood & Cancer. 2007;48(7):673–677. doi: 10.1002/pbc.21184. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO guidance note: Comprehensive cervical cancer prevention and control: A healthier future for girls and women (NLM classification: WP 480) Geneva, Switzerland: 2013. [Google Scholar]
- Wulf D. In their own right: Addressing the sexual and reproductive health needs of American men. The Alan Guttmacher Institute; New York: 2002. [Google Scholar]


