Abstract
The cornea functions as the major refractive interface for vision and protects the internal eye from insult. Current understanding of innate immune responses to corneal infection derives from a synthesis of in vitro and in vivo analyses. However, monolayer cell cultures and mouse models do not accurately duplicate all aspects of innate immunity in human patients. Here, we describe a three-dimensional culture system that incorporates human cells and extracellular matrix to more completely simulate the human cornea for studies of infection. Human corneal stromal fibroblasts were mixed with type I collagen in 3-μm pore size transwell inserts, and overlayed with Matrigel to simulate a human corneal stroma and epithelial basement membrane. These were then infected with a cornea-tropic adenovirus, and exposed on their inferior side to leukocytes derived from human peripheral blood. Subsequent analyses were performed with histology, confocal microscopy, ELISA, and fluorescence-activated cell sorting (FACS). CXCL8, a neutrophil chemokine shown previously as the first cytokine induced in infection of human corneal cells, increased upon adenovirus infection of facsimiles in a dose-responsive fashion. Myeloperoxidase-positive cells infiltrated infected corneal facsimiles in a sub-Matrigel location, possibly due to CXCL8 colocalization with heparan sulfate, a Matrigel constituent. Cellular infiltration was significantly inhibited by treatment with chemical inhibitors of p38 MAPK and Src kinase, both constituents of a signaling cascade previously suggested to regulate inflammation after adenovirus infection. FACS analysis determined that both virus and corneal fibroblasts were necessary for the induction of leukocyte migration into the facsimiles. The corneal facsimile, literally a cornea in a test tube, permits mechanistic studies on human tissue in a highly tractable system.
Keywords: Keratitis, Adenovirus, In vitro model
Introduction
The cornea is the avascular, transparent tissue of the eye which refracts light, and forms part of the barrier between the internal eye and the external environment (Suzuki et al. 2010). The principal layers of the cornea, from anterior to posterior, consist of a stratified epithelium, collagenous stroma, and monolayered endothelium. The stroma comprises 90% of the corneal thickness. It contains predominantly collagen type I and is interspersed with keratocytes, specialized fibroblast-like cells that participate in repair and maintenance of the corneal extracellular matrix (West-Mays and Dwivedi 2006). Microbial infection of the cornea leads to stromal inflammation (keratitis), which in turn leads to scarring and poor vision, and can result in corneal perforation and loss of the eye.
Epidemic keratoconjunctivitis (EKC) is a hyperacute and highly contagious ocular surface infection caused by viruses within human adenovirus species D (HAdV-D) (Ford et al. 1987). Upon resolution of the acute infection, EKC is associated with a relapsing and remitting stromal keratitis, in which peripheral blood leukocytes infiltrate the subepithelial anterior corneal stroma in a multifocal pattern resulting in eye pain and reduced vision (Butt and Chodosh 2006). No specific treatment exists for the keratitis associated with EKC, and existing animal models of the infection are limited by the relative failure of human adenoviruses to replicate outside the human host (Duncan et al. 1978; Younghusband et al. 1979; Blair et al. 1989; Ginsberg et al. 1991; Chintakuntlawar et al. 2007; ). Studies performed using either primary and immortalized human cell lines in monolayer culture, or a mouse model of keratitis in which the virus induces inflammation but does not replicate, have implicated signaling pathways involving Src kinase, phosphoinositide 3 kinase, and MAP kinases, and the chemokines CXCL8 and CCL2 in the initial pathogenesis of keratitis (Chodosh et al. 2000; Natarajan et al. 2002a, b, c, 2003; Rajala et al. 2005; Xiao and Chodosh 2005; Chintakuntlawar et al. 2007, 2010; Rajaiya et al. 2008, 2009; Chintakuntlawar and Chodosh 2009).
Three-dimensional cultures are growing in popularity as an alternative approach to biological studies. These cultures can recapitulate cell polarity, differentiation, signaling, and gene expression seen in vivo (Yamada and Cukierman 2007). To improve our understanding of basic mechanisms that underlie the pathogenesis of adenovirus keratitis, we have taken a prior three-dimensional facsimile of adenovirus keratitis (Chodosh et al. 2000), in which human keratocytes, fully susceptible to HAdV-D infection, are embedded in type 1 collagen and infected, and further adapted the model by overlaying the collagen with a basement membrane prior to infection, and coculture with human leukocytes. Remarkably, this “corneal facsimile” can be manipulated to elicit leukocytic infiltrates very similar to those seen in adenovirus keratitis in human patients with EKC, and can be applied to the examination of the molecular pathways leading to keratitis.
Materials and Methods
Cells and viruses
Keratocytes were isolated from human donor corneas as previously described (Chodosh et al. 2000) and grown in DMEM with 10% FBS (Gibco, Grand Island, NY). These cells have a fibroblast phenotype after culture in serum (LaGier et al. 2013) but recover somewhat to their original biochemical phenotype when cultured in collagen (Lakshman and Petroll 2012; Thompson et al. 2013). Cells from multiple donors were pooled and the cell monolayers used at passage 2 or 3. Human peripheral blood leukocytes were prepared from discarded buffy coats obtained from the Massachusetts General Hospital Blood Component Laboratory. Briefly, human buffy coat was diluted 4:1 with room temperature phosphate buffered saline (PBS), and 20 ml layered onto Histopaque 1119 and 1077 (Sigma-Aldrich, Steinheim, Germany) (10 ml each) and centrifuged at 1,200×g for 30 min at room temperature. The leukocyte pellet was washed twice with PBS prior to use in facsimiles (see below). HAdV-D37 was obtained from American Type Culture Collection (ATCC, Manassas, VA), and purified by cesium chloride gradient prior to use. Pseudomonas aeruginosa used in this study was originally cultured from a patient with microbial keratitis. The derivation of primary corneal cells from deceased human donors and use of buffy coat cells from anonymous donors were approved by the Massachusetts Eye and Ear Infirmary Human Studies Committee, and conformed to the tenets of the Declaration of Helsinki.
Cornea facsimile
Twelve-millimeter transwell plates with 3-μm pore size (Corning Inc., Corning, NY) were precoated with a neutralized solution containing 1× phosphate buffered saline (PBS), 10% DMEM, and 50% collagen type I (BD Biosciences, Bedford, MA). The same neutralized solution was then mixed with primary human corneal fibroblasts (derived as above) to a concentration of 1×106 cells/ml, and 350 μl (containing 3.5×105 cells) of the cell-containing solution added to the precoated transwell inserts. The facsimiles were allowed to set at 37°C in 5% CO2 for 15 min, and then 75 μl of reduced growth factor-containing Matrigel (Growth Factor Reduced, BD Biosciences, San Jose, CA) was layered onto the upper surface of the collagen and allowed to set overnight at 37°C in 5% CO2. Each facsimile was then infected overnight with HAdV-D37 at a multiplicity of infection of 10–30, depending on the experiment, with dialysis buffer as a control, or with P. aeruginosa at log phase, for 2 h in 5 μl of PBS with an optical density reading of 1.0 at 600 nm. Leukocytes derived from human buffy coat (5×106 cells, purified as above) or media control were added in 600 μl of DMEM with 2% FBS to the bottom chamber of each transwell plate for 1 h at 37°C in 5% CO2. In select experiments, cells were treated overnight with the signaling inhibitors PP2 or SB203580 (10 μM each) (Calbiochem, La Jolla, CA) prior to incubation with human peripheral blood leukocytes.
Enzyme-linked immunosorbent assay
Supernatants from the bottom chambers of transwell plates containing infected or mock-infected corneal facsimiles were collected after overnight infection, and levels of CXCL8 were quantified by sandwich enzyme-linked immunosorbent assay (ELISA). Anti-human CXCL8, biotin-conjugated anti-human CXCL8 antibodies, and recombinant CXCL8 standards were purchased from BD PharMingen (San Diego, CA). The lower detection limit of the ELISA was determined during optimization to be 30 pg/ml. ELISA plates were read on a SpectraMax M2 microplate and analyzed with SOFTmax analysis software reader (Molecular Devices, Sunnyvale, CA).
Confocal microscopy
Facsimiles were fixed in 0.05% para-formaldehyde for 10 min, washed in PBS containing 2% FBS, permeabilized in 0.1% Triton X-100 for 1 h, blocked for 1 h in 3% FBS in PBS, and then incubated in 5 μg/ml of anti-CXCL8 or anti-myeloperoxidase antibodies (Thermo Fisher, Pittsburg, PA) overnight at 4°C. After washing in 1× PBS containing 2% FBS, facsimiles were fixed in 2% paraformaldehyde, and mounted using Vectashield (Vector labs, Burlingame, CA) mounting medium containing DAPI. Images were taken in a Leica SP5 confocal microscope using a 40× objective (Leica Microsystems, Buffalo Grove, IL). Neutrophil infiltration into facsimiles, as indicated by the intensity of myeloperoxidase staining, was quantified in three randomly chosen frames from each of three experiments for each treatment group using ImageJ software (http://imagej.nih.gov/ij/).
FACS analysis
Leukocyte infiltrates in virus- or bacteria-infected facsimiles were studied by fluorescence-activated cell sorting (FACS) analysis. Facsimiles were harvested 1 h after addition of leukocytes and digested with 1 mg/ml collagenase type I (Sigma, St. Louis, MO) and 0.5 mg/ml DNase (Roche, Indianapolis, IN) for 5 min at room temperature. Single cell suspensions were washed twice in PBS, incubated on ice for 15 min with 2 μl anti-human Fc (BD Pharmingen, San Diego, CA) in a total volume of 100 μl PBS with 1% BSA, centrifuged at 300×g for 5 min, and resuspended in 10% FBS for an additional 15 min on ice. Cells were then labeled with 5 μl FITC-conjugated anti-human CD45 (BD Pharmingen) and incubated in the dark on ice for 30 min. Following incubation, the cells were washed three times with PBS-1% BSA and resuspended in 400 μl PBS. Flow cytometry was performed on a Cytomics FC500 (Beckman Coulter, Brea, CA) for CD45 positive events, representing the numbers of leukocytes present in infected and control facsimiles.
Histology
Facsimiles were removed from transwell plates, rinsed in PBS, and fixed in 4% paraformaldehyde for 24 h at room temperature, 70% alcohol for 24 h at room temperature, and embedded in paraffin. Samples were cut into 5 μm-thick sections, mounted on positively charged slides and air-dried overnight. After deparaffinization and rehydration, slides were stained with hematoxylin and eosin for histology, coverslipped, and photographed using a 40× objective (Axiovert 135; Carl Zeiss, Thornwood, NY).
Statistics
Comparisons between means were performed with ANOVA with Scheffe’s comparison test. Statistical significance was set at p<0.05.
Results
Cornea facsimile model
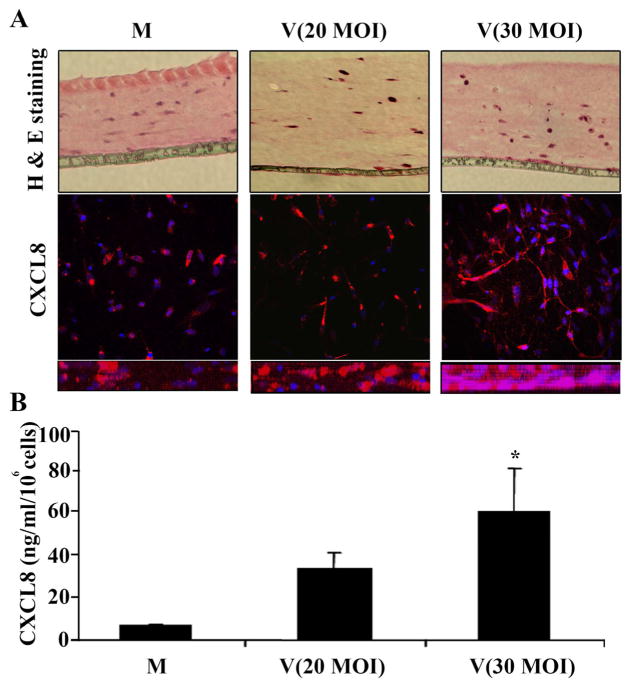
The cornea facsimile model of stromal keratitis after HAdV infection was generated as shown in Fig. 1. In brief, cultured human corneal fibroblasts from cadaveric donor eyes were embedded in type I collagen set on tissue culture inserts with a 3-μm pore size membrane, infected overnight with HAdV-D37, and then cocultured with leukocytes for 1 h from fresh human peripheral blood. The histology of mock and HAdV-37-infected facsimiles 1 d post infection and prior to coculture with leukocytes is shown in Fig. 2A, in which keratocytes in facsimiles at two different multiplicities of infection (20 and 30) appear to show cell rounding consistent with viral cytopathic effect. We previously demonstrated robust expression of CXCL8 by HAdV-infected human corneal fibroblasts in monolayer culture, log units in excess of CXCL8 expression by cultured human corneal epithelial cells (Chodosh et al. 2000). To confirm whether HADV-D37-infected human corneal fibroblasts will express CXCL8 when embedded in collagen, we next applied confocal microscopy (Fig. 2A). CXCL8 expression by embedded fibroblasts was evident and appeared more intense at the higher multiplicity of infection. When compared by ELISA of facsimile supernatants, CXCL8 expression in infected facsimiles was higher at the higher multiplicity of infection (p<0.05), consistent with a dose response to infection (Fig. 2B).
Figure 1.
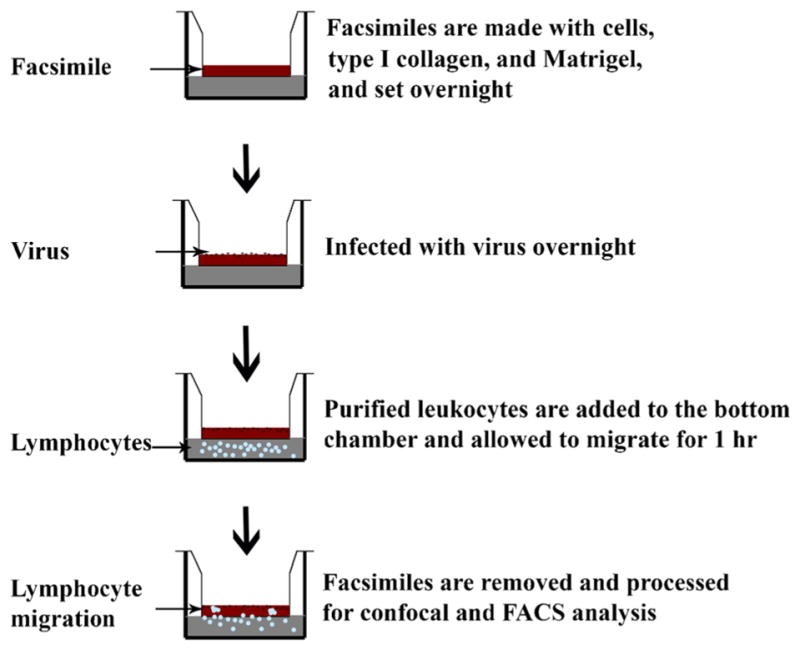
A schematic for cornea facsimile preparation.
Figure 2.
Corneal facsimile appearance and CXCL8 expression after HAdV-D37 infection, but prior to coculture with leukocytes. Facsimiles were stained with hematoxylin and eosin (H&E) after mock infection or with two different multiplicities of infection (MOI of 20 or 30) (A, upper panel). Fibroblasts in facsimiles infected with HAdV-D37 show increasing cytopathic effect. Confocal microscopy images with antibody against CXCL8 (A, middle and lower panels) demonstrate increased cellular expression of the chemokine at the higher MOI (middle panel: en face view, and lower panel: cross section). By ELISA (B), facsimile supernatants showed a MOI-dependent increase in expression of CXCL8. *p<0.05 by ANOVA with Scheffe’s comparison test.
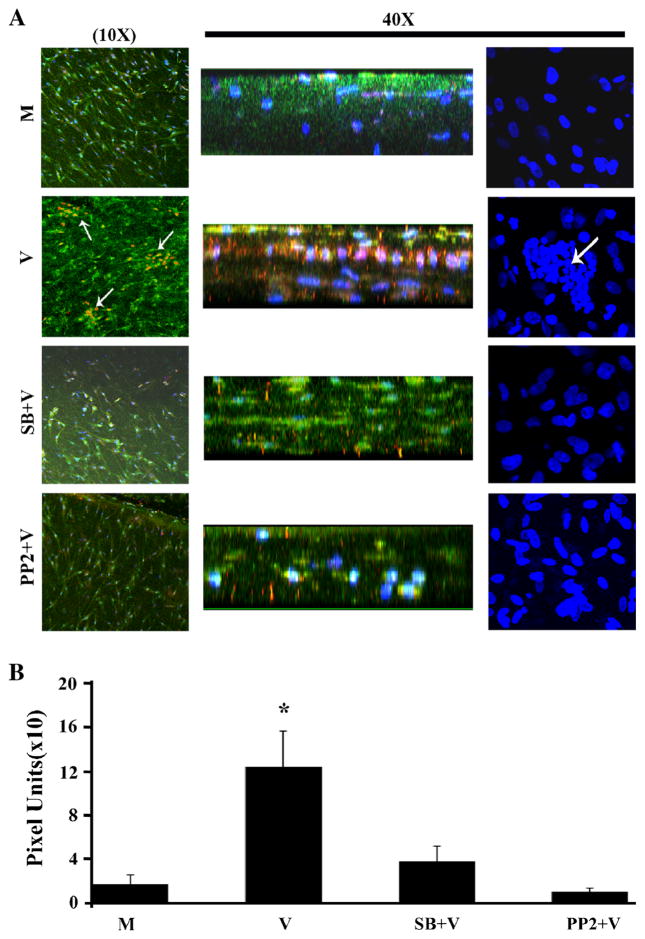
Multifocal infiltration of leukocytes into the subepithelial corneal stroma is characteristic of EKC (Butt and Chodosh 2006). We next tested the capacity of the corneal facsimile to model the infiltration by leukocytes into an infected corneal stroma-like matrix. Leukocytes derived from human buffy coat were placed in the lower chamber of HAdV-D37-infected or mock-infected facsimiles in transwell plates and incubated for 1 h. Infiltration by these cells into the collagen matrix could only occur against gravity and therefore would require chemokine activity to induce cell migration, similar to movement in a Boyden chamber (Boyden 1962). As shown in Fig. 3A, myeloperoxidase-positive cells, presumably neutrophils, aggregated in the sub-Matrigel collagen of infected but not mock-infected facsimiles. To determine if the cornea facsimile model could be used to study the role of specific signal transduction pathways in infected cells on subsequent leukocyte infiltration into the corneal stroma, we also pretreated the facsimiles with chemical inhibitors of two kinases previously shown to participate in the intracellular signaling leading to CXCL8 expression by monolayer cultured human corneal fibroblasts: p38 MAP kinase (SB203580) and Src kinase (PP2) (Natarajan et al. 2003; Rajaiya et al. 2008). In HAdV-D37-infected facsimiles, myeloperoxidase-positive cell migration was significantly abrogated by pretreatment with chemical inhibitors of p38 and Src (Fig. 3B).
Figure 3.
Cornea facsimiles were stained with antibody against myeloperoxidase to demonstrate granulocyte (neutrophil) infiltration after HAdV-D37 infection and leukocyte coculture. As shown (A), mock-infected facsimiles (M, row 1) showed no myeloperoxidase staining, indicative of absence of infiltrating neutrophils. In virus-infected facsimiles (V, row 2), aggregations of myeloperoxidase-positive cells (arrows) were observed in en face and cross sectional views, and also seen with DAPI staining (right column). Pretreatment with inhibitors to p38 (SB203580) and Src (PP2) blocked migration of leukocytes into infected facsimiles (SB+V, row 3, and PP2+V, row 4). Myeloperoxidase (red) signal was subsequently quantified in ImageJ by analysis of three randomly chosen fields in each of three experiments for each treatment group (B). Compared to DMSO, pretreatment of facsimiles with either SB203580 or PP2 prior to infection significantly reduced myeloperoxidase staining. *p<0.05 by ANOVA with Scheffe’s comparison test.
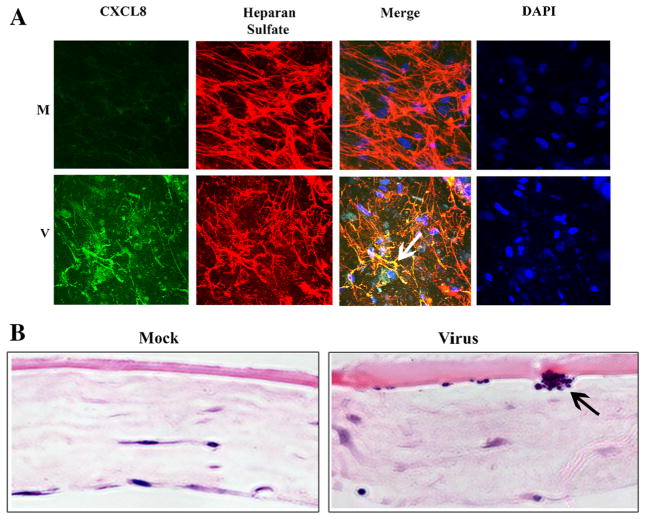
Subepithelial corneal infiltrates are the sine qua non of human stromal keratitis after adenovirus infection (Butt and Chodosh 2006). To more closely examine the apparent propensity for human leukocytes to migrate specifically to a sub-Matrigel (“subepithelial”) location in the corneal facsimile model, we double stained HAdV-D37-infected and mock-infected facsimiles for CXCL8 and heparan sulfate. The latter is a prominent component of corneal epithelial basement membranes (Torricelli et al. 2013), a constituent of Matrigel (Benton et al. 2011), and was shown previously to bind CXCL8 (Webb et al. 1993; Middleton et al. 1997; Pichert et al. 2012). By confocal microscopy, CXCL8 was expressed in HAdV-D37-infected but not in mock-infected facsimiles (Fig. 4A), and colocalized with infiltrating cells (as shown by DAPI staining), and with heparan sulfate. Histology of virus-infected facsimiles confirmed a sub-Matrigel location for focal leukocyte infiltration (Fig. 4B), very similar to the subepithelial infiltrates in EKC.
Figure 4.
Confocal microscopy (A) and histology (B) of mock (M) versus HAdV-D37 (V)-infected corneal facsimiles after coculture with leukocytes. Facsimiles were treated with antibodies against CXCL8 and heparan sulfate, and with DAPI. Merged confocal microscopic images of virus-infected facsimiles (A, second row, third column) demonstrates colocalization (arrow) of CXCL8 and heparan sulfate, the latter a component of Matrigel basement membrane. Hematoxylin and eosin stained facsimile cross sections (B) showed cellular aggregation (arrow) at the level of the Matrigel basement membrane only in virus-infected facsimiles.
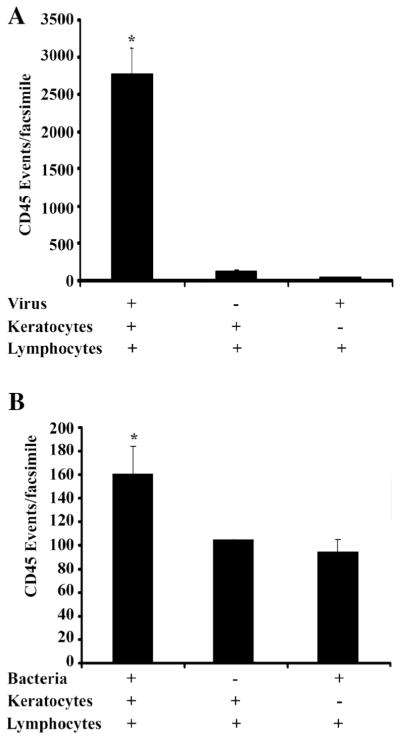
Finally, we performed FACS analysis of corneal facsimiles infected with either HAdV-D37 or P. aeruginosa, the latter a major cause of infectious keratitis (Alexandrakis et al. 2000). Virus particles are not intrinsically chemotactic, while bacterial cells can induce the migration of inflammatory cells into even devitalized tissue (Chusid and Davis 1985; Khandoga et al. 2009; Kahle et al. 2013). In HAdV-D37-infected corneal facsimiles analyzed by FACS, the presence of both virus and keratocytes was necessary for CD45+ cell infiltration (Fig. 5A). The cornea facsimile model degraded when cultured for more than 2 h with even small quantities of P. aeruginosa at log phase (data not shown), consistent with enzymatic degradation of the extracellular matrix by bacterial collagenases (Bergan 1981). At 2 h post infection, the presence of both P. aeruginosa and keratocytes in the facsimile led to more leukocyte infiltration than either alone (Fig. 5B), suggesting the presence of the human cells amplified the intrinsic chemotactic activity of the bacteria.
Figure 5.
Flow cytometry for CD45 positive cells (leukocytes) in HAdV-D37 (A) and P. aeruginosa (B)-infected facsimiles. Overnight virus infection dramatically increased CD45-positive cell infiltration only in the presence of keratocytes, consistent with the necessity of infected cells for virus-induced chemotactic activity. However, P. aeruginosa infection of facsimiles was limited by bacterial degradation of the matrix (data not shown). At 2 h post infection with bacteria and 1 h coculture with leukocytes (B), facsimiles containing both P. aeruginosa and keratocytes had more CD45 positive cells than the matrices with either one alone. *p<0.05 by ANOVA with Scheffe’s comparison test.
Discussion
CXCL8 is a member of the C-X-C chemokine family, and a potent activator and chemoattractant of neutrophils (Harada et al. 1994). Previous work demonstrated that CXCL8 establishes degradation-resistant tissue reservoirs when bound to endothelial basement membrane, providing persistent signals for migration of immune cells into tissue (Middleton et al. 1997). In microarray studies, we previously demonstrated expression of CXCL8 mRNA by HAdV-infected human corneal fibroblasts within 1 h of infection; CXCL8 was among the very first genes upregulated (Natarajan et al. 2002a, b, c, 2003). Similarly, in HAdV-infected human corneal fibroblasts in monolayer cultures, CXCL8 was the first proinflammatory protein expressed after infection. Mitogen-activated protein kinases (MAPK) respond to proinflammatory cytokines and cellular stresses (Kyriakis and Avruch 2012) and are activated by phosphorylation of upstream kinases, including Src (Kim et al. 2009). In human corneal fibroblasts, the expression of CXCL8 upon HAdV infection is controlled by a signaling cascade including the Src kinase and p38 MAPK (Natarajan et al. 2003; Rajaiya et al. 2008), and is initiated within minutes after HAdV binding to the cells.
Growing cells within an extracellular protein matrix, such as collagen, can preserve normal in vivo morphology and behavior (Fallica et al. 2012). Because the use of monolayer cultures to mimic in vivo infection might be misleading, we previously tested whether human corneal fibroblasts would express CXCL8 into a three-dimensional collagenous extra-cellular matrix when infected with HAdV (Chodosh et al. 2000). By immunohistochemistry, CXCL8 protein in infected constructs increased upon infection but appeared diffusely distributed throughout the matrix. Therefore in the current study, we added a basement membrane-like layer to provide potential binding sites for expressed chemokines. After infection with HAdV-D37, we cocultured the facsimiles with leukocytes derived from peripheral blood of human donors to determine if we could mimic the leukocyte infiltrates seen in human adenovirus keratitis. By confocal microscopy, CXCL8 colocalized with heparan sulfate, a component of Matrigel, in infected facsimiles. More remarkably, leukocytes migrated against gravity into HAdV-D37-infected facsimiles to form sub-Matrigel foci comparable to the subepithelial infiltrates seen in adenovirus infection of human eyes. Therefore, the cornea facsimile model recapitulates several fundamental features of adenovirus keratitis not faithfully reproduced in other models. The cells of the cornea facsimile are derived from human corneas and support HAdV-D replication. Virus-infected cells within the facsimile express CXCL8, which binds to epithelial basement membrane, forming chemoattractant reservoirs—foci for subsequent leukocyte infiltration. Subsequent infiltrates are multifocal and form at the level of the epithelial basement membrane, mimicking subepithelial infiltrates in human adenovirus keratitis. Furthermore, the corneal facsimile model of adenovirus keratitis was easily adapted for histology, ELISA, confocal immunomicroscopy, and FACS analysis, indicating the flexibility and usefulness of the model.
Chemotaxis of immune cells is central to inflammation (Taub et al. 1996). Existing in vitro models of three-dimensional chemotaxis (Pietrosimone et al. 2013) include the Boyden and Dunn chambers. Each system places the cells of interest on one side of a filter (or agar) with pore size large enough to allow transmigration of cells into the opposite chamber, usually containing some test substance. Cells migrating across the filter can then be quantified. Although three-dimensional culture models have been used in tumor biology to more physiologically mime the cancer microenvironment (Kim 2005; Nelson et al. 2008; Weigelt and Bissell 2008), this tool has not been widely deployed to study infections. A three-dimensional model utilizing the HeLa derivative cell line INT 407 and Salmonella enterica was thought to more closely resemble in vivo infection than a two-dimensional counterpart (Nickerson et al. 2001). However, the behavior of leukocytes during infection was not studied. Compared to our studies of adenovirus infection, the application of the cornea facsimile model to P. aeruginosa keratitis was less successful. The collagen matrices disintegrated upon exposure to relatively modest quantities of bacteria, even over a relatively brief time frame, suggesting an effect of bacterial collagenases (Bergan 1981). Use of stationary phase cultures was just as unproductive (data not shown). Further experiments are needed to refine the model for bacterial keratitis studies.
As discussed above, we have previously shown that proin-flammatory cytokines in HAdV infection are expressed in a time-dependent fashion by the action of specific signaling pathways (Natarajan et al. 2003; Xiao and Chodosh 2005; Rajaiya et al. 2008) and subsequent NFκB dimer formation (Rajaiya et al. 2009). We also previously reported that Src kinase is upregulated in the mouse adenovirus keratitis model (Yousuf et al. 2013) and may play a role in CXC chemokine expression. Using chemical signaling inhibitors to study signaling and innate immunity in an animal model is a challenge, and sometimes not feasible. Mice either knocked out or transgenic for specific signaling molecules in tissue-specific and time-controlled fashion are expensive and time consuming to produce. Also, human adenoviruses do not replicate in the mouse cornea (Chintakuntlawar et al. 2007). Use of a Src kinase inhibitor in the corneal facsimile model confirmed a role for Src in adenovirus keratitis and is an example of how the corneal facsimile can be utilized to study the role of intra-cellular signaling in human keratitis.
Every institutional animal care and use committee mandates that investigators planning to use animals in their research explain why the experiments cannot be done in vitro or in silico, and investigators studying innate immunity typically respond that complex immune mechanisms cannot be modeled outside a living animal. The cornea facsimile model of adenovirus keratitis shows the potential for developing in vitro systems that faithfully recapitulate complex responses of the immune system to infection.
Acknowledgments
This work was supported by grants R01 EY021558 (JR), EY01324 (JC), and P30 EY014104 from the National Eye Institute, the Massachusetts Lions Eye Research Fund (JR), a Senior Scientific Investigator Award from Research to Prevent Blindness (JC), and the Falk Foundation.
Contributor Information
Jaya Rajaiya, Chodosh Howe Laboratory, Massachusetts Eye and Ear Infirmary, Department of Ophthalmology, Harvard Medical School, 243 Charles Street, Boston, MA 02114, USA.
Xiaohong Zhou, Chodosh Howe Laboratory, Massachusetts Eye and Ear Infirmary, Department of Ophthalmology, Harvard Medical School, 243 Charles Street, Boston, MA 02114, USA.
Irina Barequet, Chodosh Howe Laboratory, Massachusetts Eye and Ear Infirmary, Department of Ophthalmology, Harvard Medical School, 243 Charles Street, Boston, MA 02114, USA.
Michael S. Gilmore, Chodosh Howe Laboratory, Massachusetts Eye and Ear Infirmary, Department of Ophthalmology, Harvard Medical School, 243 Charles Street, Boston, MA 02114, USA
James Chodosh, Massachusetts Eye and Ear Infirmary, Howe Laboratory, 243 Charles Street, Boston, MA 02114, USA.
References
- Alexandrakis G, Alfonso EC, Miller D. Shifting trends in bacterial keratitis in south Florida and emerging resistance to fluoroquinolones. Ophthalmology. 2000;107(8):1497–1502. doi: 10.1016/s0161-6420(00)00179-2. [DOI] [PubMed] [Google Scholar]
- Benton G, Kleinman HK, George J, Arnaoutova I. Multiple uses of basement membrane-like matrix (BME/Matrigel) in vitro and in vivo with cancer cells. Inter J Cancer. 2011;128(8):1751–1757. doi: 10.1002/ijc.25781. [DOI] [PubMed] [Google Scholar]
- Bergan T. Pathogenetic factors of Pseudomonas aeruginosa. Scand J Infect Dis Suppl. 1981;29:7–12. [PubMed] [Google Scholar]
- Blair GE, Dixon SC, Griffiths SA, Zajdel ME. Restricted replication of human adenovirus type 5 in mouse cell lines. Virus Res. 1989;14(4):339–346. doi: 10.1016/0168-1702(89)90026-9. [DOI] [PubMed] [Google Scholar]
- Boyden S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J Exp Med. 1962;115:453–466. doi: 10.1084/jem.115.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt AL, Chodosh J. Adenoviral keratoconjunctivitis in a tertiary care eye clinic. Cornea. 2006;25(2):199–202. doi: 10.1097/01.ico.0000170693.13326.fb. [DOI] [PubMed] [Google Scholar]
- Chintakuntlawar AV, Chodosh J. Chemokine CXCL1/KC and its receptor CXCR2 are responsible for neutrophil chemotaxis in adenoviral keratitis. J Interferon Cytokine Res. 2009;29(10):657–666. doi: 10.1089/jir.2009.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintakuntlawar AV, Astley R, Chodosh J. Adenovirus type 37 keratitis in the C57BL/6J mouse. Invest Ophthalmol Vis Sci. 2007;48(2):781–788. doi: 10.1167/iovs.06-1036. [DOI] [PubMed] [Google Scholar]
- Chintakuntlawar AV, Zhou X, Rajaiya J, Chodosh J. Viral capsid is a pathogen-associated molecular pattern in adenovirus keratitis. PLoS Pathog. 2010;6(4):e1000841. doi: 10.1371/journal.ppat.1000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodosh J, Astley RA, Butler MG, Kennedy RC. Adenovirus keratitis: a role for interleukin-8. Invest Ophthalmol Vis Sci. 2000;41(3):783–789. [PubMed] [Google Scholar]
- Chusid MJ, Davis SD. Polymorphonuclear leukocyte kinetics in experimentally induced keratitis. Arch Ophthalmol. 1985;103(2):270–274. doi: 10.1001/archopht.1985.01050020122034. [DOI] [PubMed] [Google Scholar]
- Duncan SJ, Gordon FC, Gregory DW, McPhie JL, Postlethwaite R, White R, Willcox HN. Infection of mouse liver by human adenovirus type 5. J Gen Virol. 1978;40(1):45–61. doi: 10.1099/0022-1317-40-1-45. [DOI] [PubMed] [Google Scholar]
- Fallica B, Maffei JS, Villa S, Makin G, Zaman M. Alteration of cellular behavior and response to PI3K pathway inhibition by culture in 3D collagen gels. PLoS One. 2012;7(10):e48024. doi: 10.1371/journal.pone.0048024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford E, Nelson KE, Warren D. Epidemiology of epidemic kerato-conjunctivitis. Epidemiol Rev. 1987;9:244–261. doi: 10.1093/oxfordjournals.epirev.a036304. [DOI] [PubMed] [Google Scholar]
- Ginsberg HS, Moldawer LL, Sehgal PB, Redington M, Kilian PL, Chanock RM, Prince GA. A mouse model for investigating the molecular pathogenesis of adenovirus pneumonia. Proc Natl Acad Sci USA. 1991;88(5):1651–1655. doi: 10.1073/pnas.88.5.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, Matsushima K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukoc Biol. 1994;56(5):559–564. [PubMed] [Google Scholar]
- Kahle NA, Brenner-Weiss G, Overhage J, Obst U, Hansch GM. Bacterial quorum sensing molecule induces chemotaxis of human neutrophils via induction of p38 and leukocyte specific protein 1 (LSP1) Immunobiology. 2013;218(2):145–151. doi: 10.1016/j.imbio.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Khandoga AG, Khandoga A, Reichel CA, Bihari P, Rehberg M, Krombach F. In vivo imaging and quantitative analysis of leukocyte directional migration and polarization in inflamed tissue. PLoS One. 2009;4(3):e4693. doi: 10.1371/journal.pone.0004693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JB. Three-dimensional tissue culture models in cancer biology. Semin Cancer Biol. 2005;15(5):365–377. doi: 10.1016/j.semcancer.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Kim LC, Song L, Haura EB. Src kinases as therapeutic targets for cancer. Nat Rev Clin Oncol. 2009;6(10):587–595. doi: 10.1038/nrclinonc.2009.129. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol Rev. 2012;92(2):689–737. doi: 10.1152/physrev.00028.2011. [DOI] [PubMed] [Google Scholar]
- LaGier AJ, Gordon GM, Katzman LR, Vasiliou V, Fini ME. Mechanisms for PDGF, a serum cytokine, stimulating loss of corneal keratocyte crystallins. Cornea. 2013;32(9):1269–1275. doi: 10.1097/ICO.0b013e318296e0b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshman N, Petroll WM. Growth factor regulation of corneal keratocyte mechanical phenotypes in 3-D collagen matrices. Invest Ophthalmol Vis Sci. 2012;53(3):1077–1086. doi: 10.1167/iovs.11-8609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton J, Neil S, Wintle J, Clark-Lewis I, Moore H, Lam C, Auer M, Hub E, Rot A. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell. 1997;91(3):385–395. doi: 10.1016/s0092-8674(00)80422-5. [DOI] [PubMed] [Google Scholar]
- Natarajan K, Chodosh J, Kennedy R. Innate immunity in the cornea: a putative role for keratocytes in the chemokine response to viral infection of the human corneal stroma. Adv Exp Med Biol. 2002a;506(Pt B):745–751. doi: 10.1007/978-1-4615-0717-8_105. [DOI] [PubMed] [Google Scholar]
- Natarajan K, Ghalayini AJ, Sterling RS, Holbrook RM, Kennedy RC, Chodosh J. Activation of focal adhesion kinase in adenovirus-infected human corneal fibroblasts. Invest Ophthalmol Vis Sci. 2002b;43(8):2685–2690. [PubMed] [Google Scholar]
- Natarajan K, Shepard LA, Chodosh J. The use of DNA array technology in studies of ocular viral pathogenesis. DNA Cell Biol. 2002c;21(5–6):483–490. doi: 10.1089/10445490260099782. [DOI] [PubMed] [Google Scholar]
- Natarajan K, Rajala MS, Chodosh J. Corneal IL-8 expression following adenovirus infection is mediated by c-Src activation in human corneal fibroblasts. J Immunol. 2003;170(12):6234–6243. doi: 10.4049/jimmunol.170.12.6234. [DOI] [PubMed] [Google Scholar]
- Nelson CM, Khauv D, Bissell MJ, Radisky DC. Change in cell shape is required for matrix metalloproteinase-induced epithelial-mesenchymal transition of mammary epithelial cells. J Cell Biochem. 2008;105(1):25–33. doi: 10.1002/jcb.21821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson CA, Goodwin TJ, Terlonge J, Ott CM, Buchanan KL, Uicker WC, Emami K, LeBlanc CL, Ramamurthy R, Clarke MS, Vanderburg CR, Hammond T, Pierson DL. Three-dimensional tissue assemblies: novel models for the study of Salmonella enterica serovar Typhimurium pathogenesis. Infect Immun. 2001;69(11):7106–7120. doi: 10.1128/IAI.69.11.7106-7120.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichert A, Schlorke D, Franz S, Arnhold J. Functional aspects of the interaction between interleukin-8 and sulfated glycosaminoglycans. Biomatter. 2012;2(3):142–148. doi: 10.4161/biom.21316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrosimone KM, Bhandari S, Lemieux MG, Knecht DA, Lynes MA. In vitro assays of chemotaxis as a window into mechanisms of toxicant-induced immunomodulation. Curr Protoc Toxicol. 2013;58(Unit 18.17) doi: 10.1002/0471140856.tx1817s58. [DOI] [PubMed] [Google Scholar]
- Rajaiya J, Xiao J, Rajala RV, Chodosh J. Human adenovirus type 19 infection of corneal cells induces p38 MAPK-dependent inter-leukin-8 expression. Virol J. 2008;5:17. doi: 10.1186/1743-422X-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaiya J, Sadeghi N, Chodosh J. Specific NFkappaB subunit activation and kinetics of cytokine induction in adenoviral keratitis. Mol Vis. 2009;15:2879–2889. [PMC free article] [PubMed] [Google Scholar]
- Rajala MS, Rajala RV, Astley RA, Butt AL, Chodosh J. Corneal cell survival in adenovirus type 19 infection requires phosphoinositide 3-kinase/Akt activation. J Virol. 2005;79(19):12332–12341. doi: 10.1128/JVI.79.19.12332-12341.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Yamada A, Gilmore MS. Host-pathogen interactions in the cornea. Jpn J Ophthalmol. 2010;54(3):191–193. doi: 10.1007/s10384-010-0802-4. [DOI] [PubMed] [Google Scholar]
- Taub DD, Anver M, Oppenheim JJ, Longo DL, Murphy WJ. T lymphocyte recruitment by interleukin-8 (IL-8). IL-8-induced de-granulation of neutrophils releases potent chemoattractants for human T lymphocytes both in vitro and in vivo. J Clin Invest. 1996;97(8):1931–1941. doi: 10.1172/JCI118625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RE, Boraas LC, Sowder M, Bechtel MK, Orwin EJ. Three-dimensional cell culture environment promotes partial recovery of the native corneal keratocyte phenotype from a subcultured population. Tissue Eng Part A. 2013;19(13–14):1564–1572. doi: 10.1089/ten.tea.2012.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torricelli AA, Singh V, Santhiago MR, Wilson SE. The corneal epithelial basement membrane: structure, function, and disease. Invest Ophthalmol Vis Sci. 2013;54(9):6390–6400. doi: 10.1167/iovs.13-12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb LM, Ehrengruber MU, Clark-Lewis I, Baggiolini M, Rot A. Binding to heparan sulfate or heparin enhances neutrophil responses to interleukin 8. Proc Natl Acad Sci USA. 1993;90(15):7158–7162. doi: 10.1073/pnas.90.15.7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigelt B, Bissell MJ. Unraveling the microenvironmental influences on the normal mammary gland and breast cancer. Semin Cancer Biol. 2008;18(5):311–321. doi: 10.1016/j.semcancer.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West-Mays JA, Dwivedi DJ. The keratocyte: corneal stromal cell with variable repair phenotypes. Int J Biochem Cell Biol. 2006;38(10):1625–1631. doi: 10.1016/j.biocel.2006.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Chodosh J. JNK regulates MCP-1 expression in adenovirus type 19-infected human corneal fibroblasts. Invest Ophthalmol Vis Sci. 2005;46(10):3777–3782. doi: 10.1167/iovs.05-0724. [DOI] [PubMed] [Google Scholar]
- Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell. 2007;130(4):601–610. doi: 10.1016/j.cell.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Younghusband HB, Tyndall C, Bellett AJ. Replication and interaction of virus DNA and cellular DNA in mouse cells infected by a human adenovirus. J Gen Virol. 1979;45(2):455–467. doi: 10.1099/0022-1317-45-2-455. [DOI] [PubMed] [Google Scholar]
- Yousuf MA, Zhou X, Mukherjee S, Chintakuntlawar AV, Lee JY, Ramke M, Chodosh J, Rajaiya J. Caveolin-1 associated adenovirus entry into human corneal cells. PLoS One. 2013;8(10):e77462. doi: 10.1371/journal.pone.0077462. [DOI] [PMC free article] [PubMed] [Google Scholar]