Abstract
Background
The presence of small dense LDL is associated with obesity and type 2 diabetes and an increased risk for cardiovascular disease. Apolipoprotein C-III (apoC-III) is involved in the formation of small dense LDL, but the exact mechanisms are still not well defined. ApoC-III is a glycosylated apolipoprotein, with three major glycoforms: apoC-III0, apoC-III1, and apoC-III2 that contain zero, one, or two molecules of sialic acid, respectively. In our previous work, we reported an association among apoC-III0 and apoC-III1, but not apoC-III2 with fasting plasma triglyceride levels in obesity and type 2 diabetes.
Objective
The goal of this study was to determine the relationship between changes in the major apoC-III glycoforms and small dense LDL levels after dietary interventions.
Methods
Mass spectrometric immunoassay (MSIA) was performed on fasting plasma samples from 61 subjects who underwent either a high carbohydrate diet (n=34) or a weight loss intervention (n=27).
Results
After both dietary interventions, changes in total apoC-III concentrations were associated with changes in LDL peak particle diameter (r= −0.58, p<0.0001). Increases in total apoC-III concentrations following the high carbohydrate diet were associated with decreases in LDL size (r= −0.53, p=0.001), and decreases in apoC-III concentrations following weight loss were associated with increases in LDL peak particle diameter (r= −0.54, p=0.004). Changes in concentrations of apoC-III1 and apoC-III0, but not apoC-III2, were associated with changes in LDL peak particle diameter in both the weight loss and high carbohydrate interventions.
Conclusion
We conclude that apoC-III0 and apoC-III1, but not apoC-III2 are associated with the formation of small dense LDL.
Keywords: Low density lipoprotein, metabolism, Apolipoprotein C-III, mass spectrometry, sialylation
Introduction
In the US, about two thirds of the population is estimated to be either obese or overweight (1). Obesity is associated with increased risk of type 2 diabetes and cardiovascular disease (CVD) (2). One obesity-associated CVD risk factor is increased concentration of small dense low-density lipoprotein (LDL) particles (3). Type 2 diabetes and metabolic syndrome are known to be associated with higher incidence of the small dense LDL phenotype (4). LDL size is influenced both genetically and by dietary or weight loss interventions (5, 6). High carbohydrate diets are associated with increased plasma levels of small dense LDL(7). In contrast, weight loss interventions are associated with an increase in LDL particle size that shifts from small dense to larger and more buoyant(5).
The mechanism(s) for the formation of small dense LDLs are not well understood, however apoC-III appears to be implicated in their formation (8, 9). A high carbohydrate diet increased the production and reduced the plasma clearance of VLDL particles (10). The changes in VLDL metabolism are attributed in part to increases in plasma apoC-III concentrations (11). VLDLs are the main precursors of LDLs, and higher VLDL levels promote the production of small dense LDL at least in part by the action of hepatic lipase (9). By this mechanism, apoC-III appears to influence the formation of smaller denser LDL (8). ApoC-III rich small dense LDL are also more prone to bind arterial proteoglycans, thereby increasing the affinity of small dense LDL to arterial walls and contributing to the atherogenicity of apoC-III (12–14).
ApoC-III exists in several different glycoforms in plasma; the most common glycosylated forms are apoC-III0, apoC-III1 and apoC-III2. These glycoforms differ by their sialic acid content in the glycan motif, having zero, one and two sialic acid residues correspondingly, attached to a glycan core of N-acetylgalactosamine (Gal-NAc) and galactose (Gal) (15). Sialylation occurs in the Golgi apparatus, and is mediated by a group of sialyltransferases, whereas lysosomal neuraminidase regulates the desialylation (16). Assessment of apoC-III’s sialylated glycoforms using traditional approaches such as isoelectric focusing is time consuming and not amenable to large sample sizes. We developed a mass spectrometric immunoassay (MSIA) to assess apoC-III glycoforms in plasma (15, 17). MSIA allows for identification of 12 different apoC-III glycoforms that differ by their content of galactose (Gal), N-acteyl galactosamine (GalNAc), fucose, alanine truncations or sialic acid residues in a single assay and in a high throughput manner.
The degree of apoC-III sialylation is under metabolic control. In previous work, we reported an association of apoC-III0 and apoC-III1, but not apoC-III2 with fasting plasma triglyceride (TG) levels in a group of obese youth with high TG levels (15), and in the setting of type 2 diabetes(18). Decreased apoC-III2 to apoC-III1 ratio was also observed in adults with metabolic syndrome (19). The apoC-III2 to apoC-III1 ratio was reduced following weight loss by caloric restriction (20), or bariatric surgery (21). In contrast, increased apoC-III0 was observed after carbohydrate feeding in rats and humans (22, 23). We hypothesized that apoC-III0 and apoC-III1, but not apoC-III2 were associated with small dense LDL peak particle diameter in samples from individuals who either underwent a high carbohydrate diet (carb), or diet-induced weight loss intervention.
Materials and Methods
IRB
The studies were approved by the Lawrence Berkeley Laboratory at University of California, and by the Children’s Hospital Oakland Research Institute IRBs.
Participants
This is a post-hoc analysis of samples obtained from two dietary interventions (weight loss or high carbohydrate). The participants had no history of cardiovascular disease or other chronic diseases and were not taking medications that affect lipid metabolism, blood thinning agents or hormones. All participants had a BMI between 25 kg/m2 and 30 kg/m2, LDL cholesterol lower than 95th percentile, TG concentration <500mg/dl, fasting glucose concentration <126mg/dl, systolic blood pressure <150 mm Hg, diastolic blood pressure < 90 mm Hg, were non-smokers, and did not drink during the duration the study was performed.
Weight loss intervention
Plasma samples were obtained from a previously conducted study (5), in which over a 3-year period, 133 overweight men (BMI:25–30 kg/m2) were enrolled in a dietary intervention program designed to reduce weight by ∼9 kg (weight loss arm; n = 96) or maintain weight (control arm; n = 37). The diet was designed to provide 40% carbohydrate, 40% fat (14% saturated, 19% monounsaturated, and 7% polyunsaturated), and 20% protein over 6-day cycles. Men were recruited based on LDL subclass pattern (A-large buoyant, or B-small dense), placed on a run-in study diet for 3 weeks, and randomized into the two arms of the study. Men in the weight loss arm followed a 9-week weight loss program followed by a 4-week weight-stable period. ApoC-III measurements were performed on available plasma obtained from a subset of overweight men in the weight loss arm who converted from pattern B to pattern A LDL (n=15) and in men who continued to express pattern B LDL (n=12) after achieving a BMI of less than 25kg/m2 through diet.
High carbohydrate dietary intervention
Plasma was obtained after overnight fast from 34 men who had undergone an isocaloric dietary intervention consisting of a low carbohydrate (carb) diet followed by a high carb, and who were determined to have LDL pattern A on the low carb diet. The diets were consumed for either 3 week (n=10) or 4 week (n=24); the results did not differ for these groups, and were therefore pooled. The dietary protocol was administered on an outpatient basis as described for a previous study [7]. In brief, the low carb diet was designed to supply 45% of calories from carb and 40% from fat (13.0% saturated, 11.0% monounsaturated, 13.8% polyunsaturated, and 3.4% trans), with 15% protein. The high carb diet was designed to supply 65% of calories from carb and 20% of calories from fat (4.9% saturated, 9.9% monounsaturated, 5.1% polyunsaturated, and 2.4% trans), with 15% protein. There were no differences in dietary cholesterol and the ratio of simple:complex carbohydrate was ≈50:50 with both diets. ApoC-III measurements were performed on available plasma selected from participants who converted from pattern A to pattern B LDL (n=14) and who continued to express pattern A LDL (n=20) after completing the carb intervention.
Laboratory Measurements
Plasma samples were prepared within 2 h of collection from venous blood collected in tubes containing Na2EDTA (1.4 g/l) and a preservative cocktail of protease and bacterial inhibitors. Blood and plasma were kept at 4 °C throughout processing. Plasma total cholesterol (TC) and TG concentrations were determined by enzymatic procedures on an Express 550 Plus analyzer (Ciba Corning, Oberlin, OH). These measurements were consistently in control as monitored by the Centers for Disease Control and Prevention—National Heart, Lung, and Blood Institute standardization program. HDL cholesterol was measured after dextran sulfate precipitation of plasma. LDL cholesterol was calculated from the formula of Friedewald et al. with all plasma samples in this study having TG concentrations <400 mg/dl(24). Apolipoproteins A-I (apoA-I) and B (apoB) were measured by immunoturbidimetric assay (Express 550 Plus Analyzer; Bacton Assay Systems, San Marcos, CA).
LDL phenotype
Non-denaturing polyacrylamide gradient gel electrophoresis with lipid staining of plasma was performed as described previously for determination of peak LDL particle diameter and LDL subclass patterns A and B (7). Pattern B is characterized by a major peak of smaller, denser LDL particles (LDL-III, diameter 255A0 or less), often with skewing to larger particle diameters. Pattern A is characterized by a predominance of larger, more buoyant LDL particles (LDL-I or II diameter 264A0 or greater), often with skewing to smaller particle diameters (25).
ApoC-III ELISA
Total apoC-III concentrations were determined by sandwich ELISA. ELISA was performed as previously described (15), using apoC-III affinity purified antibodies and apoC-III protein standard from Academy Biomedical (apoC-III (33A-G2b), and HRP Goat Anti-Human apoC-III (33H-G2a)). ApoC-III antibody was tested for cross reactivity and there was no evidence that apoC-III antibody cross reacted with any of the analyzed apolipoproteins (apoC-II, apoC-I). The inter- and intra-assay coefficients of variation were less than 10%.
MSIA
Measurements of apoC-III glycoforms were performed using triplexed mass spectrometric immunoassay (MSIA) for analysis of apoC-I, apoC-II and apoC-III, as previously described (17). In short, affinity pipettes were first complexed with corresponding antibodies (0.4, 2.25 and 2.5 µg of anti-apoC-I, anti-apoC-II and anti-apoC-III per pipette respectively). Samples (1 µL plasma) were diluted 120-fold in PBS, 0.1%Tween, and then a Multimek 96-channel robot was used to capture all apoC glycoforms from each analytical sample by repeated aspirations through the pipette tip. Non-specifically bound proteins were rinsed away, whereas captured apolipoproteins were eluted directly onto a 96-well formatted MALDI target using a sinapinic acid matrix. Using an Ultraflex III MALDI-TOF instrument (Bruker, Billerica, MA) operating in positive ion mode, linear mass spectra were acquired from each sample spot. On average, 5000 laser shots mass spectra were saved for each sample. Obtained mass spectra were internally calibrated using protein calibration standard-I, and further processed with Flex Analysis 3.0 software (Bruker Daltonics). All peaks representing apolipoproteins and their glycoforms were integrated baseline-to-baseline using Zebra 1.0 software (Intrinsic Bioprobes Inc.), and the obtained peak area values were tabulated. The peak areas were corrected individually with baseline noise-bin signals. The abundance of each apoC-III glycoform was calculated as relative percentage of the total apoC-III. MSIA can detect a total of 12 apoC-III glycoforms (Supplementary Figure 1), which present with variations in the content of galactose (Gal), N-acteyl galactosamine (GalNAc), fucose, alanine truncations or sialic acid residues. Because of the higher abundance and potential functional importance of the sialylations, analysis of apoC-III was largely restricted to these glycoforms. Characteristics of apoC-III glycoforms identified by MSIA are summarized in Supplementary Table 1. The concentration of each glycoform was obtained by multiplying the relative abundance of each apoC-III glycoform obtained by MSIA with total apoC-III concentrations obtained by the ELISA. The four glycoforms analyzed in this study were apoC-III0a, and apoC-III0b, apoC-III1, apoC-III2 given the functional relevance of apoC-III sialylation and the lower abundance of the other glycoforms. ApoC-III0a corresponds to the full-length sequence of unmodified apoC-III protein (MW = 8765 Da), and apoC-III0b has galactose and GalNAc residues. Plasma apoC-III1 was greatest in abundance, followed by apoC-III0b and apoC-III2, whereas apoC-III0a concentrations were least in abundance.
Statistical Analysis
Based on a previous study that examined total apoC-III levels in triglyceride-rich particles (TRL) after a high carbohydrate diet (7), apoC-III concentrations increased by 22 µg/mL with a SD of 36. This translates into an effect size of 0.61. With 34 participants who completed the high carb dietary program, we estimated that we will have 93% power of detecting a significant change in total apoC-III after the intervention with a two-sided alpha=0.05. We expected a similar effect size for the weight loss program on decreasing apoC-III concentrations. The goal of the study was to test the association of apoC-III glycoforms with conversion or regression from small dense LDL peak particle diameter (pattern A or pattern B) during the weight loss or the high carbohydrate dietary interventions, and these two groups were pre-specified. Data were summarized as means and SD for normally distributed data, and medians and interquartile range for TG levels (not normally distributed). Changes in apoC-III glycoforms, LDL size, TG levels and HDL cholesterol were defined as the difference between the measurements at baseline and follow-up. Comparisons at baseline, as well as the changes pre- and post-treatment were made using a paired t-test or using a multivariate linear regression model to allow for adjustment of baseline variables or covariates. The dependent variable in the model was LDL particle size. The independent variables were the apoC-III glycoforms. The main covariates were TG, HDL-C, apoB and apoA-1 concentrations. Correlations coefficients were presented using Pearson (normally distributed) or Spearman correlation coefficient (skewed distributions). Statistical significance for the four apoC-III glycoforms was defined by p value <0.0125 to account for multiple comparisons (0.05/4 glycoforms examined). Statistical program R version 3.3 was used to perform the data analyses.
Results
Baseline characteristics of the study groups
ApoC-III glycoform concentrations were measured in a total of 61 samples from predominantly white middle-aged males, with no known history of cardiovascular disease or other chronic conditions. Characteristics of the two study populations before intervention (carb diet or pre-weight loss) are shown in Table 1. By design, all men in the weight loss group had pattern B LDL, whereas all the men in the high carb group had pattern A LDL, as reflected by a significant difference in peak particle diameter. As expected, the weight loss group had higher BMI, higher total cholesterol, lower HDL cholesterol, lower apoA-I concentrations, and higher apoB and LDL cholesterol concentrations when compared to the high carb group. Total plasma apoC-III concentrations were higher in participants with pattern B LDL in comparison to those with pattern A LDL
Table 1.
Characteristics of participants
| Measurement | High carb Intervention (n=34) |
Weight Loss Intervention (n=27) |
p-value |
|---|---|---|---|
| Age (years) | 47.79 (12.51) | 48 (7.57) | 0.9 |
| LDL Size (A0) | 267.98 (4.11) | 250.96 (3.31) | <.0001 |
| BMI (kg/m2) | 25.86(2.97) | 27.12 (1.11) | 0.03 |
| TG (mg/dL) | 93.00 (73,119) | 182.00 (144.4, 217) | <.0001 |
| TC (mg/dL) | 178.32 (28.71) | 201.81 (23.00) | 0.0008 |
| LDL-C (mg/dL) | 115.97 (24.85) | 123.33 (25.05) | 0.26 |
| HDL-C (mg/dL) | 42.76 (8.41) | 37.58 (8.24) | 0.02 |
| ApoB (ug/mL) | 88.39 (16.02) | 109.03 (14.98) | <.0001 |
| ApoA-I (ug/mL) | 110.09 (15.56) | 98.48 (14.28) | 0.004 |
| ApoC-III Total (ug/mL) | 77.58 (17.03) | 86.92 (11.28) | 0.012 |
| ApoC-III0a (ug/mL) | 5.57 (3.10) | 6.50 (2.76) | 0.2 |
| ApoC-III0b (ug/mL) | 14.78 (3.63) | 17.76 (2.70) | <.0001 |
| ApoC-III1 (ug/mL) | 39.38 (9.45) | 45.29 (5.82) | 0.004 |
| ApoC-III2 (ug/mL) | 10.73(3.37) | 10.60 (2.96) | 0.87 |
Means (SD) for normally distributed variables or Means (interquartile range) for non-normal distribution
Groups were compared using a Wilcoxon T-test
BMI: Body mass index. TG: Triglyceride. TC: Total cholesterol
Association between apoC-III glycoforms and LDL size at baseline
In all the study participants, a significant inverse association was observed at baseline between LDL peak particle diameter and the total apoC-III concentration (n=61, r=-0.28, p=0.02). This association was driven by apoC-III1 (n=61, r=-0.35, p=0.005) and apoC-III0b (n=61, r=-0.43, p<0.001), but not apoC-III2 (n=61, p=0.6) and apoC-III0a (n=61, p=0.7). A significant positive association between TG and total apoC-III concentration (n=61, r=0.28, p=0.02), apoC-III1(n=61, r=0.36, p=0.005) and apoC-III0b (n=61, r=0.42, p=0.0007), but not apoC-III2 (n=61, r=-0.1, p=0.4), or apoC-III0a (n=61, r=0.1, p=0.4) was observed. Using a multivariate regression analysis with LDL peak particle diameter as the dependent variable, the association with apoC-III1 (p=0.09), apoC-III0b (p=0.2) did not persist after adjusting for fasting plasma TG concentrations.
Association between the change in apoC-III glycoforms and the change in LDL size
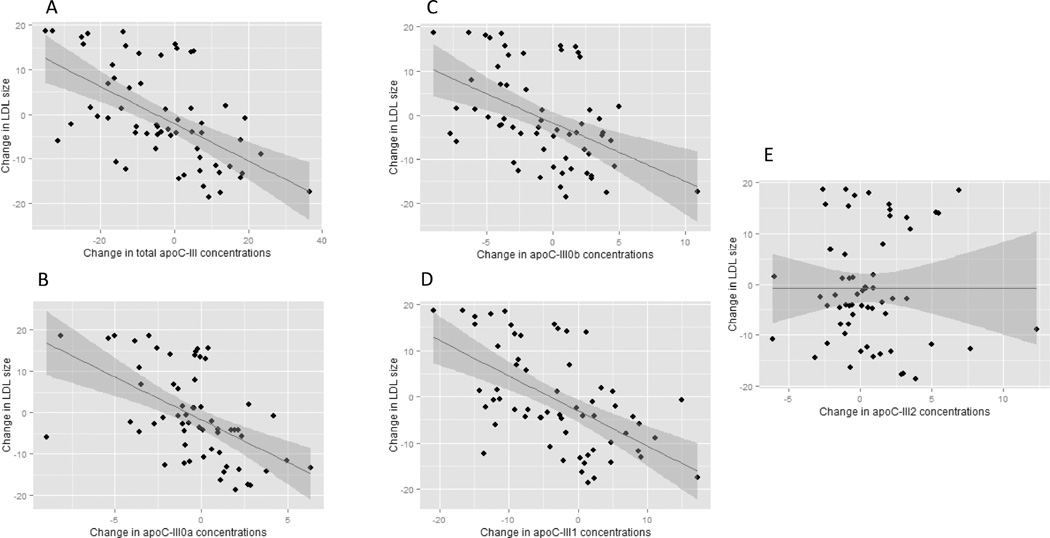
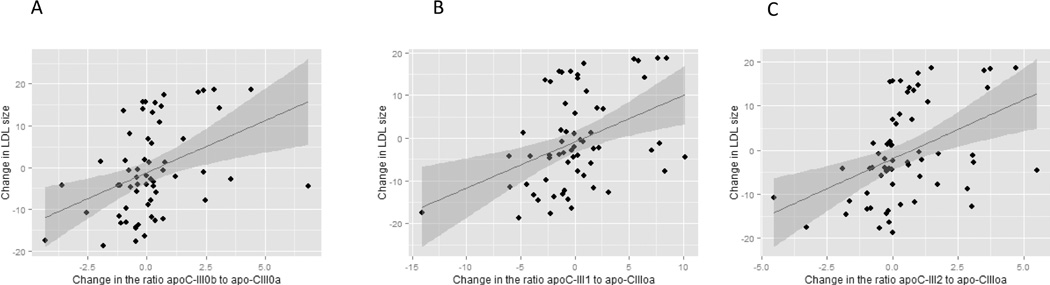
After both dietary interventions, changes in total apoC-III concentrations were associated with changes in LDL peak particle diameter (n=61, r= −0.58, p<0.0001, Figure 1A). Increases in total apoC-III concentrations following the high carb diet were associated with decreases in LDL size (n=34, r= −0.53, p=0.001), and decreases in apoC-III concentrations following weight loss were associated with increases in LDL peak particle diameter (n=27, r= −0.54, p=0.004). The changes in apoC-III glycoforms concentrations after the weight loss or high carb diet are presented in Table 2. The analysis of the individual apoC-III glycoforms revealed that changes in LDL peak particle diameter were significantly associated with changes in apoC-III0a (n=61, r=-0.57, p<0.001, Figure 1B), apoC-III0b (n=61, r=-0.48, p< 0.001, Figure 1C) and apoC-III1 (n=61, r=-0.53, p< 0.0001, Figure 1D) but not apoC-III2 (n=61, p=0.99, Figure 1E). The ratio of glycosylated apoC-III to native apoC-III was computed. Changes in apoC-III0b/ apoC-III0a (r=0.39, p=0.002, Figure 2A), apoC-III1/ apoC-III0a (r=0.41, p=0.001, Figure 2B) and apoC-III2/ apoC-III0a (r=0.43, p=0.0005, Figure 2C) were significantly associated with greater changes in LDL peak particle size, indicating that the addition of galactose, one sialic and two sialic acids attenuate the association of apoC-III with small LDL peak particle size.
Figure 1.
The association of the change in LDL size with the change in apoC-III glycoform concentrations following high carbohydrate diet and weight loss interventions (n=61). With the exception of apoC-III2, an inverse association was observed between the change in apoC-III glycoform concentrations and the change in LDL size after both interventions.
Table 2.
Effect of high carb diet and weight loss on LDL size and apoC-III proteoforms
| Low Carb Pre (n=34) |
High Carb Post (n=34) |
p- value |
Weight Loss Pre (n=27) |
Weight Loss Post (n=27) |
p- value |
|
|---|---|---|---|---|---|---|
| ApoC-III Total (ug/mL) |
77.58 (17.03) | 78.91 (17.06) | 0.6 | 86.92 (11.28) | 77.90 (5.90) | <.001 |
| ApoC-III2 (ug/mL) |
10.73(3.37) | 11.12 (4.00) | 0.5 | 10.60 (2.96) | 11.51 (3.48) | 0.2 |
| ApoC-III1 (ug/mL) |
39.38 (9.45) | 39.11 (9.91) | 0.9 | 45.29 (5.82) | 38.85 (4.60) | <.0001 |
| ApoC-III0a (ug/mL) |
5.57 (3.10) | 5.91 (3.18) | 0.5 | 6.50 (2.76) | 5.11 (2.19) | <.001 |
| ApoC-III0b (ug/mL) |
14.78 (3.63) | 14.92 (4.10) | 0.8 | 17.76 (2.70) | 15.95 (2.05) | <.001 |
| LDL Size (A0) |
267.98 (4.11) | 260.39 (7.39) | <.0001 | 250.96 (3.31) | 258.82 (10.44) | <.0001 |
| BMI (kg/m2) | 25.86(2.97) | 25.70 (2.90) | 0.6 | 27.12 (1.11) | 24.72 (1.15) | <.0001 |
| TG (mg/dL) | 98.05 (33.15) | 109.5 (40.50) | 0.2 | 204.44 (86.37) | 150.70 (68.21) | 0.002 |
| TC (mg/dL) | 178.32 (28.71) | 177.94 (30.61) | 0.9 | 201.81 (23.00) | 199 (22.72) | 0.6 |
| LDL-C. (mg/dL) |
115.97 (24.85) | 115.08 (27.75) | 0.8 | 123.33 (25.05) | 125.33 (21.31) | 0.7 |
| HDL-C (mg/dL) |
42.76 (8.41) | 40.88 (9.66) | 0.2 | 37.58 (8.24) | 43.54 (11.21) | 0.007 |
| ApoB (mg/dL) |
88.39 (16.02) | 91.41 (17.00) | 0.2 | 109.03 (14.98) | 87.48 (13.30) | <.0001 |
| ApoA-I (mg/dL) |
110.09 (15.56) | 107.29 (14.35) | 0.2 | 98.48 (14.28) | 118.92 (19.00) | <.0001 |
Means (SD)
Groups pre post were compared using a Paired T-test
BMI: Body mass index. TG: Triglyceride. TC: Total cholesterol
Figure 2.
The association of the change in LDL size with the change in the ratios of apoC-III0b, apoC-III1 and apoC-III2 to apoC-III0a glycoform following high carbohydrate diet and weight loss interventions (n=61). These results indicate that the addition of galactose and galNAC, one sialic and two sialic acids attenuate the association of greater apoC-III concentrations with lower LDL peak particle size.
Change in apoC-III glycoforms differed by LDL pattern groups
The change in apoC-III0a, apoC-III0b and apoC-III1 differed significantly between converters and non-converters in both groups (all, p<0.001). Changes in TG levels were associated with changes in apoC-III0a, apoC-III0b, and apoC-III1 concentration but not with changes in apoC-III2. In a multivariate regression analysis with peak particle diameter as the dependent variable and apoC-III glycoforms, TG, HDL, ApoA-1, apoB as covariates, changes in only apoC-III1 and HDL cholesterol were independently associated with changes in LDL peak particle diameter.
Effect of the high carb dietary intervention on apoC-III glycoforms
The changes in apoC-III glycoforms were analyzed in 14 converters to pattern B and 20 non-converters after the high carb diet (Table 3). The changes in apoC-III differed between the converters and non-converters to pattern B LDL (p=0.009), and was independent of baseline apoC-III levels (p<0.01). In the converters group, concentrations of apoC-III1, apoC-III0a and apoC-III0b were significantly increased, whereas apoC-III2 levels did not change. Converters also demonstrated increases in TG, and apoB concentrations and decreases in HDL cholesterol and apoA-I concentrations compared to non-converters. In a multivariate model with the change in LDL peak particle diameter as the dependent variable, the effect of apoC-III glycoforms on particle size was attenuated after adjusting to changes in HDL-C and TG concentrations: apoC-III1 (p=0.08), apoC-III0a (p=0.09) and apoC-III0b (p=0.04). Among the non-converters, there was a small but significant decrease in LDL peak particle diameter that was not associated with changes in apoC-III glycoforms, TG or HDL cholesterol.
Table 3.
Effect of high carb diet on LDL size and apoC-III proteoforms by LDL conversion groups
| Pattern A Low Carb (n=14) |
Pattern B High Carb (n=14) |
p-value | Pattern A Low Carb (n=20) |
Pattern A High Carb (n=20) |
p value |
|
|---|---|---|---|---|---|---|
| ApoC-III Total (ug/mL) |
72.55 (13.50) | 83.65 (18.18) | 0.004 | 81.1 (18.64) | 75.59 (15.84) | 0.11 |
| ApoC- III2 (ug/mL) |
9.32 (3.05) | 10.63 (4.54) | 0.04 | 11.72 (3.30) | 11.47 (3.65) | 0.96 |
| ApoC- III1 (ug/mL) |
38.47 (8.57) | 42.47 (10.91) | <.0001 | 40.01 (10.19) | 36.76 (8.66) | 0.17 |
| ApoC- III0a (ug/mL) |
4.63 (2.71) | 6.70 (3.29) | 0.004 | 6.23 (3.24) | 5.35 (3.06) | 0.44 |
| ApoC- III0b (ug/mL) |
13.88 (2.38) | 16.48 (3.42) | 0.008 | 15.41 (4.24) | 13.83 (4.26) | 0.26 |
| LDL Size (A0) |
266.007 (1.95) | 253.42 (5.30) | <.0001 | 269.37 (4.68) | 265.27 (3.87) | <.0001 |
| BMI (kg/m2) |
26.52 (3.09) | 26.47 (3.12) | 0.3 | 25.405 (2.87) | 25.17 (2.69) | 0.03 |
| TG (mg/dL) |
94.92 (37.66) | 143.07 (35.58) | 0.0007 | 100.25 (30.44) | 86 (23.90) | 0.126 |
| TC (mg/dL) |
169.21 (29.51) | 174.5 (30.88) | 0.07 | 184.7 (27.05) | 180.35 (30.99) | 0.778 |
| LDL-C. (mg/dL) |
110.92 (25.39) | 112.21 (27.33) | 0.147 | 119.5 (24.48) | 117.1 (28.56) | 0.05 |
| HDL-C (mg/dL) |
39.28 (6.25) | 33.57 (5.91) | <.0001 | 45.2 (9.00) | 46 (8.44) | 0.15 |
| ApoB (mg/dL) |
85.28 (18.54) | 98.21 (16.95) | <.0001 | 90.68 (13.96) | 86.65 (15.72) | 0.05 |
| ApoA-I (mg/dL) |
103.5 (12.20) | 100.5 (12.81) | 0.0012 | 114.94 (16.25) | 112.05 (13.70) | 0.13 |
Means (SD)
Groups pre and post were compared using a paired T-test
BMI: Body mass index. TG: Triglyceride. TC: Total cholesterol
Effect of the weight loss dietary intervention on apoC-III glycoforms
Changes in apoC-III glycoforms were analyzed in 15 participants who converted from pattern B to pattern A, and in 12 participants who remained at pattern B after the intervention (Table 4). The amount of weight loss did not differ between the groups, and was associated with a significant increase in LDL size, HDL-C and apoA-I concentrations, and a decrease in TG and apoB concentrations. Participants who converted to pattern A had significant decreases in TG, and increases in HDL-C and apoA-I concentrations. ApoC-III0a, apoC-III0b, and apoC-III1, but not apoC-III2, decreased after the weight loss intervention in both converters and non-converters. The change in apoC-III glycoforms differed between those who converted to pattern A, or remained at pattern B (p=0.02). ApoC-III0a, apoC-III0b, and apoC-III1 but not apoC-III2 concentrations decreased significantly more in converters compared to non-converters. Using a multivariate regression model, apoC-III1 and HDL-C (p<0.001 for both) concentrations were the only significant predictors of LDL peak particle diameter following weight loss, independent of changes in TG levels, or the other variables. Representative mass spectra from participants who converted from pattern A to pattern B after the high carb diet and pattern B to pattern A after weight loss illustrating the change in apoC-III0a, apoC-III0b, and apoC-III1 in relation to apoC-III2 are presented in Supplemental Figure 2.
Table 4.
Effect of weight loss on LDL size and apoC-III proteoforms by LDL conversion groups
| Pattern B Pre (n=15) |
Pattern A Post (n=15) |
p-value | Pattern B Pre (n=12) |
Pattern B Post (n=12) |
p-value | |
|---|---|---|---|---|---|---|
| ApoC-III Total (ug/mL) |
91.29 (10.55) | 77.7 (4.84) | 0.001 | 81.45 (10.02) | 78.15 (7.24) | 0.14 |
| ApoC-III2 (ug/mL) |
11.06 (3.45) | 12.76 (3.69) | 0.037 | 10.03 (2.21) | 9.94 (2.55) | 0.96 |
| ApoC-III1 (ug/mL) |
47.39 (5.57) | 37.77 (4.29) | <.0001 | 42.68 (5.21) | 40.21 (4.81) | 0.17 |
| ApoC-III0a (ug/mL) |
7.17 (2.97) | 4.89 (2.41) | 0.004 | 5.66 (2.34) | 5.38 (1.95) | 0.44 |
| ApoC-III0b (ug/mL) |
18.3 (2.94) | 15.55 (1.61) | 0.008 | 17.08 (2.32) | 16.46 (2.49) | 0.26 |
| LDL Size (A0) |
252.39 (2.41) | 267.47 (3.60) | <.0001 | 249.18 (3.52) | 248.01 (3.46) | <.0001 |
| BMI (kg/m2) |
26.89 (1.07) | 24.09 (0.63) | <.0001 | 27.42 (1.14) | 25.51 (1.20) | <.0001 |
| TG (mg/dL) |
172.6 (63.88) | 104.86 (23.78) | 0.0007 | 244.25 (96.58) | 208 (61.91) | 0.126 |
| TC (mg/dL) |
210.46 (23.68) | 199.2 (24.07) | 0.07 | 191 (17.52) | 198.75 (21.97) | 0.778 |
| LDL-C. (mg/dL) |
134.6 (22.09) | 127.53 (22.05) | 0.147 | 109.25 (21.76) | 122.58 (20.97) | 0.05 |
| HDL-C (mg/dL) |
41.36 (8.77) | 50.66 (8.89) | <.0001 | 32.86 (4.37) | 34.63 (6.40) | 0.16 |
| ApoB (mg/dL) |
111.53 (15.87) | 81.33 (11.96) | <.0001 | 105.91 (13.82) | 95.16 (10.95) | 0.05 |
| ApoA-I (mg/dL) |
99.6 (16.77) | 126.46 (18.62) | 0.001 | 97.08 (10.98) | 109.5 (15.44) | 0.13 |
Means (SD)
Groups pre and post were compared using a paired T-test
BMI: Body mass index. TG: Triglyceride. TC: Total cholesterol
Discussion
This study provides the first detailed characterization of apoC-III glycoforms following weight loss or high carbohydrate dietary interventions in relation to changes in peak LDL particle diameter. Our findings demonstrate that changes of apoC-III1, apoC-III0a and apoC-III0b, but not apoC-III2 were associated with changes in LDL peak particle diameter in both interventions. We have previously reported that apoC-III1 apoC-III0a and apoC-III0b but not apoC-III2 associate with fasting TG levels in a group of obese prediabetic individuals (15) and in patients with type II diabetes (18). We now demonstrate that these apoC-III glycoforms associate with the changes in small dense LDL using two interventions that promoted (high carb diet) or reversed (weight loss) small dense LDL phenotype.
Although LDL subclass pattern B is, in part, genetically influenced (26), it can also be significantly modulated by environmental factors, including dietary carbohydrate intake and adiposity (6). High carbohydrate diet impairs the clearance of VLDL particles(10). There is evidence to support that the effect of such a diet on VLDL clearance is mediated at least in part by apoC-III metabolism. High carbohydrate intake enhances the expression of apoC-III through mechanisms that involve insulin and glucose signaling pathways (27–29). Using kinetic studies, the Sacks group demonstrated that the kinetics of apoC-III in plasma determine the clearance of TRL; possibly through interactions with apoE (8). In normotriglyceridemic participants, the majority of TRL is secreted together with apoE and undergoes fast removal from the circulation by apoE-dependent liver uptake mechanisms. In contrast, in patients with hypertriglyceridemia, TRL metabolism shifts from an apoE-dominated system to an apoC-III–dominated system, which contributes to reduced VLDL clearance. Formation of small dense LDL is linked to apoC-III via increased flux of the overproduced apoC-III–containing VLDL through lipolytic pathways involving hepatic lipase.
The observation that weight loss and increased carbohydrate intake induce opposing phenotypic response on LDL size in conjunction with modulation of apoC-III glycoform metabolism indicate that sialylations may influence changes in LDL size phenotypes. Falko et al. investigated changes in apoC-III glycoforms after a high carb diet for 7 days (23). The investigators identified increases in apoC-III0 and apoC-III1 but not in apoC-III2 in both plasma and VLDL. Similar to the human study, rats fed a high carbohydrate diet selectively increased apoC-III0 and apoC-III1, but not apoC-III2 in plasma and liver perfusates (22). In a glycome biomarker study, the ratio of apoC-III2 to apoC-III1 increased 90 days after gastric bypass in 44 participants(21). Moreover, severe caloric restriction in women increased the ratio of apoC-III2 to the other apoC-III glycoforms (20). These findings support that the sialylation profile of apoC-III changes after carbohydrate feeding or weight loss interventions.
Sialylation may alter the catabolism of apoC-III glycoforms. Faster catabolism of apoC-III2-containing TRL could account for the lack of association with small LDL density patterns or with elevated TG levels. Sialylation alters the charge of apoC-III on TRL by conferring a negative charge. It is known that apoC-III inhibits apoE-mediated TRL binding and uptake by hepatic receptors (30, 31). The altered charge of apoC-III conferred by disialylation could impede its ability to compete against apoE for TRL particle binding, resulting in reduced inhibition of apoE-mediated delivery of TRL to receptors. We and others have demonstrated that apoC-III2 is a less efficient inhibitor of VLDL cellular uptake compared with apoC-III0 or apoCIII1 on hepatic receptors ex vivo (18, 32). This is also demonstrated with sialylation of apoE enhancing the uptake of HDL in HepG2 cells (33). The role of apoC-III sialylation on lipid metabolism in vivo merits additional studies.
It is interesting to note a heterogeneous response in LDL patterns to weight loss and high carb diet interventions. In the weight loss study, 58% of participants with pattern B LDL who successfully lost weight converted to pattern A(5), and in the high carb intervention, 41% participants converted from pattern A to pattern B(7). It is likely that genetic polymorphisms (such as single nucleotide polymorphisms-SNPs) influence the response to variation in dietary interventions. GWAS analysis identified significant associations of pattern B LDL with genetic variants in 14 different genes. One of the significant SNPs is rs964184, which is located in the APOA5/C3 gene locus (34). In addition, Human GWAS studies also implicate GALNT2 as an enzyme that regulates HDL and TG metabolism (35), potentially by modulating apoC-III glycosylation (36). GALNT2 enzyme carries the first step of apoC-III glycosylation by adding galactose and galNAC to native apoC-III. Carriers of GALNT2 gene variants have altered apoC-III sialylation patterns (37).
This study has some limitations. This is a post-hoc analysis of samples from previously completed studies in which eligible participants underwent dietary interventions. However, these studies were carefully designed, and samples were pre-specified from participants who responded or did not respond to the dietary interventions to better assess the association between apoC-III glycoforms and LDL peak particle size. Ou findings revealed internal consistency in the change in apoC-III glycoforms across the two groups.
A second limitation is that the study was conducted only in white men; therefore, our results may not apply to women or persons from different ethnicities. Samples for these the current analyses were stored for many years at −80 °C before measurements of apoC-III glycoforms were performed. Previous investigation of the effects of storage time and freeze/thaw cycles on these assays has indicated that the measurements are relatively stable (15, 17).
In conclusion, our study demonstrates that apoC-III1, apoC-III0a, and apoC-III0b, but not apoC-III2 are associated with changes in small dense LDL after the dietary interventions. This study was limited by virtue of the specific population recruited and the interventions used. Our findings support that sialylation of apoC-III has functional relevance to lipoprotein metabolism. Strategies that modify apoC-III should take into account its sialylation profile.
Supplementary Material
Highlights.
Apolipoprotein C-III (apoC-III) is associated with the formation of small dense LDL.
Plasma apoC-III exists in multiple glycoforms that contain zero, one, or two molecules of sialic acid.
Di-sialylated apoC-III is not associated with small dense LDL after dietary interventions.
Acknowledgments
Robin S. Rawlings for assistance with supervising clinical protocols, Harriet S. Fernstrom for devising and administering diets, and Patricia J. Blanche, Laura Holl, Joseph Orr, Bahareh Sahami, and Michael Chu for technical assistance.
Sources of support: Dr. Yassine was supported by K23HL107389 from National Institute of Heart, Lung and Blood, 15BGIA25690024 from the American Heart Association and USC CTSI pilot UL1TR000130. Mass spectrometry work was supported by Awards R01DK082542 and R24DK090958 from the National Institute of Diabetes And Digestive and Kidney Diseases. Dr. Krauss was supported from a grant from the National Dairy Council.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no conflicts of interest.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA : the journal of the American Medical Association. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Executive summary: heart disease and stroke statistics—2012 update a report from the American Heart Association. Circulation. 2012;125(1):188–197. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 3.Krauss RM. All low-density lipoprotein particles are not created equal. Arteriosclerosis, thrombosis, and vascular biology. 2014;34(5):959–961. doi: 10.1161/ATVBAHA.114.303458. [DOI] [PubMed] [Google Scholar]
- 4.Feingold KR, Grunfeld C, Pang M, Doerrler W, Krauss RM. LDL subclass phenotypes and triglyceride metabolism in non-insulin-dependent diabetes. Arteriosclerosis, thrombosis, and vascular biology. 1992;12(12):1496–1502. doi: 10.1161/01.atv.12.12.1496. [DOI] [PubMed] [Google Scholar]
- 5.Siri-Tarino PW, Williams PT, Fernstrom HS, Rawlings RS, Krauss RM. Reversal of small, dense LDL subclass phenotype by normalization of adiposity. Obesity. 2009;17(9):1768–1775. doi: 10.1038/oby.2009.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krauss RM, Blanche PJ, Rawlings RS, Fernstrom HS, Williams PT. Separate effects of reduced carbohydrate intake and weight loss on atherogenic dyslipidemia. The American journal of clinical nutrition. 2006 May 1;83(5):1025–1031. doi: 10.1093/ajcn/83.5.1025. 2006. [DOI] [PubMed] [Google Scholar]
- 7.Shin M-J, Blanche PJ, Rawlings RS, Fernstrom HS, Krauss RM. Increased plasma concentrations of lipoprotein (a) during a low-fat, high-carbohydrate diet are associated with increased plasma concentrations of apolipoprotein C-III bound to apolipoprotein B-containing lipoproteins. The American journal of clinical nutrition. 2007;85(6):1527–1532. doi: 10.1093/ajcn/85.6.1527. [DOI] [PubMed] [Google Scholar]
- 8.Zheng C, Khoo C, Furtado J, Sacks FM. Apolipoprotein C-III and the metabolic basis for hypertriglyceridemia and the dense low-density lipoprotein phenotype. Circulation. 2010 Apr 20;121(15):1722–1734. doi: 10.1161/CIRCULATIONAHA.109.875807. PubMed PMID: 20368524. Pubmed Central PMCID: 3153990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng C, Khoo C, Ikewaki K, Sacks FM. Rapid turnover of apolipoprotein C-III-containing triglyceride-rich lipoproteins contributing to the formation of LDL subfractions. Journal of lipid research. 2007 May;48(5):1190–1203. doi: 10.1194/jlr.P600011-JLR200. PubMed PMID: 17314277. Epub 2007/02/23. eng. [DOI] [PubMed] [Google Scholar]
- 10.Parks EJ, Krauss RM, Christiansen MP, Neese RA, Hellerstein MK. Effects of a low-fat, high-carbohydrate diet on VLDL-triglyceride assembly, production, and clearance. The Journal of clinical investigation. 1999;104(8):1087–1096. doi: 10.1172/JCI6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendivil CO, Zheng C, Furtado J, Lel J, Sacks FM. Metabolism of very-low-density lipoprotein and low-density lipoprotein containing apolipoprotein C-III and not other small apolipoproteins. Arteriosclerosis, thrombosis, and vascular biology. 2010;30(2):239–245. doi: 10.1161/ATVBAHA.109.197830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S-J, Campos H, Moye LA, Sacks FM. LDL containing apolipoprotein CIII is an independent risk factor for coronary events in diabetic patients. Arteriosclerosis, thrombosis, and vascular biology. 2003;23(5):853–858. doi: 10.1161/01.ATV.0000066131.01313.EB. [DOI] [PubMed] [Google Scholar]
- 13.Zheng C, Azcutia V, Aikawa E, Figueiredo J-L, Croce K, Sonoki H, et al. Statins suppress apolipoprotein CIII-induced vascular endothelial cell activation and monocyte adhesion. European heart journal. 2013;34(8):615–624. doi: 10.1093/eurheartj/ehs271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olin-Lewis K, Krauss RM, La Belle M, Blanche PJ, Barrett PHR, Wight TN, et al. ApoC-III content of apoB-containing lipoproteins is associated with binding to the vascular proteoglycan biglycan. Journal of lipid research. 2002;43(11):1969–1977. doi: 10.1194/jlr.m200322-jlr200. [DOI] [PubMed] [Google Scholar]
- 15.Yassine HN, Trenchevska O, Ramrakhiani A, Parekh A, Koska J, Walker RW, et al. The Association of Human Apolipoprotein C-III Sialylation Proteoforms with Plasma Triglycerides. PloS one. 2015;10(12):e0144138. doi: 10.1371/journal.pone.0144138. PubMed PMID: 26633899. Pubmed Central PMCID: 4669142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Millar JS. The sialylation of plasma lipoproteins. Atherosclerosis. 2001;154(1):1–13. doi: 10.1016/s0021-9150(00)00697-3. [DOI] [PubMed] [Google Scholar]
- 17.Trenchevska O, Schaab MR, Nelson RW, Nedelkov D. Development of multiplex mass spectrometric immunoassay for detection and quantification of apolipoproteins CI, C-II, C-III and their proteoforms. Methods. 2015 doi: 10.1016/j.ymeth.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koska J, Yassine H, Trenchevska O, Sinari S, Schwenke DC, Yen FT, et al. Disialylated Apolipoprotein C-III Proteoform is Associated with Improved Lipids in Prediabetes and Type 2 Diabetes. Journal of lipid research. 2016 Mar 3;:2016. doi: 10.1194/jlr.P064816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savinova OV, Fillaus K, Jing L, Harris WS, Shearer GC. Reduced Apolipoprotein Glycosylation in Patients with the Metabolic Syndrome. PloS one. 2014;9(8):e104833. doi: 10.1371/journal.pone.0104833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bosello O, Cominacini L, Zocca I, Garbin U, Ferrari F, Davoli A. Effects of severe caloric restriction on the degree of sialylation of apoprotein C-III in obese women. Annals of nutrition and metabolism. 1985;29(1):33–39. doi: 10.1159/000176951. [DOI] [PubMed] [Google Scholar]
- 21.Harvey SB, Zhang Y, Wilson-Grady J, Monkkonen T, Nelsestuen GL, Kasthuri RS, et al. O-glycoside biomarker of apolipoprotein C3: responsiveness to obesity, bariatric surgery, and therapy with metformin, to chronic or severe liver disease and to mortality in severe sepsis and graft vs host disease. Journal of proteome research. 2008;8(2):603–612. doi: 10.1021/pr800751x. [DOI] [PubMed] [Google Scholar]
- 22.Witztum JL, Schonfeld G. Carbohydrate diet-induced changes in very low density lipoprotein composition and structure. Diabetes. 1978 Dec;27(12):1215–1229. doi: 10.2337/diab.27.12.1215. PubMed PMID: 214369. Epub 1978/12/01. eng. [DOI] [PubMed] [Google Scholar]
- 23.Falko JM, Schonfeld G, Witztum JL, Kolar JB, Salmon P. Effects of short-term high carbohydrate, fat-free diet on plasma levels of Apo C-II and Apo C-III and on the Apo C subspecies in human plasma lipoproteins. Metabolism: clinical and experimental. 1980 Jul;29(7):654–661. doi: 10.1016/0026-0495(80)90110-9. PubMed PMID: 7382829. Epub 1980/07/01. eng. [DOI] [PubMed] [Google Scholar]
- 24.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical chemistry. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 25.Krauss RM, Burke DJ. Identification of multiple subclasses of plasma low density lipoproteins in normal humans. Journal of lipid research. 1982;23(1):97–104. [PubMed] [Google Scholar]
- 26.Austin MA, Newman B, Selby JV, Edwards K, Mayer EJ, Krauss RM. Genetics of LDL subclass phenotypes in women twins. Concordance, heritability, and commingling analysis. Arteriosclerosis, thrombosis, and vascular biology. 1993;13(5):687–695. doi: 10.1161/01.atv.13.5.687. [DOI] [PubMed] [Google Scholar]
- 27.Caron S, Verrijken A, Mertens I, Samanez CH, Mautino G, Haas JT, et al. Transcriptional activation of apolipoprotein CIII expression by glucose may contribute to diabetic dyslipidemia. Arteriosclerosis, thrombosis, and vascular biology. 2011;31(3):513–519. doi: 10.1161/ATVBAHA.110.220723. [DOI] [PubMed] [Google Scholar]
- 28.Li WW, Dammerman MM, Smith JD, Metzger S, Breslow JL, Leff T. Common genetic variation in the promoter of the human apo CIII gene abolishes regulation by insulin and may contribute to hypertriglyceridemia. Journal of Clinical Investigation. 1995;96(6):2601. doi: 10.1172/JCI118324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen M, Breslow JL, Li W, Leff T. Transcriptional regulation of the apoC-III gene by insulin in diabetic mice: correlation with changes in plasma triglyceride levels. Journal of lipid research. 1994 Nov;35(11):1918–1924. PubMed PMID: 7868970. Epub 1994/11/01. eng. [PubMed] [Google Scholar]
- 30.Windler E, Havel RJ. Inhibitory effects of C apolipoproteins from rats and humans on the uptake of triglyceride-rich lipoproteins and their remnants by the perfused rat liver. Journal of lipid research. 1985 May 1;26(5):556–565. 1985. [PubMed] [Google Scholar]
- 31.Kowal RC, Herz J, Weisgraber K, Mahley R, Brown M, Goldstein JL. Opposing effects of apolipoproteins E and C on lipoprotein binding to low density lipoprotein receptor-related protein. Journal of Biological Chemistry. 1990;265(18):10771–10779. [PubMed] [Google Scholar]
- 32.Mann CJ, Troussard AA, Yen FT, Hannouche N, Najib J, Fruchart JC, et al. Inhibitory effects of specific apolipoprotein C-III isoforms on the binding of triglyceride-rich lipoproteins to the lipolysis-stimulated receptor. The Journal of biological chemistry. 1997 Dec 12;272(50):31348–31354. doi: 10.1074/jbc.272.50.31348. PubMed PMID: 9395464. [DOI] [PubMed] [Google Scholar]
- 33.Marmillot P, Rao MN, Liu Q-H, Lakshman MR. Desialylation of human apolipoprotein E decreases its binding to human high-density lipoprotein and its ability to deliver esterified cholesterol to the liver. Metabolism: clinical and experimental. 1999;48(9):1184–1192. doi: 10.1016/s0026-0495(99)90136-1. [DOI] [PubMed] [Google Scholar]
- 34.Hoogeveen RC, Gaubatz JW, Sun W, Dodge RC, Crosby JR, Jiang J, et al. Small Dense Low-Density Lipoprotein-Cholesterol Concentrations Predict Risk for Coronary Heart Disease The Atherosclerosis Risk in Communities (ARIC) Study. Arteriosclerosis, thrombosis, and vascular biology. 2014;34(5):1069–1077. doi: 10.1161/ATVBAHA.114.303284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willer CJ, Sanna S, Jackson AU, Scuteri A, Bonnycastle LL, Clarke R, et al. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nature genetics. 2008;40(2):161–169. doi: 10.1038/ng.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katrine T-BS, Vakhrushev SY, Kong Y, Steentoft C, Nudelman AS, Pedersen NB, et al. Probing isoform-specific functions of polypeptide GalNAc-transferases using zinc finger nuclease glycoengineered SimpleCells. Proceedings of the National Academy of Sciences. 2012;109(25):9893–9898. doi: 10.1073/pnas.1203563109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holleboom AG, Karlsson H, Lin RS, Beres TM, Sierts JA, Herman DS, et al. Heterozygosity for a loss-of-function mutation in GALNT2 improves plasma triglyceride clearance in man. Cell Metab. 2011 Dec 7;14(6):811–818. doi: 10.1016/j.cmet.2011.11.005. PubMed PMID: 22152306. Pubmed Central PMCID: PMC3523677. Epub 2011/12/14. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.