Summary
Affinity mature B-cells require cognate antigen, retained by follicular dendritic cells (FDC), for clonal selection within germinal centers. Studies on how FDCs in lymphoid tissues acquire antigen have relied primarily on model protein antigens. To examine delivery of intact bacteria to FDC, we used inactivated Streptococcus pneumonia (SP). We found that both medullary macrophages and a subset of SIGN-R1 positive dendritic cells (DCs) in the lymph node capture SP from the draining afferent lymphatics. The presence of DCs is required for initial complement activation, opsonization of the bacteria and efficient transport of SP to FDCs. Moreover, we observed a major role for transport of bacteria to FDC by naïve B cells via a CD21-dependent pathway. We propose a mechanism by which efficient transport of SP to FDCs is dependent on DCs for initial binding and activation of complement and either direct transport to FDC or transfer to naïve B cells.
Keywords: dendritic cells, follicular dendritic cells, antigen transport, Streptococcus pneumoniae, B-cells, SIGN-R1, complement
Graphical abstract
Introduction
The Gram-positive pathogen Streptococcus pneumoniae (SP), commonly known as pneumococcus, is the predominant cause of community acquired pneumonia and causes many cases of Otis media, sinusitis, meningitis and septicemia (Musher, 1994; Tomasz, 2000; Tuomanen, 2004). SP resides in the nasopharynx as a commensal in up to 20% of healthy adults and up to 50% of healthy children. It is an opportunistic bacterium and infection often occurs after another respiratory tract infection, e.g. during influenza pandemics the leading cause of death is often a secondary infection with SP (Palese, 2004). Invasive pneumococcal disease leads to high mortality and morbidity rates, especially in young, elderly, debilitated or immunosuppressed individuals (Janoff and Rubins, 1997). Worldwide more than 1 million people die from pneumococcal infections each year, mostly in the developing world (Klein et al., 1994; Mulholland, 1979). However, the rate in which resistance of SP to antibiotics is increasing in the United States and the rest of the developed world is alarming (Appelbaum, 2002; Klugman, 2004). As of 2013, data shows that SP is resistant to one or more antibiotics in 30% of clinical cases (Centers for Disease Control and Prevention, 2013a; 2013b).
Clearance of SP is mediated through opsonization by immunoglobulin (Ig) and complement. In 1969 it was shown that complement is protective in pneumococcal disease by increasing phagocytosis (Johnston et al., 1969). Opsonization of SP with complement C3 and its breakdown product C3d, facilitates uptake of immune complexes (IC) by CD21 (Griffioen et al., 1991). Recognition and binding of SP by the C-type lectin receptor SIGN-R1 activates the classical complement pathway via C1q leading to opsonization and deposition on the Follicular Dendritic Cell (FDC), which is required for humoral immunity against SP (Brown et al., 2002; Kang et al., 2006).
Despite these elegant findings on the mechanism of antigen binding and complement activation, it remains unclear how SP is delivered to B cell follicles in draining lymph nodes (LN) for presentation in germinal centers (GC). Earlier studies tracking uptake of particulate antigen within skin draining LNs show that subcapsular sinus macrophages (SCSM) were essential in induction of a humoral response and have been referred to as guardians of the LN (Gaya et al., 2015). In similar studies, Cyster and colleagues identified one pathway by which SCSM bind C3-opsonized IC via complement receptor 3 (CR3) and shuttle it into the underlying B cell compartment where mature, non-cognate B cells take up the opsonized IC via CD21 (Phan et al., 2009; 2007). More recently, Heesters et al. reported that naïve B cells deliver C3-opsonized IC directly to FDC where it is transferred via CD21 and rapidly internalized into a cycling endosomal compartment for long term retention (Heesters et al., 2013).
FDC play a critical role in regulating the architecture of B cell follicles and maintenance of GC via secretion of chemokines, cytokines and retention of antigen (Tew et al., 1990; Wang et al., 2011; Wu et al., 2009). Studies using model protein antigens demonstrate that B cell activation in the presence of co-stimulation by T follicular helper (TFH) cells leads to formation of GC where B cells undergo class switch recombination and somatic hypermutation, which finally results in differentiation to memory and effector B cells (see Victora et al. for review) (Victora and Nussenzweig, 2012). FDC retention of antigen within GC is required for efficient clonal selection and affinity maturation as cognate B cells acquire antigen and present it to TFH (Gitlin et al., 2015; Shulman et al., 2013; Suzuki et al., 2009; Victora et al., 2010).
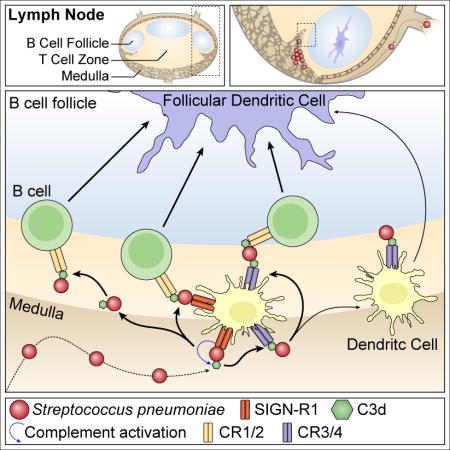
To track delivery of SP to the FDC and its subsequent acquisition by cognate B cells, we developed a model in which mice were immunized s.q. with fluorescently labeled inactivated SP. We found, unexpectedly, that SCSM did not bind SP and that macrophages were not required for humoral immunity to SP. Instead, bacteria captured and opsonized by LN resident DCs (LNDC) are either handed-off to naïve B cells in the nearby follicles or, although less common, transported directly to the FDC. We propose a mechanism in which B cells require DC mediated opsonization of SP (presumably through SIGN-R1) and in which both B cells and DCs have the capacity to transport opsonized SP to the FDC within the LN follicles.
Results
Medullary macrophages capture lymph-borne SP
In order to identify the mechanism underlying the capture and transport of lymph-borne SP in the LN, mice were injected in the footpad with fluorescent-labeled, heat-inactivated SP (strain D39, serotype 2). This results in passive drainage of the bacteria to the popliteal lymph node (pLN) (Gonzalez et al., 2011; Roozendaal et al., 2009). Imaging of cryosections of pLNs early after injection, indicate localization of SP in the medullary region and not in the subcapsular region (Figure 1 A). Capture is apparent at two hours after injection and quantification of the data shows an increase in binding in the medullary region at 12 hours but negligible binding in the subcapsular region over a 24-hour period (Figure 1 B). This was unexpected as earlier studies had identified the subcapsular sinus macrophages (SCSM) as required for humoral immunity to particulate antigens (Carrasco and Batista, 2007; Gaya et al., 2015; Iannacone et al., 2010; Junt et al., 2007). One difference from the earlier studies is that here we immunized with intact bacteria. For example, influenza virus which is taken-up by SCSM is opsonized by serum complement component mannan-binding lectin (MBL); whereas SP is not (Figure S1 A). Medullary macrophages (MM) which mark the medullary region are distinguished by staining with antibody to SIGN-R1; whereas SCSM are identified with anti-MOMA-1 and a lack of SIGN-R1 staining (Gonzalez et al., 2010; Phan et al., 2007). To further identify cell types involved in uptake of labeled SP, cryosections of pLN isolated at 2 and 12 hours post-injection in the footpad, were stained with antibody to SIGN-R1 and MOMA-1. Quantitation of the results identify a high percentage of the SP co-localize with SIGN-R1+ MM; whereas, negligible co-localization was observed with the MOMA-1+, SIGN-R1-SCSM (Figure 1 C, D).
Figure 1. Capture of Streptococcus pneumonia (SP) is exclusive to the medulla.
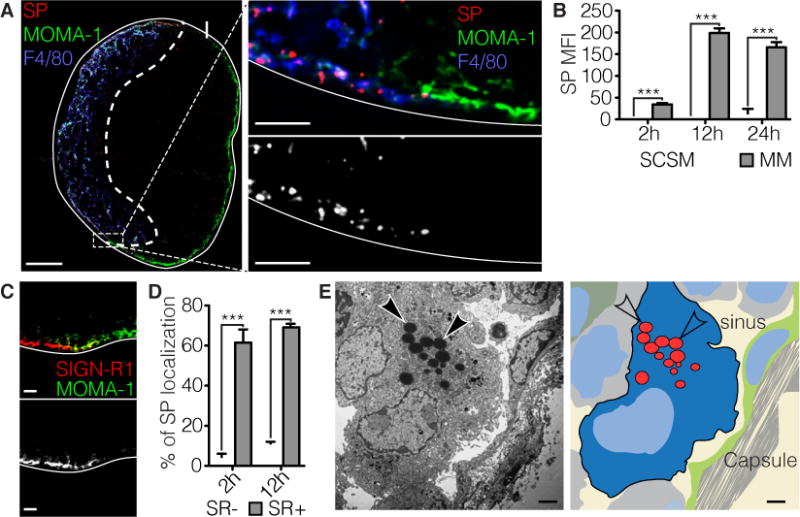
a | Left: Confocal image of the popliteal lymph node (pLN) in a mouse immunized with SP (CMTPX, red) two hours prior. Subcapsular sinus (SCS) macrophages (MOMA-1, green) mark the SCS area, while medullary macrophages (F4/80, blue) mark the medullary region. Scale bar indicates 100 μm. Right: Magnification of the transition from medulla to SCS in which single cocci can be resolved. Gray scale image of the SP channel shows selective binding to the medulla. Scale bar indicates 10 μm. b | Quantification of (a) based on mean fluorescence intensity (MFI) of SP. Arrival and accumulation of SP in the medulla is gradual over the course of 12 hours. At two hours SP was detected in the medulla, in contrast to the SCS region were no binding was detected up to 24 hours. *** p<0.001 ANOVA with Tukey’s multiple comparisons test. c | Confocal micrograph of the medulla-SCS interface shows exclusive staining for SIGN-R1 (SR1, red) in the medulla. SCS is marked with MOMA-1 (green). Grey scale image of the SR1 channel. Scale bar indicates 10 μm. d | Quantification of (c) percentage out of total identified cocci. *** p<0.001 ANOVA with Tukey’s multiple comparisons test. e | Left: Electron micrograph of a medullary macrophage (MM) that has engulfed multiple SP (arrows), adjacent to the medullary sinus. Scale bar indicates 2 μm. Right: Color-coded overlay to aid in structure identification.
Qualitative analysis of pLNs by electron microscopy (EM) following injection of SP further confirm internalization of the bacteria by macrophages within the medullary, but not the subcapsular region (Figure 1 E). At later time points SP was observed in the follicular region, co-localizing with the FDC marker 8C12, this is discussed in more detail below.
Lymph node DCs and macrophages bind SP
Flow cytometric analysis of single cell suspensions prepared from pLN was performed to determine which cell types are responsible for the binding and possible transport of SP to the FDC. Heat-inactivated fluorescent-labeled SP was injected in the footpad and the pLN was harvested at 2 hours, after which a single cell suspension was prepared that was stained with antibodies for CD11b, CD11c, CD4, CD8 and SIGN-R1. Three distinct populations of CD11c+ dendritic cells were identified as SIGN-R1+, i.e. CD11b-int SIGN-R1-int; CD11b-hi SIGN-R1-int and CD11b-hi SIGN-R1-hi (Figure 2 A). Among the three populations, CD11b-hi SIGN-R1-hi bound SP with the highest MFI (Figure 2 B). Further analysis of this SP+ population reveals a specificity for SIGN-R1+ macrophages and SIGN-R1+ CD4+ DCs (Figure 2 C). One explanation is opsonization and subsequent stabilization through complement receptors CD11b and CD11c, also known as complement receptor (CR) 3 and 4 respectively. The critical role of SIGN-R1 and complement in SP opsonization and subsequent binding by DCs is further emphasized by the lack of stable SP binding by DCs in C3−/− and C1q−/− mice (Figure S1 B). Opsonization through SIGN-R1 is mediated by C1q (Kang et al., 2006). These data suggest that stable binding of SP by DCs requires complement opsonization and possibly subsequent binding of either CR3 or CR4 (CD11b or CD11c).
Figure 2. SIGN-R1+ dendritic cells and macrophages bind SP.
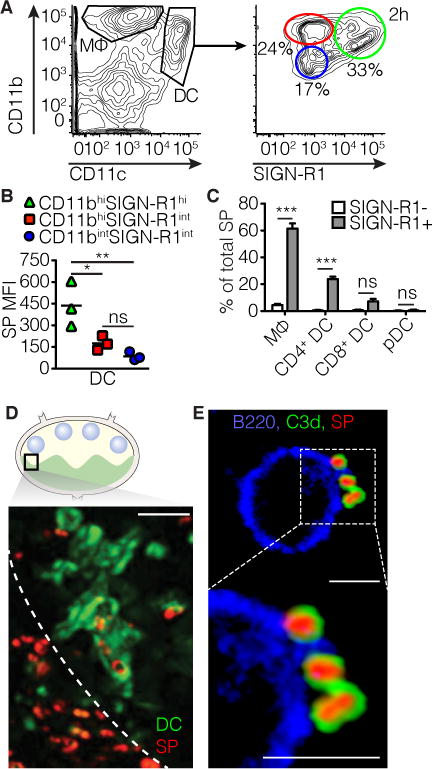
All experiments were performed 12h after injection of fluorescently labeled heat inactivated SP in the footpad. a | Left: Flow cytometry gating strategy to distinguish dendritic cells (DCs) and macrophages. Right: Based on SIGN-R1(SR1) and CD11b expression three DC subsets were identified. b | In the three groups defined the MFI of SP was determined as a correlate of binding. ** p<0.01, * p<0.05, ANOVA with Tukey’s multiple comparisons test. c | Macrophages, plasmacytoid DCs, CD4+ DCs and CD8+ DCs were divided in SR1 positive (green) and negative (blue and red) groups (negative group comprised of both CD11bhi and CD11bint, SR1int groups) and the percentage of total SP bound by these groups was determined. *** p<0.001, ns not significant, ANOVA with Tukey’s multiple comparisons test. d | Snap shot taken from multiphoton-intravital imaging of the popliteal LN in a CD11c-eYFP (green) mouse immunized with SP (red, white arrows) near the medullary border. Scale bar indicates 10 μm. e | High magnification image of an isolated B cell binding complement C3d-coated SP on its surface. Structures are marked as follows, CD45R/B220 (blue), SP (red) and C3d (green). Scale bar indicates 5 μm.
To follow the capture in the LN of SP by CD11c+ DC in real time, mice expressing eYFP under the CD11c promotor were immunized with labeled, inactivated SP and uptake was visualized by multiphoton intravital imaging (MP-IVM) of the pLN at 2 hours post-injection (Gonzalez et al., 2010; Lindquist et al., 2004) (Figure 2 D). Imaging of the interfollicular region adjacent to the B cell follicles identify eYFP+ DC in close proximity of the medullary sinus and loaded with bacteria. DCs capture bacteria in close proximity of the medullary sinus, possibly via SIGN-R1 (Figure 2 D). Notably, a similar uptake location, and involvement of SIGN-R1+ LNDC, was reported earlier by Gonzalez et al. following footpad injection of inactivated influenza A (IAV) viral particles (Gonzalez et al., 2010). Interestingly, complement C3 coated, labeled SP were also observed on the surface of B220+ cells (B cells) (Figure 2 E, Figure S2 A–D).
Lymph node DCs are required for immune response against SP
To assess whether DCs, macrophages or both are required for an immune response against SP, chimeric mice were constructed in which bone marrow (BM) from CD11c-diptheria toxin receptor (DTR) transgenic mice was transferred into lethally irradiated wild type C57BL/6 recipients (Wt). In this model, BM-derived cells within the CD11c-DTR ≫Wt chimeric mice express the diphtheria toxin receptor under control of the CD11c promoter, making dendritic cells susceptible to diphtheria toxin (DTx). As controls, irradiated WT recipients, reconstituted with either Wt BM (Wt BM≫Wt) or BM isolated from mice deficient in the alpha chain of T cell receptor (TCRα−/− BM≫WT) mice were used. After 6–8 weeks recovery, the three groups were treated with 100ng (~5 ng/g body weight) of DTx and immunized with heat inactivated SP in the footpad 48h later. IgM titers were assessed by ELISA at day 10 post-immunization (Figure 3 A–D). As expected, Wt BM≫Wt and TCRα−/− BM≫WT chimeric mice treated with DTx responded with a robust IgM titer. By contrast, the IgM response was severely diminished in CD11c-DTR BM≫Wt chimeras when administered DTx. These results support the requirement of DCs in the initiation of a T-independent humoral immune response against SP and suggests DC may participate in the transport of SP to the follicle.
Figure 3. DCs and B cells collaborate in the transport of SP to the FDC.
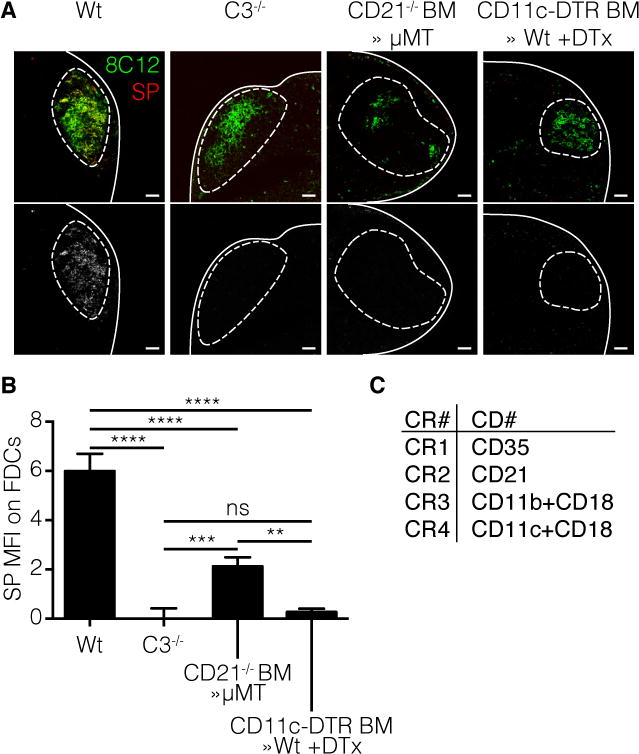
Mice were injected with fluorescent labeled SP(red) in the footpad as described earlier. Four different mouse strains were used; wild type (Wt), complement C3 knockout (C3−/−), complement receptor 2 (CD21) knockout bone marrow (BM) in μMT B cell deficient recipients (CD21−/− BM≫μMT) and CD11c-dtr BM in Wt recipients (CD11c-dtr BM≫Wt) injected with Dtx 48h prior to immunization. The FDC network was stained using 8C12 (green). a | Confocal micrographs of pLN 12h after immunization with SP. Line indicates LN capsule, dotted line indicates follicle area. Bottom row: greyscale image of the SP channel. Scale bars 100 μm b | Quantification of confocal images by Cell Profiler software. Only co-localized signal was measured. **** p<0.0001, *** p<0.001, ** p<0.01, ns not significant, ANOVA with Tukey’s multiple comparisons test. c | Overview of complement receptor (CR) nomenclature and their respective cluster of differentiation (CD) number.
To assess the contribution of sinus-lining macrophages in the immune response against SP, clodronate liposomes (CLL) were used to deplete phagocytic macrophages. Earlier studies have used this approach to eliminate sinus-lining macrophages in skin draining LNs without affecting LNDC (Gonzalez et al., 2010; Junt et al., 2007). Importantly, under the conditions used in this study, CLL does not deplete LNDCs but does deplete CD11b+ macrophages (Figure S3 F, H). Subsequently, the CLL treated group and controls (PBS only or empty CLL) were immunized with SP and their humoral response assayed by quantitating number of antibody secreting cells (ASC) in the pLN and serum IgM titers determined by ELISA. Remarkably, the CLL treatment had a negligible effect on the IgM or total Ig response relative to the control groups and did not affect the number of antibody secreting cells (ASC) in the LN (Figure S3 C–E). By contrast, a knock-down of SIGN-R1 with anti-SIGN-R1 (clone 22D1), as described by Kang et al., diminished the number of ASC in the LN (Figure S4) (Kang et al., 2003). Taken together, LNDCs, but not sinus-lining macrophages, are required for an immune response against SP.
DCs and B cells collaborate to transport SP to FDCs
As discussed above, naïve mature B cells take-up C3-opsonized IC via CD21 within the follicles and deliver them to FDC (Heesters et al., 2013; 2014; Phan et al., 2009; 2007). To examine whether transport of C3d-opsonized SP is also dependent on CD21 expression on B cells, BM chimeras were constructed in which lethally irradiated μMT mice, which lack mature B cells, were reconstituted with BM from CD21−/− mice. Thus, in the chimeric mice, the only source of mature B cells is from the donor-derived CD21−/− BM; whereas the FDC are CD21 sufficient. C3 deficient mice (C3−/−) and CD11c-DTR BM chimeric mice (CD11c-DTR BM≫Wt) were used as a negative control and Wt mice used as a positive control. The four groups of mice were injected in the hock with labeled SP. Visualization and quantification of cryosections prepared from draining LNs identified deposition of SP on FDC in the Wt but not C3 deficient and CD11c-DTR BM≫Wt control mice, as expected. Strikingly, a significant reduction in antigen deposition was observed on FDC in pLNs of the CD21−/− μMT BM chimeras (Figure 3 A, B). Interestingly, the phenotype observed in the CD21−/− μMT BM chimeras was not as severe as in C3−/− or CD11c-DTR BM≫Wt mice suggesting other C3-dependent pathways such as LNDC may be involved in SP transport (Figure 3 A, B).
Previous studies reported that deposition of C3-opsonized SP on FDCs correlates with the B cell memory response (Kang et al., 2006). To determine if DCs (CD11c+) can transport SP directly to the FDC, MP-IVM was used in combination with the CD11c-eYFP reporter mice. Visualization of early events following subcutaneous injection of labeled SP identified direct transfer of SP from the LNDC to the FDC (movie stills; Figure S3 G, Movie S1). However, given the limits of real time imaging of the draining LN, quantitation of the event was not possible by MP-IVM. Therefore, an ex vivo FDC approach was established. Cd11c-eYFP+ cells were sorted by FACS and loaded with complement-opsonized SP. Subsequently, the cells were magnetically sorted with anti-CD45 beads to remove unbound SP and co-cultured with FDCs previously isolated and plated on cover slips (Heesters et al., 2013) (Figure 4 A, B). After washing, co-cultures were imaged and SP binding to FDC was quantified (Figure 4 C, D). Results show that DCs are capable of transfer of SP to FDCs.
Figure 4. DCs can transfer SP directly to FDCs.
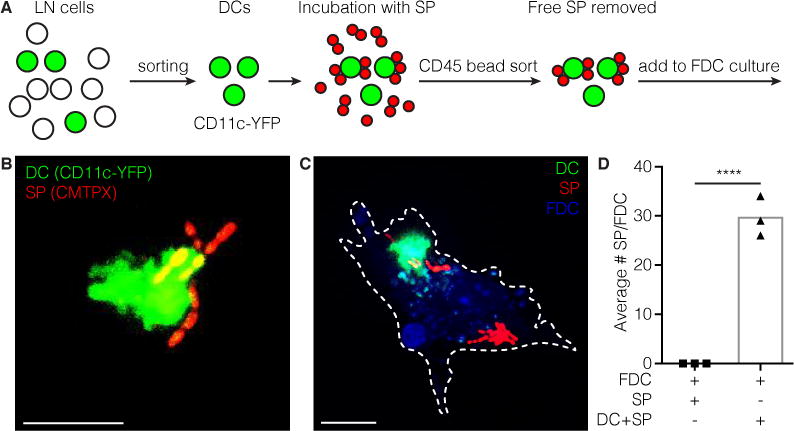
a | Schematic of experimental setup. LN single cell suspension from CD11c-YFP mouse is made. YFP positive DCs are FACS sorted. Sorted DCs are incubated with SP in the presence of complement components. Then DCs are sorted again using CD45 magnetic beads to remove unbound SP. DCs carrying SP are added to FDC cultures isolated 3 days prior. b | Sorted CD11c-YFP DC multiple carrying SP (CMTPX) on its surface. Scale bar 10 μm. c | FDC (blue) loaded with SP (CMTPX) and a DC (YFP) transferring SP. Scale bar 10 μm. d | Quantification of SP transferred to FDCs through DCs. **** p<0.0001, Students t-test.
Our combined results suggest a model in which both LNDCs and B cells are necessary for the efficient binding and transport of C3-opsonized SP to the FDC. Although naïve non-cognate B cells provide the major pathway for transport of opsonized SP to the FDC, LNDCs are crucial in the initial binding of SP that drain into the LN and subsequently either transport the bacteria directly to the FDC or transfer opsonized bacteria to naïve B cells in a CD21-dependent mechanism. In our model SP enters the lymph node through the afferent lymphatics after passive drainage from the injection site. Due to its thick glycan capsule SP has evaded complement so far and thus does not bind complement receptor 3 (CR3, CD11b) on SCS macrophages. Cells in the medulla are capable of binding SP through the SIGN-R1 receptor, which recruits C1q and activates the classical complement pathway. Macrophages can then endocytose and digest the bacterium, while DCs can use CR3 (or complement receptor 4, CR4, CD11c) to stabilize SP. Then there are multiple possible pathways: 1. Opsonized SP can be released and bound by B cells through CR2, which transports SP to the FDC. 2. The DC can transfer the opsonized SP from either SIGN-R1 or CR3 or CR4 to CR2 on a B cell, which transports SP to the FDC. 3. The DC can transport opsonized SP to the FDC by itself and transfer it to CR1/CR2 on the FDC (Graphical Abstract). The FDC then incorporates SP into a non-degradative cycling endosomal compartment, periodically displaying the antigen to cognate B cells in the germinal center reaction.
Discussion
Early studies with Streptococcus pneumonia (SP) reported that efficient clearance and host protection to the encapsulated bacteria was dependent on complement C3 and immunoglobulin (Brown et al., 2002; Griffioen et al., 1991; Neufeld, 1904; Ward and Enders, 1933). Kang et al. made the unexpected observation that C3 opsonization of SP by LN macrophages was dependent on recognition and binding of mannan residues on the capsular surface by the C-type lectin SIGN-RI (Kang et al., 2004). Moreover, they demonstrated that SP deposition on FDC within the follicles of draining LNs was C3 dependent and correlated with protective humoral immunity. These elegant studies raised the general question of the underlying mechanism for transport of C3-opsonized SP from the afferent lymphatics and delivery to the FDC.
To address this important question, we developed a model in which heat-inactivated SP were fluorescent labeled and injected in the hind limb (hock region) of Wt and reporter mice. Labeled SP was tracked both in real-time in CD11c-eYFP reporter mice using MP-IVM and by confocal imaging of cryosections prepared from the pLNs at varying time points. Strikingly, we found that the bacteria drained passively via afferent lymphatics into the medullary sinus where they were rapidly taken-up by MM and underlying LNDC. Unexpectedly, we observed that LNDC migrated into the B cell follicles and directly transferred labeled SP to FDC. Delivery was impaired by selective elimination of LNDC in CD11c-DTR BM chimeras with DTx ablation. A limitation of this study is that DTx depletion also affects a small subset of macrophages and neutrophils (Bennett and Clausen, 2007; Tittel et al., 2012). As a control, Wt mice were also treated with DTx. Further support for the importance of LNDCs came from our results showing that humoral immunity to SP immunization was significantly reduced following DTx ablation. Notably, BM chimeras (CD21−/−BM≫ μMT) in which mature B cells were deficient in the CD21 receptor also showed a significant reduction in deposition of SP on FDC following SP immunization. Based on the importance of both LNDC and CD21+ B cells in delivery of SP to FDC, we propose a mechanism by which lymph borne SP is bound by SIGN-R1+ LNDC and opsonized with C3. CR3 and CR4 expressed on LNDC may stabilize retention of the C3-opsonized complexes. Subsequently, opsonized SP are either delivered directly to FDC or transferred to naïve B cells which then deliver the complexes to FDC in a CD21-dependent pathway. These results do not rule out other pathways of delivery. For example, at later time points it seems probable that SP at the site of injection is phagocytosed by migratory DC that deliver the bacteria into the draining LNs. Although, recent studies from our lab using the ultra-violet inactivated influenza A virus (UV-IAV) model found that passive drainage of the antigen into the LN was sufficient to support a protective IgG memory response (Woodruff et al., 2014).
The finding that sinus lining macrophages are not required to support humoral immunity to SP and that SCSM do not appear to take-up the bacteria was unexpected based on earlier studies (Carrasco and Batista, 2007; Gaya et al., 2015; Iannacone et al., 2010; Junt et al., 2007). One explanation for the difference is that the nature and state of opsonization of the particulate antigen dictates in part the pathway for uptake and processing. SP is recognized by SIGN-R1 which is expressed by MM and LNDC but not SCSM and earlier opsonization is unlikely due to SP’s thick glycocoat. In an earlier study, we found that UV-inactive IAV which also is decorated with mannan oligosaccharides is bound by both SCSM and MM (as well as LNDC). Notably in the IAV model, SCSM binding was not essential in the humoral response; whereas LNDC uptake was required (Gonzalez et al., 2010). This is consistent with recent findings by Gaya et al. where only live virus, bacteria or TLR ligands disrupted the SCSM layer, but UV-inactive IAV did not (Gaya et al., 2015). Interestingly, we did not observe any interaction with SCSM, nor did depletion with liposomes disrupt the immune response. Our data emphasize that the mechanism of antigen capture in the LN might be more complex than earlier reported. We hypothesize that differences between pathogens, mainly their ability to evade complement fixation, dictate how they are processed in the LN.
Transport of SP to FDCs was shown to be a collaborative effort between DCs and B cells, which depends on C3 opsonization most likely mediated by SIGN-R1 on DCs. We hypothesize that SP is bound by SIGN-R1 and then opsonized with C3d after which CD11b or CD11c (CR3 or CR4) mediates stabilization of the immune complex. Recent structural studies identified the site on C3d that is bound by CR3 and found that it did not interfere with the known site bound by CD21 (Bajic et al., 2013; van den Elsen et al., 2002). The CR4 binding site does not seem to interfere with CD21 (CR2) binding (Bajic, personal communication). Thus, SP opsonized with C3d can be recognized by both CR3/CR4 and CD21 concurrently and this could explain how the bacteria can be transferred from LNDC to CD21+ B cells or CD21+ FDC. Although the impaired humoral response to SP seems due to a defect in deposition on the FDC, we do not exclude a role for T follicular helper cells, which might function as a regulatory agent.
It will be important in future studies to confirm the importance of CR3/CR4 in LNDC transport of SP and gain a better understanding of the relative importance of direct transport o the bacteria by LNDC versus hand-off to naïve B cells.
Materials and Methods
Mice
Mice were bred in house or were from Jackson Laboratories or Charles River Laboratory. The following strains were used: wild type (C57BL/6), CD11c-eYFP, C3−/−, C1q−/−, CD21−/−, TCRα−/−, μMT and CD11c-DTR (Gack et al., 2007; Lindquist et al., 2004). All mice were on the C56BL/6 background. Mice were maintained in specific pathogen–free facilities at Immune Disease Institute, Dana Farber Cancer Institute or Harvard Medical School. All animal experiments were in accordance with protocols approved by the Subcommittee on Research Animals Care at Harvard Medical School and The Immune Disease Institute, and were in accordance with guidelines set by the National Institutes of Health.
Bacterial strains and growth conditions
Streptococcus pneumoniae strain D39 was grown in Todd Hewitt broth supplemented with yeast extracts and horse blood until log phase (OD650=1.5) (Restrepo et al., 2005). SP was then labeled by incorporation of CMTPX dye and heat-inactivated at 65°C for 30 minutes.
Antibodies
The following antibodies were purchased from BioLegend: anti-B220 (RA3-6B2), anti-CD11c (N418), anti-CD11b (M1/70). CD169 (36.112 and MOMA-1) was acquired from AbD Serotec. Anti-CD35 (8C12), anti-SIGN-R1 (22D1 and ERTR-9), anti-CD11b (M1/70) and anti-F4/80 (HB-198; American Type Culture Collection) were produced in-house and were purified by affinity chromatography. Secondary antibodies, including streptavidin–Alexa Fluor 488 (S11223), streptavidin–Alexa Fluor 568 (S11226), streptavidin–Alexa Fluor 633 (S21375) and Alexa Fluor 633– conjugated anti-rat (A21094; all from Invitrogen). Purified antibodies were labeled with Alexa Fluor 488 (A10235), Alexa Fluor 568 (A10238), Alexa Fluor 633 (A20170) or Pacific blue (P30012) according to the manufacturer’s instructions (Invitrogen).
Mouse pretreatment
For labeling of FDCs, mice received 5 μg 8C12 antibody intraperitoneally 24 h before MP-IVM. For labeling of SSMs in vivo, 1 μg fluorescence-labeled CD169 was injected into the footpad 3–5 h before MP-IVM.
Enzyme-linked immunosorbent and immunospot assays
Mice were immunized with 1 × 105 bacteria subcutaneously. At day 10, blood was collected and serum was obtained. Enzyme-linked immunosorbent assays were done as described (Barrington et al., 2006).
Flow cytometry and data analysis
A FACSCanto II (BD Biosciences) was used for flow cytometry. Dead cells were excluded by sytox blue (Invitrogen). Data were analyzed with FlowJo software Version 9 (Tristar).
Statistical analysis
Samples were analyzed with two-tailed Student’s t test or two-way ANOVA followed by Tukey corrections to correct for multiple comparisons. p<0.05 between groups were considered significant. Statistical analysis was performed using GraphPad Prism Version 6 (GraphPad). Data are presented as data points with lines representing mean values ± SD or as bar graphs with mean ± SD.
Bacteria injection
Anesthetized mice were injected with 1 × 105 heat-inactivated CMTPX labeled bacteria in the hock (in a volume of 10 μl). At various time points, draining and non-draining lymph nodes were isolated for analysis of bacterial trafficking and SP strain D39-specific immune responses.
Histology and microscopy
Cryosections of lymph nodes embedded in optimal cutting temperature compound (TissueTek) were prepared, then sections were washed with Hank’s balanced salt solution and incubated with anti-FcR (2.4G2) and 2% BSA (Bovine Serum Albumine) before incubation with antibody as described (Roozendaal et al., 2009). Transmission electron microscopy of lymph nodes injected with SP was performed as described (Roozendaal et al., 2009). MP-IVM was performed as described (Gonzalez et al., 2010). For all mouse pretreatments, the hocks were injected with a volume of no more than 10 μl. Data was analyzed by the open source Fiji and Cell profiler software packages.
In Vivo Imaging
Mice were pretreated by intravenous injection with fluorescent anti-CR2 to label the FDCs in vivo. After 24 hr, fluorescent SP was injected subcutaneously into the footpad. The popliteal LN was surgically exposed and MP-IVM was performed as described (Miller et al., 2002). Data was analyzed by the open source Fiji software package.
Supplementary Material
Acknowledgments
We thank M. Nussenzweig (Rockefeller University) and U. von Andrian (Harvard Medical School) for CD11c-eYFP mice; R. Steinman (Rockefeller University) for the anti-SIGN-R1 hybridoma; M. Lipsitch (Harvard School of Public Health) for Streptococcus pneumoniae strain D39; S.F. Gonzalez for general guidance and help with Electron Microscopy, E.M. Carroll for technical assistance and C.M. Frijlink for proofreading of the manuscript. Supported by the National Institutes of Health (AI039246-19).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
Conceived and designed the experiments: BAH, MCC. Performed the experiments: BAH. Analyzed the data: BAH. Wrote the paper: BAH, MCC.
References
- Appelbaum PC. Resistance among Streptococcus pneumoniae: Implications for drug selection. Clin Infect Dis. 2002;34:1613–1620. doi: 10.1086/340400. [DOI] [PubMed] [Google Scholar]
- Bajic G, Yatime L, Sim RB, Vorup-Jensen T, Andersen GR. Structural insight on the recognition of surface-bound opsonins by the integrin I domain of complement receptor 3. Proc Natl Acad Sci USA. 2013;110:16426–16431. doi: 10.1073/pnas.1311261110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrington RA, Borde M, Rao A, Carroll MC. Involvement of NFAT1 in B cell self-tolerance. J Immunol. 2006;177:1510–1515. doi: 10.4049/jimmunol.177.3.1510. [DOI] [PubMed] [Google Scholar]
- Bennett CL, Clausen BE. DC ablation in mice: promises, pitfalls, and challenges. Trends Immunol. 2007;28:525–531. doi: 10.1016/j.it.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Brown JS, Hussell T, Gilliland SM, Holden DW, Paton JC, Ehrenstein MR, Walport MJ, Botto M. The classical pathway is the dominant complement pathway required for innate immunity to Streptococcus pneumoniae infection in mice. Proc Natl Acad Sci USA. 2002;99:16969–16974. doi: 10.1073/pnas.012669199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco YR, Batista FD. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity. 2007;27:160–171. doi: 10.1016/j.immuni.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Active Bacterial Core Surveillance Report 2013a [Google Scholar]
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States 2013b [Google Scholar]
- Fernandez Gonzalez S, Jayasekera JP, Carroll MC. Complement and natural antibody are required in the long-term memory response to influenza virus. Vaccine. 2008;26(Suppl 8):I86–I93. doi: 10.1016/j.vaccine.2008.11.057. [DOI] [PubMed] [Google Scholar]
- Gack MU, Shin YC, Joo CH, Urano T, Liang C, Sun L, Takeuchi O, Akira S, Chen Z, Inoue S, Jung JU. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature. 2007;446:916–920. doi: 10.1038/nature05732. [DOI] [PubMed] [Google Scholar]
- Gaya M, Castello A, Montaner B, Rogers N, Reis e Sousa C, Bruckbauer A, Batista FD. Host response. Inflammation-induced disruption of SCS macrophages impairs B cell responses to secondary infection. Science. 2015;347:667–672. doi: 10.1126/science.aaa1300. [DOI] [PubMed] [Google Scholar]
- Gitlin AD, Mayer CT, Oliveira TY, Shulman Z, Jones MJK, Koren A, Nussenzweig MC. HUMORAL IMMUNITY. T cell help controls the speed of the cell cycle in germinal center B cells. Science. 2015;349:643–646. doi: 10.1126/science.aac4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez SF, Degn SE, Pitcher LA, Woodruff M, Heesters BA, Carroll MC. Trafficking of B Cell Antigen in Lymph Nodes. Annu Rev Immunol. 2011;29:215–233. doi: 10.1146/annurev-immunol-031210-101255. [DOI] [PubMed] [Google Scholar]
- Gonzalez SF, Lukacs-Kornek V, Kuligowski MP, Pitcher LA, Degn SE, Kim YA, Cloninger MJ, Martinez-Pomares L, Gordon S, Turley SJ, Carroll MC. Capture of influenza by medullary dendritic cells via SIGN-R1 is essential for humoral immunity in draining lymph nodes. Nat Immunol. 2010;11:427–434. doi: 10.1038/ni.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffioen AW, Rijkers GT, Janssens-Korpela P, Zegers BJ. Pneumococcal polysaccharides complexed with C3d bind to human B lymphocytes via complement receptor type 2. Infect Immun. 1991;59:1839–1845. doi: 10.1128/iai.59.5.1839-1845.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesters BA, Chatterjee P, Kim YA, Gonzalez SF, Kuligowski MP, Kirchhausen T, Carroll MC. Endocytosis and recycling of immune complexes by follicular dendritic cells enhances B cell antigen binding and activation. Immunity. 2013;38:1164–1175. doi: 10.1016/j.immuni.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesters BA, Myers RC, Carroll MC. Follicular dendritic cells: dynamic antigen libraries. Nat Rev Immunol. 2014;14:495–504. doi: 10.1038/nri3689. [DOI] [PubMed] [Google Scholar]
- Iannacone M, Moseman EA, Tonti E, Bosurgi L, Junt T, Henrickson SE, Whelan SP, Guidotti LG, von Andrian UH. Subcapsular sinus macrophages prevent CNS invasion on peripheral infection with a neurotropic virus. Nature. 2010;465:1079–1083. doi: 10.1038/nature09118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoff EN, Rubins JB. Invasive pneumococcal disease in the immunocompromised host. Microb Drug Resist. 1997;3:215–232. doi: 10.1089/mdr.1997.3.215. [DOI] [PubMed] [Google Scholar]
- Johnston RB, Klemperer MR, Alper CA, Rosen FS. The enhancement of bacterial phagocytosis by serum. The role of complement components and two cofactors. J Exp Med. 1969;129:1275–1290. doi: 10.1084/jem.129.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junt T, Moseman EA, Iannacone M, Massberg S, Lang PA, Boes M, Fink K, Henrickson SE, Shayakhmetov DM, Di Paolo NC, van Rooijen N, Mempel TR, Whelan SP, von Andrian UH. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450:110–114. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- Kang YS, Do Y, Lee HK, Park SH, Cheong C, Lynch RM, Loeffler JM, Steinman RM, Park CG. A dominant complement fixation pathway for pneumococcal polysaccharides initiated by SIGN-R1 interacting with C1q. Cell. 2006;125:47–58. doi: 10.1016/j.cell.2006.01.046. [DOI] [PubMed] [Google Scholar]
- Kang YS, Kim JY, Bruening SA, Pack M, Charalambous A, Pritsker A, Moran TM, Loeffler JM, Steinman RM, Park CG. The C-type lectin SIGN-R1 mediates uptake of the capsular polysaccharide of Streptococcus pneumoniae in the marginal zone of mouse spleen. Proc Natl Acad Sci USA. 2004;101:215–220. doi: 10.1073/pnas.0307124101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YS, Yamazaki S, Iyoda T, Pack M, Bruening SA, Kim JY, Takahara K, Inaba K, Steinman RM, Park CG. SIGN-R1, a novel C-type lectin expressed by marginal zone macrophages in spleen, mediates uptake of the polysaccharide dextran. Int Immunol. 2003;15:177–186. doi: 10.1093/intimm/dxg019. [DOI] [PubMed] [Google Scholar]
- Klein A, Krishna M, Varki NM, Varki A. 9-O-acetylated sialic acids have widespread but selective expression: analysis using a chimeric dual-function probe derived from influenza C hemagglutinin-esterase. Proceedings of the National Academy of Sciences. 1994;91:7782–7786. doi: 10.1073/pnas.91.16.7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugman K. Clinical relevance of antibiotic resistance in pneumococcal infections 2004 [Google Scholar]
- Lindquist RL, Shakhar G, Dudziak D, Wardemann H, Eisenreich T, Dustin ML, Nussenzweig MC. Visualizing dendritic cell networks in vivo. Nat Immunol. 2004;5:1243–1250. doi: 10.1038/ni1139. [DOI] [PubMed] [Google Scholar]
- Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 2002;296:1869–1873. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]
- Mulholland K. Strategies for the control of pneumococcal diseases. Vaccine. 1979;17(Suppl 1):1999. doi: 10.1016/s0264-410x(99)00112-7. [DOI] [PubMed] [Google Scholar]
- Musher D. Streptococcus pneumoniae. Principles and Practice of Infectious Diseases. (4th) 1994 [Google Scholar]
- Neufeld F. Ueber die Antikorper des streptokokken-und pneumokokken-Immunserums. Dtsch Med Wochenschr 1904 [Google Scholar]
- Palese P. The great influenza The epic story of the deadliest plague in history. J Clin Invest. 2004;114:146–146. doi: 10.1172/JCI22439. [DOI] [Google Scholar]
- Phan TG, Green JA, Gray EE, Xu Y, Cyster JG. Immune complex relay by subcapsular sinus macrophages and noncognate B cells drives antibody affinity maturation. Nat Immunol. 2009;10:787–794. doi: 10.1038/ni.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan TG, Grigorova I, Okada T, Cyster JG. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat Immunol. 2007;8:992–1000. doi: 10.1038/ni1494. [DOI] [PubMed] [Google Scholar]
- Restrepo AV, Salazar BE, Agudelo M, Rodriguez CA, Zuluaga AF, Vesga O. BMC Microbiol. 2005;5:34. doi: 10.1186/1471-2180-5-34. 1471-2180-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal R, Mempel TR, Pitcher LA, Gonzalez SF, Verschoor A, Mebius RE, von Andrian UH, Carroll MC. Conduits mediate transport of low-molecular-weight antigen to lymph node follicles. Immunity. 2009;30:264–276. doi: 10.1016/j.immuni.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman Z, Gitlin AD, Targ S, Jankovic M, Pasqual G, Nussenzweig MC, Victora GD. T follicular helper cell dynamics in germinal centers. Science. 2013;341:673–677. doi: 10.1126/science.1241680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Grigorova I, Phan TG, Kelly LM, Cyster JG. Visualizing B cell capture of cognate antigen from follicular dendritic cells. J Exp Med. 2009;206:1485–1493. doi: 10.1084/jem.20090209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tew JG, Kosco MH, Burton GF, Szakal AK. Follicular dendritic cells as accessory cells. Immunol Rev. 1990;117:185–211. doi: 10.1111/j.1600-065x.1990.tb00573.x. [DOI] [PubMed] [Google Scholar]
- Tittel AP, Heuser C, Ohliger C, Llanto C, Yona S, Hämmerling GJ, Engel DR, Garbi N, Kurts C. Functionally relevant neutrophilia in CD11c diphtheria toxin receptor transgenic mice. Nat Methods. 2012;9:385–390. doi: 10.1038/nmeth.1905. [DOI] [PubMed] [Google Scholar]
- Tomasz A. Streptococcus pneumoniae: molecular biology & mechanisms of disease. Mary Ann Liebert; 2000. [Google Scholar]
- Tuomanen E. The pneumococcus. Amer Society for Microbiology 2004 [Google Scholar]
- van den Elsen JMH, Martin A, Wong V, Clemenza L, Rose DR, Isenman DE. X-ray crystal structure of the C4d fragment of human complement component C4. J Mol Biol. 2002;322:1103–1115. doi: 10.1016/s0022-2836(02)00854-9. [DOI] [PubMed] [Google Scholar]
- Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- Victora GD, Schwickert TA, Fooksman DR, Kamphorst AO, Meyer-Hermann M, Dustin ML, Nussenzweig MC. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010;143:592–605. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Cho B, Suzuki K, Xu Y, Green JA, An J, Cyster JG. Follicular dendritic cells help establish follicle identity and promote B cell retention in germinal centers. Journal of Experimental Medicine. 2011;208:2497–2510. doi: 10.1084/jem.20111449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward HK, Enders JF. AN ANALYSIS OF THE OPSONIC AND TROPIC ACTION OF NORMAL AND IMMUNE SERA BASED ON EXPERIMENTS WITH THE PNEUMOCOCCUS. J Exp Med. 1933;57:527–547. doi: 10.1084/jem.57.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff MC, Heesters BA, Herndon CN, Groom JR, Thomas PG, Luster AD, Turley SJ, Carroll MC. Trans-nodal migration of resident dendritic cells into medullary interfollicular regions initiates immunity to influenza vaccine. Journal of Experimental Medicine. 2014;211:1611–1621. doi: 10.1084/jem.20132327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, El Shikh MEM, El Sayed RM, Best AM, Szakal AK, Tew JG. IL-6 produced by immune complex-activated follicular dendritic cells promotes germinal center reactions, IgG responses and somatic hypermutation. Int Immunol. 2009 doi: 10.1093/intimm/dxp041. dxp041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


