Abstract
Purpose
Haploidentical and human leukocyte antigen (HLA)-identical sibling hematopoietic stem transplantation are two main ways used in allogeneic hematopoietic stem cell transplantation (allo-HSCT). In recent years, remarkable progress has been made in haploidentical allo-HSCT (HID-SCT), and some institutions found HID-SCT had similar outcomes as HLA-identical sibling allo-HSCT (ISD-SCT). To clarify if HID-SCT has equal effects to ISD-SCT in hematologic malignancies, we performed this meta-analysis.
Methods
Relevant articles published prior to February 2017 were searched on PubMed. Two reviewers assessed the quality of the included studies and extracted data independently. Odds ratio (OR) and 95% confidence intervals (CIs) were calculated for statistical analysis.
Results
Seven studies including 1919 patients were included. The rate of platelet engraftment is significantly lower after HID-SCT versus ISD-SCT while there is no difference in neutrophil engraftment (OR = 2.58, 95% CI = 1.70–3.93, P < 0.00001). The risk of acute graft-versus-host disease (GVHD) is significantly higher after HID-SCT versus ISD-SCT (OR = 1.88, 95% CI = 1.42–2.49, P < 0.00001), but the relapse rate is lower in HID-SCT group (OR = 0.70, 95% CI = 0.55–0.90, P = 0.005). The incidence rates of overall survival (OS) and disease-free-survival/leukemia-free survival/relapse-free survival (DFS/LFS/RFS) after ISD-SCT are all significantly superior to HID-SCT (OR = 1.32, 95% CI = 1.08–1.62, P = 0.006; OR = 1.25, 95% CI = 1.03–1.52, P = 0.02). There is no significant difference in transplantation related mortality (TRM) rate after HID-SCT and ISD-SCT.
Conclusion
After myeloablative conditioning, patients receiving ISD-SCT have a faster engraftment, lower acute GVHD and longer life expectancy compared to HID-SCT with GVHD prophylaxis (cyclosporine A, methotrexate, mycophenolate mofetil and antithymoglobulin; CsA + MTX + MMF + ATG). Currently, HID-SCT with GVHD prophylaxis (CsA + MTX + MMF + ATG) may not replace ISD-SCT when HLA-identical sibling donor available.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) with human leukocyte antigen (HLA)-identical sibling or unrelated donor is the main way for treatment for high-risk hematological malignancies. For patients without a suitable donor, especially those in urgent need of transplantation, haploidentical allo-HSCT (HID-SCT) is an option [1]. HID-SCT was unsuccessful for many years because of graft rejection and high incidence of acute graft-versus-host disease (GVHD), but the progress in GVHD prophylaxis and conditioning regimen has made HID-SCT possible [2,3]. A multicenter phase-2 study from the Chinese Bone Marrow Transplant Cooperative Group (CBMTCG) showed that the combination of cyclosporine A, methotrexate, mycophenolate mofetil (CsA + MTX + MMF) for GVHD prophylaxis significantly decreased the incidence of acute GVHD without an increase in relapse or any adverse impact on survival in standard-risk patients compared with historical controls in ISD-SCT [4]. Similarly, ATG deletes T lymphocytes chronically in vivo, and prevents GVHD without increasing the risks of relapse [5,6]. Some institutions demonstrated HID-SCT using conditioning regimen including ATG yielded similar outcomes to ISD-SCT for hematological malignancies [7,8]. Some studies also indicated similar outcomes after HID-SCT compared to HLA-identical allo-HSCT [9,10]. At present, HID-SCT has been accepted by many transplantation centers. Over the past decades, much progress has been made to improve the outcomes of transplantation, including in the conditioning regimen; prophylaxis; lower cumulative incidence rates of GVHD, transplantation-related mortality/ no-relapse mortality (TRM/NRM) and relapse; higher rates of OS and DFS/LFS/PFS. However, there haven’t been well-controlled studies to compare the efficacy of HID-SCT and ISD-SCT. Therefore, our meta-analysis aims to investigate whether HID-SCT has similar outcomes compared with ISD-SCT.
Methods
Identification and study selection
Two reviews independently identified relevant studies by searching PubMed. Search terms included “haploidentical stem cell transplantation”, “haploidentical” and “identical”. All studies published prior to February 2017 were eligible. The title and abstracts of all potentially relevant publications were reviewed. Studies that met the inclusion criteria were selected for the analysis. The reference lists from the selected articles were then hand-searched to identify further relevant trials.
Inclusion and exclusion criteria
This meta-analysis included hematologic malignancies who received HSCT (HID-SCT or ISD-SCT). T cell replete HID-SCT for hematologic malignancies using GVHD prophylaxis (CsA + MMF + MTX + ATG) was included. Studies with data concerning grades 3–4 acute GVHD were included if no suitable data of grades 2–4 acute GVHD were available. The inclusion criteria were as follows: randomized/nonrandomized studies that compared the outcomes of HID-SCT versus ISD-SCT; the key outcomes including engraftment, OS, DFS/LFS/RFS, acute/chronic-GVHD, relapse and NRM/TRM; myeloablative conditioning regimens and HID group GVHD prophylaxis (CsA + MMF + MTX + ATG).
We excluded ongoing studies or studies with data inaccessible. If the same authors had more than one publication based on same population, only the most recent or most complete report was included.
Quality assessment
Two reviewers independently assessed the quality of the included studies using Newcastle-Ottawa scale [11]. Studies scoring more than 5 stars were considered acceptable. Data were independently abstracted by each reviewer. Any disagreement between the two reviewers was solved by a third person who extracted the data again.
Statistical analysis
The extracted information was analyzed on the Cochrane statistical program Review Manager 5.3. For the key outcomes, odds ratio (OR) and 95% confidence intervals (CI) were calculated for each trial. Dichotomous outcomes were determined by the number of participants with events and the total number of participants in HID-SCT and ISD-SCT. Heterogeneity was checked by Q-test and defined as P < 0.1. Heterogeneity was quantified using the I2 metric (I2<50% acceptable level of heterogeneity; I2>50%, large or extreme). We performed meta-analysis using a fixed or random effect model (Mantel–Haenszel method for dichotomous data). A funnel plot was applied to detect the presence of publication bias.
Result
Description of included studies
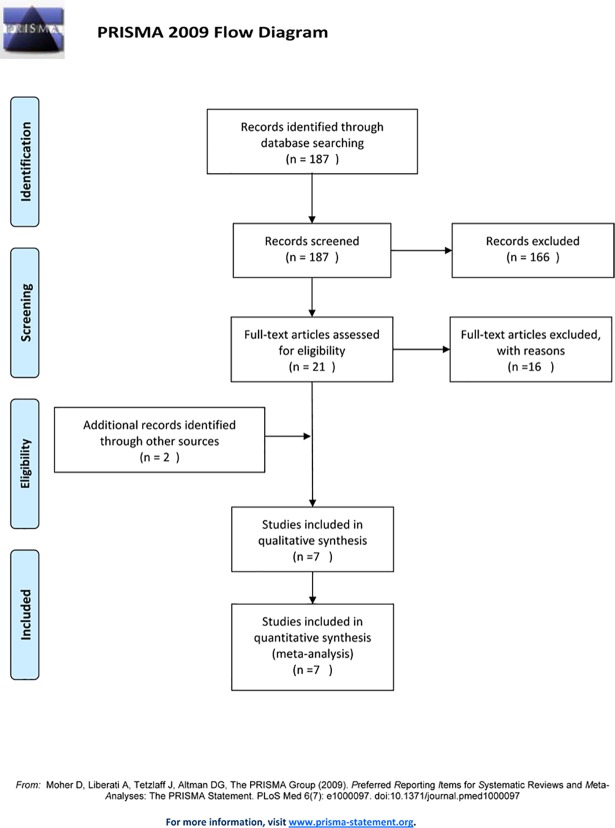
A total of 187 potentially relevant publications were retrieved from our initial search (Fig 1). Among these, 46 publications were excluded for review, 120 studies were excluded for not fulfilling the inclusion criteria and 16 were excluded for meeting the exclusion criteria. Additionally, 2 articles were included through searching the reference lists. Finally, 7 studies including 1919 patients were included [7,8,12–16] (Table 1). Specifically, 936 patients were treated with HID-SCT and 983 patients received ISD-SCT. Both non-randomized and non-blinded comparative studies were included. All data from the comparative studies of HID-SCT versus ISD-SCT were clinical trials. The key outcomes were neutrophil and platelet engraftment, OS, DFS/LFS/RFS, acute/chronic-GVHD, relapse and NRM/TRM, myeloablative conditioning regimen and HID group with GVHD prophylaxis (CsA + MMF + MTX + ATG) were also included.
Fig 1. Flowchart of selection of studies for inclusion in meta-analysis.
Table 1. Study quality.
| Study | Selection | Comparability | Outcome |
|---|---|---|---|
| QF Liu(2015) | ** | ** | ** |
| Xh Chen(2009) | ** | ** | ** |
| Yi Luo(2014) | ** | ** | ** |
| Y Wang(2016) | ** | ** | ** |
| SuJian Yu(2016) | ** | ** | ** |
| YU Wang(2016) | ** | ** | ** |
| Daopei Lu(2006) | ** | ** | ** |
A study can be awarded a maximum of one star for each numbered item within the Selection and Outcome categories if it meets the criteria. A maximum of two stars can be given for Comparability.
Selection: 1) Representativeness of the exposed cohort: truly/somewhat representative of the average in the community; 2) Selection of the non exposed cohort: drawn from the same community as the exposed cohort; 3) Ascertainment of exposure: secure record (eg surgical records) or structured interview; 4) Demonstration that outcome of interest was not present at start of study.
Comparability: 1) Comparability of cohorts on the basis of the design or analysis: a) stdy controls for; b) study controls for additional factor.
Outcome: 1) Assessment of outcome: independent blind assessment or record linkage; 2) The follow-up was long enough for outcomes to occur; 3) Adequacy of follow up of cohorts: complete follow up–all subjects accounted for, or subjects lost to follow up unlikely to introduce bias.
Characteristics of the included studies and subgroups
The characteristics of all included studies are shown in Table 2. Different hematologic diseases were included, such as acute myelogenous leukiemia (AML), acute lymphocytic leukemia (ALL), myelodysplastic syndromes (MDS), chronic myelogenous leukemia (CML), and lymphoma. Subgroups were divided by the follow up time ≤ 3 year and ≥ 4 year. The outcomes of each study are shown in Table 3.
Table 2. Characteristics of included studies.
| Trials | Type | Number | age | Diagnosis | Risk Status | Conditioning regimen | GVHD prophylaxis | |
|---|---|---|---|---|---|---|---|---|
| QF Liu(2015)[12] | HID | 231 | 28(15–57) | AML(231) | CR(231) | BUCY(231) | MAC | CsA+MMF+MTX+ATG |
| ISD | 219 | 40(17–60) | AML(231) | CR(219) | BUCY(219) | MAC | CsA+MMF+MTX | |
| Xh Chen(2009)[13] | HID | 46 | 25(5–54) | AML(15);ALL(14);CML(17) | CR+CP(39);Ad(7) | TBI-based(34);BUCY(12) | MAC | CsA+MMF+MTX+ATG |
| ISD | 52 | 30(15–52) | AML(12);ALL(11);CML(29) | CR+CP(44);Ad(8) | TBI-based(21);BUCY(31) | MAC | CsA+MMF+MTX | |
| Yi Luo(2014)[7] | HID | 99 | 25(9–55) | AML(42);ALL(32);CML(5);MDS(7); NHL(4) |
CR(78);Ad(6) | BUCY(99) | MAC | CsA+MMF+MTX+ATG |
| ISD | 90 | 33.5(16–56) | AML(29);ALL(50);CML(5);MAPL(2); MDS(10);NHL(3) |
CR(71);Ad(7) | BUCY(90) | MAC | CsA+MMF+MTX | |
| Y Wang(2016)[14] | HID | 226 | 30-35(4–61) | MDS(226) | CR(21);Ad(49) | BUCY(226) | MAC | CsA+MMF+MTX+ATG |
| ISD | 228 | 40(4–61) | MDS(228) | CR(40);Ad(51) | BUCY(228) | MAC | CsA+MMF+MTX | |
| SuJian Yu(2016)[15] | HID | 96 | 25(12–54) | AML(40);ALL(40);ABL(10);CML(6) | CR(58);Ad(38) | BUCY(35);BUFlu(2); TBI-based(59) |
MAC | CsA+MMF+MTX+ATG |
| ISD | 153 | 31(12–61) | AML(72);ALL(62);ABL(13);CML(6) | CR(122);Ad(31) | BUCY(34);BUFlu(25); TBI-based(94) |
MAC | CsA/CsA+MTX | |
| YU Wang(2016)[16] | HID | 121 | 26(18–59) | ALL(121) | CR(121) | BUCY(121) | MAC | CsA+MMF+MTX+ATG |
| ISD | 89 | 38(18–59) | ALL(121) | CR(89) | TBI-based(21);BUCY(62) | MAC | CsA+MMF+MTX | |
| Daopei Liu(2006)[8] | HID | 135 | 24 (3–50) | CML(43);AML(30);ALL (53);MDS(10) | CR1+CP(68);Ad(67) | BUCY(135) | MAC | CsA+MMF+MTX+ATG |
| ISD | 158 | 37 (5–50) | CML(68);AML(39);ALL (39);MDS(12) | CR1+CP(100);Ad(58) | BUCY(158) | MAC | CsA+MMF+MTX CsA+MMF+MTX+ATG(7) |
|
HID = haploidentical donor, ISD = HLA-identical sibling donor, CML = chronic myeloid leukemia, AML = acute myeloid leukemia, ALL = acute lymphoid leukemia, MDS = myelodysplastic syndrome, MPAL = acute mixed phenotypic leukemia, NHL = non-Hodgkin's lymphoma, ABL = acute biphenotypic leukemia, CR = complete remission, CP = chronic phase, Ad = advance disease(no-CR,no-CP), BU = busulfan, CY = cyclophosphamide, TBI = total body irradiation,MAC = myeloablative conditioning, CsA = cyclosporine A, MMF = mycophenolate mofetil, MTX = methotrexate, ATG = antithymoglobulin, Flu = fludarabine.
Table 3. Outcome of included studies.
| Trails | type | Patients (n) |
Neutrophil Engraftment(n) |
Platelet engraftment(n) |
acute GVHD(n) | Chronic GVHD (n) |
Relapse (n) | NRM(n) | TRM(n) | DFS/LFS/RFS (n) | OS (n) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| QF Liu(2015)[12] | HID | 231 | 30d(231) | 100d(208) | Grades 3–4 100d(24) | 1y(97) | 3y(35) | 3y(30) | - | DFS 3y(171) | 3y(183) |
| ISD | 219 | 30d(219) | 100d(211) | Grades 3–4 100d(7) | 1y(33) | 3y(33) | 3y(18) | - | DFS 3y(171) | 3y(180) | |
| Xh Chen(2009)[13] | HID | 46 | 30d(46) | 30d(46) | Grades 3–4 100d(0) | (5) | 2y(11) | - | 2y(4) | LFS 2y(33) | 2y(36) |
| ISD | 52 | 30d(52) | 30d(52) | Grades 3–4 100d(0) | (18) | 2y(10) | - | 2y(5) | LFS 2y(38) | 2y(40) | |
| Yi Luo(2014)[7] | HID | 99 | 30d(99) | 30d(95) | Grades 3–4 3m(17) | 2y(41) | 5y(14) | 5y(31) | - | DFS 5y(58) | 5y(61) |
| ISD | 90 | 30d(90) | 30d(86) | Grades 3–4 3m(5) | 2y(22) | 5y(31) | 5y(5) | - | DFS 5y(58) | 5y(70) | |
| Y Wang(2016)[14] | HID | 226 | 28d(216) | 100d(182) | Grades 3–4 100d(17) | 4y(91) | 4y(15) | 4y(73) | - | RFS 4y(136) | 4y(136) |
| ISD | 228 | 28d(216) | 100d(208) | Grades 3–4 100d(16) | 4y(117) | 4y(23) | 4y(37) | - | RFS 4y(162) | 4y(167) | |
| Sijian Yu(2016)[15] | HID | 96 | - | - | Grades 3–4 100d(39) | 2y(51) | 5y(19) | - | 5y(26) | DFS 5y(58) | 5y(58) |
| ISD | 153 | - | - | Grades 3–4 100d(36) | 2y(66) | 5y(41) | - | 5y(27) | DFS 5y(90) | 5y(99) | |
| YU Wang(2016)[16] | HID | 103 | 30d(102) | 100d(91) | Grades 3–4 100d(7) | 3y(40) | 3y(19) | 3y(14) | - | DFS 3y(70) | 3y(78) |
| ISD | 83 | 30d(82) | 100d(81) | Grades 3–4 100d(2) | 3y(21) | 3Y(20) | 3y(10) | - | DFS 3y(54) | 3y(59) | |
| Daopei Liu(2006)[8] | HID | 135 | - | - | Grades 2–4 100d(54) | 2y(66) | 2y(18) | - | 2y(20) | LFS 2y(87) | 2y(96) |
| ISD | 158 | - | - | Grades 2–4 100d(51) | 2y(83) | 2y(29) | - | 2y(23) | LFS 2y(113) | 2y(114) |
d = day, m = month, y = year, NRM = no relapse mortality, DFS = diseae free survival, LFS = leukemia free survival, RFS = relapse free survival, TRM = treatment-related mortality, OS = overall survival, GVHD = graft versus host disease
Effects of interventions
Neutrophil and platelet engraftment
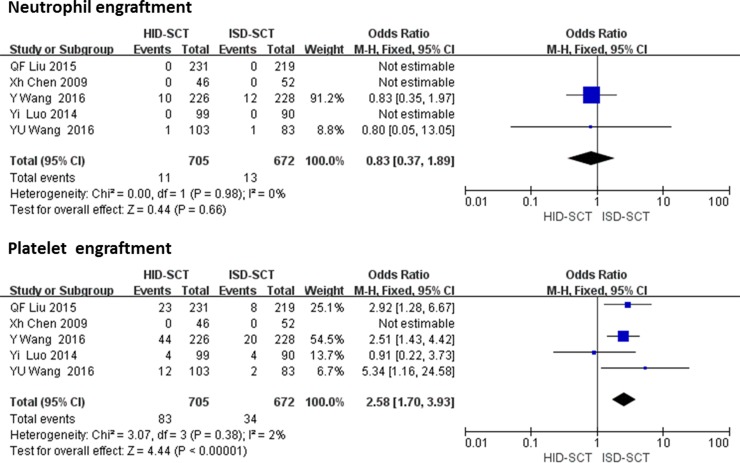
Five studies reported neutrophil and platelet engraftment rates, including 705 HID-SCT-treated patients and 672 ISD-SCT-treated patients. For neutrophil engraftment, a fixed effect model was used. There were no significances differences in the incidence rates of neutrophil engraftment between the two groups (OR = 0.83, 95% CI = 0.37–1.89, P = 0.66, I2 = 0). The platelet engraftment was significantly faster following ISD-SCT than HID-SCT (OR = 2.58, 95% CI = 1.70–3.93, P < 0.00001, I 2 = 30%) (Fig 2).
Fig 2. Forest plot of comparisons between HID-SCT and ISD-SCT: Neutrophil and platelet engraftment.
GVHD
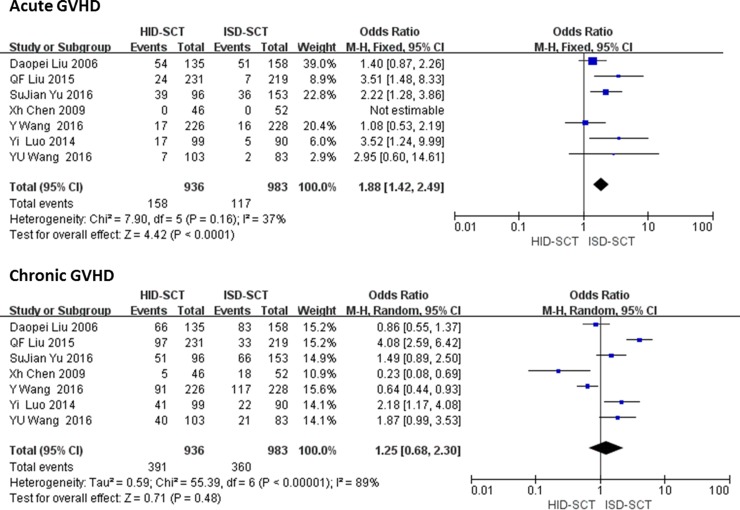
There is a significant difference between HID-SCT and ISD-SCT regarding the incidence rates of acute GVHD, but not chronic GVHD. We extracted data regarding acute GVHD from seven studies including 1919 patients. The fixed effect model was used, results showed the risk of acute GVHD after HID-SCT was significantly higher than ISD-SCT (OR = 1.88, 95% CI = 1.42–2.49, P < 0.00001, I2 = 37%). No significant difference was found in the incidence rates of chronic GVHD between HID-SCT and ISD-SCT (OR = 1.25, 95% CI = 0.68–2.30, P = 0.48, I2 = 89%) (Fig 3).
Fig 3. Forest plot of comparisons between HID-SCT and ISD-SCT: Acute GVHD and chronic GVHD.
Relapse and NRM/TRM
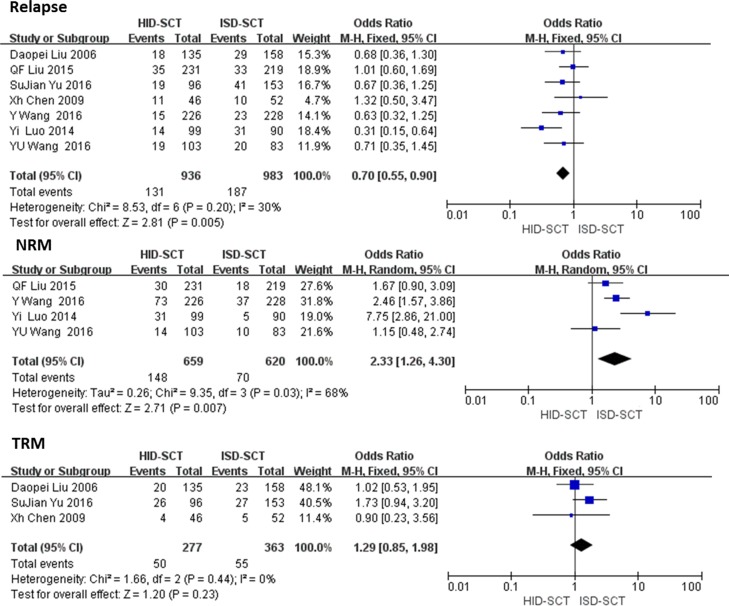
Seven studies regarding relapse involving 1919 patients were analyzed with the fixed effect model. The risk of relapse after HID-SCT was significantly lower than ISD-SCT (OR = 0.70, 95% CI = 0.55–0.90, P = 0.005, I2 = 30%). Four studies reported the NRM rates and three reported TRM rates, respectively. The risk of TRM was not significantly different between HID-SCT-treated and ISD-SCT-treated patients (OR = 1.29, 95% CI = 0.85–1.98, P = 0.23, I2 = 0). Significant difference in TRM was found, but it was unreliable because of unacceptable heterogeneity (OR = 2.33, 95% CI = 2.16–4.30, P = 0.007, I2 = 68%) (Fig 4).
Fig 4. Forest plot of comparisons between HID-SCT and ISD-SCT: Relapse and NRM/TRM.
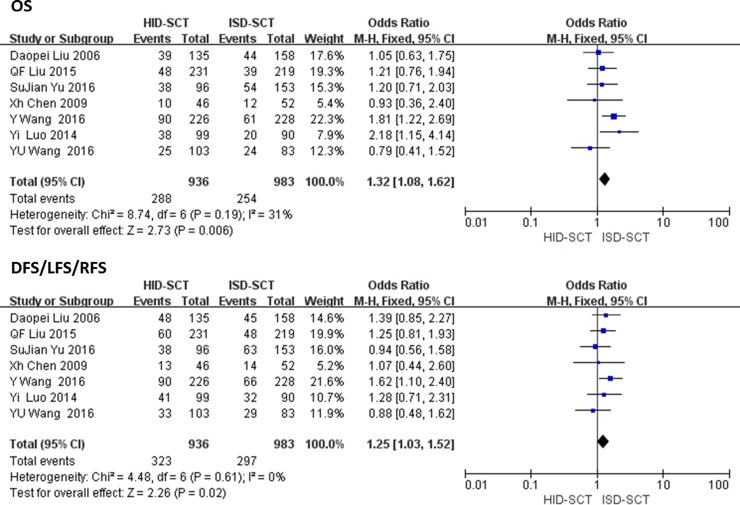
DFS/LFS/RFS and OS
All seven studies reported DFS/LFS/RFS and OS. Fixed effect model showed the heterogeneity of outcomes was acceptable. The rates of DFS/LFS/RFS after HID-SCT were significantly lower compared with ISD-SCT (OR = 1.25, 95% CI = 1.03–1.52, P = 0.02, I2 = 0). Significant differences in OS and longer life expectancy were found between ISD-SCT-treated and HID-SCT-treated group (OR = 1.32, 95% CI = 1.08–1.62, P = 0.006, I2 = 31%) (Fig 5).
Fig 5. Forest plot of comparisons between HID-SCT and ISD-SCT: DFS/LFS/RFS and OS.
Meta regression and subgroup analysis
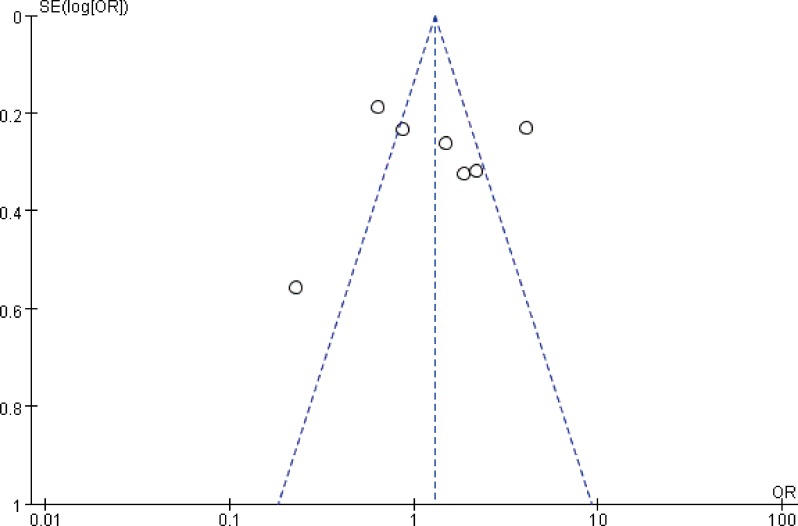
As mentioned above, we found significant heterogeneity in chronic GVHD and NRM rates. Thus, subgroup analysis by follow-up time was performed to further investigate NRM. It was found the cumulative incidence rates of NRM for HID-SCT and ISD-SCT were similar at ≤ 3 year, but not at ≥ 4year (P = 0.13, I2 = 0; P = 0.01, I 2 = 77%) (Table 4). For chronic GVHD, publication bias might cause the heterogenity(Fig 6).
Table 4. P value and 95% CI of subgroup for NRM.
| outcome | subgroup | studies | patients | OR | 95% CI | P | I2 |
|---|---|---|---|---|---|---|---|
| NRM | |||||||
| Follow-up time | ≤3 years | 2 | 636 | 1.47 | 0.89–2.43 | 0.13 | 0 |
| ≥4 years | 3 | 643 | 4.00 | 1.31–12.2 | 0.01 | 77 |
NRM = transplantation related mortality, OR = odds ratio, CI = confidence intervals
Fig 6. Funnel plot of comparisons between HID-SCT and ISD-SCT: Chronic GVHD.
Discussion
To our knowledge, this is the first meta-analysis that compares HID-SCT with ISD-SCT in an unselected population of hematological malignancies. The results indicate that HID-SCT is associated with a higher risk for acute GVHD, a lower rate of platelets engraftment, and worse OS and DFS/LFS/RFS. No significant difference was found in TRM rate and neutrophil engraftment. Previous studies reported that HID-SCThad lower neutrophil engraftment rate which led to higher TRM. Also the median time to engraftment after HID-SCT was significantly longer than after ISD-SCT [7,8,9,12,15]. HID-SCT without ATG resulted in similar median time for neutrophil engraftment, and longer platelet engraftment compared with ISD-SCT [17]. ATG is associated with delayed immune reconstitution, which is often accompanied by severe infections and higher TRM. But we found no significant difference in neutrophil engraftment (according to the follow-up time (30 days)) or cumulative incidence of TRM between the two groups. Although the median time to engraftment in HID-SCT group was significantly longer than in the ISD-SCT group, it may indicate neutrophil engraftment within 30d, did not increase the risk of TRM. For platelet engraftment, whatever median time or 100d cumulative incidence, HID-SCT was thought to have lower engraftment rate than ISD-SCT [7,12,14–16]. Our meta-analysis indicates HID-SCT is associated with a higher risk of acute GVHD. However, the reduced intensity transplantation (RIC) with HID-SCT (post-transplantation cyclophosphamide PT-Cy) has showed similar risks of severe acute GVHD and 3-year rates of NRM, relapse, OS and PFS compared with ISD-SCT [18]. Similarly, HID-SCT (T-cell depleted grafts or CsA + MMF + Cyclophosphamide) was associated with a similar risk of severe acute GVHD [17,19]. However, some studies reported a higher risk of grade 3–4 of acute GVHD following HID-SCT with ATG compared to ISD-SCT [7,12,15] while some other studies found a similar risk of grade 3–4 of GVHD between HID-SCT with ATG and ISD-SCT [13,14]. Our meta-analysis indicates HID-SCT with ATG has a higher risk of serious acute GVHD than ISD-SCT. Further studies are needed to uncover whether ATG can reduce the risk of severe acute GVHD compared with T-cell depleted grafts or PT-Cy. Higher risk for GVHD is associated with more mortality, correspondingly, the low rate of relapse is supposed to improve DFS/LFS/RFS, even OS. It should be mentioned that relevant studies also suggested in high risk acute leukemia, HID-SCT has lower relapse rate and longer OS than ISD-SCT [20]. Many studies showed the cumulative risk of relapse was similar or lower after HID-ISD than ISD-SCT [7,8,12,14–16]. However, this finding is not fully consistent with the conclusions of our meta-analysis. We found a lower risk for relapse after HID-SCT, but did not find higher rates for DFS/LFS/RFS or OS. HID-SCT may be superior to ISD-SCT in terms of high risk acute leukemia but no evidence suggests the low risk of relapse could lead to better DFS/LFS/RFS or OS.
For the incidence rates of NRM after HID-SCT and ISD-SCT, the outcomes of NRM were opposite between the follow up durations ≤ 3 years and ≥ 4 years. We did not find significant differences between the two groups at the follow up time ≤ 3 years. Related research also showed no significant differences in the incidence of NRM at one year [20]. However, the 2-year incidence of NRM was significantly lower among haploidentical related recipients compared to HLA-matched related recipients for relapsed or refractory Hodgkin Lymphoma [21]. Over the past decades, new approaches (such as GVHD prophylaxis, conditioning regimen) were applied to HID-SCT which have effectively controlled intense alloreactivity, resulting in improved outcomes, but our results indicate ISD-SCT is still the preferred option in all transplantations, and other promising HID-SCT strategies should be pursued.
Our meta-analysis has some limitations, such as different risk status and diagnosis among the included studies, long time interval, and different follow up durations. To obtain better conclusion, we need more randomized controlled studies.
Supporting information
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work is supported by Peak of Six Talent in Jiangsu Province (2015-WSN-075).
References
- 1.Ballen K, Gluckman E, Broxmeyer HE. Umbilical cord blood transplantation: the first 25 years and beyond. Blood.2013; 122(4):491–498. doi: 10.1182/blood-2013-02-453175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008; 14(6): 641–650. doi: 10.1016/j.bbmt.2008.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di BP, Santarone S, De AG, Picardi A, Cudillo L, Cerretti R, et al. Haploidentical, unmanipulated, G-CSF-primed bone marrow transplantation for patients with high-risk hematologic malignancies. Blood. 2013; 121(5):849–857. doi: 10.1182/blood-2012-08-453399 [DOI] [PubMed] [Google Scholar]
- 4.Lai Y, Ma J, Schwarzenberger P, Li W, Cai Z, Zhou J, et al. Combination of CsA, MTX and Low–dose, short-course mycophenolate mofetil for GVHD prophylaxis. Bone Marrow Transplant.2009; 43(1):61–67. doi: 10.1038/bmt.2008.265 [DOI] [PubMed] [Google Scholar]
- 5.Duggan P, Booth K, Chaudhry A, Stewart D, Ruether JD, Glück S, et al. Unrelated donor BMT recipients given pretransplant lowdose antithymocyte globulin have outcomes equivalent to matched sibling BMT: a matched pair analysis. Bone Marrow Transplant.2002; 30(10):681–686. doi: 10.1038/sj.bmt.1703674 [DOI] [PubMed] [Google Scholar]
- 6.Basara N, Baurmann H, Kolbe K, Yaman A, Labopin M, Burchardt A, et al. Antithymocyte globulin for the prevention of graft-versushost disease after unrelated hematopoietic stem cell transplantation for acute myeloid leukemia: results from the multicenter German cooperative study group. Bone Marrow Transplant.2005; 35(10):11–1018. [DOI] [PubMed] [Google Scholar]
- 7.Luo Y, Xiao H, Lai X, Shi J, Tan Y, He J, et al. T-cell-replete haploidentical HSCT with low-dose anti-T-lymphocyte globulin compared with matched sibling HSCT and unrelated HSCT. Blood. 2014; 124(17):2735–43. doi: 10.1182/blood-2014-04-571570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu DP, Dong L, Wu T, Huang XJ, Zhang MJ, Han W, et al. Conditioning including antithymocyte globulin followed by unmanipulated HLA- mismatched/haploidentical blood and marrow transplantation can achieve comparable outcomes with HLA-identical sibling transplantation. Blood. 2006; 107(8):3065–73. doi: 10.1182/blood-2005-05-2146 [DOI] [PubMed] [Google Scholar]
- 9.Raiola AM, Dominietto A, di Grazia C, Lamparelli T, Gualandi F, Ibatici A, et al. Unmanipulated haploidentical transplants compared with other alternative donors and matched sibling grafts. Biol Blood Marrow Transplant.2014; 20(10):1573–1579. doi: 10.1016/j.bbmt.2014.05.029 [DOI] [PubMed] [Google Scholar]
- 10.Di Stasi A, Milton DR, Poon LM, Hamdi A, Rondon G, Chen J, et al. Similar transplantation outcomes for acute myeloid leukemia and myelodysplastic syndrome patients with haploidentical versus 10/10 human leukocyte antigen-matched unrelated and related donors. Biol Blood Marrow Transplant.2014; 20(12):1975–1981. doi: 10.1016/j.bbmt.2014.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The Newcastle-Ottawa Quality Assessment Scale (NOS). http://www.ohri.ca/programs/clinicalepidemiology/oxford.asp.
- 12.Wang Y, Liu QF, Xu LP, Liu KY, Zhang XH, Ma X, et al. Haploidentical versus identical-sibling transplant for AML in remission: a multi-centre, prospective study. Blood. 2015; 125(25):3956–3962. doi: 10.1182/blood-2015-02-627786 [DOI] [PubMed] [Google Scholar]
- 13.Chen XH, Zhang C, Zhang X, Gao L, Gao L, Kong PY, et al. Role of Antithymocyte Globulin and Granulocyte Colony Stimulating Factor-Mobilized Bone Marrow in Allogeneic Transplantation for Patients with Hematologic Malignancies. Biol Blood Marrow Transplant. 2009; 15(2):266–73. doi: 10.1016/j.bbmt.2008.11.029 [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Wang HX, Lai YR, Sun ZM, Wu DP, Jiang M, et al. Haploidentical transplant for myelodysplastic syndrome: Registry-based comparison with identical-sibling transplant. Leukemia. 2016; 30(10):2055–2063. doi: 10.1038/leu.2016.110 [DOI] [PubMed] [Google Scholar]
- 15.Yu S, Fan Q, Sun J, Fan Z, Zhang Y, Jiang Q, et al. Haploidentical Transplantation Without In Vitro T-Cell Depletion Results in Outcomes Equivalent to Those of Contemporaneous Matched Sibling and Unrelated Donor Transplantation for Acute Leukemia.Medicine (Baltimore). 2016; 95(11):e2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Liu QF, Xu LP, Liu KY, Zhang XH, Ma X, et al. Haploidentical versus Matched-Sibling Transplant in Adults with Philadelphia-negative high-risk Acute Lymphoblastic Leukemia: A biologically phase 3 randomized study. Clin Cancer Res. 2016; 22(14):3467–76. doi: 10.1158/1078-0432.CCR-15-2335 [DOI] [PubMed] [Google Scholar]
- 17.Raiola AM, Dominietto A, di Grazia C, Lamparelli T, Gualandi F, Ibatici A, et al. Unmanipulated Haploidentical Transplants Compared with Other Alternative Donors and Matched Sibling Grafts. Biol Blood Marrow Transplant. 2014;20(10):1573–9. doi: 10.1016/j.bbmt.2014.05.029 [DOI] [PubMed] [Google Scholar]
- 18.Ghosh N, Karmali R, Rocha V, Ahn KW, DiGilio A, Hari PN, et al. Reduced-Intensity Transplantation for Lymphomas Using Haploidentical Related Donors Versus HLA-Matched Sibling Donors: A Center for International Blood and Marrow Transplant Research Analysis. J Clin Oncol.2016; 34(26):3141–9. doi: 10.1200/JCO.2015.66.3476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ringdén O, Labopin M, Ciceri F, Velardi A, Bacigalupo A, Arcese W, et al. Is there a stronger graft-versus-leukemia effect using HLA-haploidentical donors than with HLA-identical siblings? Leukemia. 2016; 30(2):447–55. doi: 10.1038/leu.2015.232 [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Liu DH, Xu LP, Liu KY, Chen H, Chen YH, et al. Superior graft-versus leukemia effect associated with transplantation of haploidentical compared with HLA-identical sibling donor grafts for high-risk acute leukemia: an historic comparison. Biol Blood Marrow Transplant.2011; 17(6): 821–830. doi: 10.1016/j.bbmt.2010.08.023 [DOI] [PubMed] [Google Scholar]
- 21.Burroughs LM, O’Donnell PV, Sandmaier BM, Storer BE, Luznik L, Symons HJ, et al. Comparison of Outcomes of HLA-Matched Related, Unrelated, or HLA-Haploidentical Related Hematopoietic Cell Transplantation following Nonmyeloablative Conditioning for Relapsed or Refractory Hodgkin Lymphoma. Biol Blood Marrow Transplant. 2008; 14(11):1279–87. doi: 10.1016/j.bbmt.2008.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.