Abstract
Background
The prognostic role of human epidermal growth factor receptor 2 (HER2) in ovarian cancer has been investigated in previous studies, but the results remain controversial. Here we present a meta-analysis to systematically review the association between HER2 expression and ovarian cancer prognosis.
Method
Observational studies published until July 2017 were searched in Pubmed, Embase, and Cochrane library databases. Hazard ratios (HRs) for survival with 95% confidence intervals (CIs), subgroup analyses, publication bias and sensitivity analyses were implemented under a standard manner. Estimates of overall survival (OS), progress-free survival (PFS) and disease-free survival (DFS) were weighted and pooled using Der Simonian-Laird random-effect model.
Result
Thirty-four studies that include 5180 ovarian cancer patients were collected for analysis. Expression of HER2 was negatively correlated with clinical prognosis of overall survival (HR = 1.57, 95% CI: 1.31 to 1.89, P < 0.001) and disease-free survival / progress-free survival (HR = 1.26, 95% CI = 1.06 to 1.49) in ovarian cancers. The association between HER2 expression and poor ovarian cancer prognosis in overall survival was also statistically significant in subgroups of unclassified ovarian cancer, Caucasian population and Asian population, while irrespective of detection method.
Conclusion
HER2 expression was related with poor prognosis in ovarian cancer patients and can be used as a predicting cancer prognostic biomarker in ovarian cancer patients.
Introduction
Ovarian cancer is the leading cause of gynecologic cancer death in women and impacts female life and health all over the world [1]. It is reported that ovarian cancer affects 238,719 women and causes over 150,000 deaths annually owing to that patients are diagnosed in late stages of the disease [2, 3]. Although radical surgical tumor debulking and platinum plus paclitaxel-based chemotherapy are currently established therapy of ovarian cancer patient, the prognosis of 5-year survival rate is still around 40% [4]. Hence, it is of great clinical value to identify applicable prognosis biomarkers to predict the outcomes of ovarian cancer patients.
Human epidermal growth factor receptor 2 (HER2), located on chromosome 17q12-21 [5], is a tyrosine kinase receptor in the epidermal growth factor (EGF) family and play a pivotal role in cell proliferation and tumor cell metastasis [6]. HER2 overexpression has been detected in various cancer types, including 30% of breast cancers [7], 35%-45% of pancreatic carcinomas [8], which seemed to be a poor predictor for cancer. Until now, the association between HER2 expression and ovarian cancer has been widely studied, but the results are still controversial [6, 9–41]. Most recent reports demonstrated that the expression of HER2 was a predictor of poor prognosis for ovarian cancer [12, 16, 20, 26, 34–35, 37–38, 41], while others showed that the HER2 expression had no influence on the survival in ovarian cancer patients [6, 11, 13–15, 17–19, 21–25, 27–33, 36, 39–40]. All studies assessed HER2 protein expression by immunohistochemistry or HER2 gene amplification. Therefore, to clarify a better understanding of the relationship between HER2 expression and ovarian cancer, we performed a meta-analysis combining 34 studies (5180 patients) as well as subgroups analysis, aiming to gain insights into the clinical implications.
Materials and methods
Search strategy
Pubmed, Embase and Cochrane library were comprehensively searched for relevant studies published from 1980 to July 2017 with the following keywords: “ovarian cancer”, “ovarian tumor”, “ovarian neoplasm”, “ovarian carcinoma” or “ovarian malignance” and “HER2”, “HER-2”, “HER 2”, “human epidermal growth factor receptor 2”, “erbB-2” or “neu” and “prognosis”, “survival” and “outcome”. No time and language restrictions were imposed. Additionally, the relevant literatures including all of the identified studies, reviews and editorials were also reviewed. All candidate studies were carried out by two independent reviewers (Luo H and Xu XH) and discrepancies were resolved by consensus.
Criteria for inclusion and exclusion
Studies that fulfilled the following criteria were considered eligible and selected into this article: (1) the publication explored the relation between HER2 expression and ovarian cancer prognosis, such as overall survival (OS), progress-free survival (PFS), disease-free survival (DFS) and recurrence-free survival (RFS), (2) sufficient data were either reported directly or there was sufficient data to calculate HR with 95% confidence interval (CI). (3) studies were written in English. (4) exclusion of reviews, letters to the editor, case reports and conference papers without original data. When duplicate or overlapped studies were retrieved, we included the most informative and recent articles.
Data extraction
Two independent investigators reviewed the publications and extracted the data by aid of predefined standardized extraction forms: the first author’s name, year of publication, country of origin, histological type and stage, number of patients, detection method, age, number of HER2 expression patients and controls, follow-up time, outcome endpoint, univariate or multivariate hazard ratio (HR) and the 95% confidence interval (95% CI) for HER2 positive-expression versus HER2 negative-expression. If univariate and multivariate HR and 95%CI were both reported, multivariate results were selected in an individual study. If the article had Kaplan-Meier curves, we used Engauge Digitizer 4.1 to digitize and extract survival information from the Kaplan-Meier curves. Discrepancies were resolved by a joint consensus and discussion.
Quality assessment
Owing to the included studies were observational studies, a Newcastle-Ottawa Scale (NOS) was used to evaluate the quality. It was used to appraise the methodological quality, which has an eight-item instrument to judge on three broad perspectives: the selection of studies; study comparability; and the ascertainment of the outcome of interest. Using the awarding of points or “stars”, we considered studies awarded with 6 or higher were classified as high-quality studies.
Statistical analysis
MetaHR (pooled HR in the survival analysis) and 95%CI were applied to assess the association between HER2 expression and outcomes of ovarian cancer patients. Outcome endpoints were divided into two groups, OS and DFS/PFS, based on the data acquired in the current study and previous report. Statistical heterogeneity was assessed by H and I-square statistics [42], random-effects model [43–44] was used in the paper. Subgroup analysis and sensitivity analysis were performed to explore the source of heterogeneity. Publication bias was evaluated by a funnel plot with Begg’s test, if a P < 0.05, publication bias was probably existed. Statistical analyses were conducted Stata version 12.0 (StataCrop LP, Texas). All the statistical tests were two-sided, P < 0.05 was considered statistically significant.
Results
Eligible studies
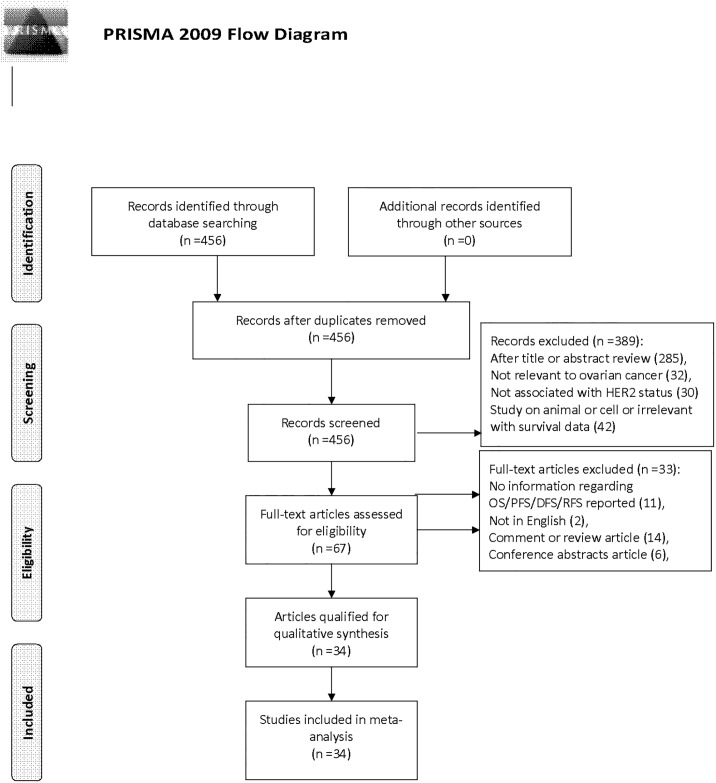
A total of 456 records were retrieved from three databases by the initial search. Then 389 articles were excluded because of obvious lack of relevance. After carefully reviewing the full texts based on the inclusive criteria, 33 articles were excluded (11 had no information regarding OS/DFS/PFS, 2 studies were not written in English, 14 articles were review or comment, 6 were conference articles). Finally, 34 observational studies were selected for the present meta-analysis. A flow chart showing the study selection was presented in Fig 1.
Fig 1. PRISMA flow chart of literature search and study selection.
Demographic characteristics of included studies
The main characteristics of the 34 studies were presented in Table 1. These studies were published between 1990 and 2017. These studies were conducted in 19 countries (6 cohorts were Asian populations, 26 cohorts were Caucasian populations and 2 cohorts were mix populations). A total of 5180 patients were included with a range from 40 to 783. 27 investigations detected the HER2 status by immunohistochemistry (IHC), 3 studies used fluorescence in situ hybridization (FISH), 1 paper used chromogenic in situ hybridization (CISH), 1 research used enzyme-linked immunosorbent assay (ELISA), 1 trail used polymerase chain reaction (PCR) and the remaining 1 research used southern blot. A total of 34 studies described the association of overall survival (OS) and HER2 expression, while 14 trials involved disease-free survival (DFS) / progress-free survival (PFS). The quality of the included studies, as assessed by the Newcastle-Ottawa Scale (NOS), ranged from six to nine scores, revealing a high quality across all studies. Detailed features were recorded in Table 1.
Table 1. Characteristics of the included studies.
| Study & year | Country | Ethnic | Histological type | Stage | Sample size | Detection method | Age (min-max) |
HER2 (positive/all) |
Follow up (months) |
Out-comes | HR (95%CI) |
Method for data collection | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shang 2017 [9] |
China | Asian | Unclassified | NA | 136 | IHC | 54(21–83) median |
41/136 | 48 (all) |
OS | 1.81 (1.16–2.83) |
Directly | 7 |
| Wang 2016 [10] |
China | Asian | Unclassified | I-IV | 111 | IHC | 51.3(24–78) (mean) |
35/111 | NA | OS | 1.92 (1.12–3.26) |
Directly | 7 |
| Shandiz 2016 [11] |
Iran | Asian | Unclassified | I-IV | 47 | IHC | 51.6(19–71) (mean) |
12/47 | 27.7(6–60) (median) |
OS | 0.82 (0.66–1.02) | Indirectly | 7 |
| Shandiz 2016 [11] |
Iran | Asian | Unclassified | I-IV | 47 | IHC | 51.6(19–71) (mean) |
12/47 | 27.7(6–60) (median) |
DFS | 0.53 (0.04–7.57) |
Indirectly | 7 |
| Zhang 2015 [12] |
China | Asian | Unclassified | I-IV | 161 | IHC | NA | NA | 60 (all) |
OS | 3.46 (1.84–6.52) |
Directly | 8 |
| Despierre 2015 [13] |
Belgium | Caucasian | Unclassified | I-IV | 106 | FISH | 59(31–85) (median) |
53/106 | NA | OS | 0.97 (0.49–1.89) |
Directly | 8 |
| Despierre 2015 [13] |
Belgium | Caucasian | Unclassified | I-IV | 106 | FISH | 59(31–85) (median) |
53/106 | NA | PFS | 1.51 (0.87–2.63) |
Directly | 8 |
| Corkery 2015 [14] |
Canada | Caucasian | Serous | NA | 103 | IHC | NA | NA | NA | OS | 4.41 (1.95–9.95) |
Indirectly | 6 |
| Corkery 2015 [14] |
Canada | Caucasian | Serous | NA | 103 | IHC | NA | NA | NA | DFS | 1.54 (0.91–2.6) |
Indirectly | 6 |
| Demir 2014 [16] |
Sweden | Caucasian | Unclassified | I-IV | 82 | IHC | 54(24–80) (median) |
15/82 | NA | OS | 4.9 (2–12.04) |
Indirectly | 7 |
| Cai 2015 [6] |
China | Asian | Unclassified | I-IV | 95 | IHC | NA | 32/95 | NA | OS | 1.34 (0.77–2.32) |
Indirectly | 8 |
| Matsuo 2014 [15] |
USA | Mix | Serous | I-IV | 120 | IHC | 62.6±10.6 (mean) |
32/120 | NA | OS | 1.19 (0.67–2.11) |
Directly | 6 |
| Matsuo 2014 [15] |
USA | Mix | Serous | I-IV | 120 | IHC | 62.6±10.6 (mean) |
32/120 | NA | PFS | 1.04 (0.63–1.71) |
Directly | 6 |
| De Toledo 2013 [17] |
Brazil | Caucasian | Unclassified | I-IV | 152 | IHC | 55.2±12.3 (mean) |
19/152 | 43.6 (mean) |
OS | 1.46 (0.42–5.04) |
Directly | 7 |
| De Toledo 2013 [17] |
Brazil | Caucasian | Unclassified | I-IV | 152 | IHC | 55.2±12.3 (mean) |
19/152 | 43.6 (mean) |
DFS | 1.57 (0.39–6.22) |
Directly | 7 |
| Chay 2013 [18] |
Singapore | Mix | Serous | I-IV | 113 | IHC | 48.3(15.8–89) (median) | 31/113 | 2.8(0–19.99) (median) |
OS | 0.56 (0.21–1.52) |
Directly | 8 |
| Chay 2013 [18] |
Singapore | Mix | Serous | I-IV | 113 | IHC | 48.3(15.8–89) (median) | 31/113 | 2.8(0–19.99) (median) |
PFS | 0.5 (0.2–1.22) |
Directly | 8 |
| Steffensen 2011 [19] |
Denmark | Caucasian | Unclassified | I-IV | 139 | Elisa | 64(32–84) (median) |
NA | 39.6 (median) |
OS | 1.11 (0.68–1.79) |
Indirectly | 7 |
| Steffensen 2011 [19] |
Denmark | Caucasian | Unclassified | I-IV | 139 | Elisa | 64(32–84) (median) |
NA | 39.6 (median) |
PFS | 1.23 (0.64–2.39) |
Indirectly | 7 |
| Liu 2010 [20] |
China | Asian | Unclassified | I-IV | 116 | IHC | 49(30–76) (median) |
26/116 | 43(5–93) (median) |
OS | 2.83 (1.39–5.79) |
Indirectly | 9 |
| Liu 2010 [20] |
China | Asian | Unclassified | I-IV | 116 | IHC | 49(30–76) (median) |
26/116 | 43(5–93) (median) |
PFS | 1.92 (1–3.69) |
Indirectly | 9 |
| Pfisterer 2009 [21] |
Germany | Caucasian | Unclassified | IIB-IV | 359 | IHC | ≥18 | 22/359 | 57.5(46–64.3) (median) |
OS | 0.71 (0.42–1.18) |
Directly | 8 |
| Garcia-Velasco 2008 [23] |
Spain | Caucasian | Unclassified | NA | 72 | IHC | 57(28–82) (median) |
4/72 | 33 (median) |
OS | 2.28 (0.12–4.2) |
Directly | 7 |
| Garcia-Velasco 2009 [23] |
Spain | Caucasian | Unclassified | NA | 72 | IHC | 57(28–82) (median) |
4/72 | 33 (median) |
PFS | 2.82 (0.38–20.9) |
Directly | 7 |
| Graeff 2008 [24] |
Netherland | Caucasian | Unclassified | I-IV | 230 | IHC | 57.8(22–90) (median) |
12/230 | NA | OS | 1.02 (0.48–2.2) |
Directly | 6 |
| Graeff 2008 [24] |
Netherland | Caucasian | Unclassified | I-IV | 230 | IHC | 57.8(22–90) (median) |
12/230 | NA | PFS | 0.98 (0.46–2.1) |
Directly | 6 |
| Tomsova 2008 [22] |
Czech Republic | Caucasian | Unclassified | I-IV | 116 | IHC | 53(27–82) (median) |
10/116 | 39(1–120) (median) |
OS | 1.9 (0.79–4.58) |
Indirectly | 7 |
| Tuefferd 2007 [25] |
France | Caucasian | Unclassified | I-IV | 320 | IHC | 58(25–77) median |
41/320 | 24.9 (median) |
OS | 0.95 (0.51–1.74) |
Directly | 7 |
| Tuefferd 2007 [25] |
France | Caucasian | Unclassified | I-IV | 320 | IHC | 58(25–77) median |
41/320 | 24.9 (median) |
PFS | 0.81 (0.54–1.19) |
Directly | 7 |
| Steffensen 2007 [26] |
Denmark | Caucasian | Unclassified | II-IV | 160 | IHC | 54.5(29–70) median |
57/160 | 120 (all) |
OS | 1.5 (1.02–2.2) |
Directly | 8 |
| Pils 2007 [27] |
Austria | Caucasian | Unclassified | I-IV | 128 | IHC | 59.2 (mean) |
35/128 | 43.7(0.4–168.7) (median) |
OS | 1.92 (0.94–3.94) |
Indirectly | 7 |
| Malamou-Mitsi 2007[28] |
Greece | Caucasian | Unclassified | I-III | 95 | IHC | NA | 17/95 | 66(0.4–89.3) (median) |
OS | 1.85 (0.93–4.12) |
Indirectly | 7 |
| Malamou-Mitsi 2007[28] |
Greece | Caucasian | Unclassified | I-III | 95 | IHC | NA | 17/95 | 66(0.4–89.3) (median) |
PFS | 1.44 (0.79–2.63) |
Indirectly | 7 |
| Brozek 2006 [31] |
Gdansk | Caucasian | Unclassified | I-IV | 53 | FISH | NA | 10/53 | NA | OS | 2.44 (0.79–7.52) |
Indirectly | 6 |
| Surowiak 2006 [29] |
Germany | Caucasian | Unclassified | I-III | 43 | IHC | 51 (mean) |
21/43 | 0–52 |
OS | 0.85 (0.17–4.33) |
Indirectly | 7 |
| Castellvi 2006 [30] |
Spain | Caucasian | Unclassified | I-IV | 75 | IHC | 55(20–87) (mean) |
23/75 | 31(24–80) (mean) |
OS | 1.12 (0.49–2.54) |
Indirectly | 7 |
| Verri 2005 [32] |
Italy | Caucasian | Unclassified | I-IV | 194 | IHC | 57(25–90) median |
53/194 | 45(1–161) (median) |
OS | 1.36 (0.76–2.42) |
Directly | 8 |
| Verri 2005 [32] |
Italy | Caucasian | Unclassified | I-IV | 194 | IHC | 57(25–90) median |
53/194 | 45(1–161) (median) |
PFS | 1.61 (0.94–2.73) |
Directly | 8 |
| Lassus 2004 [34] |
Sweden | Caucasian | Serous | I-IV | 401 | CISH | NA | 66/401 | NA | OS | 2.14 (1.34–3.42) |
Directly | 7 |
| Nielsen 2003 [33] |
Denmark | Caucasian | Unclassified | I-IV | 783 | IHC | 58(13–91) (median) |
272/783 | NA | OS | 0.95 (0.66–1.36) |
Directly | 7 |
| Camilleri-Broet 2004 [35] |
France | Caucasian | Unclassified | IIIA-IV | 95 | IHC | 59(23–74) median |
15/95 | 68 (median) |
OS | 2.12 (1.13–3.98) |
Directly | 7 |
| Camilleri-Broet 2004 [35] |
France | Caucasian | Unclassified | IIIA-IV | 95 | IHC | 59(23–74) median |
15/95 | 68 (median) |
PFS | 1.99 (1.12–3.54) |
Directly | 7 |
| Skirnisdottir 2001[36] |
Sweden | Caucasian | Unclassified | IA-IIC | 106 | IHC | 60(26–82) mean |
20/106 | 87(57–125) mean |
OS | 2.28 (0.67–7.82) |
Indirectly | 8 |
| Davidson 2000 [37] |
Norway | Caucasian | Unclassified | NA | 75 | IHC | 56.9(30–84) mean |
35/75 | 70(8–224) mean |
OS | 1.92 (1.1–3.37) |
Indirectly | 8 |
| Wang 1999 [38] |
USA | Caucasian | Unclassified | NA | 40 | FISH | 61(35–83) median |
10/40 | 1–56 all |
OS | 4 (1.2–13.9) |
Indirectly | 8 |
| Medl 1995 [39] |
Austria | Caucasian | Unclassified | I-IV | 196 | PCR | 59.6(15–88) median |
79/196 | 59 mean |
OS | 1.08 (0.73–1.6) |
Indirectly | 8 |
| Fajac 1995[40] |
France | Caucasian | Unclassified | I-IV | 65 | South blot | 52 mean |
9/65 | 71 (10–43) median |
OS | 1.8 (0.75–4.33) |
Indirectly | 7 |
| Berchuck 1990 [41] |
USA | Caucasian | Unclassified | NA | 73 | IHC | 63.5 (median) |
23/73 | 1–100 (all) |
OS | 4.39 (2.13–9.06) |
Inirectly | 8 |
Abbreviations: HR: hazard ratio, CI: confidence interval, IHC: immunohistochemistry, FISH: fluorescence in situ hybridization. ELISA: enzyme-linked immunosorbent assay, CISH: chromogenic in situ hybridization, PCR: polymerase chain reaction, NA: not available, OS: overall survival, DFS/PFS: disease-free survival/ progress-free survival, Serous: serous ovarian cancer, Unclassified: serous, mucinous, clear cell, endometrioid, transitional cell, undifferentiated, differentiated, and others.
Association of HER2 expression with overall survival and its subgroup analysis
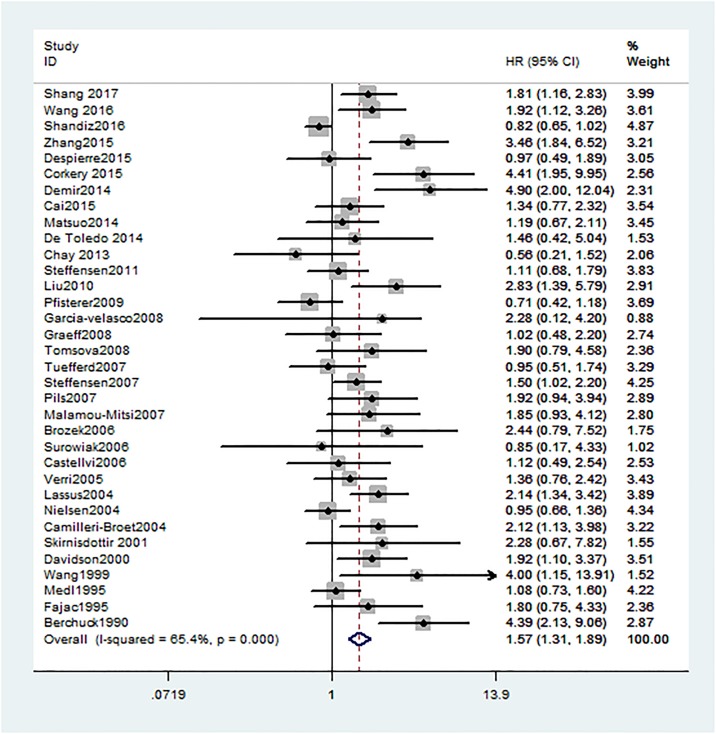
All 34 studies investigating OS were showed that HER2 positive expression in ovarian cancer patients was significantly associated with worse OS (HR = 1.57, 95% CI: 1.31 to 1.89, H2 = 1.7). As severe heterogeneity was observed (I2 = 65.4%, 95%CI: 50% to 76%), a random-effects model was determined for the pooled HR and 95% CI and subgroup meta-analysis was conducted to investigate the possible source of the heterogeneity among studies (Fig 2).
Fig 2. Forest plots of HR and 95%CI for overall survival in ovarian cancer according to presence of HER2.
Random-effects model was used.
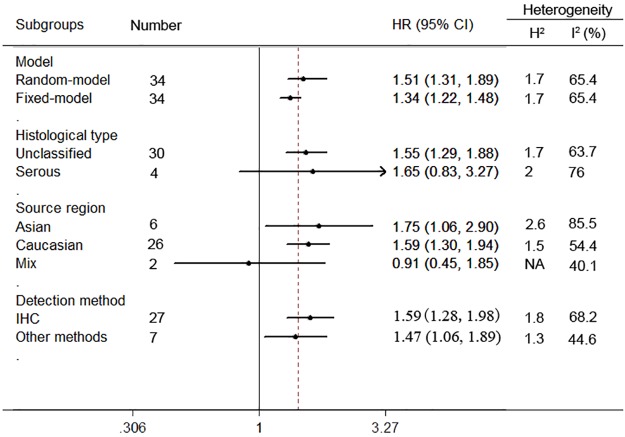
In the stratified analysis by histological type, HER2 expression was associated with worse OS of unclassified ovarian cancer (n = 30, HR = 1.55, 95% CI = 1.29 to 1.88, H2 = 1.7; I2 = 63.7%, 95%CI = 46% to 75%), while HER2 expression implied no significant association in serous ovarian cancer (n = 4, HR = 1.65, 95% CI = 0.83 to 3.27, H2 = 2; I2 = 76%, 95%CI = 34% to 91%) (Fig 3).
Fig 3. Subgroup analyses of the relationship between HER2 expression and overall survival of ovarian cancer.
When sub-grouped by ethnicity, a worse overall survival was strong linked to HER2 positivity in Asian populations (n = 6, HR = 1.75, 95% CI = 1.06 to 2.9, H2 = 2.6; I2 = 85.5%, 95%CI = 70% to 93%) as well as Caucasian populations (n = 26, HR = 1.59, 95% CI = 1.3 to 1.94, H2 = 1.5; I2 = 54.4%, 95%CI = 29% to 71%). Nevertheless, HER2 positivity was irrelevant to OS of ovarian cancer in Mix populations (n = 2, HR = 0.91, 95% CI = 0.45 to 1.85, I2 = 40.1%).
With regard to different detection methods of HER2 in ovarian cancer, positive HER2 expression status was a worse prognostic marker of overall survival in immunohistochemistry (IHC) group (n = 27, HR = 1.59, 95% CI = 1.28 to 1.98, H2 = 1.8; I2 = 68.2%, 95% CI = 54% to 79%). Similarly, HER2 expression was also associated with OS by using other detection methods (n = 7, HR = 1.47, 95% CI = 1.06 to 1.89, H2 = 1.3; I2 = 44.6%, 95% CI = 0% to 77%).
Association of HER2 expression with disease-free survival / progress-free survival and its subgroup analysis
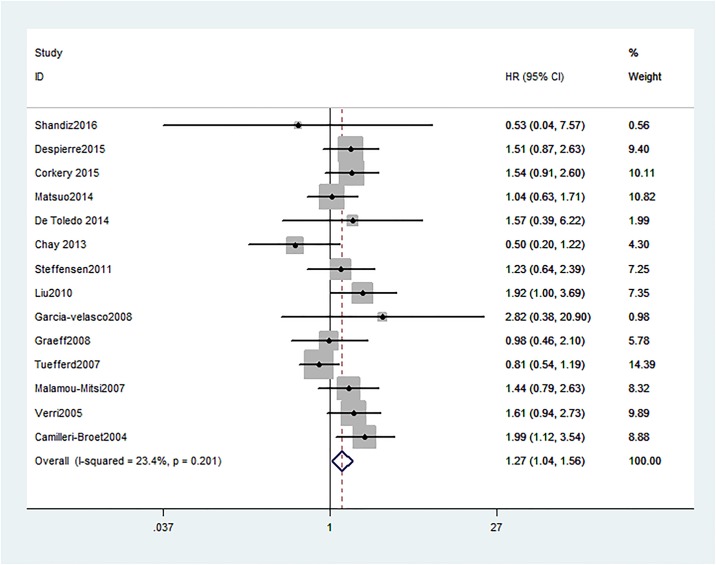
Pooled HRs and 95% CI for disease-free survival (DFS) / progress-free survival (PFS) were conducted in 14 studies, the pooling analysis showed an increased risk of disease progression in patients with HER2 positive group (HR = 1.27, 95% CI = 1.04 to 1.56), along with a moderate heterogeneity of the data (I2 = 23.4%, 95% CI = 0% to 59%) (Fig 4).
Fig 4. Forest plots of HR and 95%CI for disease-free survival / progress-free survival in ovarian cancer according to presence of HER2.
Random-effects model was used.
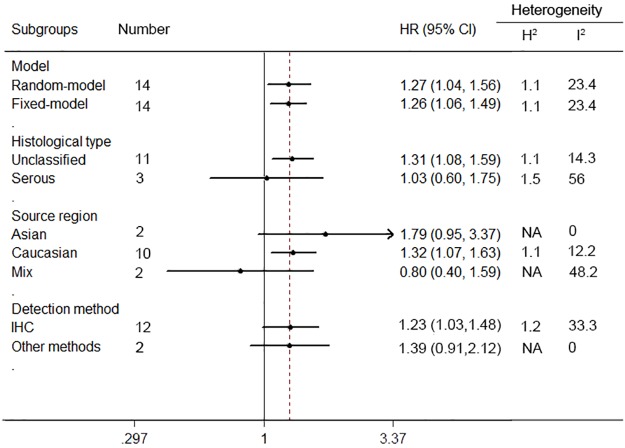
When considering differences in histological types of cancers, high levels of HER2 were significantly associated with a poorer DFS/PFS of unclassified ovarian cancer patients (n = 11, HR = 1.34, 95% CI = 1.08 to 1.67, H2 = 1.1; I2 = 14.3%, 95% CI = 0% to 55%), but not in serous ovarian cancer patients (n = 3, HR = 1.03, 95% CI = 0.6 to 1.75, H2 = 1.5; I2 = 56%, 95% CI = 0% to 87%) (Fig 5).
Fig 5. Subgroup analyses of the relationship between HER2 expression and disease-free survival / progress-free survival of ovarian cancer.
Subgroup analyses by ethnicity revealed that HER2 was an unfavorable predictor of DFS/PFS in Caucasian populations (n = 10, HR = 1.32, 95% CI = 1.07 to 1.63, H2 = 1.1; I2 = 12.2%, 95% CI = 0% to 53%). However, not significant association between positive HER2 expression and poor DFS/PFS was found in Asian populations (n = 2, HR = 1.79, 95% CI = 0.95 to 3.37, I2 = 0%) or Mix populations (n = 2, HR = 0.8, 95% CI = 0.4 to 1.59, I2 = 48.2%).
Among the subgroups determined by detection approaches, HER2 over-expression in IHC detection group was related to a significantly worse DFS/PFS (n = 12, HR = 1.23, 95% CI = 1.03 to 1.48, H2 = 1.2, I2 = 33.3%, 95% CI = 0% to 66%), whereas, there was no significant association between HER2 expression and DFS/PFS among patients in the other detection methods groups (n = 2, HR = 1.39, 95% CI = 0.91 to 2.12, I2 = 0%).
Publication bias
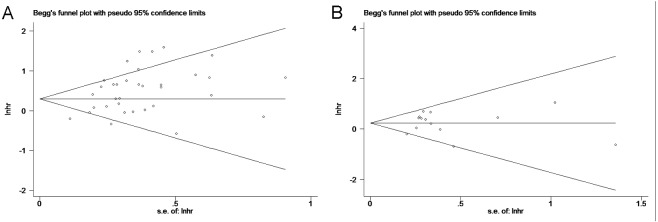
Begg’s test was used to investigate publication bias. No evidence of publication bias was observed for OS (P = 0.192) or DFS/PFS (P = 0.827) analyses (Fig 6A and 6B).
Fig 6.
6A. Begg’s publication bias plot of the studies assessing HER2 expression and overall survival in ovarian cancer. 6B. Begg’s publication bias plot of the studies assessing HER2 expression and disease-free survival / progress-free survival in ovarian cancer. Visual inspection of the funnel plot did not identify substantial asymmetry.
Sensitivity analysis
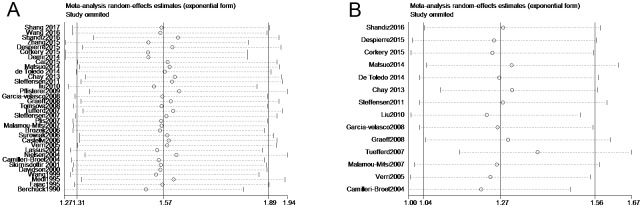
A leave-one-out sensitivity analysis by removing sequential study per time was adopted to assess the influence of each study on the pooled HR (Fig 7A and 7B). The result was not obviously changed when any single study was elided.
Fig 7.
7A. Sensitivity analysis of 34 studies included in this meta-analysis for overall survival. 7B. Sensitivity analysis of 14 studies included in this meta-analysis for disease-free survival / progress-free survival. Leave-one-out method was used to confirm the stability of the results.
Discussion
To our knowledge, this is the most comprehensive meta-analysis of the current literature on HER2, although our result is consistent with the only previous study to explore the prognostic role of HER2 in ovarian cancer in 2013 [45]. Notably, our research included almost four times more patients than the previously reported one, and the studies employed more subgroups and patients with longer follow-ups. Therefore, our meta-analysis was able to show a more reliable result.
In the current meta-analysis, we systematically evaluated survival data from 34 studies, which including 5180 patients. We demonstrated that the expression of HER2 was an indicator of a poor prognosis of ovarian cancer, with consistent results of OS and DFS/PFS. HER2 expression was low in normal ovarian epithelium while expressed highly in a variable percentage of epithelial ovarian cancer (11%-66%) [39, 46]. Either gene amplification or overexpression may lead to the dysregulation of HER2 signaling in ovarian cancer, then result in faster cell growth, DNA damage and increasing tumor progression [47]. Such effects may partially explain the negative relationship between HER2 expression and survival rate of ovarian cancer patients.
Ovarian cancers consist of many histological subtypes, including those of serous, mucinous, endometrioid and clear cell cancer [9]. The expression of tumor biomarkers was different according to clinicopathological features, including histological types. Shang et al. [9] found HER2 positivity was much higher in serous (29%) and mucinous carcinoma (38%) than that in endometrioid (20%) and clear cell carcinoma (23.1%). In the present study, we found that with respect to histological types, increased levels of HER2 had a negative influence on OS and DFS/PFS in the unclassified ovarian cancers. Corkery et al. [14] and Lassus et al. [34] presented an association of HER2 with poor survival in serous ovarian carcinoma, but the pooled four articles [14–15, 18, 34] showed that HER2 expression was related to neither OS nor DFS/PFS in serous type of ovarian cancer. Therefore, we suggest that the expression of HER2 may be a prognostic biomarker in non-serous ovarian cancer rather than serous ovarian cancer.
Regarding the ethnicity/race, HER2 expression was correlated with poorer OS of ovarian cancer patients in Asian group and Caucasian group but not in mix populations. Nevertheless, HER2 expression implied a worse PFS/DFS trend in Caucasian populations and showed no significant association in Asian populations or mix populations. It seemed that certain genes exerted different effects on cancer risk and prognosis across ethnic group. For instance, patient with high expression of HER2 lle655Val polymorphism have a negative prognosis among Caucasian subgroup, while no significant associations were observed in the Asian and African groups [48]. These maybe caused by genetic background, life style and environmental effect differed from ethnic regions.
Subgroup analysis showed that expression of HER2 had a negative influence on clinical outcome in the immunohistochemical technology group, with consistent results of OS and DFS/PFS. Nevertheless, in other detection method, HER2 expression implied poor OS outcome while showed no association with DFS/PFS/RFS. However, it was still difficult to draw a conclusion because the result was based on small numbers and required confirmation in large studies. These two studies detected HER2 using fluorescence in situ hybridization (FISH) and enzyme-linked immunosorbent assay (ELISA). FISH was a molecular based technique that detected HER2 gene amplification, but HER2 protein overexpression was attributable to gene amplification, what’s more, copy number intensity of signal was reflective of the quality of HER2 protein, on the other hand, FISH was a valid and supplement method to reflect HER2 overexpression to recommend trastuzumab therapy [49].
Immunohistochemical staining was widely used to detect the distribution and localization of biomarkers and protein expression status in the biological tissue and contributes to decisions on prognosis.
There are several important implications for the clinical management of ovarian cancer. First, it shows that HER2 expression is associated with worse outcome of ovarian cancer, implicating HER2 maybe a potential prognostic indicator for ovarian cancer patients. Second, it identifies a subgroup of ovarian cancer with histological type, source region and detection technology to analyze the heterogeneity. Finally, publication bias tests and plots are only relevant if studies are more than 10 otherwise underpowered to detect much and tend to lead to conclusions [50], in our study, there were 34 studies and it was considerable strength, which indicated the statistical results of the analyses were robust.
Some limitations in this meta-analysis have to be mentioned. First, it based on population-level data rather than individual patient-level data. Second, some of the HRs and 95% CIs were extracted indirectly from growth curve or formula computing, which could result in bias of outcome in certain extent. Third, due lack of detailed data, we only performed the sub-group analysis between HER2 and ovarian cancer with OS or PFS/DFS. Therefore, further investigations are needed to address these shortcomings.
Conclusion
In summary, our study suggests that HER2 may be a potential marker to predict the poor prognosis of ovarian cancer patients, especially for patients with unclassified ovarian cancer and Caucasian region. Additionally, immunohistochemistry is an effective method for predicting ovarian cancer clinical outcomes when evaluate HER2 expression.
Supporting information
(DOC)
Acknowledgments
This study was supported by grants from Key Lab of Wenzhou city-Gynecological Oncology (No. ZD201603).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by grants from Key Lab of Wenzhou city-Gynecological Oncology (No. ZD201603).
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61(2):69–90. doi: 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 2.Chudecka-Glaz AM. ROMA, an algorithm for ovarian cancer. Clin Chim Acta. 2015; 440:143–151. doi: 10.1016/j.cca.2014.11.015 [DOI] [PubMed] [Google Scholar]
- 3.Jacobs IJ, Menon U. Progress and challenges in screening for early detection of ovarian cancer. Mol Cell Proteomics. 2004;3(4):355–366. doi: 10.1074/mcp.R400006-MCP200 [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Matulonis UA. New strategies in ovarian cancer: translating the molecular complexity of ovarian cancer into treatment advances. Clin Cancer Res. 2014;20(20):5150–5156. doi: 10.1158/1078-0432.CCR-14-1312 [DOI] [PubMed] [Google Scholar]
- 5.Schluter B, Gerhards R, Strumberg D, Voigtmann R. Combined detection of Her2/neu gene amplification and protein overexpression in effusions from patients with breast and ovarian cancer. J Cancer Res Clin Oncol. 2010,136(9):1389–1400. doi: 10.1007/s00432-010-0790-2 [DOI] [PubMed] [Google Scholar]
- 6.Cai Y, Wang J, Zhang L, Wu D, Yu D, Tian X, et al. Expressions of fatty acid synthase and HER2 are correlated with poor prognosis of ovarian cancer. Med Oncol (Northwood, London, England). 2015;32(1):391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Revillion F, Bonneterre J, Peyrat JP. ERBB2 oncogene in human breast cancer and its clinical significance. Eur J Cancer. 1998;34(6):791–808. [DOI] [PubMed] [Google Scholar]
- 8.Stoecklein NH, Luebke AM, Erbersdobler A, Knoefel WT, Schraut W, Verde PE, et al. Copy number of chromosome 17 but not HER2 amplification predicts clinical outcome of patients with pancreatic ductal adenocarcinoma. J Clin Oncol. 2004;22(23):4737–4745. doi: 10.1200/JCO.2004.05.142 [DOI] [PubMed] [Google Scholar]
- 9.Shang AQ, Wu J, Bi F, Zhang YJ, Xu LR, Li LL, et al. Relationship between HER2 and JAK/STAT-SOCS3 signaling pathway and clinicopathological features and prognosis of ovarian cancer. Cancer biology & therapy. 2017:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang D, Zhu H, Ye Q, Wang C, Xu Y. Prognostic value of KIF2A and HER2-Neu overexpression in patients with epithelial ovarian cancer. Medicine. 2016;95(8): e2803 doi: 10.1097/MD.0000000000002803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shandiz FH, Kadkhodayan S, Ghaffarzadegan K, Esmaeily H, Torabi S, Khales SA. The impact of p16 and HER2 expression on survival in patients with ovarian carcinoma. Neoplasma. 2016;63(5):816–821. doi: 10.4149/neo_2016_520 [DOI] [PubMed] [Google Scholar]
- 12.Zhang M, Zhuang G, Sun X, Shen Y, Zhao A, Di W. Risk prediction model for epithelial ovarian cancer using molecular markers and clinical characteristics. J Ovarian Res. 2015; 8:67 doi: 10.1186/s13048-015-0195-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Despierre E, Vergote I, Anderson R, Coens C, Katsaros D, Hirsch FR, et al. Epidermal growth factor receptor (EGFR) pathway biomarkers in the randomized phase III trial of erlotinib versus observation in ovarian cancer patients with no evidence of disease progression after first-line platinum-based chemotherapy. Targeted Oncol. 2015; 10(4):583–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corkery DP, Le Page C, Meunier L, Provencher D, Mes-Masson AM, Dellaire G. PRP4K is a HER2-regulated modifier of taxane sensitivity. Cell Cycle. 2015;14(7):1059–1069. doi: 10.1080/15384101.2015.1007775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuo K, Sheridan TB, Mabuchi S, Yoshino K, Hasegawa K, Studeman KD, et al. Estrogen receptor expression and increased risk of lymphovascular space invasion in high-grade serous ovarian carcinoma. Gynecol Oncol. 2014;133(3):473–479. doi: 10.1016/j.ygyno.2014.03.563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demir L, Yigit S, Sadullahoglu C, Akyol M, Cokmert S, Kucukzeybek Y, et al. Hormone receptor, HER2/NEU and EGFR expression in ovarian carcinoma—is here a prognostic phenotype? Asian Pac J Cancer Prev. 2014;15(22):9739–9745. [DOI] [PubMed] [Google Scholar]
- 17.de Toledo MC, Sarian LO, Sallum LF, Andrade LL, Vassallo J, de Paiva Silva GR, et al. Analysis of the contribution of immunologically-detectable HER2, steroid receptors and of the "triple-negative" tumor status to disease-free and overall survival of women with epithelial ovarian cancer. Acta Histochem. 2014;116(3):440–447. doi: 10.1016/j.acthis.2013.09.010 [DOI] [PubMed] [Google Scholar]
- 18.Chay WY, Chew SH, Ong WS, Busmanis I, Li X, Thung S, et al. HER2 amplification and clinicopathological characteristics in a large Asian cohort of ovarian cancer. PLoS One. 2013;8(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steffensen KD, Waldstrom M, Brandslund I, Jakobsen A. Prognostic impact of prechemotherapy serum levels of HER2, CA125, and HE4 in ovarian cancer patients. Int J Gynecol Cancer. 2011;21(6):1040–1047. doi: 10.1097/IGC.0b013e31821e052e [DOI] [PubMed] [Google Scholar]
- 20.Liu N, Wang X, Sheng X. The clinicopathological characteristics of ‘triple-negative’ epithelial ovarian cancer. J Clin Pathol. 2010;63(3):240–243. doi: 10.1136/jcp.2009.071985 [DOI] [PubMed] [Google Scholar]
- 21.Pfisterer J, Du BA, Bentz E, Kommoss F, Harter P, Huober J, et al. Prognostic value of human epidermal growth factor receptor 2 (Her-2)/neu in patients with advanced ovarian cancer treated with platinum/paclitaxel as first-line chemotherapy: a retrospective evaluation of the AGO-OVAR 3 Trial by the AGO OVAR Germany. Int J Gynecol Cancer. 2009;19(1):109–115. [DOI] [PubMed] [Google Scholar]
- 22.Tomsova M, Melichar B, Sedlakova I, Steiner I. Prognostic significance of CD3+ tumor-infiltrating lymphocytes in ovarian carcinoma. Gynecol Oncol. 2008;108(2):415–420. doi: 10.1016/j.ygyno.2007.10.016 [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Velasco A, Mendiola C, Sanchez-Munoz A, Ballestin C, Colomer R, Cortes-Funes H. Prognostic value of hormonal receptors, p53, ki67 and HER2/neu expression in epithelial ovarian carcinoma. Clin Transl Oncol. 2008;10(6):367–371. [DOI] [PubMed] [Google Scholar]
- 24.de Graeff P, Crijns AP, Ten Hoor KA, Klip HG, Hollema H, Oien K, et al. The ErbB signalling pathway: protein expression and prognostic value in epithelial ovarian cancer. Brit J Cancer. 2008;99(2):341–349. doi: 10.1038/sj.bjc.6604471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tuefferd M, Couturier J, Penault-Llorca F, Vincent-Salomon A, Broet P, Guastalla JP, et al. HER2 status in ovarian carcinomas: a multicenter GINECO study of 320 patients. PLoS One. 2007;2(11): e1138 doi: 10.1371/journal.pone.0001138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steffensen KD, Waldstrom M, Jeppesen U, Jakobsen E, Brandslund I, Jakobsen A. The prognostic importance of cyclooxygenase 2 and HER2 expression in epithelial ovarian cancer. Int J Gynecol Cancer. 2007;17(4):798–807. doi: 10.1111/j.1525-1438.2006.00855.x [DOI] [PubMed] [Google Scholar]
- 27.Pils D, Pinter A, Reibenwein J, Alfanz A, Horak P, Schmid BC, et al. In ovarian cancer the prognostic influence of HER2/neu is not dependent on the CXCR4/SDF-1 signalling pathway. Brit J Cancer. 2007,96(3):485–491. doi: 10.1038/sj.bjc.6603581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malamou-Mitsi V, Crikoni O, Timotheadou E, Aravantinos G, Vrettou E, Agnantis N, et al. Prognostic significance of HER-2, p53 and Bcl-2 in patients with epithelial ovarian cancer. Anticancer Re. 2007;27(2):1157–1165. [PubMed] [Google Scholar]
- 29.Surowiak P, Materna V, Kaplenko I, Spaczynski M, Dietel M, Lage H, et al. Topoisomerase 1A, HER/2neu and Ki67 expression in paired primary and relapse ovarian cancer tissue samples. Histol Histopathol. 2006;21(7):713–720. doi: 10.14670/HH-21.713 [DOI] [PubMed] [Google Scholar]
- 30.Castellvi J, Garcia A, Rojo F, Ruiz-Marcellan C, Gil A, Baselga J, et al. Phosphorylated 4E binding protein 1: a hallmark of cell signaling that correlates with survival in ovarian cancer. Cancer. 2006;107(8):1801–1811. doi: 10.1002/cncr.22195 [DOI] [PubMed] [Google Scholar]
- 31.Brozek I, Kardas I, Ochman K, Debniak J, Stukan M, Ratajska M, et al. HER2 amplification has no prognostic value in sporadic and hereditary ovarian tumours. Hered Cancer Clin Pract. 2006;4(1):39–42. doi: 10.1186/1897-4287-4-1-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verri E, Guglielmini P, Puntoni M, Perdelli L, Papadia A, Lorenzi P, et al. HER2/neu oncoprotein overexpression in epithelial ovarian cancer: evaluation of its prevalence and prognostic significance. Clinical study. Oncology. 2005;68(2–3):154–161. doi: 10.1159/000086958 [DOI] [PubMed] [Google Scholar]
- 33.Nielsen JS, Jakobsen E, Holund B, Bertelsen K, Jakobsen A. Prognostic significance of p53, Her-2, and EGFR overexpression in borderline and epithelial ovarian cancer. Int J Gynecol Cancer. 2004;14(6):1086–1096. doi: 10.1111/j.1048-891X.2004.14606.x [DOI] [PubMed] [Google Scholar]
- 34.Lassus H, Leminen A, Vayrynen A, Cheng G, Gustafsson JA, Isola J, et al. ERBB2 amplification is superior to protein expression status in predicting patient outcome in serous ovarian carcinoma. Gynecol Oncol. 2004;92(1):31–39. [DOI] [PubMed] [Google Scholar]
- 35.Camilleri-Broet S, Hardy-Bessard AC, Le Tourneau A, Paraiso D, Levrel O, Leduc B, et al. HER-2 overexpression is an independent marker of poor prognosis of advanced primary ovarian carcinoma: a multicenter study of the GINECO group. Ann Oncol. 2004;15(1):104–112. [DOI] [PubMed] [Google Scholar]
- 36.Skirnisdottir I, Sorbe B, Seidal T. The growth factor receptors HER-2/neu and EGFR, their relationship, and their effects on the prognosis in early stage (FIGO I-II) epithelial ovarian carcinoma. Int J Gynecol Cancer. 2001;11(2):119–129. [DOI] [PubMed] [Google Scholar]
- 37.Davidson B, Gotlieb WH, Ben-Baruch G, Nesland JM, Bryne M, Goldberg I, et al. E-Cadherin complex protein expression and survival in ovarian carcinoma. Gynecol Oncol. 2000;79(3):362–371. doi: 10.1006/gyno.2000.5964 [DOI] [PubMed] [Google Scholar]
- 38.Wang ZR, Liu W, Smith ST, Parrish RS, Young SR. c-myc and chromosome 8 centromere studies of ovarian cancer by interphase FISH. Exp Mol Pathol. 1999;66(2):140–148. doi: 10.1006/exmp.1999.2259 [DOI] [PubMed] [Google Scholar]
- 39.Medl M, Sevelda P, Czerwenka K, Dobianer K, Hanak H, Hruza C, et al. DNA amplification of HER-2/neu and INT-2 oncogenes in epithelial ovarian cancer. Gynecol Oncol. 1995;59(3):321–326. [DOI] [PubMed] [Google Scholar]
- 40.Fajac A, Benard J, Lhomme C, Rey A, Duvillard P, Rochard F, et al. c-erbB2 gene amplification and protein expression in ovarian epithelial tumors: evaluation of their respective prognostic significance by multivariate analysis. Int J Cancer. 1995;64(2):146–151. [DOI] [PubMed] [Google Scholar]
- 41.Berchuck A, Kamel A, Whitaker R, Kerns B, Olt G, Kinney R, et al. Overexpression of HER-2/neu is associated with poor survival in advanced epithelial ovarian cancer. Cancer Res. 1990;50(13):4087–4091. [PubMed] [Google Scholar]
- 42.Hardy RJ, Thompson SG. Detecting and describing heterogeneity in meta-analysis. Stat Med. 1998;17(8):841–856. [DOI] [PubMed] [Google Scholar]
- 43.Brockwell SE, Gordon IR. A comparison of statistical methods for meta-analysis. Stat Med. 2001;20(6):825–840. doi: 10.1002/sim.650 [DOI] [PubMed] [Google Scholar]
- 44.Kontopantelis E, Reeves D. Performance of statistical methods for meta-analysis when true study effects are non-normally distributed: A simulation study. Stat Methods Med Res. 2012;21(4):409–426. doi: 10.1177/0962280210392008 [DOI] [PubMed] [Google Scholar]
- 45.Zhao D, Zhang F, Zhang W, He J, Zhao Y, Sun J. Prognostic role of hormone receptors in ovarian cancer: a systematic review and meta-analysis. Int J Gynecol Cancer. 2013;23(1):25–33. [DOI] [PubMed] [Google Scholar]
- 46.Bookman MA, Darcy KM, Clarke-Pearson D, Boothby RA, Horowitz IR. Evaluation of monoclonal humanized anti-HER2 antibody, trastuzumab, in patients with recurrent or refractory ovarian or primary peritoneal carcinoma with overexpression of HER2: a phase II trial of the gynecologic oncology group. J Clin Oncol. 2003;21(2):283–290. doi: 10.1200/JCO.2003.10.104 [DOI] [PubMed] [Google Scholar]
- 47.Bartsch R, Wenzel C, Zielinski CC, Steger GG. HER-2-positive breast cancer: hope beyond trastuzumab. BioDrugs. 2007;21(2):69–77. doi: 10.2165/00063030-200721020-00001 [DOI] [PubMed] [Google Scholar]
- 48.Chen W, Yang H, Tang WR, Feng SJ, Wei YL. Updated meta-analysis on HER2 polymorphisms and risk of breast cancer: evidence from 32 studies. Asian Pac Cancer Prev. 2014;15(22):9643–9647. [DOI] [PubMed] [Google Scholar]
- 49.Bahreini F, Soltanian AR, Mehdipour P. A meta-analysis on concordance between immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) to detect HER2 gene overexpression in breast cancer. Breast Cancer. 2015;22(6):615–625. doi: 10.1007/s12282-014-0528-0 [DOI] [PubMed] [Google Scholar]
- 50.Steme JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epodemiol. 2000;53(11):1119–1129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.