Abstract
OBJECTIVES:
To identify factors associated with longer length of stay (LOS) and higher 30-day hospital revisit rates for children hospitalized with bacterial tracheostomy–associated respiratory tract infections (bTARTIs).
METHODS:
This was a multicenter, retrospective cohort study using administrative data from the Pediatric Health Information System database between 2007 and 2014 of patients 30 days to 17 years old with a principal discharge diagnosis of bTARTI or a principal discharge diagnosis of bTARTI symptoms with a secondary diagnosis of bTARTI. Primary outcomes of LOS (in days) and 30-day all-cause revisit rates (inpatient, observation, or emergency department visit) were analyzed by using a 3-level hierarchical regression model (discharges within patients within hospital).
RESULTS:
We included 3715 unique patients and 7355 discharges. The median LOS was 4 days (interquartile range: 3–8 days), and the 30-day revisit rate was 30.5%. Compared with children 1 to 4 years old, children aged 30 days to 12 months had both longer LOS (adjusted length of stay [aLOS] = +0.9 days; 95% confidence interval [CI]: 0.6 to 1.3) and increased hospital revisit risk (adjusted odds ratio [aOR] = 1.5; 95% CI: 1.3 to 1.7). Other factors associated with longer LOS included public insurance (aLOS = +0.5 days; 95% CI: 0.2 to 0.8), 3 or more complex chronic conditions (CCCs), mechanical ventilation (acute or chronic), and empirical anti-Pseudomonas aeruginosa antibiotics (aLOS = +0.6 days; 95% CI: 0.3 to 0.9). Other factors associated with 30-day revisit included 4 or more CCCs (aOR = 1.3; 95% CI: 1.1 to 1.6) and chronic ventilator dependency (aOR = 1.1; 95% CI: 1.0 to 1.3).
CONCLUSIONS:
Ventilator-dependent patients <12 months old with at least 4 CCCs are at highest risk for both longer LOS and 30-day revisit after discharge for bTARTIs. They may benefit from bTARTI prevention strategies and intensive care coordination while hospitalized.
Over 4000 children undergo tracheostomy placement each year in the United States.1,2 Because children with tracheostomy may be chronically colonized with bacteria and often have impaired airway clearance, they have high incidence rates of bacterial tracheostomy–associated respiratory tract infections (bTARTIs), such as bacterial pneumonia.3 Despite this, there are no national guidelines for prevention, diagnosis, or treatment of bTARTIs in children with tracheostomies,4–7 causing wide hospital-level care variations in the treatment of these patients.8 Research using administrative data to study pediatric community-acquired pneumonia has revealed that younger age, public insurance, and the presence of a complex chronic condition (CCC) are associated with longer length of stay (LOS) and higher readmission rates.9–11 However, no previous studies have identified risk factors for longer LOS and hospital revisit in pediatric patients admitted with bTARTIs. Identifying the factors that are associated with LOS and revisit rates may help providers to recognize patients who will benefit from interventions to mitigate these differences. Thus, the current study sought to examine factors associated with increased LOS and 30-day all-cause revisit rates for pediatric patients with tracheostomies admitted for bTARTIs.
Methods
Data Source
We conducted a multicenter retrospective cohort study of patients discharged from 1 of 42 not-for-profit, freestanding children’s hospitals contributing to the Pediatric Health Information System (PHIS) database between 2007 and 2014. This database represents 15% of the national and 46.4% of the children’s hospital total volume, and it also provides deidentified resource use data that are subjected to a number of reliability and validity checks before being included in the database.12 The study was reviewed and granted an exemption per 45 Code of Federal Regulations 46.101[b][4] by the Children’s Hospital Los Angeles Institutional Review Board.
Patient Selection
We included children 30 days to 17 years of age at admission with an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code consistent with the presence of tracheostomy (V44.0, V55.0, 519.00, 519.01, 519.02, and 519.09) and either: (1) an ICD-9-CM code consistent with a principal diagnosis of bTARTI (eg, acute bacterial tracheitis); or (2) a principal diagnosis of a symptom of a respiratory tract infection (eg, cough) with a secondary diagnosis of a bTARTI. We identified pediatric patients with tracheostomy with ICD-9-CM codes used in previous studies of administrative data.1,3,8,13 To identify bTARTIs, in addition to the ICD-9-CM codes previously used for bacterial community-acquired pneumonia,14 we included codes for aspiration pneumonia (507.0, 507.8) and acute bacterial tracheitis (464.1×), as used in previous studies (see Supplemental Table 4 for a complete list).8 Principal diagnoses were categorized as (1) bacterial pneumonia, (2) bacterial tracheitis, (3) aspiration pneumonia, and (4) other (eg, symptom of respiratory tract infections, such as viral pneumonia (Supplemental Table 4).
We excluded the following: (1) any patient hospitalized over 30 days, because this represented an extreme outlier in the entire population before applying any exclusion criteria; (2) any patient transferred from an inpatient status at an outside hospital given our interest in LOS as an outcome (n = 1278); (3) any patient who did not receive antibiotics within 48 hours of admission, to minimize the chance of including patients not initially admitted with a bTARTI but who developed one later during the hospitalization (n = 280); and (4) any discharge that ended in the patient’s death (n = 40; <0.1% of the original sample), because these patients could not have a subsequent revisit.
Outcomes
Our primary outcomes of interest were discharge LOS and postdischarge 30-day all-cause revisit, defined as any hospital readmission to the inpatient unit, readmission to the observation unit, or an emergency department visit. We chose to examine all-cause revisit, rather than hospital readmission or respiratory infection-specific readmission, because it represents urgent hospital resource use, even if not tied to original bTARTI hospitalization, and because it is difficult to assess if the revisit was related to the index hospitalization by using administrative data alone.
Covariates
Demographic covariates of interest included admission age (categorized as 30 days–12 months, 1–4 years, 5–12 years, and 13–17 years), race and/or ethnicity (categorized as non-Hispanic white, non-Hispanic black Hispanic, and other), and payer status (categorized as public versus other). Medical covariates included individual CCCs (as defined by Feudtner et al’s15 classification system), gastrostomy tube status (ICD-9-CM procedure codes 43.1× and 97.02, diagnosis codes V44.1, V55.1, and 536.4×),16 and chronic ventilator dependency (presence of an ICD-9-CM code of V46.1×). The ventilator dependency and mechanical ventilation variables were collapsed into a single variable because of multicollinearity; patients were then categorized as having (1) chronic ventilator dependence, (2) acute ventilator use, or (3) no ventilation use. We also identified receipt of antibiotics targeting P aeruginosa on hospital day 0 or 1 (see Supplemental Table 5).
Statistical Methodology
The relationship between individual risk factors and outcome measures was first examined via bivariate logistic regression predicting revisit within 30 days through unadjusted odds ratios (95% confidence intervals [CIs]) and via bivariate linear regression examining LOS through unadjusted estimates measured in the number of days (95% CI). A hierarchical multivariate regression analysis was used to incorporate all risk factors that either significantly predicted 1 or both of the outcome measures in the bivariate analyses or had medical justification for inclusion in the model, while adjusting for all covariates. The final model was a 3-level, hierarchical multivariate model in which conditions unique to a patient’s discharge or that changed over time were modeled at level 1 (eg, comorbid conditions, patient age), demographic factors that were the same over time within the same patient were modeled at level 2 (eg, race), and factors common to all patients within the same hospital were modeled at level 3 (eg, region). This hierarchical framework allowed for the use of clustering groups to account for the variability explained at each of the levels while assessing outcomes. We reported adjusted length of stay (aLOS) change, in days, and reported adjusted odds ratios (aORs) for hospital revisit rates.
Eight additional multivariate, hierarchical models were run to supplement the final model and generate predicted values that revealed the relationship of the individual CCCs with LOS and readmission within 30 days. To allow the most accurate estimates of the unique effects of each CCC, these multivariate models were adjusted for all covariates but were run independently of other CCCs. In all analyses, we used 2-tailed tests with a significance level of 0.05. Data analyses were performed by using Mplus (version 7.11; Muthén and Muthén, Los Angeles, CA).
Results
Discharge-Level Demographic Data
The sample consisted of 3715 unique patients and 7355 discharges across 42 hospitals. The median number of admissions per patient was 1 (interquartile range [IQR]: 1–2, range: 1–27) (Table 1). Over 12.4% of patients included had 3 or more discharges included in the data set. Of the discharges, 59% (n = 4343) were male and 38.6% (n = 2839) were non-Hispanic white. The majority of discharges had a public payer (73.3%; n = 5391). Nearly 74% (n = 5422) of discharges were associated with 3 or more CCCs. The most common CCCs included respiratory (100%; n = 7355), gastrointestinal (83.8%; n = 6160), and neuromuscular (52.4%; n = 3856) conditions. Discharges were associated with a high level of medical technology dependency, with 77.1% (n = 5672) of patients having gastrostomy tube dependency and nearly 32% (n = 2346) of patients having ventilator dependency. Primary discharge diagnoses were largely bacterial pneumonia (39.4%; n = 2901) or bacterial tracheitis (48.6%; n = 3573). With respect to clinical care during the hospitalizations, 51.4% (n = 3779) of patients received mechanical ventilation at some point during their hospitalization and 36.5% (n = 2688) were admitted to an ICU at some point during their hospitalization. Over 72% (n = 5336) received empirical antibiotic coverage targeting P aeruginosa. Over 78% (n = 5771) were discharged from the hospital without home nursing.
TABLE 1.
Demographic Characteristics of Pediatric Admissions for bTARTIs in the Study Population, Stratified by 30-Day Revisit Rate (n = 3715 Unique Patients)
| Variable | Total Sample | Hospital Revisit Within 30 d | |
|---|---|---|---|
| No | Yes | ||
| 7355 (100) | 5113 (69.5) | 2242 (30.5) | |
| Discharge-level variables | |||
| Admission age, y, median (IQR) | 3 (1–9) | 4 (2–9) | 2 (1–7) |
| Infant, n (%) | 860 (11.7) | 465 (9.1) | 395 (17.6) |
| Early childhood, n (%) | 3300 (44.9) | 2226 (43.5) | 1074 (47.9) |
| Late childhood, n (%) | 2240 (30.5) | 1719 (33.6) | 521 (23.2) |
| Adolescence, n (%) | 955 (13.0) | 703 (13.7) | 252 (11.2) |
| Insurance, n (%) | |||
| Public | 5391 (73.3) | 3697 (72.3) | 1694 (75.6) |
| Private, self-pay, other, missing | 1964 (26.7) | 1416 (27.7) | 548 (24.4) |
| CCCs | |||
| Total no., median (IQR) | 3 (2–4) | 3 (2–4) | 3 (2–4) |
| Respiratory, n (%) | 7355 (100) | 5113 (100) | 2242 (100) |
| Gastrointestinal, n (%) | 6160 (83.8) | 4275 (83.6) | 1885 (84.1) |
| Neuromuscular, n (%) | 3856 (52.4) | 2703 (52.9) | 1153 (51.4) |
| Congenital, n (%) | 2445 (33.2) | 1672 (32.7) | 773 (34.5) |
| Cardiovascular, n (%) | 1228 (16.7) | 782 (15.3) | 446 (19.9) |
| Metabolic, n (%) | 532 (7.2) | 365 (7.1) | 167 (7.4) |
| Renal, n (%) | 486 (6.6) | 307 (6.0) | 179 (8.0) |
| Hematologic, n (%) | 275 (3.7) | 184 (3.6) | 91 (4.1) |
| Oncological, n (%) | 161 (2.2) | 110 (2.2) | 51 (2.3) |
| No. CCCs, n (%) | |||
| 1 | 450 (6.1) | 318 (6.2) | 132 (5.9) |
| 2 | 1483 (20.2) | 1051 (20.6) | 432 (19.3) |
| 3 | 3169 (43.1) | 2247 (43.9) | 922 (41.1) |
| 4 or more | 2253 (30.6) | 1497 (29.3) | 756 (33.7) |
| Other comorbidities, n (%) | |||
| Gastrostomy tube | 5672 (77.1) | 3969 (77.6) | 1703 (76.0) |
| Ventilator dependency | 2346 (31.9) | 1560 (30.5) | 786 (35.1) |
| Disposition, n (%) | |||
| Home without home nurse | 5771 (78.5) | 4042 (79.1) | 1729 (77.1) |
| Other | 1584 (21.5) | 1071 (20.9) | 513 (22.9) |
| Mechanical ventilation during admission, n (%) | |||
| Chronic ventilator dependency | 2281 (31.0) | 1513 (29.6) | 768 (34.3) |
| Mechanical ventilation only during admission | 1498 (20.4) | 1045 (20.4) | 453 (20.2) |
| No mechanical ventilation | 3576 (48.6) | 2555 (50.0) | 1021 (45.5) |
| Admission diagnosis, n (%) | |||
| Bacterial pneumonia | 2901 (39.4) | 2105 (41.2) | 796 (35.5) |
| Bacterial tracheitis | 3573 (48.6) | 2372 (46.4) | 1201 (53.6) |
| Aspiration pneumonia | 589 (8.0) | 404 (7.9) | 185 (8.3) |
| Other | 292 (4.0) | 232 (4.5) | 60 (2.7) |
| LOS, d, median (IQR) | 4 (3–8) | 4 (3–8) | 5 (3–8) |
| Admitted to ICU during hospitalization, n (%) | 2688 (36.5) | 1855 (36.3) | 833 (37.2) |
| Anti-Pseudomonas antibiotic on hospital day 0–1, n (%) | 5336 (72.5) | 3725 (72.9) | 1611 (71.9) |
| Patient-level variables, n (%) | |||
| Sex | |||
| Male | 4343 (59.0) | 2980 (58.3) | 1363 (60.8) |
| Female | 3012 (41.0) | 2133 (41.7) | 879 (39.2) |
| Race | |||
| White | 2839 (38.6) | 1981 (38.7) | 858 (38.3) |
| Black | 1414 (19.2) | 995 (19.5) | 419 (18.7) |
| Hispanic | 1893 (25.7) | 1310 (25.6) | 583 (26.0) |
| Other | 1209 (16.4) | 827 (16.2) | 382 (17.0) |
| Hospital-level variables, n (%) | |||
| Region | |||
| West | 1810 (24.6) | 1266 (24.8) | 544 (24.3) |
| Midwest | 2142 (29.1) | 1409 (27.6) | 733 (32.7) |
| Northeast | 886 (12.0) | 604 (11.8) | 282 (12.6) |
| South | 2517 (34.2) | 1834 (35.9) | 683 (30.5) |
Primary Outcomes
The median LOS for the entire cohort was 4 days (IQR = 3–8 days). When we analyzed the intraclass correlation for the hierarchical, multivariate linear regression model for LOS (Table 2), 83.4% of the variance in LOS was caused by experiences unique to each discharge, 11.2% within the patient level, and 5.4% within the hospital level. The 30-day all-cause revisit rate was 30.5% (95% CI: 29.4% to 31.5%). Of those with a revisit, 70.6% (n = 1583) had inpatient status, 4.4% (n = 98) had observation status, and 25% (n = 561) went to the emergency department. Variability in the hierarchical, multivariate logistic regression model for 30-day all-cause revisit was primarily caused by experiences unique to each discharge (92.3%), with the patient and hospital levels accounting for 6.8% and 0.9% of the variance, respectively (Table 3).
TABLE 2.
Hierarchical, Multivariate Linear Regression of Factors Associated With LOS for Pediatric Patients Admitted With bTARTIs
| Variable | Unadjusted Change in LOS (95% CI) | P | Adjusted Change in LOS (95% CI) | P |
|---|---|---|---|---|
| Age | ||||
| Infant, 30 d–12 mo | 0.55 (0.18 to 0.94) | .004 | 0.81 (0.45 to 1.19) | <.001 |
| Early childhood, 1–4 y | Ref | — | Ref | — |
| Late childhood, 5–12 y | 0.63 (0.32 to 0.89) | <.001 | 0.28 (−0.002 to 0.54) | .052 |
| Adolescence, 13–17 y | 1.32 (0.92 to 1.74) | <.001 | 0.68 (0.29 to 1.07) | <.001 |
| Male | −0.20 (−0.49 to 0.06) | .13 | −0.18 (−0.44 to 0.07) | .08 |
| Race | ||||
| White | Ref | — | Ref | — |
| Black | 0.13 (−0.25 to 0.49) | .49 | 0.24 (−0.14 to 0.63) | .22 |
| Hispanic | −0.01 (−0.34 to 0.36) | .97 | −0.04 (−0.36 to 0.45) | .85 |
| Other | 0.39 (−0.05 to 0.77) | .07 | 0.25 (−0.15 to 0.64) | .26 |
| Public insurance | 0.61 (0.34 to 0.88) | <.001 | 0.50 (0.23 to 0.79) | .002 |
| Total no. CCCs | ||||
| 1 | Ref | — | Ref | — |
| 2 | 0.63 (0.13 to 1.08) | .01 | 0.23 (−0.26 to 0.75) | .37 |
| 3 | 1.53 (1.06 to 1.92) | <.001 | 0.87 (0.40 to 1.37) | .004 |
| 4 or more | 2.52 (2.01 to 2.91) | <.001 | 1.59 (1.12 to 2.12) | <.001 |
| Disposition: other than home without nursing | 1.43 (1.17 to 1.76) | <.001 | 1.05 (0.73 to 1.36) | <.001 |
| Mechanical ventilation during admission | ||||
| Chronic ventilator dependency | 1.84 (1.55 to 2.13) | <.001 | 0.62 (0.32 to 0.90) | <.001 |
| Mechanical ventilation only during admission | 3.14 (2.82 to 3.48) | <.001 | 1.80 (1.47 to 2.14) | <.001 |
| No mechanical ventilation | Ref | — | Ref | — |
| Principal diagnosis | ||||
| Bacterial pneumonia | Ref | — | Ref | — |
| Bacterial tracheitis | −1.09 (−1.35 to −0.83) | <.001 | −0.88 (−1.13 to −0.63) | <.001 |
| Aspiration pneumonia | 0.67 (0.24 to 1.10) | <.001 | 0.86 (0.45 to 1.28) | <.001 |
| Other | 2.79 (2.26 to 3.40) | <.001 | 2.23 (1.69 to 2.81) | <.001 |
| Admitted to ICU during hospitalization | 3.17 (2.93 to 3.41) | <.001 | 2.18 (1.90 to 2.45) | <.001 |
| Anti-Pseudomonas antibiotic, hospital day 0–1 | 0.88 (0.61 to 1.13) | <.001 | 0.57 (0.30 to 0.85) | <.001 |
| Hospital region | ||||
| West | Ref | — | Ref | — |
| Midwest | −0.50 (−1.41 to 0.51) | .31 | −1.17 (−2.39 to 0.05) | .06 |
| Northeast | −0.51 (−1.54 to 0.58) | .36 | −1.49 (−2.79 to −0.16) | .01 |
| South | 0.29 (−0.72 to 1.23) | .55 | 0.10 (−0.99 to 1.10) | .85 |
| Hospital-level empirical anti-Pseudomonas antibiotic, % total admissions | 1.35 (−0.36 to 2.97) | .16 | 1.56 (−0.76 to 3.82) | .15 |
| No. cases, unit change per 100 | −0.04 (−0.14 to 0.05) | .46 | −0.04 (−0.16 to 0.07) | .44 |
| Census, unit change per 100 | 0.12 (−0.34 to 0.56) | .61 | 0.02 (−0.48 to 0.55) | .93 |
—, not applicable.
TABLE 3.
Hierarchical, Multivariate Logistic Regression of Factors Associated With 30-Day All-Cause Hospital Revisit for Pediatric Patients Admitted With bTARTIs
| Variables | Unadjusted Odds Ratio (95% CI) | P | aOR (95% CI) | P |
|---|---|---|---|---|
| Age | ||||
| Infant, 30 d–12 mo | 1.49 (1.36 to 1.66) | <.001 | 1.49 (1.33 to 1.67) | <.001 |
| Early childhood, 1–4 y | Ref | — | Ref | — |
| Late childhood, 5–12 y | 0.74 (0.68 to 0.80) | <.001 | 0.71 (0.65 to 0.78) | <.001 |
| Adolescence, 13–17 y | 0.81 (0.72 to 0.89) | <.001 | 0.76 (0.68 to 0.86) | <.001 |
| Male | 1.07 (1.00 to 1.15) | .09 | 1.07 (1.00 to 1.16) | .07 |
| Race | ||||
| White | Ref | — | Ref | — |
| Black | 1.04 (0.93 to 1.19) | .54 | 1.04 (0.92 to 1.16) | .52 |
| Hispanic | 1.11 (0.97 to 1.24) | .09 | 1.09 (0.98 to 1.24) | .12 |
| Other | 1.04 (0.92 to 1.19) | .54 | 0.98 (0.88 to 1.12) | .71 |
| Public insurance | 1.09 (1.02 to 1.16) | .01 | 1.08 (0.99 to 1.18) | .07 |
| Total no. CCCs | ||||
| 1 | Ref | — | Ref | — |
| 2 | 0.99 (0.90 to 1.12) | .87 | 1.04 (0.88 to 1.32) | .67 |
| 3 | 0.97 (0.88 to 1.10) | .61 | 1.10 (0.96 to 1.38) | .22 |
| 4 or more | 1.10 (0.99 to 1.23) | .07 | 1.29 (1.11 to 1.61) | <.001 |
| Disposition, other than home without nursing | 1.05 (0.98 to 1.14) | .15 | 1.01 (0.92 to 1.11) | .78 |
| Mechanical ventilation during admission | ||||
| Chronic ventilator dependency | 1.13 (1.03 to 1.21) | .004 | 1.16 (1.04 to 1.27) | .01 |
| Mechanical ventilation during admission | 1.03 (0.95 to 1.11) | .47 | 1.07 (0.96 to 1.19) | .22 |
| No mechanical ventilation | Ref | — | Ref | — |
| Admission diagnosis | ||||
| Bacterial pneumonia | Ref | — | Ref | — |
| Bacterial tracheitis | 1.13 (1.05 to 1.21) | <.001 | 1.08 (0.99 to 1.17) | .09 |
| Aspiration pneumonia | 1.13 (0.99 to 1.29) | .08 | 1.07 (0.94 to 1.22) | .28 |
| Other | 0.78 (0.67 to 0.91) | <.001 | 0.76 (0.62 to 0.91) | .004 |
| Admitted to ICU during hospitalization | 1.01 (0.94 to 1.09) | .91 | 0.95 (0.86 to 1.03) | .23 |
| LOS | 1.01 (1.00 to 1.02) | .01 | 1.01 (1.00 to 1.02) | .04 |
| Anti-Pseudomonas antibiotic on hospital day 0–1 | 0.91 (0.85 to 0.97) | <.001 | 0.94 (0.86 to 1.02) | .12 |
| Hospital region | ||||
| West | Ref | — | Ref | — |
| Midwest | 1.24 (1.01 to 1.62) | .04 | 1.28 (1.03 to 1.60) | .03 |
| Northeast | 1.09 (0.90 to 1.38) | .43 | 1.15 (0.90 to 1.46) | .26 |
| South | 1.01 (0.84 to 1.24) | .96 | 1.05 (0.87 to 1.31) | .65 |
| Hospital-level empirical anti-Pseudomonas antibiotic | 1.07 (0.69 to 1.60) | .72 | 0.92 (0.63 to 1.41) | .67 |
| No. cases, unit change per 100 | 1.01 (0.99 to 1.03) | .65 | 1.00 (0.98 to 1.03) | .72 |
| Census, unit change per 100 | 1.02 (0.94 to 1.11) | .63 | 0.99 (0.90 to 1.08) | .87 |
—, not applicable.
Demographic and Social Factors
In our hierarchical, multivariate analysis of factors associated with LOS (Table 2) and 30-day revisit (Table 3), when compared with patients with admission age of 1 to 4 years, those with admission age 30 days to 12 months had longer LOS (aLOS = +0.8 days; 95% CI: 0.5 to 1.2) and higher odds of revisit (aOR = 1.5; 95% CI: 1.3 to 1.7), whereas admission ages of 13 to 17 years were associated with longer LOS (aLOS = +0.7 days; 95% CI: 0.3 to 1.1) but lower odds of 30-day all-cause revisit (aOR = 0.8; 95% CI: 0.7 to 0.9). Public insurance was associated with increased LOS (aLOS = +0.5 days; 95% CI: 0.2 to 0.8) but with no difference in 30-day revisit odds. Other demographic factors, including sex and race and/or ethnicity, were not associated with LOS or hospital revisit.
Medical Covariates
On hierarchical analysis, 4 or more CCCs were associated with longer LOS (aLOS = +1.6; 95% CI: 1.1 to 2.1) and higher revisit odds (aOR = 1.3; 95% CI: 1.1 to 1.6). Chronic ventilator dependence, when compared with those not receiving mechanical ventilation during the hospitalization, was also associated with increased LOS (aLOS = +0.6 days; 95% CI: 0.3 to 0.9) and higher odds of 30-day revisit (aOR = 1.2; 95% CI: 1.04 to 1.3). Those receiving mechanical ventilation acutely during hospitalization had a longer LOS (aLOS = +1.8 days; 95% CI: 1.5 to 2.1) but showed no differences in 30-day revisit rates.
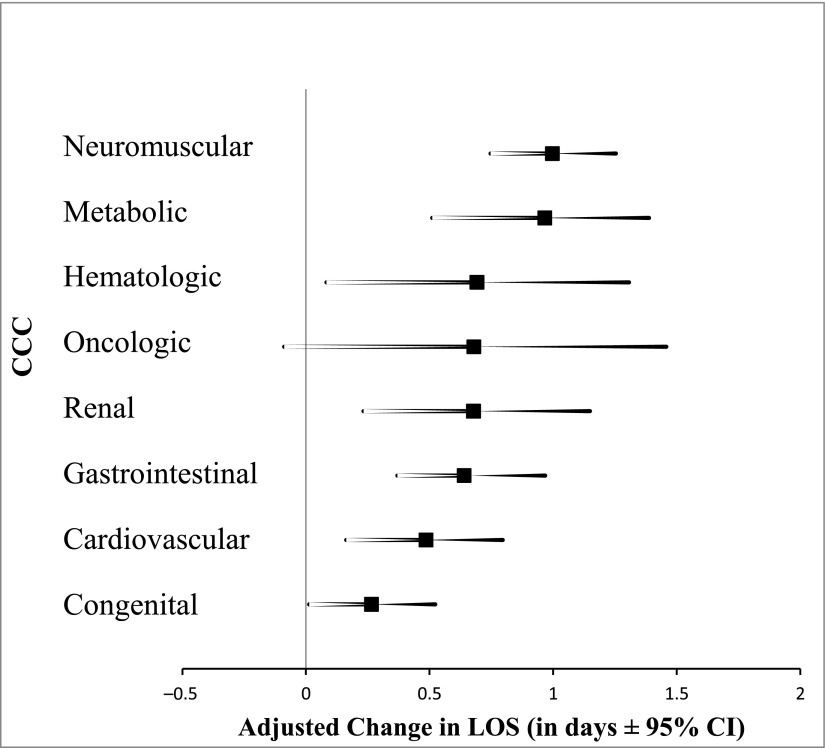
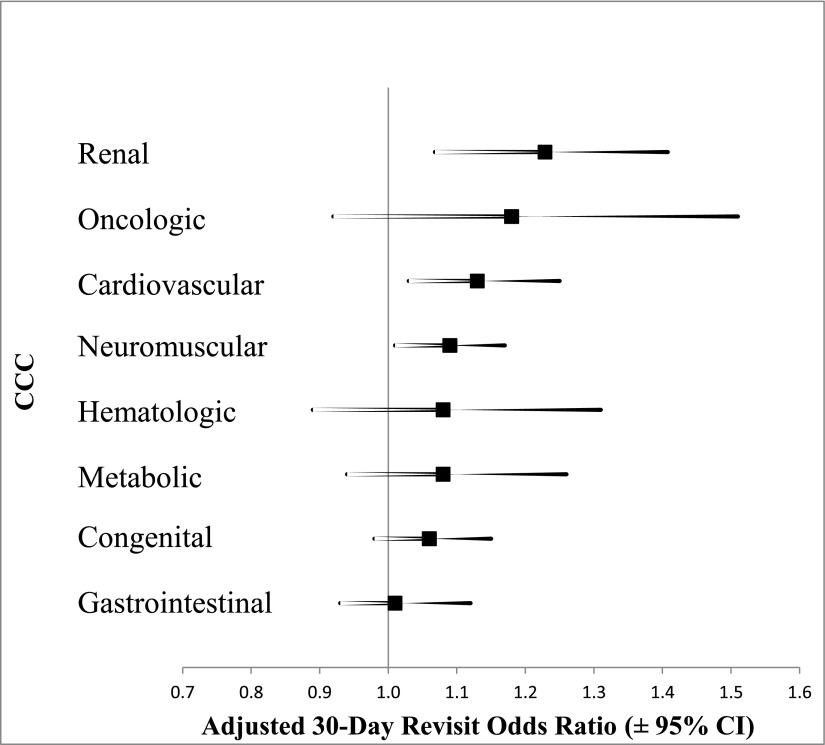
In supplementary hierarchical, multivariate analyses to assess the impact of individual CCCs on the 2 primary outcomes (Supplemental Tables 6 and 7), all CCCs except oncological conditions were associated with increased LOS (Fig 1). Neuromuscular (aLOS = +1 days; 95% CI: 0.8 to 1.3) and metabolic (aLOS = +1 day; 95% CI: 0.5 to 1.4) conditions showed the strongest associations. In contrast to the LOS analysis, only renal (aOR = 1.2; 95% CI: 1.1 to 1.4), cardiovascular (aOR = 1.1; 95% CI: 1.03 to 1.3), and neuromuscular (aOR = 1.1; 95% CI: 1.01 to 1.7) conditions were associated with increased odds of 30-day revisit (Fig 2).
FIGURE 1.
Association between individual CCCs and LOS. LOS is adjusted for all variables in Table 2, except for the total number of CCCs.
FIGURE 2.
Association between individual CCCs and 30-day all-cause hospital revisits. Thirty-day all-cause hospital revisit rates are adjusted for all variables in Table 3, except for the total number of CCCs.
Principal Diagnosis
Compared with bacterial pneumonia as a principal diagnosis, bacterial tracheitis was associated with shorter LOS (aLOS = −0.9; 95% CI: −1.1 to −0.6) but no difference in revisit odds, whereas a principal diagnosis categorized as other was associated with longer LOS (aLOS = +2.2; 95% CI: 1.7 to 2.8) and lower revisit odds (aOR = 0.8; 95% CI: 0.6 to 0.9). Those with a principal diagnosis of aspiration pneumonia had a longer LOS (aLOS = +0.9; 95% CI: 0.5 to 1.3) but showed no differences in revisit rate.
Hospital and Other Characteristics
Patients discharged from hospitals in the Northeast had a shorter LOS (reference group: West; aLOS: −1.5 days; 95% CI: −2.8 to −0.2) without any difference in 30-day revisit rate. Conversely, patients discharged from hospitals in the Midwest had higher 30-day revisit rates (reference group: West; aOR: 1.3; 95% CI: 1.03 to 1.6) but no differences in LOS. With respect to other hospitalization characteristics and association with LOS, admission to the ICU at some point during the hospitalization (aLOS = +2.2 days; 95% CI: 1.9 to 2.5) and patient empirical use of antibiotics that target P aeruginosa (aLOS = +0.6 days; 95% CI: 0.3 to 0.9) were associated with longer LOS, but not 30-day revisit. Those discharged from the hospital and taken to a setting other than home with no home nursing had a longer LOS (aLOS = +1.1; 95% CI: 0.7 to 1.4) but showed no differences in revisit rates. Other hospital factors, such as hospital percentage use of empirical anti-Pseudomonas antibiotics, hospital average daily census, and number of bTARTI discharges were not associated with LOS or 30-day revisit.
Discussion
In our multicenter study of a cohort of children hospitalized with bTARTIs, we have demonstrated that specific hospital and patient factors are associated with increased LOS and higher odds of 30-day all-cause revisit after hospitalization for a bTARTI, with most variation occurring at the discharge level. An admission age <12 months, 4 or more CCCs, and chronic ventilator dependency were associated with both longer LOS and increased odds for 30-day revisit. Other variables (eg, public insurance, ICU admission, acute mechanical ventilation, and patient-level empirical anti-Pseudomonas antibiotics) were associated with longer LOS but not with increased revisit odds. Specific factors not associated with LOS or revisit included patient race, size of the hospital, number of cases, and hospital-level use of empirical anti-Pseudomonas antibiotics
The median LOS of 4 days seen in our study is higher than both the 2-day LOS seen in patients without CCCs hospitalized with community-acquired pneumonia10,14,17 and the 3-day LOS seen in patients with CCCs hospitalized with community-acquired pneumonia.10 The LOS nadir seen at ages of 1 to 4 years may reveal different indications for tracheostomy placement, lower physiologic reserves, or a lower threshold for hospitalization in children <12 months, although older patients may have more morbidity from their chronic medical problems. The 30-day overall hospital revisit rate of 30.5% and the 30-day readmission rate of 22.9% in this cohort is markedly higher than the 1% to 7% 30-day hospital readmission rates seen for cases of community-acquired pneumonia in children both with and without CCCs.10,11 The inverse relationship seen between higher admission age and lower readmission odds may be caused by increasing parental knowledge and comfort gained through managing their children throughout years. Not surprisingly, patients with 4 or more CCCs had significantly longer LOS and higher odds of readmission, after controlling for other variables. Similarly, patients with chronic ventilator dependency represent another population with high medical complexity18–21 and, therefore, are at a high risk for readmission and health care use.22 Overall, our findings are consistent with those from previous studies, which revealed longer LOS and higher revisit rates in publicly insured and medically complex patients admitted with community-acquired pneumonia10,11,14,23 and aspiration pneumonia.24
We found an association between patient receipt of empirical P aeruginosa antibiotics and increased LOS. The authors of recent work have demonstrated that P aeruginosa is a common respiratory isolate in pediatric patients admitted with aspiration pneumonia25,26 and in pediatric patients after tracheotomy.27,28 This result complements recent work revealing an association between P aeruginosa isolation and poorer outcomes, including readmission for a bTARTI,28 ICU admission, and intubation.26 Receiving antibiotics targeting P aeruginosa may be a proxy for higher illness severity or increased likelihood of having multidrug-resistant organisms. Given the limited enteral alternatives for P aeruginosa treatment, patients may require longer hospitalization for intravenous antibiotics. With our current findings, we add to the literature produced to highlight the need for additional research on interventions in the prevention and treatment of respiratory infections caused by multidrug-resistant organisms and on the increased roles of antimicrobial stewardship programs to limit the unnecessary use of broad-spectrum antibiotics.
The associations among principal diagnoses, LOS, and revisit odds should be further explored. We found a shorter LOS without increased revisit rates in patients diagnosed with bacterial tracheitis. Patients diagnosed with bacterial tracheitis may have a shorter LOS because this condition may not be associated with the hypoxemia seen in pneumonia and because antibiotic courses for the condition may be shorter.29 Similarly, those with a primary diagnosis of a symptom of bacterial illness or viral infection may have a LOS because of delays in diagnosis or because these patients began with a viral infection and subsequently developed a bacterial superinfection that required treatment. However, the longer LOS in this group was balanced with statistically lower odds of readmission.
The current study had the limitations associated with conducting an observational, retrospective study by using administrative data, which included being able to demonstrate associations without being able to make a causal link. We could only study patients hospitalized at participating PHIS hospitals; there are missing children hospitalized for bTARTIs at non-PHIS–contributing children’s hospitals or at community hospitals not included in PHIS. In addition, we could not track readmission to a non-PHIS hospital; therefore, we may not have captured all patient revisits. With the use of administrative data, we relied on accurate coding and translation of existing data; by using our inclusion criteria, we may have missed patients who were hospitalized for a bTARTI but whose discharges were not assigned the representative ICD-9-CM codes, or we may have included patients incorrectly coded as having a bTARTI. Additionally, we did not include patients with a primary diagnosis associated with respiratory failure and a secondary diagnosis of a respiratory infection, which limits generalizability for patients who may have a higher illness severity. Because we used an administrative database, we were unable to obtain actual test results (eg, respiratory cultures, Gram-stain results) that could have been used as objective information to differentiate between acute infection and chronic colonization in those cases assigned ICD-9-CM codes for bacterial infection. Thus, there may have been an overdiagnosis of bacterial respiratory infections in this population, which may have led to increased health care use and costs. This may also have led to an overestimation of the total burden of bTARTIs in pediatric patients when using administrative data. Finally, given the retrospective nature of the study, there may have been unmeasured confounders that led to the observations noted.
Notwithstanding these limitations, this is the first study in which the authors identify specific factors associated with longer LOS and increased odds for 30-day hospital revisit in pediatric patients admitted with bTARTIs. We have found that patients <12 months old with ≥4 CCCs and chronic ventilator dependency are at the highest risk for longer LOS and for hospital revisit. Providers can use this information when counseling families about possible adverse outcomes after tracheotomy. Given the identified factors, the authors of future studies should (1) identify a patient population that may benefit from bTARTI prevention strategies (eg, prophylactic or suppressive inhaled antibiotic therapy for Pseudomonas colonization); (2) develop evidence-based guidelines for diagnosis and management of children with bTARTIs, including differentiation between acute infection and chronic colonization; and (3) develop interventions for specific populations (eg, younger patients, patients requiring chronic mechanical ventilation, and patients with neuromuscular, cardiovascular, or renal comorbidities) to minimize longer LOS and revisit risk.
Acknowledgments
We thank Sharis Shahnazarian, MPH, and Eugene Nguyen, BA, for their assistance with data management and Vivian Lee, MD, Margaret Trost, MD, and other members of the Children’s Hospital Los Angeles PHIS Research Group for providing thoughtful input on study design and data interpretation.
Footnotes
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Dr Russell is a KL2 Scholar awarded under the KL2 Mentoring Research Career Development Award through Southern California Clinical and Translational Science Institute at the University of Southern California, Keck School of Medicine. As part of his career development, Dr Russell was supported by the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through grant Award KL2TR000131. The content is solely the responsibility of the author(s) and does not necessarily represent the official view of the NIH. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
Dr Russell conceptualized the study, designed the study, and drafted the analytic plan and the initial manuscript; Drs Mamey and Schrager conducted the statistical analyses and revised the manuscript; Drs Koh, Neely, and Wu reviewed and critically revised the manuscript; and all authors approved the final manuscript as submitted.
References
- 1.Berry JG, Graham RJ, Roberson DW, et al. Patient characteristics associated with in-hospital mortality in children following tracheotomy. Arch Dis Child. 2010;95(9):703–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis CW, Carron JD, Perkins JA, Sie KC, Feudtner C. Tracheotomy in pediatric patients: a national perspective. Arch Otolaryngol Head Neck Surg. 2003;129(5):523–529 [DOI] [PubMed] [Google Scholar]
- 3.Zhu H, Das P, Roberson DW, et al. Hospitalizations in children with preexisting tracheostomy: a national perspective. Laryngoscope. 2015;125(2):462–468 [DOI] [PubMed] [Google Scholar]
- 4.Brook I. Treatment of aspiration or tracheostomy-associated pneumonia in neurologically impaired children: effect of antimicrobials effective against anaerobic bacteria. Int J Pediatr Otorhinolaryngol. 1996;35(2):171–177 [DOI] [PubMed] [Google Scholar]
- 5.Cline JM, Woods CR, Ervin SE, Rubin BK, Kirse DJ. Surveillance tracheal aspirate cultures do not reliably predict bacteria cultured at the time of an acute respiratory infection in children with tracheostomy tubes. Chest. 2012;141(3):625–631 [DOI] [PubMed] [Google Scholar]
- 6.Graf J, Stein F. Tracheitis in pediatric patients. Semin Pediatr Infect Dis. 2006;17(1):11–13 [DOI] [PubMed] [Google Scholar]
- 7.Rusakow LS, Guarín M, Wegner CB, Rice TB, Mischler EH. Suspected respiratory tract infection in the tracheostomized child: the pediatric pulmonologist’s approach. Chest. 1998;113(6):1549–1554 [DOI] [PubMed] [Google Scholar]
- 8.Russell CJ, Mack WJ, Schrager SM, Wu S. Care variations and outcomes for children hospitalized with bacterial tracheostomy-associated respiratory infections. Hosp Pediatr. 2017;7(1):16–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pati S, Lorch SA, Lee GE, Sheffler-Collins S, Shah SS. Health insurance and length of stay for children hospitalized with community-acquired pneumonia. J Hosp Med. 2012;7(4):304–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leyenaar JK, Lagu T, Shieh MS, Pekow PS, Lindenauer PK. Management and outcomes of pneumonia among children with complex chronic conditions. Pediatr Infect Dis J. 2014;33(9):907–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neuman MI, Hall M, Gay JC, et al. Readmissions among children previously hospitalized with pneumonia. Pediatrics. 2014;134(1):100–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pediatric Health information System. PHIS. Avaiable at: https://www.childrenshospitals.org/programs-and-services/data-analytics-and-research/pediatric-analytic-solutions/pediatric-health-information-system. Accessed December 14, 2017
- 13.Berry JG, Graham DA, Graham RJ, et al. Predictors of clinical outcomes and hospital resource use of children after tracheotomy. Pediatrics. 2009;124(2):563–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brogan TV, Hall M, Williams DJ, et al. Variability in processes of care and outcomes among children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31(10):1036–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feudtner C, Christakis DA, Connell FA. Pediatric deaths attributable to complex chronic conditions: a population-based study of Washington State, 1980-1997. Pediatrics. 2000;106(1 pt 2):205–209 [PubMed] [Google Scholar]
- 16.Barnhart DC, Hall M, Mahant S, et al. Effectiveness of fundoplication at the time of gastrostomy in infants with neurological impairment. JAMA Pediatr. 2013;167(10):911–918 [DOI] [PubMed] [Google Scholar]
- 17.Leyenaar JK, Lagu T, Shieh MS, Pekow PS, Lindenauer PK. Variation in resource utilization for the management of uncomplicated community-acquired pneumonia across community and children’s hospitals. J Pediatr. 2014;165(3):585–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edwards JD, Kun SS, Keens TG. Outcomes and causes of death in children on home mechanical ventilation via tracheostomy: an institutional and literature review. J Pediatr. 2010;157(6):955–959.e2 [DOI] [PubMed] [Google Scholar]
- 19.Com G, Kuo DZ, Bauer ML, et al. Outcomes of children treated with tracheostomy and positive-pressure ventilation at home. Clin Pediatr (Phila). 2013;52(1):54–61 [DOI] [PubMed] [Google Scholar]
- 20.Benneyworth BD, Gebremariam A, Clark SJ, Shanley TP, Davis MM. Inpatient health care utilization for children dependent on long-term mechanical ventilation. Pediatrics. 2011;127(6). Available at: www.pediatrics.org/cgi/content/full/127/6/e1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edwards JD, Houtrow AJ, Lucas AR, et al. Children and young adults who received tracheostomies or were initiated on long-term ventilation in PICUs. Pediatr Crit Care Med. 2016;17(8):e324–e334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kun SS, Edwards JD, Ward SL, Keens TG. Hospital readmissions for newly discharged pediatric home mechanical ventilation patients. Pediatr Pulmonol. 2012;47(4):409–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Millman AJ, Finelli L, Bramley AM, et al. Community-acquired pneumonia hospitalization among children with neurologic disorders. J Pediatr. 2016;173:188–195.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomson J, Hall M, Ambroggio L, et al. Aspiration and non-aspiration pneumonia in hospitalized children with neurologic impairment. Pediatrics. 2016;137(2):e20151612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ashkenazi-Hoffnung L, Ari A, Bilavsky E, Scheuerman O, Amir J, Prais D. Pseudomonas aeruginosa identified as a key pathogen in hospitalised children with aspiration pneumonia and a high aspiration risk. Acta Paediatr. 2016;105(12):e588–e592 [DOI] [PubMed] [Google Scholar]
- 26.Gerdung CA, Tsang A, Yasseen AS, III, Armstrong K, McMillan HJ, Kovesi T. Association between chronic aspiration and chronic airway infection with Pseudomonas aeruginosa and other gram-negative bacteria in children with cerebral palsy. Lung. 2016;194(2):307–314 [DOI] [PubMed] [Google Scholar]
- 27.McCaleb R, Warren RH, Willis D, Maples HD, Bai S, O’Brien CE. Description of respiratory microbiology of children with long-term tracheostomies. Respir Care. 2016;61(4):447–452 [DOI] [PubMed] [Google Scholar]
- 28.Russell CJ, Simon TD, Mamey MR, Newth CJL, Neely MN. Pseudomonas aeruginosa and post-tracheotomy bacterial respiratory tract infection readmissions. Pediatr Pulmonol. 2017;52(9):1212–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Filice GA, Drekonja DM, Thurn JR, Hamann GM, Masoud BT, Johnson JR. Diagnostic errors that lead to inappropriate antimicrobial use. Infect Control Hosp Epidemiol. 2015;36(8):949–956 [DOI] [PubMed] [Google Scholar]