Key Points
Question
Does selection and immortal-time bias influence the findings in studies of use of statin therapy that report reduced early cancer-specific and all-cause mortality in patients with recently diagnosed prostate, breast, colorectal, or bladder cancer?
Findings
In patients with cancer identified through the SEER-Medicare database, the hazard ratios of mortality were 1 in comparisons between those who began statin therapy and those who did not. Strong, apparently beneficial effects of statin therapy use estimated by previous observational studies are likely owing to selection bias and immortal-time bias, biases that can be prevented with an appropriate study design.
Meaning
An explicit target trial emulation helps avoid misleading estimates and found no evidence that initiation of statin therapy improves 3-year survival in patients with cancer.
Abstract
Importance
Patients with cancer who use statins appear to have a substantially better survival than nonusers in observational studies. However, this inverse association between statin use and mortality may be due to selection bias and immortal-time bias.
Objective
To emulate a randomized trial of statin therapy initiation that is free of selection bias and immortal-time bias.
Design, Setting, and Participants
We used observational data on 17 372 patients with cancer from the Surveillance, Epidemiology, and End Results (SEER)-Medicare database (2007-2009) with complete follow-up until 2011. The SEER-Medicare database links 17 US cancer registries and claims files from Medicare and Medicaid in 12 US states. We included individuals with a new diagnosis of colorectal, breast, prostate, or bladder cancer who had not been prescribed statins for at least 6 months before the cancer diagnosis. Individuals were duplicated, and each replicate was assigned to either the strategy “statin therapy initiation within 6 months after diagnosis” or “no statin therapy initiation.” Replicates were censored when they stopped following their assigned strategy, and the potential selection bias was adjusted for via inverse-probability weighting. Hazard ratios (HRs), cumulative incidences, and risk differences were calculated for all-cause mortality and cancer-specific mortality. We then compared our estimates with those obtained using the same analytic approaches used in previous observational studies.
Exposures
Statin therapy initiation within 6 months after cancer diagnosis.
Main Outcomes and Measures
Cancer-specific and all-cause mortality using SEER-Medicare data and data from previous studies.
Results
Of the 17 372 patients whose data were analyzed, 8440 (49%) were men, and 8932 (51%) were women (mean [SD] age, 76.4 [7.4] years; range, 66-115 years). The adjusted HR (95% CI) comparing statin therapy initiation vs no initiation was 1.00 (0.88-1.15) for cancer-specific mortality and 1.07 (0.93-1.21) for overall mortality. Cumulative incidence curves for both groups were almost overlapping (the risk difference never exceeded 0.8%). In contrast, the methods used by prior studies resulted in an inverse association between statin use and mortality (pooled hazard ratio 0.69).
Conclusion and Relevance
After using methods that are not susceptible to selection bias from prevalent users and to immortal time bias, we found that initiation of therapy with statins within 6 months after cancer diagnosis did not appear to improve 3-year cancer-specific or overall survival.
This SEER-Medicare database study of patients with colorectal, breast, prostate, or bladder cancer compares mortality between those who began therapy with statins within 6 months after cancer diagnosis and those who did not by emulating a trial free of selection bias.
Introduction
Patients with cancer who use statins seem to have a better survival within several months of diagnosis than nonusers. In 3 recent meta-analyses of observational studies of prostate,1 breast,2 and colorectal cancer,3 the cancer survival was 18% to 44% greater among statin users than among nonusers. The magnitude of this inverse association is stronger than the beneficial effect of statins on all-cause mortality (14%) in randomized trials of individuals without cancer,4 which has led to calls for further research.5
It is conceivable that the strong association between statin use and early survival in patients with cancer might be the result of a biologically beneficial effect of statins. For example, it has been hypothesized that statins may inhibit the proliferation of cancer cells.6 On the other hand, this association might also be explained by bias in the observational studies. At least 3 types of biases could explain the inverse association: (1) confounding due to incomplete adjustment by prognostic factors associated with statin therapy initiation and discontinuation after initiation, ie, healthy-user bias; (2) selection bias due to the inclusion of prevalent statin users at the start of follow-up, ie, at the time of cancer diagnosis; and (3) immortal-time bias due to defining statin users as those who survive during part of the follow-up.7 The possibility of the first type of bias (confounding) is inherent to observational studies. However, the other 2 biases (selection bias due to prevalent users8 and immortal-time bias) are the consequence of flaws in study design and analysis.
We explored whether the association between statin use and survival in patients with cancer may be explained by selection bias and immortal-time bias rather than by an unexpected beneficial effect of statins. We present estimates of the effect of statin therapy initiation on the survival of patients with cancer that are unaffected by these biases and compare our estimates with those obtained by naïve analyses likely affected by prevalent user bias and immortal-time bias.
Methods
Study Population
The Surveillance, Epidemiology, and End Results (SEER)-Medicare database is a linkage of patient demographic and tumor-specific variables collected by 17 SEER cancer registries across 12 states of the United States with claim files from the Centers for Medicare & Medicaid Services.9 For our analyses, cancer data were retrieved from the Patient Entitlement and Diagnosis Summary File (PEDSF) and linked to claims from the Outpatient (OUTSAF), Durable Medical Equipment (DME), Inpatient (ie, Medical Provider Analysis and Review [MEDPAR]), and National Claims History (NCH) files. All patient demographics, tumor features, and census tract features were retrieved from the PEDSF file.9 Diagnostic codes of comorbidities (hypertension,10 hyperlipidemia,10 anemia,11,12 depression,13 tobacco use,14 obesity,15 and a composite cardiovascular event of myocardial infarction, stroke, congestive heart failure, or peripheral vascular disease) as well as the number of preventive visits within the 2 years preceding cancer diagnosis were extracted from inpatient and outpatient claims. We calculated the Charlson-Klabunde index,16 not including cancer diagnoses, to quantify the comorbidity burden. Cancer-specific therapies were extracted from the NCH, OUTSAF, MEDPAR, and DME files. Data on statin medication was retrieved from the Prescription Drug Event file (PDE). All definitions and codes used are available in eTable 1 in the Supplement. The institutional review board of the Harvard T.H. Chan School of Public Health determined that the study met the criteria for exemption.
Eligibility Criteria
Our analyses include all microscopically confirmed cases of stages I to III colorectal, breast, prostate, and bladder cancer in individuals diagnosed with their first cancer at age 66 years or older who had complete information on stage in the SEER-Medicare database from 2007 to 2009. All individuals had been enrolled in Medicare, parts A and B, but not in a health maintenance organization (HMO), during at least 1 year and in part D at least 6 months before cancer diagnosis, and had not been prescribed statins for at least 6 months before diagnosis (since data on prescription were available from January 2007, the earliest date of cancer diagnosis in our study was July 1, 2007). We excluded individuals with cancer diagnoses from nursing homes, hospices, autopsy or death certificates, those who died within the same calendar month as the cancer diagnosis, and those with missing Charlson-Klabunde index at cancer diagnosis.
End Points and Follow-up
We considered 2 outcomes: cancer-specific mortality and all-cause mortality. (For exact definitions, see eTable 1 in the Supplement.) All patient records were followed from date of cancer diagnosis (baseline) until whichever of the following events occurred first: (1) death, (2) loss to follow-up (due to enrollment with an HMO or discontinuation of enrollment in Medicare, part A, B, or D), or (3) the administrative end of follow-up (December 31, 2011, for all-cause mortality and December 31, 2010, for cancer-specific mortality because cause-specific mortality was available only through 2010).
Treatment Strategies
We compared the following 2 strategies:
Initiation of therapy with any statin (simvastatin, atorvastatin, fluvastatin, rosuvastatin, lovastatin, pitavastatin, pravastatin, or combination therapy including any of these) at any dose within 6 months after cancer diagnosis. Individuals assigned to this strategy might discontinue statin therapy at any time when clinically indicated.
No initiation of statin therapy during the follow-up.
Statistical Analysis
We explicitly emulated a target trial of statin therapy initiation among patients with cancer using observational data from the SEER-Medicare database. To do so, we created a data set with 2 copies of each eligible individual at baseline and assigned each of the replicates to 1 of the treatment strategies.17 Replicates assigned to the statin strategy were artificially censored at 6 months if they had not initiated statin therapy by then. Replicates assigned to the no-statin strategy were censored if and when they initiated statin therapy at any time during the follow-up period. This approach avoided both selection bias due to the inclusion of prevalent statin users and immortal-time bias due to defining statin therapy initiation among survivors.17
We fit pooled logistic models for each outcome (cancer-specific and all-cause mortality) including an indicator for the statin strategy (initiation vs no initiation), month of follow-up (linear and quadratic term), and the following baseline covariates: sex, cancer stage, cancer site, race, year of diagnosis, marriage status, Charlson-Klabunde index, a composite measure of previous cardiovascular disease, tobacco use, obesity, depression, hyperlipidemia, hypertension, and anemia based on claims during the year before diagnosis. These variables were selected because they are potential confounders for the effect of statin initiation on survival. Additional adjustment for census tract features did not materially alter the results. Because the outcome of the models is rare at all times, the odds ratio from this model approximates the hazard ratio (HR).18
For the 2 outcomes, we also estimated absolute risks by fitting the pooled logistic models with product (“interaction”) terms between the strategy indicator and the month of follow-up variables. The models’ predicted values were then used to estimate the cumulative incidence of cancer-specific and all-cause death from baseline. The cumulative incidence curves were standardized to the baseline variables.19
Because the censoring required by our analytic approach has the potential to introduce selection bias due to postbaseline variables, we estimated inverse probability weights for the outcome models. We estimated the denominator of the weights in the original study population via 2 pooled logistic models for statin therapy initiation that included the baseline covariates, month of follow-up (linear and quadratic term), and the following postbaseline (time-varying) covariates: Charlson-Klabunde index (0, 1, 2, 3, 4, ≥5, or missing), tobacco use, obesity, depression, hypertension, anemia, surgery, radiotherapy, chemotherapy, and a composite measure of cardiovascular disease. The first model was restricted to the first 6 months of follow-up (eTable 2 in the Supplement), and the second model to subsequent months (eTable 2 in the Supplement). For the statin arm, we used the first model to estimate the probability of initiating therapy with statins at 6 months if it had not been initiated previously (ie, the probability of remaining uncensored at the last month); during the first 5 months, the probabilities were set to 1, and after month 6, the weights remained constant (ie, were multiplied by 1). See eTable 3 in the Supplement for month-specific construction of the weights. For the no statin arm, we used the first model to estimate the probability of not having initiated statin therapy during each of the first 6 months, and the second model for the remaining months. Weights were truncated at the 99.5th percentile. We used a nonparametric bootstrap with 500 samples to appropriately compute the 95% confidence intervals (CIs) for the HR and cumulative incidence estimates from the expanded data set. All analyses were conducted using SAS software, version 9.4 (SAS Institute Inc).
Comparison With Previous Observational Studies
We identified observational studies included in the most recently published meta-analyses of statin use in patients with prostate,1 breast,2 colorectal,3 or urological cancer.20 Eligible studies for our analysis reported HRs, or other measures of relative risk, of cancer-specific mortality or all-cause mortality for statin vs no statin use among patients with stages I to III cancer followed up from time of cancer diagnosis. Because of substantial heterogeneity across studies, these relative risk estimates cannot be pooled using a fixed-effect approach. We therefore pooled the study-specific estimates using a standard random-effects approach21 that estimates the average of the distribution of the estimates without imposing the assumption that they are all equal. These analyses were conducted using Stata 14 software (StataCorp LP). We classified these observational studies into 3 categories, as listed in Table 1.22,23,24,25,26,27,28,29,30,31,32,33,34
Table 1. Observational Studies of Statins and Mortality Among Patients With Cancer Included in Recent Meta-analyses1,2,3,20.
| Source | Publication Year | Cancer Location | HR (95% CI) | |
|---|---|---|---|---|
| Cancer-Specific Mortality | All-Cause Mortality | |||
| Category 1, Statin Users vs Nonusers During Follow-up (Risk of Immortal-Time Bias) | ||||
| Murtola et al22 | 2014 | Breast | 0.35 (0.28-0.45) | 0.39 (0.33-0.46) |
| Katz et al23 | 2010 | Prostate (radical prostatectomy) | NA | 0.35 (0.21-0.58) |
| Katz et al23 | 2010 | Prostate (radiation therapy) | NA | 0.59 (0.37-0.94) |
| Lakha et al24 | 2012 | Colorectal | 0.54 (0.19-1.50) | 0.61 (0.26-1.41) |
| Pooled estimate | NA | NA | 0.35 (0.27-0.44) | 0.39 (0.33-0.45) |
| Category 2, Prevalent Statin Users vs Nonusers at Cancer Diagnosis (Risk of Prevalent-User/Selection Bias) | ||||
| Geybels et al25 | 2013 | Prostate | 0.19 (0.06-0.56) | NA |
| Desai et al26 | 2015 | Breast | 0.91 (0.60-1.37) | NA |
| Brewer et al27 | 2013 | Breast | 0.95 (0.58-1.56) | 1.00 (0.63-1.60) |
| Caon et al28 | 2014 | Prostate | 0.77 (0.55-1.08) | NA |
| Lakha et al24 | 2012 | Colorectal | 0.60 (0.33-1.32) | 0.59 (0.28-1.24) |
| Nielsen et al29 | 2012 | All cancer forms | 0.85 (0.82-0.87) | 0.85 (0.83-0.87) |
| Siddiqui et al30 | 2009 | Colorectal | NA | 0.70 (0.60-0.90) |
| Shao et al31 | 2015 | Colorectal | 0.77 (0.68-0.88) | 0.82 (0.74-0.92) |
| Murtola et al22 | 2014 | Breast | 0.60 (0.46-0.77) | 0.58 (0.49-0.70) |
| da Silva et al32 | 2013 | Bladder | 1.04 (0.84-1.28) | NA |
| Hoffmeister et al33 | 2015 | Colorectal | 1.11 (0.82-1.50) | 1.10 (0.85-1.41) |
| Pooled estimate | NA | NA | 0.77 (0.64-0.89) | 0.78 (0.67-0.90) |
| Category 3, Mixed Prevalent and Incident Statin Users vs Nonusers (Risk of Prevalent-User/Selection Bias) | ||||
| Desai et al26 | 2015 | Breast | 0.59 (0.32-1.06) | NA |
| Chan et al34 | 2015 | Prostate | NA | 0.84 (0.71-0.99) |
| Pooled estimate | NA | NA | 0.59 (0.32-1.06) | 0.84 (0.71-0.99) |
| Overall pooled estimate | NA | NA | 0.73 (0.58-0.88) | 0.69 (0.55-0.84) |
Abbreviations: HR, hazard ratio; NA, not applicable.
The first category included studies that classified individuals with a cancer diagnosis into 2 groups: those who used statin therapy at some point during the follow-up and those who did not (category 1 in Table 1). The comparison of these 2 groups may introduce immortal-time bias.17,35,36 We conducted similar analyses in our data by classifying eligible individuals as initiators if they initiated statin therapy anytime during the first 6 months of follow-up and as noninitiators otherwise. In separate analyses, we varied the definition of initiators as those who initiated statin therapy during the first 12, 18, or 24 months, or at any time during the follow-up. That is, we did not duplicate individuals and assign each replicate to one of the strategies, but rather classified individuals as initiators or not at baseline based on postbaseline statin therapy initiation within different time frames. We then fitted unweighted pooled logistic models identical to the ones described above to estimate the HRs.
The second category of studies (Table 1) compared current (ie, prevalent) statin users vs nonusers at cancer diagnosis. The comparison of these 2 groups may introduce selection bias.37 To replicate this analysis in our data, we eliminated the exclusion criterion “no previous claim of statin use” and classified individuals at baseline as “current users” or “nonusers.” Then, we fitted unweighted pooled logistic models to estimate the HRs.
Finally, the third category of observational studies compared a mixture of prevalent users at cancer diagnosis plus new users during the follow-up vs nonusers.
Results
Patients From the SEER-Medicare Database
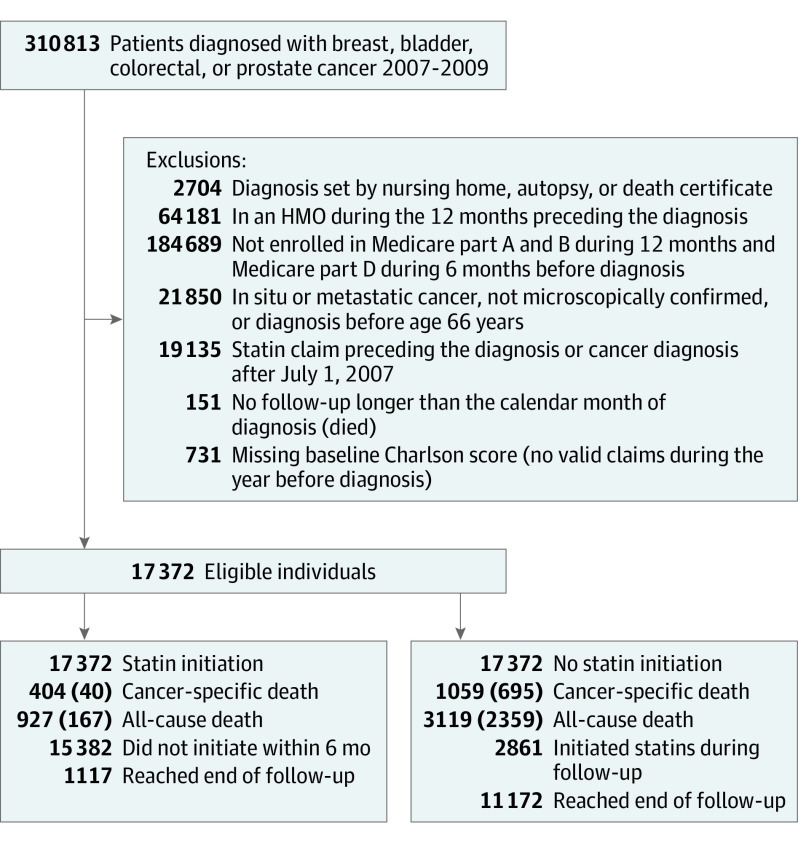
There were 17 372 eligible individuals (Figure 1) with a median (interquartile range) of 33 (25-42) months of follow-up for all-cause mortality (maximum follow-up, 52 months) and 23 (15-31) months for cancer-specific mortality (maximum follow-up, 39 months). Of these, 1825 (10.5%) were censored owing to loss of follow-up. Table 2 summarizes the distribution of baseline variables.
Figure 1. Flowchart of Eligible Individuals, SEER-Medicare 2007-2009.
Numbers in parentheses represent unique deaths. HMO indicates health maintenance organization; SEER, Surveillance, Epidemiology, and End Results database.
Table 2. Characteristics of the 17 372 Eligible Individuals, SEER-Medicare 2007-2009a.
| Characteristic | All Eligible Individuals Month 0 | Statin Initiators Month 5 | Statin Noninitiators Month 5 |
|---|---|---|---|
| Total No. | 17 372 | 897 | 15 328 |
| Cancer location | |||
| Colon | 4201 (24) | 192 (21) | 3491 (23) |
| Breast | 6053 (35) | 289 (32) | 5547 (36) |
| Prostate | 6071 (35) | 367 (41) | 5471 (36) |
| Bladder | 1047 (6) | 49 (5) | 819 (5) |
| Stage | |||
| 1 | 5196 (30) | 252 (28) | 4656 (30) |
| 2 | 9618 (55) | 534 (60) | 8501 (55) |
| 3 | 2558 (15) | 111 (12) | 2171 (14) |
| Sex | |||
| Male | 8440 (49) | 481 (54) | 7419 (48) |
| Female | 8932 (51) | 416 (46) | 7909 (52) |
| Race | |||
| White | 14 799 (85) | 750 (84) | 13 055 (85) |
| Black | 1582 (9) | 76 (8) | 1345 (9) |
| Other | 1057 (6) | 71 (8) | 928 (6) |
| Marriage status | |||
| Single | 1635 (10) | 93 (10) | 1404 (9) |
| Married | 8318 (48) | 449 (50) | 7458 (49) |
| Divorced | 103 (1) | Maskedb | 97 (1) |
| Widowed | 1383 (8) | 66 (7) | 1206 (8) |
| Unmarried or domestic partner | 4582 (26) | 225 (25) | 3948 (26) |
| Unknown | 1351 (8) | Maskedb | 1215 (8) |
| Year of diagnosis | |||
| 2007 | 2752 (16) | 185 (21) | 2342 (15) |
| 2008 | 7823 (45) | 403 (45) | 6948 (45) |
| 2009 | 6797 (39) | 309 (34) | 6038 (39) |
| Charlson-Klabunde index | |||
| 0 | 11 352 (65) | 525 (59) | 10 245 (67) |
| 1 | 3712 (21) | 212 (23) | 3219 (21) |
| 2 | 1345 (7) | 87 (9) | 1121 (7) |
| 3 | 494 (3) | 36 (4) | 398 (3) |
| 4 | 274 (2) | 20 (2) | 207 (1) |
| ≥5 | 195 (1) | 17 (2) | 138 (1) |
| Comorbidities | |||
| Tobacco use | 307 (2) | 19 (2) | 266 (2) |
| Obesity | 308 (2) | 22 (2) | 262 (2) |
| Depression | 838 (5) | 53 (6) | 698 (4) |
| Hyperlipidemia | 4238 (24) | 373 (42) | 3648 (24) |
| Hypertension | 9016 (52) | 530 (59) | 7812 (51) |
| Anemia | 2458 (14) | 138 (15) | 2025 (13) |
| Cardiovascular disease | 2127 (12) | 133 (15) | 1720 (11) |
Abbreviation: SEER, Surveillance, Epidemiology, and End Results database.
Unless otherwise indicated, data are reported as number (percentage) of study patients.
Masked because counts fewer than 11 cannot be specified according to the data user agreement.
Of 932 individuals who initiated statin therapy within 6 months after cancer diagnosis, 447 (48%) had at least 1 statin prescription during their last year of follow-up. Of the 16 440 individuals who did not initiate statin therapy within 6 months after diagnosis, 14 511 (88%) did not initiate statin therapy later during the follow-up. Statin therapy initiation was more likely in those with hyperlipidemia, hypertension, prior cardiovascular events, and prostate cancer diagnosis (eTable 2 in the Supplement).
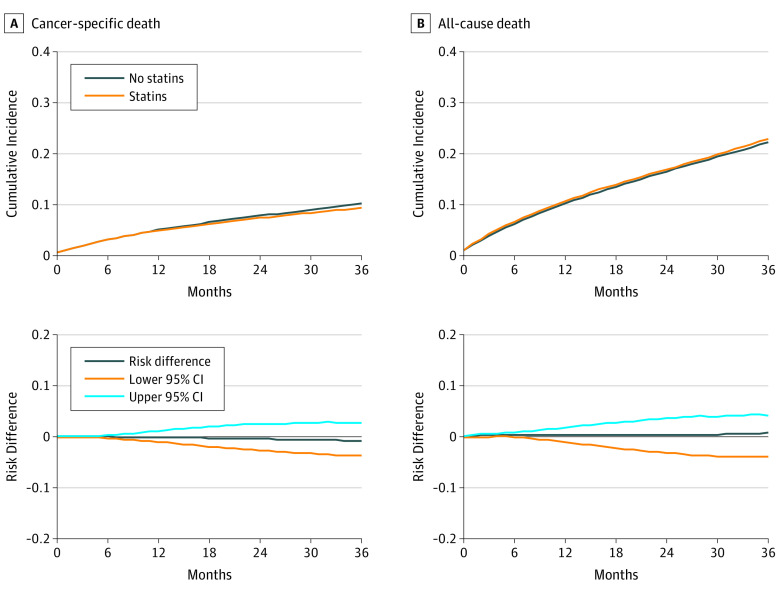
A total of 1099 individuals died of cancer during follow-up. The HR (95% CI) of cancer-specific mortality for statin therapy initiation vs no initiation was 0.94 (0.89-1.00) in the absence of any adjustment, 0.96 (0.90-1.02) after adjustment for baseline covariates, and 1.00 (0.88-1.15) after adjustment for baseline and postbaseline covariates. The fully adjusted 3-year risk of cancer death was 10.2% in noninitiators and 9.4% in initiators; the risk difference was −0.8% (−3.7% to 2.7%).
Altogether, 3286 individuals died over the follow-up period. The HR (95% CI) of all-cause mortality for initiation vs no initiation of statin therapy was 1.00 (0.96-1.05) in the absence of any adjustment, 1.02 (0.97-1.07) after adjustment for baseline covariates, and 1.07 (0.93-1.21) after adjustment for baseline and postbaseline covariates. The fully adjusted 3-year risk of death was 22.3% in noninitiators and 23.0% in initiators; the risk difference was 0.7% (−4.0 to 4.1).
In 2734 individuals who died before 2011 (when specific cause of death was available), the recorded primary cause of death was colorectal cancer in 22%, breast cancer in 10%, prostate cancer in 4%, bladder cancer in 9%, other cancers in 8%, cardiovascular disease in 21%, and chronic obstructive pulmonary disease or pneumonia in 12%. The cumulative incidence curves for cancer-specific and all-cause death are presented in Figure 2.
Figure 2. Three-Year Cumulative Incidence and Risk Differences, SEER-Medicare 2007-2009.
SEER indicates Surveillance, Epidemiology, and End Results database.
Comparison With Previous Observational Studies
In contrast with our null estimates, the pooled estimates from previous studies showed an association between statin use and survival (Table 1). Similarly, we also found an association when we replicated the analytic approach of previous studies.
When the eligible individuals were classified in the statin therapy initiation group if they ever initiated statin in the first 6 months of follow-up (similar to the analyses used in category 1 studies of Table 1), the HRs (95% CIs) of cancer-specific mortality for initiation vs no initiation were 0.62 (0.45-0.85) in the absence of any adjustment, and 0.67 (0.49-0.93) after adjustment for baseline covariates. The HRs (95% CIs) of all-cause mortality were 0.83 (0.71-0.97) in the absence of any adjustment and 0.86 (0.74-1.01) after adjustment for baseline covariates. The HR estimates became stronger (ie, further from 1) as the postbaseline period used to define statin therapy initiation increased (Table 3), which supports the existence of immortal-time bias.
Table 3. Mortality HRs for Statin Initiators vs Noninitiators When Statin Initiation Is Defined Within Increasingly Longer Periods Since Baseline, SEER-Medicare 2007-2009a.
| Time From Baseline of Statin Initiation, mo | Cancer-Specific Death | All-Cause Death | ||
|---|---|---|---|---|
| Unadjusted | Adjustedb | Unadjusted | Adjustedb | |
| 0-6 | 0.62 (0.45-0.85) | 0.67 (0.49-0.93) | 0.83 (0.71-0.97) | 0.86 (0.74-1.01) |
| 0-12 | 0.49 (0.38-0.65) | 0.56 (0.42-0.73) | 0.71 (0.63-0.81) | 0.75 (0.66-0.86) |
| 0-18 | 0.37 (0.28-0.48) | 0.43 (0.33-0.56) | 0.62 (0.55-0.69) | 0.67 (0.60-0.76) |
| 0-24 | 0.30 (0.23-0.39) | 0.36 (0.28-0.47) | 0.56 (0.50-0.63) | 0.62 (0.55-0.69) |
| Ever during follow-up | 0.25 (0.20-0.33) | 0.31 (0.24-0.40) | 0.51 (0.46-0.57) | 0.57 (0.51-0.63) |
Abbreviations: HR, hazard ratio; SEER, Surveillance, Epidemiology, and End Results database.
All data reported as HR (95% CI).
Adjusted for; sex, cancer stage, cancer site, race, year of diagnosis, marriage status, Charlson-Klabunde index and a composite measure of previous cardiovascular disease.
When we compared the 17 458 prevalent statin users with the 17 372 nonusers at baseline (similar to the analyses used in category 2 of Table 1), the HRs (95% CIs) of cancer-specific mortality for prevalent users vs nonusers were 0.76 (0.69-0.83) in the absence of any adjustment and 0.83 (0.76-0.91) after adjustment for baseline covariates. The corresponding HRs (95% CIs) of all-cause mortality were 0.86 (0.82-0.90) and 0.83 (0.79-0.87).
Discussion
Our findings suggest that statin therapy initiation after cancer diagnosis does not improve the early survival of patients with cancer. In an analysis that was susceptible to neither selection bias nor immortal-time bias, the HRs were essentially null. This finding is consistent with the results from previous meta-analyses of randomized trials, which did not find an effect of statins on cancer incidence or survival.38,39
In contrast, analyses of our data inspired by previously published studies resulted in surprisingly strong HR estimates. A causal interpretation of these estimates as showing the existence of a large benefit of statins is unwarranted because these analyses are likely affected by selection bias and immortal-time bias. In particular, we found a clear trend toward an apparently more beneficial effect of statins (up to a 69% reduction in cancer-specific mortality) as the period used to define statin therapy initiation, and therefore the potential for immortal-time bias, increased.
Our analysis of the observational data explicitly emulated a (hypothetical) target trial of statin therapy initiation in patients with cancer. To do so, we set time zero of our analysis to correspond with time zero in the target trial, that is, the time when both (1) eligibility criteria are met and (2) treatment strategies are assigned. In contrast, previous observational studies used a time zero that violated condition 2. Some studies classified individuals as statin users based on statin use that took place before time zero (prevalent users), which may result in selection bias. Other studies assigned the treatment strategies based on information that was not available at time zero. This approach effectively ensures (1) that those defined as statin therapy initiators have a guaranteed period of survival (ie, an immortal time) between baseline and initiation and (2) that early deaths occurring before statin therapy initiation has been considered will be assigned to the noninitiators group.
Limitations
Limitations of our study include a relatively short follow-up period, which prevents studying the effect of statin therapy initiation beyond 3 years after cancer diagnosis. The study was also limited by the use of data based on diagnostic codes, which may introduce measurement error in the definition of initiation and discontinuation of statin therapy and in clinical diagnoses, and the possibility of residual confounding. However, none of these limitations affect the main conclusion of our analysis.
Conclusions
In summary, when applying a methodological approach that minimizes immortal-time and selection bias, we found no suggestion that initiation of statin therapy after cancer diagnosis improves the early survival in patients with stages I though III cancer. We hope that our analysis will contribute to the prevention of self-inflicted biases in future observational research using real world data.
eAppendix. Inclusion criteria and codes from the PEDSF-file
eTable 1. Codes used to define the outcome and the covariates
eTable 2. Parameter estimates from regression model for statin treatment initiation
eTable 3. Construction of inverse probability weights
References
- 1.Meng Y, Liao YB, Xu P, Wei WR, Wang J. Statin use and mortality of patients with prostate cancer: a meta-analysis. Onco Targets Ther. 2016;9:1689-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manthravadi S, Shrestha A, Madhusudhana S. Impact of statin use on cancer recurrence and mortality in breast cancer: a systematic review and meta-analysis. Int J Cancer. 2016;139(6):1281-1288. [DOI] [PubMed] [Google Scholar]
- 3.Gray RT, Coleman HG, Hughes C, Murray LJ, Cardwell CR. Statin use and survival in colorectal cancer: results from a population-based cohort study and an updated systematic review and meta-analysis. Cancer Epidemiol. 2016;45:71-81. [DOI] [PubMed] [Google Scholar]
- 4.Chou R, Dana T, Blazina I, Daeges M, Jeanne TL. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316(19):2008-2024. [DOI] [PubMed] [Google Scholar]
- 5.Caporaso NE. Statins and cancer-related mortality—let’s work together. N Engl J Med. 2012;367(19):1848-1850. [DOI] [PubMed] [Google Scholar]
- 6.Spampanato C, De Maria S, Sarnataro M, et al. Simvastatin inhibits cancer cell growth by inducing apoptosis correlated to activation of Bax and down-regulation of BCL-2 gene expression. Int J Oncol. 2012;40(4):935-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lévesque LE, Hanley JA, Kezouh A, Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ. 2010;340:b5087. [DOI] [PubMed] [Google Scholar]
- 8.Danaei G, Tavakkoli M, Hernán MA. Bias in observational studies of prevalent users: lessons for comparative effectiveness research from a meta-analysis of statins. Am J Epidemiol. 2012;175(4):250-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8)(suppl):IV-3-IV-18. [DOI] [PubMed] [Google Scholar]
- 10.Black L, Runken MC, Eaddy M, Shah M. Chronic disease prevalence and burden in elderly men: an analysis of Medicare medical claims data. J Health Care Finance. 2007;33(4):68-78. [PubMed] [Google Scholar]
- 11.Herzog CA, Muster HA, Li S, Collins AJ. Impact of congestive heart failure, chronic kidney disease, and anemia on survival in the Medicare population. J Card Fail. 2004;10(6):467-472. [DOI] [PubMed] [Google Scholar]
- 12.Sandgren PE, Murray AM, Herzog CA, et al. Anemia and new-onset congestive heart failure in the general Medicare population. J Card Fail. 2005;11(2):99-105. [DOI] [PubMed] [Google Scholar]
- 13.Spettell CM, Wall TC, Allison J, et al. Identifying physician-recognized depression from administrative data: consequences for quality measurement. Health Serv Res. 2003;38(4):1081-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CCW Task Order 10 Inter-Agency Panel working document and input from subject matter experts. https://ccwdata.org/cs/groups/public/documents/document/clin_cond_refer.pdf. Accessed August 15, 2016.
- 15.Hedley Dodd A, Gleason P. Using the MAX-NHANES Merged Data to Evaluate the Association of Obesity and Medicaid Costs [2013]. https://www.cms.gov/Research-Statistics-Data-and-Systems/Computer-Data-and-Systems/MedicaidDataSourcesGenInfo/Downloads/MAX_IB16_MAX_NHANES.PDF. Accessed August 15, 2016.
- 16.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258-1267. [DOI] [PubMed] [Google Scholar]
- 17.Hernán MA, Sauer BC, Hernández-Díaz S, Platt R, Shrier I. Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses. J Clin Epidemiol. 2016;79:70-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson WA Jr. On the treatment of grouped observations in life studies. Biometrics. 1977;33(3):463-470. [PubMed] [Google Scholar]
- 19.Hernan M, Robins J. Causal Inference. Boca Raton, FL: Chapman & Hall/CRC; 2017. [Google Scholar]
- 20.Luo Y, She DL, Xiong H, Fu SJ, Yang L. The prognostic effect of statin use on urologic cancers: an updated meta-analysis of 35 observational studies. Medicine (Baltimore). 2015;94(36):e1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188. [DOI] [PubMed] [Google Scholar]
- 22.Murtola TJ, Visvanathan K, Artama M, Vainio H, Pukkala E. Statin use and breast cancer survival: a nationwide cohort study from Finland. PLoS One. 2014;9(10):e110231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katz MS, Carroll PR, Cowan JE, Chan JM, D’Amico AV. Association of statin and nonsteroidal anti-inflammatory drug use with prostate cancer outcomes: results from CaPSURE. BJU Int. 2010;106(5):627-632. [DOI] [PubMed] [Google Scholar]
- 24.Lakha F, Theodoratou E, Farrington SM, et al. Statin use and association with colorectal cancer survival and risk: case control study with prescription data linkage. BMC Cancer. 2012;12:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geybels MS, Wright JL, Holt SK, Kolb S, Feng Z, Stanford JL. Statin use in relation to prostate cancer outcomes in a population-based patient cohort study. Prostate. 2013;73(11):1214-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desai P, Lehman A, Chlebowski RT, et al. Statins and breast cancer stage and mortality in the Women’s Health Initiative. Cancer Causes Control. 2015;26(4):529-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brewer TM, Masuda H, Liu DD, et al. Statin use in primary inflammatory breast cancer: a cohort study. Br J Cancer. 2013;109(2):318-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caon J, Paquette M, Hamm J, Pickles T. Does statin or ASA affect survival when prostate cancer is treated with external beam radiation therapy? Prostate Cancer. 2014;2014(4):184297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med. 2012;367(19):1792-1802. [DOI] [PubMed] [Google Scholar]
- 30.Siddiqui AA, Nazario H, Mahgoub A, Patel M, Cipher D, Spechler SJ. For patients with colorectal cancer, the long-term use of statins is associated with better clinical outcomes. Dig Dis Sci. 2009;54(6):1307-1311. [DOI] [PubMed] [Google Scholar]
- 31.Shao YY, Hsu CH, Yeh KH, et al. Statin use is associated with improved prognosis of colorectal cancer in Taiwan. Clin Colorectal Cancer. 2015;14(3):177-184.e4. [DOI] [PubMed] [Google Scholar]
- 32.da Silva RD, Xylinas E, Kluth L, et al. Impact of statin use on oncologic outcomes in patients with urothelial carcinoma of the bladder treated with radical cystectomy. J Urol. 2013;190(2):487-492. [DOI] [PubMed] [Google Scholar]
- 33.Hoffmeister M, Jansen L, Rudolph A, et al. Statin use and survival after colorectal cancer: the importance of comprehensive confounder adjustment. J Natl Cancer Inst. 2015;107(6):djv045. [DOI] [PubMed] [Google Scholar]
- 34.Chan JM, Kenfield SA, Paciorek A, Platz EA, Giovannucci EL, Stampfer MJ. Postdiagnostic statin use and the risk of lethal prostate cancer in the health professionals follow-up study. Cancer Epidemiol Biomarkers Prev. 2015;24(10):1638-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suissa S, Dell’aniello S, Vahey S, Renoux C. Time-window bias in case-control studies: statins and lung cancer. Epidemiology. 2011;22(2):228-231. [DOI] [PubMed] [Google Scholar]
- 36.Hernández-Díaz S. Name of the bias and sex of the angels. Epidemiology. 2011;22(2):232-233. [DOI] [PubMed] [Google Scholar]
- 37.Hernán MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol. 2016;183(8):758-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dale KM, Coleman CI, Henyan NN, Kluger J, White CM. Statins and cancer risk: a meta-analysis. JAMA. 2006;295(1):74-80. [DOI] [PubMed] [Google Scholar]
- 39.Emberson JR, Kearney PM, Blackwell L, et al. ; Cholesterol Treatment Trialists’ (CTT) Collaboration . Lack of effect of lowering LDL cholesterol on cancer: meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS One. 2012;7(1):e29849. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Inclusion criteria and codes from the PEDSF-file
eTable 1. Codes used to define the outcome and the covariates
eTable 2. Parameter estimates from regression model for statin treatment initiation
eTable 3. Construction of inverse probability weights